Abstract
The aquatic plant Nymphaea, a model genus of the early flowering plant lineage Nymphaeales and family Nymphaeaceae, has been extensively studied. However, the availability of chloroplast genome data for this genus is incomplete, and phylogenetic relationships within the order Nymphaeales remain controversial. In this study, 12 chloroplast genomes of Nymphaea were assembled and analyzed for the first time. These genomes were 158,290–160,042 bp in size and contained 113 non-repeat genes, including 79 protein-coding genes, 30 tRNA genes, and four rRNA genes. We also report on codon usage, RNA editing sites, microsatellite structures, and new repetitive sequences in this genus. Comparative genomics revealed that expansion and contraction of IR regions can lead to changes in the gene numbers. Additionally, it was observed that the highly variable regions of the chloroplast genome were mainly located in intergenic regions. Furthermore, the phylogenetic tree showed the order Nymphaeales was divided into three families, and the genus Nymphaea can be divided into five (or three) subgenera, with the subgenus Nymphaea being the oldest. The divergence times of nymphaealean taxa were analyzed, with origins of the order Nymphaeales and family Nymphaeaceae being about 194 and 131 million years, respectively. The results of the phylogenetic analysis and estimated divergence times will be useful for future evolutionary studies of basal angiosperm lineages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Angiosperms are plants that bear flowers and fruits that encase seeds are the most diverse land plants with about 300,000 species (Singh and Singh 2022). The basal angiosperms are essential for studying the origin and evolution of flowering plants (Xi et al. 2014). They contain some ancestral morphological and structural features that have attracted extensive interest from botanists (Yamada et al. 2001). Studying basal angiosperms has profound implications for understanding angiosperm diversity, adaptation, genome evolution, and development (Li et al. 2021a; Qiu et al. 1999). Nymphaeales is the second-most basal lineage after Amborellales (Soltis et al. 2000), and plays an important role in the study of the origin of early angiosperm lineages (Zanis et al. 2002). Although it is now generally thought that the order Nymphaeales is divided into three families, namely Nymphaeaceae, Cabombaceae, and Hydatellaceae (Gruenstaeudl et al. 2017; Saarela et al. 2007), the phylogenetic relationships within Nymphaeales were controversial (He et al. 2018; Sun et al. 2021).
Nymphaea is a perennial aquatic herbaceous plant, commonly known as the water lily. It is the largest and most widely distributed genus in the Nymphaeaceae with approximately 45–50 species (Borsch et al. 2007), and not only provides food and habitat for aquatic animals, but also reduces water turbidity and stabilizes stream sediments in shallow freshwater ecosystems, both tropical and temperate (Dalziell et al. 2020; Parveen et al. 2022). In addition, most water lilies have considerable ornamental value (Borsch et al. 2007) and many are well-known because of their variety of flower colors, long flowering period and strong adaptability (Bhandarkar and Khan 2004). In addition to being ornamental, water lilies are added as ingredients in numerous products, including cosmetics, soaps, perfumes and traditional medicines (Bhandarkar and Khan 2004). Several kinds of water lilies are also used for sewage purification (Lavid et al. 2001; Lu et al. 2012). The genus Nymphaea is phenotypically diverse and exhibits high levels of inter-specific polymorphism (Heslop-Harrison 1955). Although various molecular marker methods such as inter-simple sequence repeats (ISSR), amplified fragment length polymorphism (AFLP), and random amplified polymorphic DNA (RAPD) have been applied to study aquatic plant evolution (Hu et al. 2012; Koga et al. 2007; Kumar et al. 2016), the diversity and structure of the Nymphaea genome should be further explored.
As an important genetic element in plant cells, the chloroplast genome deserves to be studied in depth because of its diversity (Brunkard et al. 2015). The DNA of higher plant chloroplasts is a double-stranded covalently closed circular molecule whose length varies among species. It is composed of four sequences, including two inverted repeats (IRa and IRb), one long single copy sequence (LSC), and one short single copy (SSC) sequence (Odintsova and Yurina 2003). Chloroplast genomes have been widely used in the study of molecular evolution and phylogeny due to their moderate size for sequencing and the good collinearity among chloroplast genomes of different plant groups for comparative analysis (Olmstead and Palmer 1994). Since the first complete plastid genome was applied to plant phylogeny, plastid phylogenomics has been widely used to resolve phylogenetic relationships in photosynthetic eukaryotes, including green algae (Sun et al. 2016) and land plants (Ruhfel et al. 2014). The increased availability of complete plastid genomes has considerably increased the genomic resources for analyses of evolutionary relationships, including those that are controversial such as among the early-diverging angiosperms (Gruenstaeudl et al. 2017).
In this study, the chloroplast genomes of 12 Nymphaea species were sequenced and assembled for the first time and submitted to the National Center for Biotechnology Information (NCBI). We analyzed these sequences to further reveal genomic differentiation and interspecific evolutionary relationships in early flowering plants by: (1) comparing the structure and content of these genomes; (2) analyzing their relative synonymous codon usage (RSCU) and RNA editing sites; (3) examining the pattern of repeat sequences and microsatellites; (4) comparing genomic variation levels; (5) identifying highly variable loci that are suitable as molecular markers; (6) evaluating the phylogenetic relationships of these and other closely related species; (7) estimating their divergence times. These results not only enriched the genetic information of Nymphaea for the utilization of germplasm resources but also clarified the phylogenetic relationships among basal angiosperm lineages.
Material and methods
Data preprocessing
All plastid genomes sequenced for this investigation were obtained from the NCBI public database (Supplementary Table S1) (Gruenstaeudl et al. 2017). Approximately 2.4 GB of high-quality raw reads for each species were obtained and used to assemble the complete chloroplast genome of Nymphaea. Raw data were pre-processed using Trimmomatic v0.39 software (Bolger et al. 2014), including the removal of adapter sequences and other special sequences from sequencing, such as reads with many ‘N’ bases. The quality of newly produced clean short reads was evaluated using FASTQC v0.11.9 (Andrews 2014) and MULTIQC v.1.7 (Ewels et al. 2016) software to select reads with Phred scores averaging above 35.
Assembly and annotation of chloroplast genomes
We used two types of genome assembly. First, de novo splicing software GetOrganelle v 1.7.4 (Jin et al. 2020) was used to assemble the chloroplast genome sequence from the clean reads. Second, chloroplast-like (cp) fragments were selected from clean reads by comparing them to the reference sequence of Nymphaea colorata (MT107631) using BLAST (Camacho et al. 2009). The reads were assembled with SPAdes v 3.12.0 (Bankevich et al. 2012) by setting k-mer values as 35, 44, 71, and 101. The coverage of each sample was measured using Geneious software (Supplementary Fig. S1). The new complete chloroplast genome was annotated by the combined results from Plastid Genome Annotator (PGA) (Qu et al. 2019) and GeSeq v1.42 (Tillich et al. 2017). RNAmmer v.1.2 (Lagesen et al. 2007) and tRNAscan-SE v 1.21 (Lowe and Eddy 1996) were used to validate RNA genes with default settings. As a final check, GB2Sequin (Lehwark and Greiner 2019) was used to manually check the boundaries of introns or exons and the positions of start or stop codons, with the reference sequence in the GenBank database. Chloroplot (Zheng et al. 2020) was used to draw the circular map of the genomes. The 12 new chloroplast genomes of Nymphaea have been deposited in GenBank under accession numbers MW802262–MW802273.
