Abstract
The endometrium is one of the most dynamic organs in the human body. Until now, cell lines have furthered the understanding of endometrial biology and associated diseases, but they failed to recapitulate the key physiological aspects of the endometrium, especially as it relates to its complex architecture and functions. Organoid culture systems have become an alternative approach to reproduce biological functions of tissues in vitro. Endometrial organoids have now been established from stem/progenitor cells and/or differentiated cells by several methods, which represents a promising tool to gain a deeper understanding of this dynamic organ. In this review, we will discuss the establishment, characteristics, applications, and potential challenges and directions of endometrial organoids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endometrium
The endometrium is a complex tissue that lines the inside of the uterine cavity. It is morphologically divided into the functionalis and basal layers. The functionalis layer contributes to two thirds of the endometrial thickness, and it comprises of several cell types including luminal and glandular epithelial cells, stromal cells, immune cells, and vascular cells forming the spiral arterioles [1,2,3]. In reproductive-aged women, the endometrium follows a precisely programmed series of morphologic and physiologic events under the control of the sex steroid hormones and paracrine-secreted molecules from neighboring cells during the menstrual cycle [4]. It is characterized by growth, secretory differentiation, and in the absence of conception, degeneration, and regeneration. Proliferation, secretion, and degeneration are confined to the upper functionalis layer, while the regenerative capacity of this organ lies in the basal layers [1, 5]. This highly dynamic tissue is cyclically shed, repaired, regenerated, and remodeled. Any dysregulation in these processes can lead to the development of various types of endometrial associated disorders which affect the health and quality of life for a considerable number of women. Those include infertility, pregnancy disorders, endometriosis, and endometrial cancers as well as other endometrial pathologies such as adenomyosis, endometrial hyperplasia, and a thin unresponsive endometrium [6,7,8,9,10]. Although much is known about the pathologies of these diseases, the molecular and the cellular mechanisms associated with these disorders are still not resolved. Thus, dissecting the cellular and molecular mechanisms involved in both physiological and pathological conditions of the endometrium could help to understand this dynamic organ and its associated diseases as well aid in the development of new therapeutics.
To study the cellular and molecular mechanisms related to endometrial biology, both appropriate in vivo and in vitro models are indispensable. For in vivo studies, the non-human primates and rodents have been used most extensively [11, 12]. Non-human primates offer a physiologically relevant model and provide an opportunity to study the transformative processes that are unique to menstruating species [13]. But the high cost, technical skillset, and required infrastructure are the major limitations associated with the use of non-human primate models [14]. Rodent species, which permit tissue-specific gene manipulation, are advantageous and are commonly used experimental models for studies on endometrial receptivity and embryo implantation [15,16,17,18]. Rodent models have been instrumental in contributing to our understanding of endometrial biology [19,20,21,22]. However, these models cannot accurately recapitulate all the characteristics of human endometrial development and function. Take decidualization as an example. In primates the endometrium undergoes spontaneous decidualization in a cyclic manner [13], whereas in rodents decidualization of the endometrium occurs only in the presence of an implanting embryo or in response to mechanical injury [23]. Thus, findings obtained with rodent models often cannot be directly translated to humans. Two-dimensional (2D) monolayer cells cultures are used for in vitro studies in most instances of cell biology research. Primary endometrial stromal cells such as human uterine fibroblasts (HuF) cells are used extensively for in vitro decidualization studies [24,25,26]. However, primary cells do not expand in long-term culture, and endometrial biopsies obtained are limited in size. Primary endometrial epithelial cells grown in monolayer cultures do not passage efficiently and easily and lose their columnar phenotype and polarity in culture. Immortalized endometrial cell lines, such as Ishikawa cells (derived from endometrial cancer epithelial cells) [27, 28] or 12Z cells (derived from endometriotic epithelial cells) [29] are easily cultured for long periods, but these cell lines do not faithfully mimic the in vivo situation, in particular because of their transformed phenotype. Furthermore, the architecture and cell-to-cell interactions that occur in three dimensions are lost in 2D monolayer cultures, resulting in distorted cell function and cell phenotype. Taken together, these limitations encouraged biologists to find a more suitable model system of the human endometrium. The emergence of three-dimensional (3D) organoid culture systems has enabled researchers to study endometrial cells in a context much closer to normal physiology.
What Is an Organoid?
The techniques of 3D cell culture are boosted by a deeper understanding of materials that have been developed mainly related to extracellular matrix (ECM) biology and a bioengineering approach. The terminology “organoid” has previously been used for a range of 3D culture systems that resemble the modelled organ. The organoid was initially defined as an in vitro 3D cellular cluster derived from stem/progenitor cells, embryonic stem cells (ESCs), or induced pluripotent stem cells (iPSCs) capable of self-renewal and self-organization that recapitulates the function of the tissue of origin [30,31,32,33]. The definition was extended by combining differentiated cells, often epithelial cells, or including other types of cells [34,35,36]. Many organoids contain both differentiated cells and tissue-specific stem or progenitor cells [37]. In vivo, cells are in complex microenvironments and are subject extensive signaling communications and interactions which are key in establishing, maintaining, and regulating cellular phenotypes and functions [33]. Conversely, interactions are limited to the horizontal plane in 2D monolayer cultures, and cells are exposed to a uniform concentration of factors because of direct contact with the culture medium. These examples partly explain the failure of 2D cell culture systems to recapitulate drug screening outcomes that are observed in vivo [33, 38, 39]. Compared with the 2D cell culture system, the cells in the 3D organoid culture system more closely resemble architectural and functional characteristics of in vivo tissues. Thus, organoids represent a promising model system that may bridge the gap between 2D culture and in vivo models [40]. They provide a reductionist model of in vivo biology which makes it possible to study tissue function in diverse contexts and to conduct high-throughput screens, drug testing, and biobanking [41].
Endometrial Organoids
Establishment of Endometrial Organoids
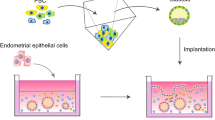
Endometrial organoids (EO), as the name suggests, are self-organizing 3D aggregations of endometrial cells that represent the structure and function of endometrium. According to the number of cell types included, endometrial organoids can be divided into two categories: monocellular endometrial organoids and multicellular endometrial organoids (Fig. 1).
