Abstract
The production of an all-triploid population by mating tetraploid males with diploid females is the best and most fundamental method for the large-scale production of triploid oysters. Obtaining a stable tetraploid population is essential for guaranteed production in industrialized triploid cultivation. C. hongkongensis and C. sikamea are important oyster breeding species in southern China, and have great economic value. However, there are not any published data on inducing tetraploid C. hongkongensis or C. sikamea. Therefore, we investigated tetraploid induction in these two oyster species by inhibiting the PB1 release in diploid fertilized eggs using Cytochalasin B (CB) under 31 °C, 15 ‰ salinity. The results confirmed that the optimal tetraploid induction conditions for C. hongkongensis were a CB concentration of 0.50 mg/L with induction starting at 9.0 min after fertilization, and stopping at 21.0 min after fertilization; the induction efficiency index reached 0.123 under these conditions. The optimal tetraploid induction conditions for C. sikamea were a CB concentration of 0.50 mg/L, with induction starting at 7.5 min after fertilization and stopping at 18 min after fertilization; the induction efficiency index could be as high as 0.281 under these conditions. However, we confirmed that the tetraploid rate decreased with larval growth, and no tetraploids were detected in the juvenile period of either C. hongkongensis or C. sikamea. This may be attributed to the very low survival of the tetraploid larvae induced by this method, especially as most tetraploid larvae died during the first three days. In summary, it is simple to directly induce tetraploid C. hongkongensis and C. sikamea larvae by inhibiting the PB1 release of diploid zygotes, but the low survival rate makes it challenging to obtain viable juvenile tetraploids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since Stanley et al. (1981) first successfully induced triploid Crassostrea virginica by inhibiting polar body 2 (PB2) release of diploid zygotes, many experts have demonstrated that triploid shellfish show fast growth, poor fertility, high survival rates, and good meat quality (Barreto-Hernández et al. 2018; Degremont et al. 2012; Hand et al. 2004; Qin et al. 2019; Wu et al. 2019). Based on these advantages, triploid breeding has become an important part of shellfish breeding (Callam et al. 2016; Francesc et al. 2009; Nell 2002). However, the physical and chemical methods (i.e., CB, 6-DMAP, caffeine and cold shock.) for directly inducing triploid oysters often result in low triploid rates, low D larval rates and unstable ploidy levels, and are complex to execute (Allen and Bushek 1992; Gerard et al. 1994; Qin et al. 2017). So, the production of 100% triploids by the interploid crossing of tetraploid sperm and diploid eggs is the most practical method for obtaining large numbers of triploids (Francesc et al. 2009; Guo 1991; Guo and Allen 1994; Nell 2002). Therefore, tetraploid breeding stocks are critical for the commercial production and breeding of triploid oysters. However, viable tetraploid oysters are particularly difficult to obtain. Since Guo and Allen (1994) successfully induced tetraploid C. gigas, many scientists have made substantial efforts to induce tetraploid shellfish, but few have obtained viable juvenile tetraploids (Benabdelmouna and Ledu 2015; Eudeline et al. 2000b; Peachey and Allen 2016; Tan et al. 2017; Yang and Guo 2006b; Yang et al. 2000).
To date, there are three recognized methods for inducing tetraploid shellfish, although the commercial application of tetraploid technology is still limited to C. gigas and C. virginica. The first method of inducing tetraploid shellfish is to inhibit PB1 release of diploid fertilized eggs. This method was first proposed by Guo (1991), but he did not obtain viable tetraploid juvenile oysters (Guo 1991; Guo et al. 1992a, b). The lack of viable tetraploids was attributed to the “cell-number deficiency” hypothesis, which was based on the fact that a tetraploid cell would have to contain twice as much nuclear material as a diploid cell. However, the abnormal nucleus/cytoplasm ratio in a diploid cell would cause abnormal mitosis and a reduction in cell number (Francesc et al. 2009; Guo 1991; Tan et al. 2017; Yang et al. 2019). Since then, many scientists have used this method to induce tetraploids in other shellfish, but the number of tetraploids surviving to the juvenile stage is typically low (Allen et al. 1994; Peruzzi and Guo 2002; Tan et al. 2017; Yang and Guo 2006a; Yang et al. 2000). However, based on the above method, Benabdelmouna and Ledu (2007, 2015) succeeded in generating tetraploid larvae that survived to the larval period and produced viable and fertile autotetraploid C. gigas. These workers did not believe that egg size was a limiting factor for successful tetraploid induction. The mechanism of this method is that diploid fertilized eggs undergo multipolar division after the PB1 release is successfully inhibited, and then, some eggs form triploids (Fig. 1), with others forming tetraploids (Benabdelmouna and Ledu 2015; Guo 1991; Guo et al. 1992a; McCombie et al. 2009; Peachey and Allen 2016; Yang et al. 2019).