Sequence structure analysis
Relative synonymous codon usage was calculated by the Computer Codon Usage Bias function in MEGA 7 (Kumar et al. 2016). Information regarding genome length, GC content, and gene number of each region in the chloroplast genomes was obtained using Geneious Prime software (Kearse et al. 2012). A microsatellite identification tool (Beier et al. 2017) was used to find Simple Sequence Repeats (SSRs). The minimum number of repeats for motif lengths of 1, 2, 3, 4, 5, and 6 were 10, 4, 4, 3, 3, and 3, respectively. REPuter (Kurtz et al. 2001) was used to calculate four types of repeats with the following parameter settings: (1) Hamming distance of 3; (2) sequence identity higher than 90%; and (3) minimum repeat size of 20 bp. Predictive RNA Editor for Plants (PREP) was used to examine RNA editing sites from protein-coding sequences (CDS) with a cutoff value set to 0.8.
Comparative analysis
The IRscope tool (Amiryousefi et al. 2018) was used to detect and visualize the contraction and expansion sequence of inverted repeats of the Nymphaea chloroplast genomes. To compare chloroplast genomic differences between species, sequence comparisons were performed on the online comparison tool mVISTA (Frazer et al. 2004), setting up the Shuffle-LAGAN (Brudno et al. 2003) method, and selecting N. lotus as the reference species. We also used DNAsp v6 software (Rozas et al. 2017) to calculate the nucleotide diversity (Pi) with 1000 bp window length and 50 bp step size KaKs_Calculator v2.0 (Wang et al. 2008) was used to calculate the ratio of non-synonymous mutation and synonymous mutation.
Phylogenetic analysis and divergence time estimation
To construct the phylogenetic relationships of basal angiosperms, we selected 42 species of Nymphaeales and the basal angiosperm Amborella trichopoda (Yang et al. 2020) as outgroups (Supplementary Table S2). Two types of tree were constructed using the complete chloroplast genome sequence data as well as 84 protein-coding genes to frame the maximum likelihood (ML) topologies, respectively. MAFFT-X (Katoh and Standley 2013) was used to perform the multiple gene alignment MrBayes v3.1.2 (Ronquist and Huelsenbeck 2003) was used to conduct Bayesian inference (BI) analyses. Modeltest v3.7 (Posada and Buckley 2004) was used to find the best models by the Akaike information criterion (Posada and Crandall 1998). The GTR + G + I model was selected to construct the ML tree in MEGA X (Kumar et al. 2018), with the bootstrap value set to 1000. Well-supported clades were defined by having a posterior probability above 0.95 (PPBI > 0.95) and a bootstrap value above 70% (BSML > 70%) (Alfaro and Holder 2006).
To estimate the evolutionary timescale of Nymphaeales, we calibrated a relaxed molecular clock using one molecular dating and four fossil-based ages throughout the tree (more fossils are shown in Sect. “Phylogenetic analysis and divergence time estimation of Nymphaeales”). Gblocks v0.91 (Castresana 2000) was used to remove all gap positions, most variable sites, and ambiguous sites in the multiple alignments. The base mutation rates were calculated using BASEML from the PAML v4.8 package (Yang 2007). The divergence times were calculated using mcmctree (Puttick 2019), with samples drawn every 400 steps over a total of 10 million following the burn-in of 3 million steps. We checked for convergence and sufficient sampling by running the analysis in duplicate. Finally, Tracer v1.7.2 (Rambaut et al. 2018) was used to verify that the effective sample size value was above 200.
Results
General characteristics analysis
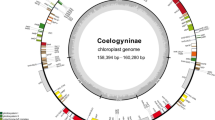
The complete chloroplast genome of Nymphaea had a typically circular structure consisting of four sequences, including a large single copy (LSC), a small single copy (SSC), and two inverted repeats (IRa/IRb) (Fig. 1). The genome size of the 12 species of Nymphaea varied from 158,290 to 160,042 bp, among which N. immutabilis was the largest (160,042 bp) and N. tenerinervia was the smallest (158,290 bp). The total GC content varied between 39.1% and 39.2% (Table 1), this great similarity indicating their high degree of affinity. The LSC region ranged from 88,499 bp (N. tenerinervia) to 90,118 bp (N. immutabilis) in size, and its total GC content was around 37.8%. The SSC region was between 19,283 bp (N. rudgeana) and 19,594 bp (N. immutabilis), and the total GC content ranged between 34.2% and 34.4%. The IR regions were between 50,300 bp (N. sp. NY590) and 50,396 bp (N. gracilis), and their GC content was about 43.4%. The proportion of coding regions was highest (nearly 70%) in the chloroplast genome (Table 1).
Circular maps of Nymphaea amazonum. The various functional groups are depicted by the colored bars. Small single-copy regions (SSC) and large single-copy regions (LSC) of the genome are separated by the range of inverted repeats (IRa and IRb), which are represented by dark areas. Genes drawn outside the circle are transcribed counterclockwise, whereas genes drawn inside the circle are transcribed clockwise. AT content is represented by the light gray inner circle, while GC content is represented by the dark gray inner circle
According to the annotated results, considerable similarities in the type and number of genes were found between all 12 species of Nymphaea. The chloroplast genomes of most Nymphaea species had a total gene number of 130, except for N. sp. NY701, which had one more copy of rps15. The chloroplast genome of Nymphaea contained 113 non-repeating genes, including 79 protein-coding genes, 30 tRNA genes, and four rRNA genes. These genes can be roughly divided into three categories: chloroplast self-replication genes, photosynthesis-related genes, and other genes (Table 2). Introns were found in 12 genes (one intron: trnA-UGC, trnG-GCC, trnI-GAU, trnK-UUU, trnV-UAC, rps12, rpl2, rpoC1, ndhA, ndhB, atpF; two introns: clpP, ycf3). The 17 repeating genes were all located in the IR region, including four rRNA genes, seven tRNA genes, and six or seven protein-coding genes.
Codon usage and RNA editing sites analysis
Since N. immutabilis had the largest chloroplast genome, we used it as an example to calculate the codon usage bias and RSCU values of 84 CDS genes (Fig. 2; Supplementary Table S3). The results showed that among all the codons of N. immutabilis, 31 had an RSCU value greater than 1, indicating that these were preferred codons. Moreover, 30 codons ended with A/U. Among these codons, UUA, UCU, ACU, GCU, and AGA showed strong bias (RSCU ≥ 1.6). However, the RSCU values of codon AUG encoding methionine and codon UGG encoding tryptophan were both 1, indicating that there is no bias in terms of the usage of methionine and tryptophan in N. immutabilis. A total of 26,338 codons were found in these coding regions. The most common amino acids were leucine (2679 codons), isoleucine (2197 codons) and serine (2079 codons), while cysteine had the fewest codons (309).
RNA editing refers to the replacement, insertion, and deletion of nucleotides in the process of RNA maturation after transcription. We used the PREP suite to analyze the RNA editing sites of 26 protein-coding genes in the cp genome of N. amazonum. A total of 96 RNA editing sites were detected, most of which involved the conversion of serine to leucine (Supplementary Table S4). The most frequently edited genes were rpoC2 (13 sites), ndhA (10 sites) and ndhB (9 sites). All the observed changes occurred at the first or second nucleotide site in each codon. Most RNA editing sites resulted in the conversion of polar amino acids to nonpolar amino acids, with the commonest conversion being serine to leucine or phenylalanine.