Establishment of endometrial organoids. Monocellular endometrial organoid is developed by embedding endometrial epithelial cells in Matrigel[34, 35]. EEO forms a glandular-like organization with apicobasal polarized columnar epithelium. Multicellular endometrial organoids are developed by co-culturing stromal cells and EEO fragments in the porous collagen scaffolds [42]. The organoid shows apicobasal polarized epithelial cells lining the outer surface surrounded by stromal cells. Multicellular endometrial organoids can be also developed by seeding both epithelial and stromal cells into micro-molded agarose gel plate [43]. The organoid exhibits distinct organization with polarized epithelial cells lining the outer surface and stromal cells in the center of the organoid. Images are created with BioRender. EEO endometrial epithelial organoid
Monocellular Endometrial Organoids
The monocellular endometrial organoid is developed by embedding endometrial epithelial cells or progenitor/stem cells in Matrigel [34, 35, 44, 45]. The first monocellular organotypic model of the endometrium can be traced back to 1986[46]. Endometrial gland fragments were isolated and embedded within a collagen gel which resulted in glandular structures consisting of a single, polarized layer of epithelial cells which could respond to steroid hormone treatments [46]. Subsequently, in 1988, monocellular endometrial organoid models began to take shape. Rinehart et al. [47] developed a method by suspending gland fragments in Matrigel and cultured in Matrigel precoated dishes. The cells eventually formed glandular structures consisting of polarized columnar epithelial cells surrounding a lumen. These organoid structures could produce secretory vesicles and could be passaged for up to 6 months. However, what was first considered as true endometrial organoid were published independently by Turco et al. and Boretto et al. in 2017 (Fig. 1) [34, 35]. The protocol using Matrigel was established as follows. Briefly, the tissue samples were minced and dissociated by collagenase and mechanical agitation. Following centrifugation, washing, and filtration, the glandular pellets were gently resuspended in a mix of 70% Matrigel in medium. The suspension was plated in pre-warmed 48-well plates and after solidification, culture medium containing growth factors and other components were added (Table 1). Medium was refreshed every 2–3 days, and organoids were enzymatically dissociated and passaged every 7–10 days. For freezing organoids, Matrigel was removed using cell recovery solution, and organoids were resuspended in recovery cell culture freezing medium and then frozen down following a cell freezing protocol. This protocol is suitable for single-cell types, especially for endometrial epithelial cells, because the primary endometrial epithelial cells are very difficult to maintain in primary culture and their polarity is lost in 2D monolayer cultures. Recently, this protocol was also successfully used to develop trophoblast organoids with villous cytotrophoblasts from placental tissues to recapitulate the developmental program of the early human placenta [36, 48].
Multicellular Endometrial Organoids
Both epithelial and stromal cells are the main components of endometrium. Generation of more complex endometrial organoids including both cell types may enable the study of stromal–epithelial interactions and communication and their roles in endometrial biological functions. The first 3D endometrial culture system combining endometrial epithelial and stromal cells was established by Bentin-Ley et al. [49]. In this model, endometrial stromal cells were embedded in a collagen matrix and separated from the endometrial epithelial cells by Matrigel. The epithelial cells when grown on top of the Matrigel had a polarized columnar epithelial phenotype and displayed functional characteristics. Another organotypic culture system consisting of both epithelial and stromal cells was developed in 2005 [50]. In this study, endometrial stromal cells were grown on the plate in monolayer while epithelial cells were cultured as organoids within Matrigel in tissue culture inserts. This model was useful to study the effects of steroids on epithelial cell proliferation in the presence or absence of stromal cells. However, the first complete endometrial organoid containing both epithelial and stromal cells was generated by Wiwatpanit et al. in 2020 (Fig. 1) [43]. One unique feature of this endometrial organoid system is that no exogenous basement membrane matrix was used but endometrial organoids were generated in micro-molded agarose gel instead. Briefly, both epithelial and stromal cells were isolated from endometrial tissues and then resuspended in MammoCultTM growth medium at similar densities. Epithelial and stromal cells were mixed at a 3:1 ratio by volume and 50 uL of epithelial-stromal cell suspension was seeded into 1.5% (w/v) micro-molded agarose gel. Cells were cultured in MammoCultTM growth medium for at least 7 days to allow organoid formation prior to further treatment. These organoids exhibited distinct organization with polarized epithelial cells lining the outer surface and stromal cells in the center of the organoids. The scaffold-free multicellular endometrial organoids hold promise for studying endometrial processes and diseases where the crosstalk between epithelial and stromal cells occurs.
Soon after another group [42] published a method to develop multicellular endometrial organoids based on the Matrigel endometrial epithelial organoids (EEO). They used 3D porous collagen scaffolds produced with controlled lyophilization to direct cellular organization of both EEO and stromal cells [42]. Briefly, the process was as follows. Firstly, porous collagen scaffolds were produced by lyophilization. The internal pore structure of the scaffold was optimized for stromal cells with an optimal average pore size of 101 μm. Next, the stromal cells and EEO were co-cultured in scaffolds. Scaffolds were first seeded with stromal cells by the following process. Specifically, the scaffolds were cut with an 8-mm biopsy punch and sectioned into 750-μm thick slices. Each scaffold was submerged in Eppendorf tube using 250 μl Advanced DMEM/F-12 containing 500,000 stromal cells. The tube was placed in a hybridizer oven at 37 °C for 45 min with continuous rotation. The scaffolds were transferred to a 24-well plate, covered with 1 ml of stromal cell medium, and were incubated for 2 days. Subsequently, scaffolds were washed and EEO fragments were seeded on the center of each scaffold and cultured up to 10 days. EEO organized to form a luminal-like epithelial layer on the surface of the scaffold. Epithelial cells polarized with their apical surface facing the pore cavities and their basal surface attached to the scaffold. It was also demonstrated that both cell types could be easily removed from the scaffold for downstream analysis. This model can potentially be used to study stromal–epithelial interactions.