Schematic diagram of triploid formation by inhibiting the polar body 1 or the polar body 2 release of diploid fertilized eggs during tetraploid induction. A Schematic diagram of triploid formation by inhibiting the PB1 release of diploid zygotes. B Schematic diagram of triploid formation by inhibiting the PB2 release of diploid zygotes
Subsequently, Guo and Allen (1994) proposed a method to induce tetraploids using eggs from triploids that were fertilized with sperm from diploids along with PB1 inhibition. These workers obtained viable tetraploid juvenile C. gigas using this method. However, there are two difficulties: the poor fertility of triploids and the low tetraploid rate (Allen et al. 1994; Brake et al. 2004; Eudeline et al. 2000b; Francesc et al. 2009; Peachey and Allen 2016). The third method is a complementary approach that enriches tetraploid genetic diversity. The indispensable condition for using this method is to first confirm the presence of tetraploid sperm. In this method, tetraploids are induced by inhibiting PB2 release after crossing tetraploid males and diploid females. McCombie et al. (2005) used this method to obtain viable tetraploid juvenile C. gigas, but other researchers considered that inducing tetraploids by this method was a challenge because of the low survival rate of the tetraploids originated from diploid eggs (Francesc et al. 2009; Yang et al. 2019).
C. hongkongensis and C. sikamea are native estuarine species in southern China, and the animals have high economic value. Previously, we confirmed the growth and survival advantages of triploid C. hongkongensis and C. sikamea, but not any published papers were available regarding tetraploid induction in these species (Qin et al. 2019; Wu et al. 2019). Moreover, the effectiveness of tetraploid induction methods varies depending on the species, and determining which method will be most effective usually requires trials and optimization experiments (Francesc et al. 2009; Gerard et al. 1999; Yang and Guo 2006b).
The application of an appropriate and highly effective method is a key requirement for obtaining a successful breeding population of tetraploids. In this study, we designed different CB concentrations and duration time gradients to confirm the optimal induction conditions for tetraploid C. hongkongensis and C. sikamea by inhibiting PB1 release from diploid fertilized eggs. Furthermore, we assessed the variations in the ploidy profiles of larvae and juvenile oysters under optimal conditions. Based on these experiments, we provided a basis for the successful induction of tetraploid C. hongkongensis and C. sikamea.
Results
Optimization of tetraploid induction by inhibiting the polar body 1 release of diploid zygotes
Since the parent oysters have a substantial impact on the results of the experiments, it is recommended to compare the results that originated from the same batch of parental oysters. The cleavage rate was measured at 1.5 h; the corresponding D larval rate was measured at 18.0 h, and the tetraploid rate was identified at 36.0 h all after fertilization. Based on the results, we confirmed that the optimal induction conditions for tetraploid C. hongkongensis was a CB concentration of 0.50 mg/L with induction starting at 9.0 min and stopping at 21.0 min both after fertilization. Under these conditions, the induction efficiency index was highest (0.123), the cleavage rate was 78.71 ± 13.56%, the D larval rate was 67.43 ± 0.71%, and the tetraploid rate was 18.22 ± 0.68% (Table 1). Different treatment durations produced different proportions of tetraploids. Delayed induction start and stop times led to decreases in the cleavage rate and the D larval rate, which led to a reduction in the induction efficiency index. The lowest induction efficiency index was 0.15, and most eggs were stunted and deformed under these conditions (Table 1). The cleavage rate and D larval rate of the control group were significantly higher than those of the experimental groups.