SSRs and long repeats analysis
Six types of SSR were measured, namely mononucleotide, dinucleotide, trinucleotide, tetranucleotide, pentanucleotide, and hexanucleotide repeats. A total of 1464 SSR markers, ranging in length from 8 to 183 bp, were detected in the chloroplast genomes of the 12 Nymphaea species. According to the data, the first four repeat contents were significant, while the number of pentanucleotide and hexanucleotide repeats was relatively low or even non-existent. The number of SSRs in the chloroplast genomes of the 12 species ranged from 117 (N. immutabilis) to 126 (N. heudelotii and N. sp. NY701) (Fig. 3A; Supplementary Table S5). The number of mononucleotide repeats was the largest, accounting for about 49.81% of the total number of SSRs. 89.72% of these were A/T repeats, and a few tandem cytosine and guanine were found. The dinucleotide repeats were the second most common; 59.46% of these were AT/AT repeats, and the rest were AG/CT and AC/GT repeats. The number of trinucleotide repeats accounted for 3.42% of all SSRs. There were three types of trinucleotide repeats (AAG/CTT, AAT/ATT, and AGG/CCT) in all species except for N. sp. NY590. There were six different types of tetranucleotide repeats of these the chloroplast genomes of all 12 species all had AAAG/CTTT and AATC/ATTG repeats, while the other four tetranucleotide repeats were rare or found in only a few species.
The number of long repeats detected in this experiment ranged from 20 (N. heudelotii) to 49 (N. amazonum), and the types and numbers of long repeats varied widely from species to species (Fig. 3B). The number of forward and palindromic repeats was higher than that of reverse and complementary repeats. Indeed, only four species (N. amazonum, N. sp. NY668, N. sp. NY701, and N. tenerinervia) contained complementary repeats. Moreover, in four types of repeats, the long repeats were the most abundant, and the complementary repeats were the least abundant. The length of repeats ranged from 20 to 24 bp (Fig. 3C). The distribution of repetitive sequences was also region-specific, with the largest number of repetitive sequences in the LSC region (Fig. 3D).
The chloroplast genome is made up of four regions (LSC, SSC, IRa, and IRb), thus forming four boundaries. In genome evolution, the differences between species of the same genus are often associated with the expansion and contraction of IR regions (Wang et al. 2008). We conducted a comprehensive comparative analysis of four junctions (JLA, JLB, JSA, and JSB) between two IR regions (IRa and IRb) and two single-copy regions (LSC and SSC) in 12 Nymphaea species (Fig. 4). Although the genomes of IR regions in the 12 Nymphaea species were similar in size, ranging from 25,150 to 25,198 bp, some expansion and contraction could be observed. The JSA boundary was spanned by the ycf1 gene, with a length difference of less than 2 bp between different species. The ndhF gene was located near the JSB boundary, with the ndhF of N. immutabilis being the closest to the JSB line (27 bp distant) and that of N. tenerinervia being the farthest (82 bp distant). The rpl2 and trnH genes were located in the JLA boundary, which was spanned by the trnH gene. trnH was mainly located in the LSC region and extended to the IRa region by 2–9 bp. The rps19 and rpl2 genes were located in the JLB boundary, where the rpl2 was 25–32 bp away from the junction. Expansion and contraction of the IR regions were presumably the main reason for the variation in chloroplast genome size, which is consistent with the previous reports (Asaf et al. 2016; Li et al. 2018).
Comparative analysis
The chloroplast genomes were highly conserved in terms of structure and gene sequence (Fig. 5). The variation in the IR region was considerably lower than that of the LSC and SSC regions. Moreover, the difference in the non-coding sequence region was greater than that in the coding region, as the former had more mutations than the latter (Widmer and Baltisberger 1999). Regions with significant variation in the chloroplast genome of Nymphaea generally appeared in the intergenic spacer regions (IGS), such as between trnH-GUG and psbA, trnK-UUU and rps16, rps16 and trnQ-UUG, trnS-GCU and trnG-UCC, atpF and atpH, atpH and atpI, rpoB and trnC-GCA, trnC-GCA and petN, petN and psbM, psbM and trnD-GUC, trnD-GUC and trnY-GUA, trnT-GGU and psbD, and psbD and rpoA. The results also showed that the protein-coding sequence regions were highly conserved, especially the rRNA gene, with almost no variation observed.
Comparison of chloroplast genomes of 12 Nymphaea species. The direction of the gene is indicated by the gray arrow above the comparison. Exons are shown in dark blue, untranslated regions (UTR) in light blue, and conserved non-coding sequences (CNS) in pink. The percentage consistency on the Y-axis ranges from 50% to 100%
Select pressure and nucleotide diversity analysis
Non-synonymous substitution (Ka), synonymous substitution (Ks), and their ratio (Ka/Ks) are important indicators for understanding the direction of evolution and selection (Li et al. 2009). Therefore, we looked at these variables in 79 protein-coding genes (PCGs) (Supplementary Table S6). Ka/Ks > 1 indicates positive selection, Ka/Ks < 1 indicates negative selection, and Ka/Ks≈1 indicates neutral evolution (Li et al. 2009). Compared with other genes, the Ka/Ks ratios of photosystem I and photosystem II genes were either equal to or close to 0. This indicated that these genes were highly conserved and showed an elevated level of purification selection. The genes with positive selection were atpF (except N. sp. NY590), clpP (N. amazonum, N. rudgeana, N. tenerinervia, and N. sp. NY590), ndhA (N. amazonum, N. conardii, N. glandulifera, N. tenerinervia, and N. sp. NY590), ycf2 (N. gracilis, N. heudelotii, N. sp. NY566, N. sp. NY668, and N. sp. NY701), and ycf3 (N. heudelotii, N. immutabilis, N. sp. NY566, N. sp. NY668, and N. sp. NY701). The Ka/Ks ratio of the atpF gene was greater than 1.35 in all Nymphaea species except N. sp. NY590. This suggested the presence of beneficial mutations and rapid development of the atpF gene in these species. On the other hand, the Ka/Ks ratios of the atpE, clpP, ndhA, ndhD, ndhK, petB, and ycf3 genes in some species were equal to or close to 1, indicating that these genes had neutral evolution.
The nucleotide diversity value (Pi value) reflects the base diversity level of the population genome. The Pi value was used to analyze base polymorphism. The lower the level of polymorphism, the higher the degree of selection. To fully understand the differences between sequences, we performed slicing window analysis to visualize the nucleotide variation values. A total of 17 regions with high divergence values (Pi > 0.07) were identified and designated as hypervariable regions. Among the 17 highly variable regions, 10 were located in the LSC region, seven in the SSC region, and none in the IR regions. It can also be clearly seen from Supplementary Fig. S2 that the SSC and LSC regions were more distinctly different than IR regions, and the Pi value of the IR region was considerably lower.
Phylogenetic analysis and divergence time estimation of Nymphaeales
To infer the taxonomy and phylogeny of Nymphaeales, we constructed two phylogenetic trees using 79 CDS genes and the complete chloroplast genomes, respectively. Our results are consistent with the view that Nymphaeales includes three families, namely Hydatellaceae, Cabombaceae, and Nymphaeaceae (Fig. 6A). There are five subgenera in Nymphaea, namely Brachyceras, Anecphya, Lotos, Hydrocallis, and Nymphaea. Twelve species with newly sequenced genomes were placed in the subgenera Brachyceras (five species), Anecphya (one species), and Hydrocallis (six species). Notably, the phylogenetic tree indicated that Victoria and Euryale were placed within Nymphaea, as sister taxa to the subgenera of Lotos and Hydrocallis. In addition, there were some differences between the two trees, such as sister relationships and bootstrap values, and especially the position of the subgenus Nymphaea. The subgenus Nymphaea was sister to a clade comprising all other subgenera plus Victoria and Euryale in the 79 CDS tree (Fig. 6B), whereas it was sister to a clade comprising Hydrocallis, Lotos, Euryale and Victoria in the complete chloroplast genome tree (Fig. 6C).