Collectively, each of the three methods has its own advantages. The Matrigel culture system has been the most extensively used, and it is suitable for epithelial biology study and bio-banking. The porous collagen scaffolds combined with the Matrigel culture system can be used to study stromal–epithelial interactions. The micro-molded agarose gel culture system does not require any exogenous scaffold materials, and different cell types can specifically organize themselves. In the future, the complexity of endometrial organoids could be eventually expanded to integrate with other cell types, including endothelial, immune, and myometrial cells to truly mimic human endometrium physiology.
Characterization of Endometrial Organoids (EO)
EOs Express Specific Makers of the Original Tissue
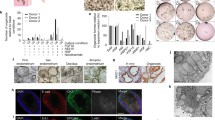
The EOs generated with endometrial epithelium strongly expressed epithelial cell markers (such as E-cadherin, EPCAM, FOXA2, MUC1) and steroid hormone receptors like estrogen receptor α (ERα) [34, 35]. In the organoids which combined epithelial and stromal cells [43], E-cadherin, pan-Cytokeratin, and FOXA2 were found on the periphery of the organoids, while cells in the center were positive for the stromal cell marker vimentin.
EOs Reveal a Glandular-like Morphology
The mouse EOs showed a lumen surrounded by an epithelial (pan Cytokeratin and E-cadherin positive) layer. These cells were polarized in an apicobasal manner with the microvilli towards the lumen and mucus in the lumen [34]. Similarly, human EOs generated from the endometrium [34, 35] formed a glandular-like organization with apicobasal polarized columnar epithelium having microvilli as well as cilia in the apical side. A major component of endometrial glandular secretions, glycogen, was visualized in the lumen of EOs. In the porous collagen scaffolds combined with the Matrigel culture system, the epithelial cells polarized with their apical surface carrying microvilli and cilia that face the pore cavities and their basal surface attaching to the scaffold with the formation of extracellular matrix proteins [42]. In the micro-molded agarose gel culture system when epithelial cells were cultured with stromal cells, organoids exhibited distinct organization with epithelial cells lining the outer surface and stromal cells in the center of the organoids [43]. Epithelial cells were polarized with nuclei localized at the basolateral end of the cell closest to the stroma and could secrete mucins towards outside, while the stromal cells in the center of the endometrial organoid could secrete collagen [43].
EOs Show Physiological Responses to Hormone Treatment
In mouse EOs, in response to estradiol (E2), the number of proliferating cells increased and E2 responsive markers (such as Epidermal growth factor, Insulin-like growth factor 1 and lactoferrin) were significantly upregulated [34]. Treatment with progesterone (P) downregulated Estrogen receptor 1 (ESR1) expression in organoids as it does in vivo. Mouse EOs, transplanted under the kidney capsule of ovariectomized immunodeficient nonobese diabetic/severe combined immunodeficiency (NOD/SCID) Gamma mice, expanded and assembled into an organized structure with glandular type protuberances when treated with E2 and P[34]. The results described above demonstrate that mouse EOs are responsive to hormonal regulation in a manner that resembles the behavior of the endometrial epithelium in vivo. The human EOs also respond to steroid hormones to mimic the menstrual cycle [34]. Characteristics of the proliferative phase such as increased epithelial cell proliferation and expression of specific markers such as thyrotropin-releasing hormone (TRH) are reproduced in the EOs after E2 treatment. Subsequent P exposure induces features of the secretory phase, as occurs in vivo, including enhanced folding and tortuosity, formation of columnar epithelium with subnuclear vacuolation, mucus production, ciliogenesis, downregulation of ERα, and expression of secretory phase-specific markers such as progestogen-associated endometrial protein (PAEP). Finally, hormone withdrawal triggers a phenotype that is reminiscent of the menstrual phase, including disruption of tissue structure and shedding of dying cells. Moreover, Turco et al. [35] found that high expression of both ERα and progesterone receptor (PGR) in most organoids after exposure to E2 and P and the addition of cyclic adenosine monophosphate (cAMP) treatment could enhance the expression of differentiation markers such as PAEP and Human Osteopontin (SPP1). Moreover, human EOs could be further stimulated by differentiation medium containing E2+P+cAMP, chorionic gonadotropin, and human placental lactogen prolactin or conditioned media from decidualized stromal cells in vitro, which mimic signals from the placenta and decidualized stroma in vivo.
In terms of the development of EOs with epithelial and stromal cells in the micro-molded agarose gel [43], only a small portion of organoids displayed distinct organization in the absence of hormones. In most cases, without hormones, stromal and epithelial cells were intermingled randomly within the organoids. However, when organoids were exposed to stepwise E2 and testosterone treatments mimicking the follicular phase of the menstrual cycle, the majority of the organoids exhibited distinct structural architecture with a clear demarcation of epithelial and stromal layers. The findings demonstrated the important role that hormones play in promoting the organization of the organoids containing both epithelial and stromal cells. While in the porous collagen scaffolds with co-culturing both epithelial and stromal cells on the scaffold, hormone stimulation resulted in epithelial differentiation and stromal cell decidualization [42]. Collectively, both monocellular and multicellular EOs are physiologically responsive to hormones.
EOs Recapitulate Genomic Signatures of the Tissue of Origin
The global gene expression profiles were analyzed to assess the similarity between organoids and the tissue of origin [35]. The global gene expression profiles were compared between the established organoid lines, initial glandular digests, and cultured stromal cells from the same biopsy. Hierarchical clustering analysis based on 15,475 probes showed that the organoid cultures cluster more closely to glands than to stroma, confirming their glandular epithelial nature. The genomic characteristics of EOs were also explored by Fitzgerald et al. [51] highlighting the composition of epithelial cell types. They performed a comprehensive analysis of EEO under the influence of E2 and P in an unbiased approach using both bulk RNA-seq and single-cell RNA-seq technologies. Importantly, the organoids expressed similar gene patterns compared with that of uterine glands and consisted of the different epithelial cell types found in the in vivo endometrium.
In summary, EOs closely recapitulate the molecular and functional characteristics of the tissue of origin and serve as an effective in vitro model system for studying the endometrium.