The optimal induction condition for tetraploid C. sikamea was a CB concentration of 0.50 mg/L with induction starting at 7.5 min and stopping at 18 min after fertilization. The cleavage rate was 65.09 ± 5.23%, the D larval rate was 53.29 ± 0.94%, the tetraploid rate was 52.81 ± 4.33%, and the induction efficiency index (0.281) was highest under the optimal conditions (Table 2). Similarly, a delayed treatment start time and extended induction time caused decreases in the cleavage rate, D larval rate, and induction efficiency index. The lowest induction efficiency index was 0.022 (when the induction start and stop times were extended by 3 min and 6 min, respectively); whereas, the cleavage rate (21.55 ± 1.41%) and tetraploid rate (10.33 ± 1.44%) were significantly lower than those in the other experimental groups (Table 2). After CB treatment, partially induced eggs suffered from abnormal development (some fertilized eggs did not develop normally to the D larvae), and all experimental groups showed significantly lower cleavage rates and D larval rates than the control group. In addition, the optimal CB treatment concentration for the two oysters was the same, but the optimal start induction and stop induction times were quite different between C. hongkongensis and C. sikamea.
Variations in the tetraploid rate after optimal induction treatment
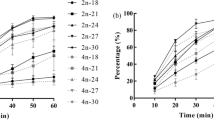
After determining the optimal conditions for tetraploid induction by inhibiting the PB1 release of C. hongkongensis diploid zygotes, we induced four batches of tetraploid groups under these conditions (the same source but different batches of parent oysters were used), followed by the identification of their DNA ploidy levels on the first, third, fifth, seventh, tenth, thirteenth and thirtieth days after fertilization. Based on the experimental results, we found that the ploidy composition of the larvae changed considerably over time, and the tetraploid rate gradually decreased. On the first day, the diploid rate was 39.84 ± 1.91%, the triploid rate was 32.62 ± 6.64%, the tetraploid rate was 22.82 ± 6.56% and the pentaploid rate was 4.72 ± 0.96%. Moreover, on the thirteenth day, the diploid rate was 57.37 ± 1.01%, the triploid rate was 40.79 ± 1.59% and the tetraploid rate was 1.84 ± 0.64%. However, on the thirtieth day, we identified 120 individual oysters, and could not detect the presence of any tetraploids; only 59.87 ± 4.01% diploids and 40.13 ± 4.01% triploids were identified (Fig. 2, Table 3).
As with C. hongkongensis, we identified the ploidy profiles of C. sikamea on the first, second, fifth, seventh, ninth, fifteenth and thirtieth days after fertilization (Fig. 3). The ploidy profiles of C. sikamea larvae were analyzed by a CyFlow Ploidy Analyser. All larvae were diploid in the control group. The ploidy profile of the larvae varied greatly over time, and the decrease in the tetraploid rate was obvious. On the first day after fertilization, the larval ploidy profile consisted of 2 N 24.34 ± 5.78%, 3 N 10.51 ± 5.09%, 4 N 55.49 ± 8.66%, and others 8.74 ± 2.99%. On the second day, the proportion of cells in each ploidy class was classified as 46.82 ± 5.40% diploid, 20.57 ± 6.48% triploid, 28.02 ± 6.18% tetraploid, and 3.61 ± 3.90% other. However, on the fifteenth day, the diploid rate increased to 71.96 ± 5.62%, the triploid rate increased to 26.33 ± 4.50%, and the tetraploid rate decreased to 1.71 ± 1.43%. In addition, on the thirtieth day, there were 80.00 ± 6.15% diploids and 19.99 ± 6.15% triploids of the 120 juvenile individuals, and no tetraploids were detected in any of the replicates (Fig. 3, Table 4).