Phylogenetic relationships and divergence times of the Nymphaeales. A Phylogenetic tree constructed using 78 protein-coding genes. B Phylogenetic tree constructed from protein-coding genes. C Phylogenetic tree constructed from complete chloroplast genes. D A time evolutionary tree constrained by a relaxed molecular clock
To estimate the divergence times of taxa within the order Nymphaeales, a time tree was constructed using a relaxed molecular clock calibrated using one molecular-based and four fossil-based ages (Fig. 6D). The result of the time estimation had the following noteworthy points (Fig. 6D; Supplementary Table S7): (a) Amborella trichopoda, considered to be the oldest angiosperm, diverged around 228.52 million years ago (Ma). (b) Nymphaeales originated about 200 million years ago, Hydatellaceae about 194.01 Ma, Cabombaceae about 163.17 Ma, and Nymphaeaceae about 131.68 Ma. (c) Nymphaea and Barclaya diverged about 99.76 Ma. (d) Diversification of the five subgenera of Nymphaea occurred 69.49 Ma, with the Nymphaea being the oldest subgenus having originated about 66.97 Ma and subgenera Brachyceras and Anecphya being the youngest having originated about 27.82 Ma. It is also noteworthy that Victoria and Euryale, as two genera of Nymphaeaceae, diverged about 57.49 Ma, meaning that they have a later origin than the subgenus Nymphaea.
Discussion
Plastome features
In most higher plants, plastids are maternally inherited and exhibit highly conserved structures, showing little recombination (Birky 1995). With the development of sequencing technology, several comparative studies have revealed that chloroplast genomes exhibit similarities in terms of genome size, GC content, and gene type (Song et al. 2022a; Yang et al. 2022). For example: (i) the chloroplast genome is a circular double-stranded DNA molecule, ranging in size between 120 and 180 kb; (ii) the GC content in the IR region is relatively higher than that in the LSC and SSC regions; (iii) the number of genes is estimated to be around 120–140, including a protein-coding genome of 70–80 genes, around 37 tRNA genes, and approximately eight rRNA genes. A previous study found gene loss in the chloroplast genomes of Nymphaea, such as ycf1, ndhF, and rpoC2 (Sun et al. 2021). However, no genes were lost in our 12 chloroplast genomes of Nymphaea, all of which contained 113 unique genes. These genes formed a circle, with a pair of inverted repeat sequences dividing it into four parts (Sato et al. 1999). We found that the high GC content of the IR region was attributed to the abundance of tRNA and rRNA genes, which were predominantly composed of GC base pairs (Doorduin et al. 2011).
Relative synonymous codon usage (RSCU) is an indicator used to evaluate the preferences for evaluating 59 synonymous codons to study variations in synonymous codon use between genes. The high relative similarity in codon usage among different species suggested that a similar environmental selection may have been experienced (Yang et al. 2014). In the genome, we found a tendency to use codon endings with A/T in high-use codons (RSCU > 1), which was also frequently observed in angiosperms (Mehmood et al. 2020). Our analysis revealed few differences in the RSCU of water lily chloroplast genes, suggesting that codon usage was conserved (Liu and Xue 2005; Zhou et al. 2008). It has been shown that RNA editing usually occurs at the first or second base of the codon, which tends to shift amino acids from hydrophilic to hydrophobic and from polar to non-polar, resulting in an increase in the hydrophobicity of the protein (Chen et al. 2011; Han et al. 2022). Our data support this view, with a large amount of serine (hydrophilic) converted to leucine (hydrophobic) and phenylalanine (hydrophobic).
Repeated sequences in the chloroplast genome may have the potential to promote gene rearrangement and recombination (Zhou et al. 2019). Among the 12 Nymphaea species, the most common type of repeat was mononucleotide, and the majority of these repeats were AT-enriched. This observation is consistent with the finding that chloroplast SSRs typically consist of short polyA or polyT repeats (Ye et al. 2018). It is noteworthy that the repetitive sequences rarely contained G-C bases, possibly due to the fact that G-C bases form three hydrogen bonds, which makes them more resistant to disruption and less conducive to genetic recombination (Li et al. 2021b). We detected long repeats in nine Nymphaea species, most of which were located in the LSC region. Repeat sequences play a role in various processes, such as gene activity, genome organization, DNA replication, recombination, and repair (He et al. 2018).
Comparative genomics
In angiosperms, the border positions of IR/LSC are not conserved, having frequent contractions and expansions (He et al. 2018), which is the main reason for the variation of chloroplast genome length (Chumley et al. 2006). Furthermore, the expansion and contraction of the region are associated with the structure of the chloroplast, leading to changes in gene number and position (Yang et al. 2022). For example, an alteration of the IR region makes an extra copy of the rps19 gene in Musa chloroplasts (Song et al. 2022b). In the present study, the IR region also showed expansion and contraction, with the presence of an additional copy of the rps15 gene in N. sp. NY701 and a change in the distance between the ndhF gene and the JSB boundary. In addition, previous studies found the absence of the ndhF gene in N. odorata and of the ycf1 gene in N. tetragona due to changes in the IR region (Sun et al. 2021). However, gene loss was not found in the present study, which may be related to the selective pressure of the habitats of the 12 Nymphaea species (Cheng et al. 2021).
Chloroplasts were relatively stable in nucleotide content and highly conserved in gene structure, but there were hotspots of variation. These hotspots with relatively high mutation rates can be used as DNA barcoding for plant identification (Ge et al. 2019; Kuang et al. 2011). In our study, the comparison of 12 Nymphaea species revealed higher variation in the non-coding regions than in the coding regions. Therefore, the speed of molecular evolution in non-coding regions provide a good basis for phylogenetic inference (Shi et al. 2022; Zhu and Ge 2005). In addition, the nucleotide diversity results showed that most of the highly variable regions were located in the SC, and the IR regions were highly conserved (Smith 2015). By comparing the chloroplast genome sequences, it is possible to find similarities and homologies between species and predict evolutionary relationships between sequences (Wu and Chaw 2015).
The ratio of synonymous (Ks) and non-synonymous (Ka) substitutions is important for inferring evolutionary rates and for understanding adaptive development among species (Fay and Wu 2003). In chloroplast protein-coding genes, synonymous substitutions are usually more frequent than nonsynonymous substitutions, with ratios of KA/KS < 1 (Abdullah et al. 2020). The Maturases, Ubiquinol Cytochrome C reductase, and Cytochrome C biogenesis genes also exhibit Ka/Ks ratios below 1, indicating a negative selection (Cheng et al. 2021). In the present study, most Ks were much higher than Ka, implying a relatively slow evolution of Nymphaea species. However, the Ka/Ks ratios of atpF, ndhA, clpP, ycf2, and ycf3 were greater than 1, meaning that these genes had undergone positive selection by the environment. The six ATP synthase genes, atpA, atpB, atpE, atpH, and atpI had very low Ka/Ks values, and only atpF had a Ka/Ks value above 1. Neutral theory shows that most of the mutations at the molecular level, such as nucleotide substitution in gene spacers and introns, and the pseudogenes that are not translated into proteins, are neutral or nearly neutral, meaning they were not subject to natural selection (Fay and Wu 2001).