Applications of Endometrial Organoids
Organoids allow cell-cell and ECM interactions in three dimensions and can more accurately recapitulate the structural and the functional properties of the in vivo tissue compared to 2D monolayer cell culture. EOs have provided an alternate physiologic model of the endometrium that can be adapted to the investigation of endometrial physiology and pathology in vitro.
Endometrial Development
The human endometrium, which is mainly composed of endometrial stromal and epithelial cells, undergoes dynamic cyclic tissue remodeling which is critical for reproduction. Our knowledge of its molecular and cellular regulation has been ascertained primarily from in vivo studies, primarily due to the lack of appropriate in vitro models. This is especially true with regard to our knowledge about the function of epithelial cells in vitro since they are limited in their ability to robust expansion in monolayer cell culture. The ability to reliably produce, maintain, and expand physiologically relevant epithelial organoids from the human endometrium opens exciting new avenues for endometrial biology research. The endometrial epithelium consists of secretory and ciliated epithelial cells and how changes during the menstrual cycle control the balance between the two cell types remains unclear. EOs were used by Haider et al. [52] to dissect the molecular mechanisms underlying the generation of ciliated cells. They found that generation of ciliated cells in the endometrial epithelium was orchestrated by the coordinated action of E2 and NOTCH signaling. E2 was the primary driver, but the inhibition of NOTCH signaling provided a permissive environment; however, this alone was not sufficient to trigger ciliogenesis. This study proposed that EOs are a powerful model for studying ciliogenesis in vitro. EOs were also applied to study progenitor/stem cells in regulating glandular stemness and differentiation during uterine gland development. Seishima et al. [44] identified the role of Wnt-dependent, Lgr5-expressing stem/progenitor cells in the developing uterus. They first sorted the single EPCAM-positive cells from Lgr5-2A-EGFP mice and cultured them following the established Matrigel organoid culture protocol. The cells efficiently generated organoids that exhibited a spherical phenotype and could be continually passaged in defined Rspo1/Wnt3a-supplemented media. Next, organoids were cultured from EPCAM–positive cells isolated from the uterus of Lgr5-DTR-EGFP mice which expresses an Lgr5postive-cell-driven Diphtheria toxin (DT) receptor-EGFP fusion gene. The addition of DT ablates the resident Lgr5-positive cells. A complete inhibition of organoid outgrowth following DT treatment was observed. Coincidentally, Syed and colleagues [45] employed the same method to study Axin2, a classical Wnt reporter gene, expressed in epithelial cells to regulate homeostasis and regeneration. They found that Axin2-expressing glandular cells could form fully functional EOs and ablation of Axin2 positive cells impaired endometrial regeneration. These studies suggest that the endometrial organoid culture system is a powerful tool in conjunction with transgenic mouse models to understand uterine development.
Endometrium-Embryo Crosstalk
In women the average chance of pregnancy is only 15% per cycle during their reproductive lifespan [53]. Of the pregnancies that are lost, 75% fail to implant and are not recognized clinically [54]. A successful implantation requires a competent blastocyst, a receptive endometrium, and a precise dialogue between them during a specific window of time [55]. Until now, most of the knowledge on mechanisms underlining these processes have been derived from animal models [17], but these findings cannot always be transferred to humans. Therefore, the development of an in vitro model of a receptive endometrium is essential. Luddi et al. [56] validated endometrial organoids as a suitable 3D model to study the epithelial endometrial interface for embryo implantation. They showed that EEOs developed in the Matrigel culture system responded to hormones by recapitulating morphological and ultrastructural features of the different phases of the menstrual cycle. Interestingly, EEOs mirrored the early secretory phase showing the development of pinopodes, a reliable marker of the implantation window and the expression of PAEP, a cycle-dependent marker of the endometrial receptivity. In addition, mechanosensitive ion channels are considered as one of the molecules involved in the early communication process between the blastocyst and receptive endometrium. Hennes et al. [57] found that EEOs retain a comparable ion channel expression pattern as observed in primary endometrial epithelial cells. Mechanical and chemical stimulation of EEOs induced strong calcium responses. Collectively, the present results from both studies support the fact that endometrial organoids can recapitulate the molecular and functional characteristics of the receptive endometrium, which suggest that endometrial organoids could be feasibly used to develop a 3D in vitro model for the investigation of embryo-endometrial interactions. These studies are a prerequisite for the improvement of assisted reproduction outcomes and for understanding the causes of early pregnancy loss.
A Model for Studying Extracellular Vesicles in Endometrial Biology
Extracellular vesicles (EVs) are a heterogeneous mixture of membranous structures released from cells and contain proteins, lipids, and RNAs of the cells of origin [58, 59]. They are taken up by distant cells, where they can affect cell function and behavior. Intercellular communication through EVs seems to be involved in both normal physiology and pathological conditions [60]. In the female reproductive tract, EVs act as vehicles for embryo-endometrial cross-talk and have potential roles in regulating pathological conditions [61, 62]. EVs recovered from uterine flushings and a cervical brush express endometrial makers like steroid hormone receptors and PAEP, express transcripts associated with the endometrium, and recapitulate the variability in gene expression during the menstrual cycle [63]. This study suggests that EVs from liquid biopsies can be used identify expression profiles in endometrial tissues. Given the roles of EVs in reproductive biology, an in vitro system to model EV-mediated cell communication is needed. Studies have shown that EVs isolated from 3D cultures proved to have higher similarity to the EVs isolated from patient plasma compared with the same cancer cells cultured as 2D monolayers [64, 65]. In addition, Ke et al. [66] demonstrated the ability of organoids to incorporate and respond to EVs. They demonstrated that human gastric organoids could acquire a neoplastic phenotype after exposure to tumor-derived EVs. These studies suggest that organoids could be utilized for studying EVs secretion and uptake. Interestingly, EO culture systems integrated with EVs could offer a new opportunity for exploring the role of intercellular communication in endometrial biology in vitro.