Discussion
The tetraploid rate was significantly affected by the variations of induction conditions
Polyploid breeding in oyster species has been a popular topic of study since Stanley et al. (1981) successfully induced triploid C. virginica with CB. Researchers have developed and honed many polyploid induction methods for oysters (such as CB, 6-DMAP, caffeine, salinity stimulation, heat shock), and majority researchers consider CB to be an efficient inducing reagent (Allen and Downing 1986; Arai et al. 1986; Benabdelmouna and Ledu 2015; Gerard et al. 1999; Guo et al. 1992a, b; Qin et al. 2017; Tan et al. 2017). Some researchers confirmed the feasibility of inducing tetraploid oysters by inhibiting PB1 release from diploid fertilized eggs (Guo et al. 1992a; Peruzzi and Guo 2002; Tan et al. 2017), and Benabdelmouna and Ledu (2015) successfully produced a breeding population of tetraploid C. gigas by this direct method. However, no papers have been published on inducing tetraploid of C. hongkongensis and C. sikamea. Therefore, we considered it worthwhile to study tetraploid induction in these two oyster species by inhibiting PB1 release of diploid crosses with CB.
CB mainly affects actin polymerization, which is particularly important for forming the cleavage furrow of the polar body. The effect on fertilized eggs is almost immediate, so the induction time needs to be precisely controlled (Allen et al. 1989; Barber et al. 1992; Barreto-Hernández et al. 2018). Our results confirmed that both the CB concentration and the induction duration strongly influenced the normal development of eggs and the tetraploid rates. In both C. hongkongensis and C. sikamea, compared with that at 0.50 mg/L, the tetraploid rate was much lower when the CB concentration was 0.25 mg/L, and the cleavage rate and D larval rate decreased sharply when the concentration was 0.75 or 1.00 mg/L (Tables 1, 2). If the CB concentration is excessive low, the release of PB1 is not inhibited successfully, so the induction efficiency index is low. Also, if the CB concentration is excessive high, some eggs cannot develop normally, and the deformity rate begins to rise (Barreto-Hernández et al. 2018; Ledu and McCombie 2003; Qin et al. 2017). CB has some toxic effects on developing eggs, so the D larval rates of all experimental groups are significantly lower than that of the control group (Peachey and Allen 2016; Yang and Guo 2006b).
According to previous studies, proper initiation and duration of induction times were particularly important for improving polyploid rates (Francesc et al. 2009; Nell 2002; Qin et al. 2017; Wadsworth et al. 2019). By inhibiting the PB1 release of diploid fertilized eggs to induce tetraploids, if the start induction time is early, some eggs will become deformed. If the start induction time is late, the PB1 of some eggs will have already been released, and further induction would lead to an increase in diploid and triploid rates. Also, we found that both C. hongkongensis and C. sikamea had low tetraploid rates when the treatment stop time was excessively early because most of the PB1 release was not inhibited successfully. In addition, when the stop induction time was delayed, the deformity rate and the pentaploid rate increased because both PB1 and PB2 were inhibited in some eggs. The same phenomena were also found in polyploid induction of other shellfish (Arai et al. 1986; Gerard et al. 1994; Ledu and McCombie 2003; Peachey and Allen 2016; Qin et al. 2017; Tan et al. 2017; Verdugo et al. 2000). Triploid production during induction was mainly due to the inhibition of the PB1 or PB2 release (Fig. 1).
The differences in the optimal induction conditions for tetraploid C. hongkongensis and C. sikamea
The experimental results showed that the optimal CB concentration for tetraploid induction was the same for both C. hongkongensis and C. sikamea. The results were consistent with the optimal CB concentrations for triploid induction in C. gigas, C. virginica, C. sikamea, and C. hongkongensis, suggesting that this concentration is sufficient to inhibit polar body release (Downing and Allen 1987; Qin et al. 2017; Stanley et al. 1981; Wu et al. 2019). However, the optimal start induction and stop induction times were different between C. hongkongensis and C. sikamea, which might be attributed to the difference in the releasing rate of PB1. According to previous studies, the temperature, salinity, hydration time, serotonin level, oyster source, and species of parent oysters all have effects on the dynamics of the polar body release (Eudeline et al. 2000a; Qin et al. 2018). In this experiment, the temperature, salinity and parental source were all consistent during fertilization, so the notable differences in the PB1 release could be attributed mainly to the difference in oyster species. Also, other researchers demonstrated that the polar body release in different species was quite different (Barber et al. 1992; Gerard et al. 1994; Mallia et al. 2010; Tan et al. 2017; Wu et al. 2019). Through the observation of PB1 release in C. hongkongensis and C. sikamea, we found that although the optimal start induction and stop induction times of the two species were different, the corresponding polar body release ratios were consistent (Qin et al. 2018; Wu 2019). The optimal start induction times for the two oysters both corresponded to approximately 5% of the PB1 release, and the optimal stop induction times both corresponded to approximately 60% of the PB1 release. Also, this indicated that the polar body release rate of C. sikamea was faster than that of C. hongkongensis in the same environment (Qin et al. 2017; Wu 2019; Wu et al. 2019). These two polar body release ratios may be used as initiation and termination markers of tetraploid induction by inhibiting PB1 release of diploid zygotes.