Phylogeny and evolution
The genetic and intergenic regions of the chloroplast genome have different rates of molecular evolution, which provide differing genetic variation for phylogenetic studies (Clegg et al. 1994; Jian et al. 2008). Earlier phylogenetic analyses of Nymphaeales were limited to the use of one or a few marker fragments from either the plastid or nuclear genome (Biswal et al. 2012; Les et al. 1991). With the rapidly increasing number of sequenced plastid genomes, more recent studies have used complete chloroplast genomes to analyze evolutionary relationships, but few systematic studies have been conducted on the basal taxa of flowering plants (He et al. 2018; Sun et al. 2021). Here, we used the complete chloroplast genomes and coding regions of 43 species to construct comprehensive phylogenetic relationships within Nymphaeales and estimate divergence times for early angiosperms. In previous studies, the relationship between Cabomba and Brasenia was controversial (Sokoloff et al. 2008; Sun et al. 2021). Some studies suggested that the two genera should be part of the Nymphaeaceae, while others reported that they could be members of Cabombaceae (Biswal et al. 2012; Dkhar et al. 2012; Löhne et al. 2007). The findings of the present study strongly supported the latter, implying that members of the order Nymphaeales are clearly divided into three families: Hydatellaceae, Cabombaceae, and Nymphaeaceae.
Zhang et al. (2020) concluded that Victoria and Euryale should be divided into separate genera based on phylogenetic analyses of nuclear genome data. However, Victoria, Euryale, and Nymphaea formed a clade in our phylogenetic tree. Previous studies suggested that the inconsistency between the positions of the Euryale + Victoria clade in the chloroplast tree and nuclear gene tree might be caused by chloroplast capture (Liu et al. 2017; Sun et al. 2021). Our results indicated that Victoria and Euryale should be assigned to the genus Nymphaea for the following two reasons: (a) the behavior of chloroplast capture has occurred only rarely throughout evolutionary time and has not been found in species that are closely related (Kawabe et al. 2018; Yang et al. 2021); and (b) chloroplast genomes are more stable when maternally inherited gene (Kumar et al. 2004). Conard (1905) was the first to divide Nymphaea into five subgenera, and this conclusion was further supported by the molecular findings of Borsch et al. (2007). Our phylogenetic tree also support the division of Nymphaea into five subgenera: Brachyceras, Anecphya, Lotos, Hydrocallis, and Nymphaea. However, we found that Brachyceras and Anecphya form one branch, and Lotos and Hydrocallis form another branch in the evolutionary tree. As a result, it was more parsimonious to conclude that Nymphaea should be divided into three subgenera: Brachyceras-Anecphya, Lotos-Hydrocallis, and Nymphaea, which consistent with previous studies (Dkhar et al. 2012; Löhne et al. 2007).
Angiosperms evolved a diversity of species during a relatively short geological period—Darwin’s ‘abominable mystery’ (Shi et al. 2022). In recent years, molecular time trees have been used to estimate the divergence times within angiosperms (Li et al. 2019; Wu et al. 2014; Zeng et al. 2014). We provide here for the first time a near-complete temporal framework for the evolution of Nymphaeales above the generic level. Although previous studies have acknowledged a significant gap in the fossil record of the angiosperm stem lineage (Bell et al. 2010; Magallón 2010), there is no compelling argument supporting a specific maximum age constraint for the crown node of angiosperms (Massoni et al. 2015). We restricted ourselves to conservative fossil age constraints based on the timescale of early land plant evolution (Morris et al. 2018). We used the chloroplast genome to estimate the time of divergence for angiosperms at about 228 Ma, the time of divergence for the Nymphaeales at about 194 Ma (crown group), the time of divergence for the Nymphaeaceae at about 131 Ma, and the time of divergence for the genus Nymphaea at about 69 Ma. Our divergence times were a little later than those inferred for the nuclear genome, which may be attributed to the more conserved chloroplast genome (Dong et al. 2013; Zhang et al. 2020). In general, new age estimates for species, families and orders of angiosperms are compatible with the putative fossil record attributed to each of these taxa (Foster and Ho 2017; Li et al. 2019; Ran et al. 2018). Molecular time estimation should be seen as an attempt to reduce the range of the most likely ages for nodes constrained by the age of reliable fossil records securely placed and dated (Kumar et al. 2017) and as a method to evaluate the probable ages of nodes for which there is no direct fossil record (Bouckaert et al. 2014; Puttick 2019). Therefore, we should take into account the ambiguity of our current knowledge since molecular dating approaches cannot provide unambiguous ages except for particularly fossil-rich clades (Massoni et al. 2015). Future studies of molecular dating will likely use additional fossils, which could revise several estimates supported in the current study and decrease the size of the credibility intervals.
Conclusions
Here, we sequenced and compared the chloroplast genomes of 12 Nymphaea species for the first time. The results showed that three amino acids (leucine, isoleucine, and serine) had a specific usage preference in N. immutabilis and that RNA editing sites resulted in the conversion of polar to non-polar amino acids. Contraction and expansion of the IR regions led to genome size differences, gene duplications, and deletions. Regions with high variations in the chloroplast genome of Nymphaea were generally in the intergenic spacer areas. The nucleotide diversity in the LSC and SSC regions was much higher than that in the IR region. The Ka/Ks ratios of the atpF, ndhA, clpP, ycf2, and ycf3 genes were greater than 1, meaning that these genes had undergone positive selection by the environment. The results of the phylogenetic analysis and estimated divergence time will be helpful for future evolutionary studies of the basal taxa of angiosperms.
Data Availability
The data that support the findings of this study are openly available in the GenBank database at https://www.ncbi.nlm.nih.gov/, under accession number [MW802262-MW802273].
References
Abdullah MF, Shahzadi I, Waseem S, Mirza B, Ahmed I, Waheed MT (2020) Chloroplast genome of Hibiscus rosasinensis (Malvaceae): comparative analyses and identification of mutational hotspots. Genomics 112:581–591
Alfaro ME, Holder MT (2006) The posterior and the prior in Bayesian phylogenetics. Annu Rev Ecol Evol Syst 37:19–42
Amiryousefi A, Hyvönen J, Poczai P (2018) IRscope: an online program to visualize the junction sites of chloroplast genomes. Bioinformatics 34:3030–3031
Andrews S (2014) FastQC—a quality control tool for high throughput sequence data. Babraham Bioinforma. http://www.bioinformatics.babraham.ac.uk/projects/fastqc/. Accessed 26 June 2013