Disease Modeling
Endometriosis
Patient-derived EOs can be developed for modeling endometriosis, which is characterized by the presence of endometrial-like tissue outside of the uterine cavity [67]. Endometriosis affects between 10 and 15% of all women of reproductive age, 70% of women with chronic pelvic pain, and 20–50% women with infertility [68, 69]. Current treatments provide only a temporary but not permanent cure primarily as a result of limited information of its etiology and pathogenesis. Boretto et al. [70] first established long-term expandable organoids from patient-matched eutopic endometrium and ectopic lesions from each of the different clinical stages (I−IV) based on the American Society for Reproductive Medicine revised staging system. Compared wtih organoids from eutopic and control endometrium, ectopic organoids also exhibited ERα and PGR expression, microvilli, and cilia directed towards the lumen and secreted mucus into the lumen. However, ectopic organoids contained a thicker lumen-bordering cell layer with stratified epithelium and displayed epithelial cell invasion that was not observed in normal organoids. In addition, ectopic organoids could generate implants that reproduce endometriotic features by both subrenal transplantation and peritoneal cavity injection in the NOD/SCID mice. Furthermore, ectopic organoids showed endometriosis-associated traits and cancer-linked mutations. They exhibited altered pathways and biological terms that have been previously associated with endometriotic patient samples. Signaling pathways such as integrin, PI3K−AKT, WNT and Hippo, ECM-receptor interactions, hormonal responses genes, and adhesion and invasion pathways showed altered expression levels compared with the organoids from the healthy endometrium. Finally, endometrial cancer-associated driver genes such as KRAS were identified in ectopic organoids from advanced stages of endometriosis. More recently, another group developed organoids from the eutopic endometrium of patients with endometriosis, and the organoids expressed the endometrial receptivity maker PAEP in a manner similar as that in endometrial tissues of patients [56]. The findings from both studies suggest that organoids developed in a Matrigel culture system recapitulates endometriosis heterogeneity and maintains key features of the primary tissue. Thus, endometriotic organoid biobanks will be valuable in deciphering disease-specific pathogenesis. Epithelial-stromal interactions and the crosstalk between the endometrial cells and immune cells are proposed to play important roles in endometriosis development but the underlying mechanisms are still far from understood[19, 71]. Recently, Wendel et al. developed a 3D biofabrication model of endometriosis and the endometriotic microenvironment [72]. In these 3D biofabricated constructs, the heterotypic spheroids composed of 12Z and T-HESC, an immortalized endometrial stromal cell line, self-assembled into a biologically relevant pattern, consisting of epithelial cells on the outside of the spheroids and stromal cells in the core. This study provides another novel model to study the complex interactions of multiple cell types within a biologically relevant microenvironment which promotes the development of endometriosis. Wiwatpanit et al. [43] and Abbas et al.[42] consecutively developed organoids containing both epithelial and stromal compartments for studying endometriosis. By taking advantage of the multicellular organoid culture systems, researchers have the ability to study the role of cell-cell interactions and communication in lesion development in vitro. Cell invasion is one of the key processes that are involved in endometriosis development and progression. Currently, wound healing assays and transwell assays of a single-cell type are still the primary methods to study invasion of endometrial cells in vitro. In future studies it may be possible to develop a 3D endometrial organoid invasion model based on multicellular organoid culture systems and thus more accurately simulate the cell invasion process that occurs in vivo. Collectively, endometrial organoids could be very helpful for dissecting the mechanisms associated with endometriosis development and evaluating the efficacy of therapeutic agents for endometriosis.
Endometrial Cancer
Endometrial cancer is one of most common gynecological cancers [73]. The clinical management involves surgical resection with chemotherapy and/or adjuvant radiotherapy, but the cancer often recurs [74]. The underlying pathobiology remains largely unknown, and therapeutic efficiency and overall survival rates are not substantially improving, mainly due to a lack of reliable preclinical models to study the disease [70, 75]. Turco et al. have successfully derived organoids from samples of endometrial cancer and the normal adjacent endometrium from post-menopausal women [35]. These organoids recapitulated the morphology of the primary tumor (FIGO Grade I Endometrioid Cancer) showing pleomorphic cells with hyperchromatic nuclei and disorganized epithelium, supporting the idea that this model recapitulates the histological organization and phenotype of the endometrial cancer. In addition, this model allows for the comparison of the endometrial cancer tissue to the normal adjacent endometrium providing an isogenic control tissue, without the biological “noise” that could result from the variability of an individual’s genetic background [35]. Recently, Boretto et al. [70] derived organoids from patients with low-to-high–grade endometrial cancers. Interestingly, these organoids accurately captured the heterogeneity and mutational landscape of the tumors, recapitulated disease phenotype, and displayed patient-specific drug responses. These endometrial cancer-derived organoids may be used in the future to build a biobank for drug screening and investigating the mutational changes and could possibly replace the use of patient derived xenografts (PDX) mouse models and decrease the overall costs associated with in vivo models.
Polycystic Ovary Syndrome
Polycystic ovary syndrome (PCOS) is a complex multifactorial disorder in 5–20% reproductive aged women and is associated with a number of endocrine and metabolic complications, including the increased risk of endometrial cancer [76]. It is believed that the endometrium of PCOS women becomes hyperplastic due to the absence of a complete menstrual cycle resulting from oligo or anovulation [77]. But it remains unclear as to how risk factors, like PCOS, affect the normal endometrium and contribute towards neoplastic transformation [43]. Recently, Wiwatpanit and colleagues established scaffold-free EOs comprising of epithelial and stromal cells from human endometrial tissues [43]. These organoids exhibited functional characteristics of the normal endometrium in response to follicular phase E2 and testosterone. Treatment with hormones (excess androgens mimicking PCOS) increased cell proliferation and dysregulated genes associated with proliferation and migration in EOs. By taking advantage of this scaffold-free endometrial organoid, they revealed new mechanisms of PCOS-associated risk of endometrial neoplasia.
In summary, studies described above suggest that organoids developed from tissues associated with endometrial diseases can recapitulate disease diversity and provide promising research models for deciphering disease pathogenesis and drug screening.