After the optimal induction treatment, the tetraploid rate decreased with larval development
We confirmed that the tetraploid rates decreased with larval growth, and the presence of tetraploids was not detected in the juvenile period in either C. hongkongensis or C. sikamea. This may be due to the death of tetraploids during growth, especially during the first three days of the larval stage. The low survival rate might be the result of tetraploidy and the culture conditions (Eudeline et al. 2000b; Francesc et al. 2009; Tan et al. 2017; Yang and Guo 2006a). Tetraploid cell architecture and numbers have changed, which could impose developmental and physiological hardships on larvae, and a lack of careful breeding of tetraploid larvae aggravates the disadvantages of tetraploids (Luca 2005; Francesc et al. 2009; Peachey and Allen 2016). The mechanism of inducing tetraploids by inhibiting the PB1 release of diploid fertilized eggs is that inhibiting the PB1 release will lead to the occurrence of biopolar and tripolar divisions at the PB2, resulting in the production of partial tetraploids (Benabdelmouna and Ledu 2007, 2015; Guo 1991; Guo et al. 1992a). During the induction process, triploidy is produced at the same time, which is also due to the inhibition of the PB1 or PB2 of some diploid zygotes (Fig. 1). However, the direct induction of tetraploids by inhibiting the PB1 release of diploid fertilized eggs has proven to be difficult in shellfish. Thus, many researchers attributed the failure of this method to the cell-number deficiency due to the small volume of the diploid eggs (Francesc et al. 2009; Guo and Allen 1994; Guo et al. 1992b; Miller et al. 2014; Yang et al 2019). The mismatch between a normal-volume diploid shellfish egg and a large tetraploid nucleus was more likely to lead to cell-number deficiency compared to fish eggs, where the cell-number deficiency could be compensated at the later development of zygotes (Francesc et al. 2009; Guo and Allen 1994). Also, Guo (1991) attempted to directly select large diploid eggs to induce tetraploids. However, diploid egg size varies only slightly, so it would be difficult to induce tetraploids using diploid eggs with this method.
However, Benabdelmouna and Ledu (2015), Mccombie et al. (2005, 2009) and Tan et al. (2017) obtained tetraploid oysters through diploid eggs. Moreover, Benabdelmouna and Ledu (2007, 2015) confirmed that oocyte size was not a limiting factor for the success of tetraploid induction, and they obtained a patent for successfully inducing tetraploids by inhibiting the PB1 release in diploid zygotes. McCombie et al. (2005) were the first workers to demonstrate that viable tetraploid oysters could be produced using large diploid eggs. Consequently, they obtained a patent for inducing tetraploids by inhibiting PB2 release after diploid eggs were crossed with tetraploid sperm. The optimal breeding environment and the careful cultivation of the tetraploid larvae derived from the diploid eggs might be the reasons that they were able to obtain viable tetraploid oysters (Benabdelmouna and Ledu 2007; Benabdelmouna et al. 2007; Francesc et al. 2009). In this study, we tried many times with the same source but different parental batches of C. hongkongensis and C. sikamea, and found that although tetraploids could always be identified before metamorphosis, juvenile tetraploids were never detected. The treatment effect was highly variable from egg batch to egg batch in terms of the tetraploid percentage (Tables 3, 4). In conclusion, we confirmed that tetraploid larvae induced by inhibiting PB1 release of diploid fertilized eggs had difficulty reaching metamorphosis and attachment, so it was difficult to obtain tetraploid juvenile C. hongkongensis and C. sikamea directly using diploid zygotes. Moreover, no stable tetraploid oyster population originated from diploid zygotes has yet been obtained and applied to commercial promotion, and we have decided to abandon further exploration of this method.