Asaf S, Khan AL, Khan AR, Waqas M, Kang SM, Khan MA, Lee SM, Lee IJ (2016) Complete chloroplast genome of Nicotiana otophora and its comparison with related species. Front Plant Sci 7:1–12
Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA (2012) SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19:455–477
Beier S, Thiel T, Münch T, Scholz U, Mascher M (2017) MISA-web: a web server for microsatellite prediction. Bioinformatics 33:2583–2585
Bell CD, Soltis DE, Soltis PS (2010) The age and diversification of the angiosperms re-revisited. Am J Bot 97:1296–1303
Bhandarkar MR, Khan A (2004) Antihepatotoxic effect of Nymphaea stellata willd., against carbon tetrachloride-induced hepatic damage in albino rats. J Ethnopharmacol 91:61–64
Birky CW (1995) Uniparental inheritance of mitochondrial and chloroplast genes: mechanisms and evolution. Proc Natl Acad Sci USA 92:11331–11338
Biswal DK, Debnath M, Kumar S, Tandon P (2012) Phylogenetic reconstruction in the order Nymphaeales: ITS2 secondary structure analysis and in silico testing of maturase k (matK) as a potential marker for DNA bar coding. BMC Bioinform 13:1–16
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120
Borsch T, Hilu KW, Wiersema JH, Löhne C, Barthlott W, Wilde V (2007) Phylogeny of Nymphaea (Nymphaeaceae): evidence from substitutions and microstructural changes in the chloroplast trnT-trnF region. Int J Plant Sci 168:639–671
Bouckaert R, Heled J, Kühnert D, Vaughan T, Wu CH, Xie D, Suchard MA, Rambaut A, Drummond AJ (2014) BEAST 2: a software platform for Bayesian evolutionary analysis. PLoS Comput Biol 10:e1003537
Brudno M, Malde S, Poliakov A, Do CB, Couronne O, Dubchak I, Batzoglou S (2003) Glocal alignment: finding rearrangements during alignment. Bioinformatics 19:i54–i62
Brunkard JO, Runkel AM, Zambryski PC (2015) Chloroplasts extend stromules independently and in response to internal redox signals. Proc Natl Acad Sci USA 112:10044–10049
Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL (2009) BLAST+: architecture and applications. BMC Bioinform 10:1–9
Castresana J (2000) Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol 17:540–552
Chen H, Deng L, Jiang Y, Lu P, Yu J (2011) RNA editing sites exist in protein-coding genes in the chloroplast genome of Cycas taitungensis. J Integr Plant Biol 53:961–970
Cheng Y, He X, Priyadarshani SVGN, Wang Y, Ye L, Shi C, Ye K, Zhou Q, Luo Z, Deng F, Cao L, Zheng P, Aslam M, Qin Y (2021) Assembly and comparative analysis of the complete mitochondrial genome of Suaeda glauca. BMC Genom 22:1–19
Chumley TW, Palmer JD, Mower JP, Fourcade HM, Calie PJ, Boore JL, Jansen RK (2006) The complete chloroplast genome sequence of Pelargonium × hortorum: organization and evolution of the largest and most highly rearranged chloroplast genome of land plants. Mol Biol Evol 23:2175–2190
Clegg MT, Gaut BS, Learn GH, Morton BR (1994) Rates and patterns of chloroplast DNA evolution. Proc Natl Acad Sci USA 91:6795–6801
Conard HS (1905) The waterlilies: a monograph of the genus Nymphaea, vol 4. Carnegie institution of Washington
Dalziell EL, Lewandrowski W, Merritt DJ (2020) Increased salinity reduces seed germination and impacts upon seedling development in Nymphaea L. (Nymphaeaceae) from northern Australia’s freshwater wetlands. Aquat Bot 165:103235
Dkhar J, Kumaria S, Rao SR, Tandon P (2012) Sequence characteristics and phylogenetic implications of the nrDNA internal transcribed spacers (ITS) in the genus Nymphaea with focus on some Indian representatives. Plant Syst Evol 298:93–108
Dong WP, Xu C, Cheng T, Lin K, Zhou SL (2013) Sequencing angiosperm plastid genomes made easy: a complete set of universal primers and a case study on the phylogeny of Saxifragales. Genome Biol Evol 5:989–997
Doorduin L, Gravendeel B, Lammers Y, Ariyurek Y, Chin-A-Woeng T, Vrieling K (2011) The complete chloroplast genome of 17 individuals of pest species Jacobaea vulgaris: SNPs, microsatellites and barcoding markers for population and phylogenetic studies. DNA Res 18:93–105
Ewels P, Magnusson M, Lundin S, Käller M (2016) MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics 32:3047–3048
Fay JC, Wu C-I (2001) The neutral theory in the genomic era. Curr Opin Genet Dev 11:642–646
Fay JC, Wu CI (2003) Sequence divergence, functional constraint, and selection in protein evolution. Annu Rev Genomics Hum Genet 4:213–235
Foster CSP, Ho SYW (2017) Strategies for partitioning clockmodels in phylogenomic dating: application to the angiosperm evolutionary timescale. Genome Biol Evol 9:2752–2763
Frazer KA, Pachter L, Poliakov A, Rubin EM, Dubchak I (2004) VISTA: computational tools for comparative genomics. Nucleic Acids Res 32:W273–W279
Ge Y, Dong X, Wu B, Wang N, Chen D, Chen H, Zou M, Xu Z, Tan L, Zhan R (2019) Evolutionary analysis of six chloroplast genomes from three Persea americana ecological races: insights into sequence divergences and phylogenetic relationships. PLoS ONE 14:e0221827
Gruenstaeudl M, Nauheimer L, Borsch T (2017) Plastid genome structure and phylogenomics of Nymphaeales: conserved gene order and new insights into relationships. Plant Syst Evol 303:1251–1270
Han CY, Ding R, Zong XY, Zhang LJ, Chen XH, Qu B (2022) Structural characterization of Platanthera ussuriensis chloroplast genome and comparative analyses with other species of Orchidaceae. BMC Genom 23:1–13
He DX, Gichira AW, Li ZZ, Nzei JM, Guo YH, Wang QF, Chen JM (2018) Intergeneric relationships within the early-diverging angiosperm family Nymphaeaceae based on chloroplast phylogenomics. Int J Mol Sci 19:9–11
Heslop-Harrison Y (1955) Nymphaea L. J Ecol 43:719–734
Hu J, Pan L, Liu H, Wang S, Wu Z, Ke W, Ding Y (2012) Comparative analysis of genetic diversity in sacred lotus (Nelumbo nucifera Gaertn.) using AFLP and SSR markers. Mol Biol Rep 39:3637–3647
Jian SG, Soltis PS, Gitzendanner MA, Moore MJ, Li RQ, Hendry TA, Qiu YL, Dhingra A, Bell CD, Soltis DE (2008) Resolving an ancient, rapid radiation in Saxifragales. Syst Biol 57:38–57
Jin JJ, Yu WB, Yang JB, Song Y, Depamphilis CW, Yi TS, Li DZ (2020) GetOrganelle: a fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biol 21:241
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780
Kawabe A, Nukii H, Furihata HY (2018) Exploring the history of chloroplast capture in Arabis using whole chloroplast genome sequencing. Int J Mol Sci 19:602
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A (2012) Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28:1647–1649
Koga K, Kadono Y, Setoguchi H (2007) The genetic structure of populations of the vulnerable aquatic macrophyte Ranunculus nipponicus (Ranunculaceae). J Plant Res 120:167–174
Kuang DY, Wu H, Wang YL, Gao LM, Zhang SZ, Lu L (2011) Complete chloroplast genome sequence of Magnolia kwangsiensis (Magnoliaceae): implication for DNA barcoding and population genetics. Genome 54:663–673
Kumar S, Dhingra A, Daniell H (2004) Stable transformation of the cotton plastid genome and maternal inheritance of transgenes. Plant Mol Biol 56:203–216
Kumar H, Priya P, Singh N, Kumar M, Choudhary BK, Kumar L, Singh IS, Kumar N (2016) RAPD and ISSR marker-based comparative evaluation of genetic diversity among Indian germplasms of Euryale ferox: an aquatic food plant. Appl Biochem Biotechnol 180:1345–1360