Future Directions, Challenges, and Conclusions
In the past few years, EOs have been developed from monocellular to multicellular model systems. They allow the researchers the possibility for studying endometrial development and modeling diseases in vitro. There are some promising avenues for the use of EOs for future studies. One avenue is the ability to genetically modify the cells, which is a significant benefit compared with the cost, time, and energy required to create transgenic mouse models, for example. The EOs can be manipulated genetically through various methods such as CRISPR-Cas9. The CRISPR-Cas9 technology has a series of applications including DNA base editing, RNA targeting, epigenome editing, and gene expression manipulation [78, 79]. Previous studies have successfully used CRISPR-Cas9 technology to edit the genome in organoids [78]. However, the efficiency of genome editing in organoids is still not ideal due to experimental limitations. Thus, it is necessary to find ways to select and enrich positive organoids after CRISPR-Cas9 editing. Recently, Ringel et al. developed a genome-wide pooled-library CRISPR screen approach by capturing single-guide RNA integrations in human intestinal organoids to dissect oncogenic signaling pathways [80]. This screening method would be applicable to EOs and enable researchers to improve the efficacy to understand the genetic basis and the molecular mechanisms associated with the pathology of endometrial diseases. Another avenue for the use of EOs is the possibility to screen for markers of endometrial diseases and identify novel therapeutic targets. EOs from diseased tissues offer a powerful model for drug screening and toxicology testing. Indeed, patient-specific-derived EOs may be a potential model for personalized drug screening and efficacy predication before treating the patient.
There are some obstacles that still need to be resolved regarding the efficacy of EOs. Firstly, a common obstacle in organoid research is to overcome the variability and improve the utility; secondly, to develop a culture system that can integrate other cell types that are present in the uterus, including immune, endothelial, and myometrial cells; and, thirdly, to mimic the special features of the endometrium in organoids in vitro, since the endometrium has specialized anatomical features including the basalis and functionalis layer.
In conclusion, EOs are opening new avenues for endometrial research and providing excellent opportunities to study the endometrium in an unprecedented manner, which will accelerate our understanding of the molecular and cellular mechanisms involved in endometrial development and disease. EOs will be promising tools for a wide range of biomedical applications, from disease modeling to personalized medicine. Although there remain significant challenges related to the development of a complex endometrial organoid which truly mimics endometrial physiology, there should be optimism that close multidisciplinary collaboration between biologists, bioengineers, and clinicians will certainly promote further understanding as to how this dynamic tissue functions.
Availability of Data and Material (Data Transparency)
Not applicable.
Code Availability (Software Application or Custom Code)
Not applicable.
References
Ferenczy A, Bergeron C. Histology of the human endometrium: from birth to senescence. Ann N Y Acad Sci. 1991;622:6–27.
David L, Keefe KPW. Chapter 2 – Reproductive Physiology. General Gynecology. 2007:21–41.
Hawkins SM, Matzuk MM. The menstrual cycle: basic biology. Ann N Y Acad Sci. 2008;1135:10–8.
Ruiz-Alonso M, Blesa D, Simón C. The genomics of the human endometrium. Biochim Biophys Acta. 2012;1822(12):1931–42.
Evans J, Salamonsen LA, Winship A, Menkhorst E, Nie G, Gargett CE, et al. Fertile ground: human endometrial programming and lessons in health and disease. Nat Rev Endocrinol. 2016;12(11):654–67.
Ulukus M, Cakmak H, Arici A. The role of endometrium in endometriosis. J Soc Gynecol Investig. 2006;13(7):467–76.
Senturk LM, Erel CT. Thin endometrium in assisted reproductive technology. Curr Opin Obstet Gynecol. 2008;20(3):221–8.
Benagiano G, Brosens I. The endometrium in adenomyosis. Womens Health (Lond). 2012;8(3):301–12.
Kyo S, Sato S, Nakayama K. Cancer-associated mutations in normal human endometrium: Surprise or expected? Cancer Sci. 2020.
Lessey BA, Lebovic DI, Taylor RN. Eutopic endometrium in women with endometriosis: ground zero for the study of implantation defects. Semin Reprod Med. 2013;31(2):109–24.
Stouffer RL, Woodruff TK. nonhuman primates: a vital model for basic and applied research on female reproduction, prenatal development, and women’s health. ILAR J. 2017;58(2):281–94.
Lim HJ, Wang H. Uterine disorders and pregnancy complications: insights from mouse models. J Clin Invest. 2010;120(4):1004–15.
Ochoa-Bernal MA, Fazleabas AT. Physiologic Events of Embryo Implantation and Decidualization in Human and Non-Human Primates. Int J Mol Sci. 2020;21(6).
Song Y, Joshi NR, Vegter E, Hrbek S, Lessey BA, Fazleabas AT. Establishment of an immortalized endometriotic stromal cell line from human ovarian endometrioma. Reprod Sci. 2020.
Zuberi A, Lutz C. Mouse models for drug discovery. Can new tools and technology improve translational power? ILAR J. 2016;57(2):178–85.
Fellmann C, Gowen BG, Lin PC, Doudna JA, Corn JE. Cornerstones of CRISPR-Cas in drug discovery and therapy. Nat Rev Drug Discov. 2017;16(2):89–100.
Matsumoto H. Molecular and cellular events during blastocyst implantation in the receptive uterus: clues from mouse models. J Reprod Dev. 2017;63(5):445–54.
Wu SP, Emery OM, DeMayo FJ. Molecular studies on pregnancy with mouse models. Curr Opin Physiol. 2020;13:123–7.
Rubel CA, Jeong JW, Tsai SY, Lydon JP, Demayo FJ. Epithelial-stromal interaction and progesterone receptors in the mouse uterus. Semin Reprod Med. 2010;28(1):27–35.
Filant J, Spencer TE. Endometrial glands are essential for blastocyst implantation and decidualization in the mouse uterus. Biol Reprod. 2013;88(4):93.
Vasquez YM, Wang X, Wetendorf M, Franco HL, Mo Q, Wang T, et al. FOXO1 regulates uterine epithelial integrity and progesterone receptor expression critical for embryo implantation. PLoS Genet. 2018;14(11):e1007787.
Wang X, Li X, Wang T, Wu SP, Jeong JW, Kim TH, et al. SOX17 regulates uterine epithelial-stromal cross-talk acting via a distal enhancer upstream of Ihh. Nat Commun. 2018;9(1):4421.
Finn CA, Martin L. Endocrine control of the timing of endometrial sensitivity to a decidual stimulus. Biol Reprod. 1972;7(1):82–6.