Materials and methods
Preparation of parent oysters
The sexually mature diploid C. hongkongensis and C. sikamea used in this study were collected from Zhulin in Beihai, China. The parent oysters were artificially cultivated in an open circulating system (temperature 29.0–32.0 °C, salinity 15 ‰ salinity, pH 7.8–8.1) for at least one week before being used for later experiments. During the temporary rearing period, the parent oysters were fed twice daily with plenty of Isochrysis zhanjiangensis and Chaetoceros calcitrans, and stimulation was minimized to prevent gamete discharge (Qin et al. 2019).
Gamete preparation and fertilization
The oysters selected for the experiment were dissected, and the gametes were subsequently collected. The parent oysters were carefully shucked using a scalpel knife, sexed under a light microscope, and then segregated by sex. Before fertilization, all containers used in this study were cleaned with fresh water to prevent any accidental fertilization. Eggs were separated from faeces and large tissue debris by passing through a 48 μm nylon screen, and then rinsed on a 25 μm nylon screen to wash out broken eggs and other small impurities. Then, the eggs were soaked in seawater under optimum conditions (31 °C, 15 ‰ salinity) until germinal vesicle breakdown was observed, which was a sign that meiosis was ready to resume initiation (Qin et al. 2018).
Sperm were separated from impurities by passing through a 40 μm screen and were resuspended in seawater (31 °C, 15 ‰ salinity) for activation 10 min before fertilization. Just before fertilization, the most active sperm group was selected, and the fertilization procedure was performed following the protocol of Qin et al. (2019). According to a previous study, the release of polar bodies varies significantly under different environmental conditions (Qin et al. 2018). Therefore, before the formal experiment, pre-fertilization was performed to investigate the polar body release pattern at 31 °C with 15 ‰ salinity seawater.
Experimental protocol
The optimal CB treatment conditions for inducing polyploid bivalves are usually characterized by different concentrations, start induction times and stop induction times. CB was dissolved in dimethyl sulfoxide (DMSO) and stored at − 20 °C (Qin et al. 2019). Based on the previous studies (Qin et al. 2018; Wu 2019) and the polar body release pattern from pre-fertilization, the CB concentration gradient was set to 0.25, 0.50, 0.75, and 1.00 mg/L, the gradient of start induction time was set to 6.0, 7.5, 9.0 and 10.5 min after fertilization, and the gradient of stop induction time was set to 12.0, 15.0, 18.0, 21.0 and 24.0 min after fertilization (Barber et al. 1992; Peachey and Allen 2016; Qin et al. 2017, 2018). Then, induced combinations were established as shown in Tables 1 and 2. Three replicates were established for each group, and a control group was also established.
Briefly, the soaked eggs of five females were pooled and divided into corresponding groups, then fertilized with equal and sufficient sperm from one male. Then, based on Table 1 or Table 2, the corresponding CB concentration was used at the corresponding start induction time to the corresponding stop induction time. After the induction treatment, the drugs were washed off with a 25 μm nylon screen and the eggs were cultured at 31.0 °C in 15 ‰ salinity seawater and supplied with slow aeration. Then, the cleavage rate, D larval rate and tetraploid ratio of all groups were measured. In addition, after determining the optimal CB treatment conditions for inducing tetraploids, four batches of tetraploids (the same source but different batches of parent oysters were used) were further induced using these conditions, and the variations in the tetraploid rate were tracked and measured.
DNA ploidy determination
The DNA ploidy level was identified using flow cytometry with a CyFlow Ploidy Analyser (Sysmex, Japan) according to Qin et al. (2018). In the planktonic larval stage, about 200–300 larvae of free-swimming larvae were collected on a 48 µm nylon screen, and the larval suspensions were centrifuged at 3000 r/min for 3 min. After the removal of the supernatant, 0.20 ml of nuclear extraction buffer and 0.80 ml of DAPI staining buffer (Sysmex, Japan) were added to each tube. The larvae were resuspended by gentle mixing and stained for 10 min at room temperature. Then, the samples were filtered through a 42 µm nylon screen and immediately analyzed using flow cytometry. In addition, the DNA ploidy level of juvenile oysters was individually identified by flow cytometry using a small tube of lymph extracted from each oyster and the method described above.