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549
Kumar S, Stecher G, Suleski M, Blair Hedges S (2017) TimeTree: a resource for timelines, timetrees, and divergence times. Mol Biol Evol 34:1812–1819
Kurtz S, Choudhuri JV, Ohlebusch E, Schleiermacher C, Stoye J, Giegerich R (2001) REPuter: the manifold applications of repeat analysis on a genomic scale. Nucleic Acids Res 29:4633–4642
Lagesen K, Hallin P, Rødland EA, Stærfeldt HH, Rognes T, Ussery DW (2007) RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res 35:3100–3108
Lavid N, Barkay Z, Tel-Or E (2001) Accumulation of heavy metals in epidermal glands of the waterlily (Nymphaeaceae). Planta 212:313–322
Lehwark P, Greiner S (2019) GB2sequin—a file converter preparing custom GenBank files for database submission. Genomics 111:759–761
Les DH, Garvin DK, Wimpee CF (1991) Molecular evolutionary history of ancient aquatic angiosperms. Proc Natl Acad Sci USA 88:10119–10123
Li HT, Luo Y, Gan L, Ma PF, Gao LM, Yang JB, Cai J, Gitzendanner MA, Fritsch PW, Zhang T, Jin JJ, Zeng CX, Wang H, Yu WB, Zhang R, van der Bank M, Olmstead RG, Hollingsworth PM, Chase MW, Soltis DE et al (2021a) Plastid phylogenomic insights into relationships of all flowering plant families. BMC Biol 19:232
Li HT, Yi TS, Gao LM, Ma PF, Zhang T, Yang JB, Gitzendanner MA, Fritsch PW, Cai J, Luo Y, Wang H, van der Bank M, Zhang SD, Wang QF, Wang J, Zhang ZR, Fu CN, Yang J, Hollingsworth PM, Chase MW et al (2019) Origin of angiosperms and the puzzle of the Jurassic gap. Nat Plants 5:461–470
Li J, Zhang Z, Vang S, Yu J, Wong GKS, Wang J (2009) Correlation between Ka/Ks and Ks is related to substitution model and evolutionary lineage. J Mol Evol 68:414–423
Li YT, Dong Y, Liu YC, Yu XY, Yang MS, Huang YR (2021b) Comparative analyses of Euonymus chloroplast genomes: genetic structure, screening for loci with suitable polymorphism, positive selection genes, and phylogenetic relationships within Celastrineae. Front Plant Sci 11:593984
Li ZZ, Saina JK, Gichira AW, Kyalo CM, Wang QF, Chen JM (2018) Comparative genomics of the Balsaminaceae sister genera Hydrocera triflora and Impatiens pinfanensis. Int J Mol Sci 19:1–17
Liu QP, Xue QZ (2005) Comparative studies on codon usage pattern of chloroplasts and their host nuclear genes in four plant species. J Genet 84:55–62
Liu X, Wang ZS, Shao WH, Ye ZY, Zhang JG (2017) Phylogenetic and taxonomic status analyses of the abaso section from multiple nuclear genes and plastid fragments reveal new insights into the North America origin of Populus (Salicaceae). Front Plant Sci 7:2022
Löhne C, Borsch T, Wiersema JH (2007) Phylogenetic analysis of Nymphaeales using fast-evolving and noncoding chloroplast markers. Bot J Linn Soc 154:141–163
Lowe TM, Eddy SR (1996) TRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res 25:955–964
Lu XM, Lu PZ, Chen JJ (2012) Nitrogen and phosphorus removal and morphological and physiological response in Nymphaea tetragona under various planting densities. Toxicol Environ Chem 94:1319–1330
Magallón S (2010) Using fossils to break long branches in molecular dating: a comparison of relaxed clocks applied to the origin of angiosperms. Syst Biol 59:384–399
Massoni J, Couvreur TLP, Sauquet H (2015) Five major shifts of diversification through the long evolutionary history of Magnoliidae (angiosperms) phylogenetics and phylogeography. BMC Evol Biol 15:49
Mehmood F, Abdullah SI, Ahmed I, Waheed MT, Mirza B (2020) Characterization of Withania somnifera chloroplast genome and its comparison with other selected species of Solanaceae. Genomics 112:1522–1530
Morris JL, Puttick MN, Clark JW, Edwards D, Kenrick P, Pressel S, Wellman CH, Yang Z, Schneider H, Donoghue PCJ (2018) The timescale of early land plant evolution. Proc Natl Acad Sci USA 115:E2274–E2283
Odintsova MS, Yurina NP (2003) Plastid genomes of higher plants and algae: structure and functions. Mol Biol 37:649–662
Olmstead RG, Palmer JD (1994) Chloroplast DNA systematics: a review of methods and data analysis. Am J Bot 81:1205–1224
Parveen S, Singh N, Adit A, Kumaria S, Tandon R, Agarwal M, Jagannath A, Goel S (2022) Contrasting reproductive strategies of two Nymphaea species affect existing natural genetic diversity as assessed by microsatellite markers: implications for conservation and wetlands restoration. Front Plant Sci 13:773572
Posada D, Buckley TR (2004) Model selection and model averaging in phylogenetics: advantages of Akaike information criterion and Bayesian approaches over likelihood ratio tests. Syst Biol 53:793–808
Posada D, Crandall KA (1998) Modeltest: testing the model of DNA substitution. Bioinformatics 14:817–818
Puttick MN (2019) MCMCtreeR: functions to prepare MCMCtree analyses and visualize posterior ages on trees. Bioinformatics 35:5321–5322
Qiu YL, Lee J, Bernasconi-Quadroni F, Soltis DE, Soltis PS, Zanis M, Zimmer EA, Chen Z, Savolainen V, Chase MW (1999) The earliest angiosperms: evidence from mitochondrial, plastid and nuclear genomes. Nature 402:404–407
Qu XJ, Moore MJ, Li DZ, Yi TS (2019) PGA: a software package for rapid, accurate, and flexible batch annotation of plastomes. Plant Methods 15:50
Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA (2018) Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst Biol 67:901–904
Ran JH, Shen TT, Wang MM, Wang XQ (2018) Phylogenomics resolves the deep phylogeny of seed plants and indicates partial convergent or homoplastic evolution between Gnetales and angiosperms. Proc R Soc 285:20181012
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Rozas J, Ferrer-Mata A, Sanchez-DelBarrio JC, Guirao-Rico S, Librado P, Ramos-Onsins SE, Sanchez-Gracia A (2017) DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol Biol Evol 34:3299–3302
Ruhfel BR, Gitzendanner MA, Soltis PS, Soltis DE, Burleigh JG (2014) From algae to angiosperms-inferring the phylogeny of green plants (Viridiplantae) from 360 plastid genomes. BMC Evol Biol 14:23
Saarela JM, Rai HS, Doyle JA, Endress PK, Mathews S, Marchant AD, Briggs BG, Graham SW (2007) Hydatellaceae identified as a new branch near the base of the angiosperm phylogenetic tree. Nature 446:312–315
Sato S, Nakamura Y, Kaneko T, Asamizu E, Tabata S (1999) Complete structure of the chloroplast genome of Arabidopsis thaliana. DNA Res 290:283–290
Shi C, Wang S, Cai HH, Zhang HR, Long XX, Tihelka E, Song WC, Feng Q, Jiang RX, Cai CY, Lombard N, Li X, Yuan J, Zhu JP, Yang HY, Liu XF, Xiang QP, Zhao ZT, Long CL, Schneider H et al (2022) Fire-prone Rhamnaceae with South African affinities in Cretaceous Myanmar amber. Nat Plants 8:125–135
Singh R, Singh G (2022) Association of aphids with plants belonging to order Nymphaeales, Austrobaileyales, Laurales, Magnoliales and Piperales (Angiosperms) in India. J Appl Entomol 2:54–60
Smith DR (2015) Mutation rates in plastid genomes: they are lower than you might think. Genome Biol Evol 7:1227–1234
Sokoloff DD, Macfarlane TD, Remizowa MV, Rudall PJ (2008) Classification of the early-divergent angiosperm family Hydatellaceae: one genus instead of two, four new species and sexual dimorphism in dioecious taxa. Taxon 57:179–200