Kim JJ, Jaffe RC, Fazleabas AT. Comparative studies on the in vitro decidualization process in the baboon (Papio anubis) and human. Biol Reprod. 1998;59(1):160–8.
Strakova Z, Reed J, Ihnatovych I. Human transcriptional coactivator with PDZ-binding motif (TAZ) is downregulated during decidualization. Biol Reprod. 2010;82(6):1112–8.
Strug MR, Su RW, Kim TH, Jeong JW, Fazleabas A. The notch family transcription factor, RBPJκ, modulates glucose transporter and ovarian steroid hormone receptor expression during decidualization. Reprod Sci. 2019;26(6):774–84.
Nishida M, Kasahara K, Kaneko M, Iwasaki H, Hayashi K. Establishment of a new human endometrial adenocarcinoma cell line, Ishikawa cells, containing estrogen and progesterone receptors. Nihon Sanka Fujinka Gakkai Zasshi. 1985;37(7):1103–11.
Nishida M. The Ishikawa cells from birth to the present. Hum Cell. 2002;15(3):104–17.
Zeitvogel A, Baumann R, Starzinski-Powitz A. Identification of an invasive, N-cadherin-expressing epithelial cell type in endometriosis using a new cell culture model. Am J Pathol. 2001;159(5):1839–52.
Schutgens F, Verhaar MC, Rookmaaker MB. Pluripotent stem cell-derived kidney organoids: an in vivo-like in vitro technology. Eur J Pharmacol. 2016;790:12–20.
Leibel SL, McVicar RN, Winquist AM, Niles WD, Snyder EY. Generation of complete multi-cell type lung organoids from human embryonic and patient-specific induced pluripotent stem cells for infectious disease modeling and therapeutics validation. Curr Protoc Stem Cell Biol. 2020;54(1):e118.
Naumovska E, Aalderink G, Wong Valencia C, Kosim K, Nicolas A, Brown S, et al. Direct on-chip differentiation of intestinal tubules from induced pluripotent stem cells. Int J Mol Sci. 2020;21(14).
Prior N, Inacio P, Huch M. Liver organoids: from basic research to therapeutic applications. Gut. 2019;68(12):2228–37.
Boretto M, Cox B, Noben M, Hendriks N, Fassbender A, Roose H, et al. Development of organoids from mouse and human endometrium showing endometrial epithelium physiology and long-term expandability. Development. 2017;144(10):1775–86.
Turco MY, Gardner L, Hughes J, Cindrova-Davies T, Gomez MJ, Farrell L, et al. Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat Cell Biol. 2017;19(5):568–77.
Turco MY, Gardner L, Kay RG, Hamilton RS, Prater M, Hollinshead MS, et al. Trophoblast organoids as a model for maternal-fetal interactions during human placentation. Nature. 2018;564(7735):263–7.
Deane JA, Cousins FL, Gargett CE. Endometrial organoids: in vitro models for endometrial research and personalized medicine. Biol Reprod. 2017;97(6):781–3.
Breslin S, O'Driscoll L. The relevance of using 3D cell cultures, in addition to 2D monolayer cultures, when evaluating breast cancer drug sensitivity and resistance. Oncotarget. 2016;7(29):45745–56.
Hwang TJ, Carpenter D, Lauffenburger JC, Wang B, Franklin JM, Kesselheim AS. Failure of investigational drugs in late-stage clinical development and publication of trial results. JAMA Intern Med. 2016;176(12):1826–33.
Li M, Izpisua Belmonte JC. Organoids - preclinical models of human disease. N Engl J Med. 2019;380(6):569–79.
Kim J, Koo BK, Knoblich JA. Human organoids: model systems for human biology and medicine. Nat Rev Mol Cell Biol. 2020:1–14.
Abbas Y, Brunel LG, Hollinshead MS, Fernando RC, Gardner L, Duncan I, et al. Generation of a three-dimensional collagen scaffold-based model of the human endometrium. Interface Focus. 2020;10(2):20190079.
Wiwatpanit T, Murphy AR, Lu Z, Urbanek M, Burdette JE, Woodruff TK, et al. Scaffold-free endometrial organoids respond to excess androgens associated with polycystic ovarian syndrome. J Clin Endocrinol Metab. 2020;105(3).
Seishima R, Leung C, Yada S, Murad KBA, Tan LT, Hajamohideen A, et al. Neonatal Wnt-dependent Lgr5 positive stem cells are essential for uterine gland development. Nat Commun. 2019;10(1):5378.
Syed SM, Kumar M, Ghosh A, Tomasetig F, Ali A, Whan RM, et al. Endometrial Axin2(+) cells drive epithelial homeostasis, regeneration, and cancer following oncogenic transformation. Cell Stem Cell. 2020;26(1):64–80.e13.
Kirk D, Alvarez RB. Morphologically stable epithelial vesicles cultured from normal human endometrium in defined media. In Vitro Cell Dev Biol. 1986;22(10):604–14.
Rinehart CA Jr, Lyn-Cook BD, Kaufman DG. Gland formation from human endometrial epithelial cells in vitro. In Vitro Cell Dev Biol. 1988;24(10):1037–41.
Haider S, Meinhardt G, Saleh L, Kunihs V, Gamperl M, Kaindl U, et al. Self-renewing trophoblast organoids recapitulate the developmental program of the early human placenta. Stem Cell Reports. 2018;11(2):537–51.
Bentin-Ley U, Pedersen B, Lindenberg S, Larsen JF, Hamberger L, Horn T. Isolation and culture of human endometrial cells in a three-dimensional culture system. J Reprod Fertil. 1994;101(2):327–32.
Blauer M, Heinonen PK, Martikainen PM, Tomas E, Ylikomi T. A novel organotypic culture model for normal human endometrium: regulation of epithelial cell proliferation by estradiol and medroxyprogesterone acetate. Hum Reprod. 2005;20(4):864–71.
Fitzgerald HC, Dhakal P, Behura SK, Schust DJ, Spencer TE. Self-renewing endometrial epithelial organoids of the human uterus. Proc Natl Acad Sci U S A. 2019;116(46):23132–42.