Statistical analysis
The cleavage rate is defined as the proportion of cleaving eggs of the total fertilized eggs, and the D larval rate is defined as the proportion of D larvae of the total fertilized eggs. The induction efficiency index is the product of the D larval rate and the tetraploid rate (Qin et al. 2017). All data in this paper are presented as the mean ± standard deviation (M ± SD). Multiple comparisons of the cleavage rate, D larval rate and tetraploid ratio of all groups were performed using one-way analysis of variance (ANOVA) followed by Duncan test using SPSS18. P < 0.05 was considered significant, while P < 0.01 was considered highly significant.
References
Allen S, Bushek D (1992) Large-Scale production of triploid oyster Crassostrea virginica (Gmelin), using “stripped” gametes. Aquaculture 103:241–251
Allen SK, Downing SL (1986) Performance of triploid Pacific oysters, Crassostrea gigas (Thunberg). I. Survival, growth, glycogen content, and sexual maturation in yearlings. J Exp Mar Biol Ecol 102:197–208
Allen SK, Downing SL, Chew KK (1989) Hatchery manual for producing triploid oysters. Washington Sea Grant Program
Allen SK, Shpigel M, Utting S, Spencer B (1994) Incidental production of tetraploid Manila clams, Tapes philippinarum (Adams and Reeve). Aquaculture 128:13–19
Arai K, Naito F, Fujino K (1986) Triploidization of the Pacific abalone with temperature and pressure treatments. Nippon Suisan Gakkaishi 52:417–422
Barber BJ, Mann R, Allen S (1992) Optimization of triploid induction for the oyster Crassostrea virginica (Gmelin). Aquaculture 106:21–26
Barreto-Hernández A, Velasco LA, Winkler FM (2018) Effect of three triploidy induction methods on the growth and survival of larvae and post-larvae of the Caribbean scallop Argopecten nucleus. Aquac Res 49:1578–1587
Benabdelmouna A, Ledu C (2007) Obtention de mollusques bivalves tétraploïdes à partir de géniteurs diploïdes. FR patent 2913982-A2913981
Benabdelmouna A, Ledu C (2015) Autotetraploid Pacific oysters (Crassostrea gigas) obtained using normal diploid eggs: induction and impact on cytogenetic stability. Genome 58:333–348
Benabdelmouna A, Ledu C, Gerard A (2007) Obtention de mollusques bivalves tétraploïdes à partir du croisement de femelles diploïdes et de mâles tétraploïdes. FR patent 2913983-A2913981
Brake J, Davidson J, Davis J (2004) Field observations on growth, gametogenesis, and sex ratio of triploid and diploid Mytilus edulis. Aquaculture 236:179–191
Callam BR, Allen SK, Frank-Lawale A (2016) Genetic and environmental influence on triploid Crassostrea virginica grown in Chesapeake Bay: growth. Aquaculture 452:97–106
Degremont L, Garcia C, Frank-Lawale A, Allen SK (2012) Triploid oysters in the Chesapeake Bay: comparison of diploid and triploid Crassostrea virginica. J Shellfish Res 31:21–31
Downing SL, Allen SK (1987) Induced triploidy in the Pacific oyster, Crassostrea gigas: optimal treatments with cytochalasin B depend on temperature. Aquaculture 61:1–15
Eudeline B, Allen SK, Guo XM (2000a) Delayed meiosis and polar body release in eggs of triploid Pacific oysters, Crassostrea gigas, in relation to tetraploid production. J Exp Mar Bio Ecol 248:151–161
Eudeline B, Allen SK, Guo XM (2000b) Optimization of tetraploid induction in Pacific oysters, Crassostrea gigas, using first polar body as a natural indicator. Aquaculture 187:73–84
Francesc P, Andy B, Jeanclaude FR, Martin FH, Pierrick H, Lorenzo C (2009) Polyploid fish and shellfish: production, biology and applications to aquaculture for performance improvement and genetic containment. Aquaculture 293:125–156
Gerard A, Naciri Y, Peignon JM, Ledu C, Phelipot P (1994) Optimization of triploid induction by the use of 6-DMAP for the oyster Crassostrea gigas (Thunberg). Aquacult Res 25:709–719
Gerard A, Ledu C, Phelipot P, Naciri-Graven Y (1999) The induction of MI and MII triploids in the Pacific oyster Crassostrea gigas with 6-DMAP or CB. Aquaculture 174:229–242
Guo XM (1991) Studies on tetraploid induction in the Pacific oyster, Crassostrea gigas (Thunberg). PhD thesis, University of Washington.