Soltis PS, Soltis DE, Zanis MJ, Kim S (2000) Basal lineages of angiosperms: relationships and implications for floral evolution. Int J Plant Sci 161:96–107
Song WC, Chen ZM, He L, Feng Q, Zhang HR, Du GL, Shi C (2022a) Comparative chloroplast genome analysis of wax gourd (Benincasa hispida) with three Benincaseae species, revealing evolutionary dynamic patterns and phylogenetic implications. Genes 13:461
Song WC, Ji CX, Chen ZM, Cai HH, Wu XM, Shi C, Wang S (2022b) Comparative analysis the complete chloroplast genomes of nine Musa species: genomic features, comparative analysis, and phylogenetic implications. Front Plant Sci 13:1–15
Sun C, Chen F, Teng N, Xu Y, Dai Z (2021) Comparative analysis of the complete chloroplast genome of seven Nymphaea species. Aquat Bot 170:103353
Sun L, Fang L, Zhang Z, Chang X, Penny D, Zhong B (2016) Chloroplast phylogenomic inference of green algae relationships. Sci Rep 6:20528
Tillich M, Lehwark P, Pellizzer T, Ulbricht-Jones ES, Fischer A, Bock R, Greiner S (2017) GeSeq - Versatile and accurate annotation of organelle genomes. Nucleic Acids Res 45:W6–W11
Wang RJ, Cheng CL, Chang CC, Wu CL, Su TM, Chaw SM (2008) Dynamics and evolution of the inverted repeat-large single copy junctions in the chloroplast genomes of monocots. BMC Evol Biol 8:36
Widmer A, Baltisberger M (1999) Extensive intraspecific chloroplast DNA (cpDNA) variation in the alpine Draba aizoides L. (Brassicaceae): haplotype relationships and population structure. Mol Ecol 8:1405–1415
Wu CS, Chaw SM (2015) Evolutionary stasis in cycad plastomes and the first case of plastome GC-biased gene conversion. Genome Biol Evol 7:2000–2009
Wu Z, Gui S, Quan Z, Pan L, Wang S, Ke W, Liang D, Ding Y (2014) A precise chloroplast genome of Nelumbo nucifera (Nelumbonaceae) evaluated with Sanger, Illumina MiSeq, and PacBio RS II sequencing platforms: insight into the plastid evolution of basal eudicots. BMC Plant Biol 14:289
Xi ZX, Liu L, Rest JS, Davis CC (2014) Coalescent versus concatenation methods and the placement of Amborella as sister to water lilies. Syst Biol 63:919–932
Yamada T, Imaichi R, Kato M (2001) Developmental morphology of ovules and seeds of Nymphaeales. Am J Bot 88:963–974
Yang J, Hu G, Hu G (2022) Comparative genomics and phylogenetic relationships of two endemic and endangered species (Handeliodendron bodinieri and Eurycorymbus cavaleriei) of two monotypic genera within Sapindales. BMC Genom 23:1–22
Yang X, Luo X, Cai X (2014) Analysis of codon usage pattern in Taenia saginata based on a transcriptome dataset. Parasit Vectors 7:527
Yang YY, Qu XJ, Zhang R, Stull GW, Yi TS (2021) Plastid phylogenomic analyses of Fagales reveal signatures of conflict and ancient chloroplast capture. Mol Phylogenet Evol 163:107232
Yang YZ, Sun PH, Lv LK, Wang DL, Ru DF, Li Y, Ma T, Zhang L, Shen XX, Meng FB, Jiao BB, Shan LX, Liu M, Wang QF, Qin ZJ, Xi ZX, Wang XY, Davis CC, Liu JQ (2020) Prickly waterlily and rigid hornwort genomes shed light on early angiosperm evolution. Nat Plants 6:215–222
Yang Z (2007) PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol 24:1586–1591
Ye WQ, Yap ZY, Li P, Comes HP, Qiu YX (2018) Plastome organization, genome-based phylogeny and evolution of plastid genes in Podophylloideae (Berberidaceae). Mol Phylogenet Evol 127:978–987
Zanis MJ, Soltis DE, Soltis PS, Mathews S, Donoghue MJ (2002) The root of the angiosperms revisited. Proc Natl Acad Sci USA 99:6848–6853
Zeng L, Zhang Q, Sun R, Kong H, Zhang N, Ma H (2014) Resolution of deep angiosperm phylogeny using conserved nuclear genes and estimates of early divergence times. Nat Commun 5:4956
Zhang LS, Chen F, Zhang XT, Li Z, Zhao YY, Lohaus R, Chang XJ, Dong W, Ho SYW, Liu X, Song A, Chen JH, Guo WL, Wang ZJ, Zhuang YY, Wang HF, Chen XQ, Hu J, Liu YH, Qin Y et al (2020) The water lily genome and the early evolution of flowering plants. Nature 577:79–84
Zheng S, Poczai P, Hyvönen J, Tang J, Amiryousefi A (2020) Chloroplot: an online program for the versatile plotting of organelle genomes. Front Genet 11:576124
Zhou M, Long W, Li X (2008) Patterns of synonymous codon usage bias in chloroplast genomes of seed plants. For Stud China 10:235–242
Zhou T, Ruhsam M, Wang J, Zhu H, Li W, Zhang X, Xu Y, Xu F, Wang X (2019) The complete chloroplast genome of Euphrasia regelii, pseudogenization of ndhH genes and the phylogenetic relationships within Orobanchaceae. Front Genet 10:444
Zhu QH, Ge S (2005) Phylogenetic relationships among A-genome species of the genus Oryza revealed by intron sequences of four nuclear genes. New Phytol 167:249–265
Acknowledgements
We thank Qi Feng, Hongrui Zhang, and Rixin Jiang for their help in species identification and molecular experiments. We are very grateful to Freie Universitaet Berlin for sequencing data for this study. This work was supported by the National Natural Science Foundation of China (No. 32370244), the Taishan Scholar Project (No. tsqn202306214), the Shandong Province Natural Science Foundation of China (No. ZR2023MC157), and the State Key Laboratory of Palaeobiology and Stratigraphy (No. 223123).
Author information
Authors and Affiliations
Contributions
WCS and CS conceived and designed the research. WBS and ZRZ analyzed the data. WCS, HW, ZRZ and JL completed the experiments and prepared the tables. WBS and RQT prepared the figures. WCS wrote the manuscript. SW revised the manuscript. All authors edited and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest related to this work and the manuscript has been approved by all authors for publication.
Animal and human rights statement
This article does not contain any studies with human participants or animals performed by the authors.
Additional information
Edited by Jiamei Li.
Supplementary Information
Below is the link to the electronic supplementary material.
42995_2024_242_MOESM1_ESM.pdf
Figure S1: The results of mapping the reads to the assembled Nymphaea complete chloroplast genome sequences (PDF 1481 KB)
42995_2024_242_MOESM2_ESM.pdf
Figure S2: Sliding window analysis of 12 Nymphaea chloroplast genomes (PDF 262 KB)
42995_2024_242_MOESM3_ESM.xlsx
Table S1: Information on raw sequencing data (XLSX 11 KB)
42995_2024_242_MOESM4_ESM.xlsx
Table S2: Information on the 43 species in the phylogenetic tree (XLSX 13 KB)
42995_2024_242_MOESM5_ESM.xlsx
Table S3: codon usage details (XLSX 12 KB)
42995_2024_242_MOESM6_ESM.xlsx
Table S4: RNA editing sites raw data (XLSX 17 KB)
42995_2024_242_MOESM7_ESM.xlsx
Table S5: Details of microsatellite structures (XLSX 12 KB)
42995_2024_242_MOESM8_ESM.xlsx
Table S6: Value of Ka/Ks in 12 Nymphaea species (XLSX 30 KB)
42995_2024_242_MOESM9_ESM.xlsx
Table S7: Time estimation tree with credibility intervals (XLSX 11 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Song, W., Shi, W., Wang, H. et al. Comparative analysis of 12 water lily plastid genomes reveals genomic divergence and evolutionary relationships in early flowering plants. Mar Life Sci Technol 6, 425–441 (2024). https://doi.org/10.1007/s42995-024-00242-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42995-024-00242-0