Haider S, Gamperl M, Burkard TR, Kunihs V, Kaindl U, Junttila S, et al. Estrogen signaling drives ciliogenesis in human endometrial organoids. Endocrinology. 2019;160(10):2282–97.
Hjollund NH, Jensen TK, Bonde JP, Henriksen TB, Andersson AM, Kolstad HA, et al. Spontaneous abortion and physical strain around implantation: a follow-up study of first-pregnancy planners. Epidemiology. 2000;11(1):18–23.
Wilcox AJ, Weinberg CR, O'Connor JF, Baird DD, Schlatterer JP, Canfield RE, et al. Incidence of early loss of pregnancy. N Engl J Med. 1988;319(4):189–94.
Guzeloglu-Kayisli O, Basar M, Arici A. Basic aspects of implantation. Reprod BioMed Online. 2007;15(6):728–39.
Luddi A, Pavone V, Semplici B, Governini L, Criscuoli M, Paccagnini E, et al. Organoids of human endometrium: a powerful in vitro model for the endometrium-embryo cross-talk at the implantation site. Cells. 2020;9(5).
Hennes A, Held K, Boretto M, De Clercq K, Van den Eynde C, Vanhie A, et al. Functional expression of the mechanosensitive PIEZO1 channel in primary endometrial epithelial cells and endometrial organoids. Sci Rep. 2019;9(1):1779.
Burns G, Brooks K, Wildung M, Navakanitworakul R, Christenson LK, Spencer TE. Extracellular vesicles in luminal fluid of the ovine uterus. PLoS One. 2014;9(3):e90913.
van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213–28.
Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478).
Simon C, Greening DW, Bolumar D, Balaguer N, Salamonsen LA, Vilella F. Extracellular vesicles in human reproduction in health and disease. Endocr Rev. 2018;39(3):292–332.
Kurian NK, Modi D. Extracellular vesicle mediated embryo-endometrial cross talk during implantation and in pregnancy. J Assist Reprod Genet. 2019;36(2):189–98.
Luddi A, Zarovni N, Maltinti E, Governini L, Leo V, Cappelli V, et al. Clues to non-invasive implantation window monitoring: isolation and characterisation of endometrial exosomes. Cells. 2019;8(8).
Villasante A, Marturano-Kruik A, Ambati SR, Liu Z, Godier-Furnemont A, Parsa H, et al. Recapitulating the size and cargo of tumor exosomes in a tissue-engineered model. Theranostics. 2016;6(8):1119–30.
Thippabhotla S, Zhong C, He M. 3D cell culture stimulates the secretion of in vivo like extracellular vesicles. Sci Rep. 2019;9(1):13012.
Ke X, Yan R, Sun Z, Cheng Y, Meltzer A, Lu N, et al. Esophageal adenocarcinoma-derived extracellular vesicle MicroRNAs induce a neoplastic phenotype in gastric organoids. Neoplasia. 2017;19(11):941–9.
Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362(25):2389–98.
Practice committee of the american society for reproductive medicine. Treatment of pelvic pain associated with endometriosis. Fertil Steril 2008;90(5 Suppl):S260-S269.
Endometriosis and infertility: a committee opinion. Fertil Steril. 2012;98(3):591-8.
Boretto M, Maenhoudt N, Luo X, Hennes A, Boeckx B, Bui B, et al. Patient-derived organoids from endometrial disease capture clinical heterogeneity and are amenable to drug screening. Nat Cell Biol. 2019;21(8):1041–51.
Yang HL, Zhou WJ, Chang KK, Mei J, Huang LQ, Wang MY, et al. The crosstalk between endometrial stromal cells and macrophages impairs cytotoxicity of NK cells in endometriosis by secreting IL-10 and TGF-β. Reproduction. 2017;154(6):815–25.
Wendel JRH, Wang X, Smith LJ, Hawkins SM. Three-dimensional biofabrication models of endometriosis and the endometriotic microenvironment. Biomedicines. 2020;8(11).
Braun MM, Overbeek-Wager EA, Grumbo RJ. Diagnosis and Management of Endometrial Cancer. Am Fam Physician. 2016;93(6):468–74.
Lee YC, Lheureux S, Oza AM. Treatment strategies for endometrial cancer: current practice and perspective. Curr Opin Obstet Gynecol. 2017;29(1):47–58.
Van Nyen T, Moiola CP, Colas E, Annibali D, Amant F. Modeling endometrial cancer: past, present, and future. Int J Mol Sci. 2018;19(8).
Azziz R, Carmina E, Chen Z, Dunaif A, Laven JS, Legro RS, et al. Polycystic ovary syndrome. Nat Rev Dis Primers. 2016;2:16057.
Li X, Feng Y, Lin JF, Billig H, Shao R. Endometrial progesterone resistance and PCOS. J Biomed Sci. 2014;21(1):2.
Li Y, Tang P, Cai S, Peng J, Hua G. Organoid based personalized medicine: from bench to bedside. Cell Regen. 2020;9(1):21.
Adli M. The CRISPR tool kit for genome editing and beyond. Nat Commun. 2018;9(1):1911.
Ringel T, Frey N, Ringnalda F, Janjuha S, Cherkaoui S, Butz S, et al. Genome-Scale CRISPR Screening in Human Intestinal Organoids Identifies Drivers of TGF-β Resistance. Cell Stem Cell. 2020;26(3):431–40.e8.
Acknowledgments
This study was funded by NIH Grants R01 HD042280 and HD083273 to A.T.F.
Funding
This research was supported by NIH grants RO1 HD042280 and HD083273 to A.T.F.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declared no potential conflicts of interest.
Ethics Approval (Include Appropriate Approvals or Waivers)
Not applicable.
Consent to Participate (Include Appropriate Statements)
Not applicable.
Consent for Publication (Include Appropriate Statements)
The authors declared that the work has not been published in whole or in part elsewhere.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Song, Y., Fazleabas, A.T. Endometrial Organoids: A Rising Star for Research on Endometrial Development and Associated Diseases. Reprod. Sci. 28, 1626–1636 (2021). https://doi.org/10.1007/s43032-021-00471-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-021-00471-z