Guo XM, Allen SK (1994) Viable tetraploid Pacific oyster (Crassostrea gigas Thunburg) produced by inhibiting polar body I in eggs of triploids. Mol Mar Biol Biotechnol 3:42–50
Guo XM, Hershberger WK, Cooper K, Chew KK (1992a) Genetic consequences of blocking polar body I with cytochalasin B in fertilized eggs of the Pacific oyster, Crassostrea gigas: II. Segregation of chromosomes. Bio Bullet 183:387–393
Guo XM, Cooper K, Hershberger WK, Chew KK (1992b) Genetic consequences of blocking polar body I with cytochalasin B in fertilized eggs of the Pacific oyster, Crassostrea gigas: I. Ploidy of resultant embryos. Bio Bullet 183:381–386
Hand RE, Nell JA, Thompson PA (2004) Studies on triploid oysters in Australia. XIII. Performance of diploid and triploid Sydney rock oyster, Saccostrea glomerata (Gould, 1850), progeny from a third generation breeding line. Aquaculture 233:93–107
Ledu C, Mccombie H (2003) Effects of cytochalasin B on fertilization and ploidy in the Pacific oyster Crassostrea gigas. Inverte Reprode Dev 44:131–137
Luca C (2005) The advantages and disadvantages of being polyploid. Nat Rev Genet 6:836–846
Mallia JV, Muthiah P, Thomas PC (2010) Growth of triploid oyster, Crassostrea madrasensis (Preston). Aquacult Res 37:718–724
Mccombie H, Ledu C, Phelipot P, Lapegue SP, Gerard A (2005) A complementary method for production of tetraploid Crassostrea gigas using crosses between diploids and tetraploids with cytochalasin B treatments. Mar Biotechnol 7:318–330
McCombie H, Cornette F, Beaumont AR (2009) Short sharp shock produces viable tetraploids in crosses of diploid blue mussels Mytilus edulis. Aquacult Res 40:1680–1682
Miller PA, Elliott NG, Vaillancourt RE, Kube PD, Koutoulis A (2014) Genetic diversity and pedigree assignment in tetraploid Pacific oysters (Crassostrea gigas). Aquaculture 433:318–324
Nell JA (2002) Farming triploid oysters. Aquaculture 210:69–88
Peachey BL, Allen SK (2016) Evaluation of cytochalasin B and 6-dimethylaminopurine for tetraploidy induction in the Eastern oyster, Crassostrea virginica. Aquaculture 450:199–205
Peruzzi S, Guo X (2002) Tetraploid induction by meiosis inhibition with cytochalasin B in the dwarf surfclam, Mulinia lateralis Say: effects of temperature. J Shellfish Res 21:677–684
Qin Y, Zhang Y, Zhou Y, Wu X, Peng M, Yu Z (2017) Comparative studies on triploidy induction using CB and 6-DMAP in Crassostrea hongkongensis. J Fisher China 41:250–257
Qin Y, Xiao S, Ma H, Mo R, Zhang Y, Yu Z (2018) Effects of salinity and temperature on the timing of germinal vesicle breakdown and polar body release in diploid and triploid Hong Kong oysters, Crassostrea hongkongensis, in relation to tetraploid induction. Aquacult Res 49:3647–3657
Qin Y, Zhang Y, Mo R, Zhang Y, Li J, Zhou Y, Yu Z (2019) Influence of ploidy and environment on grow-out traits of diploid and triploid Hong Kong oysters Crassostrea hongkongensis in southern China. Aquaculture 507:108–118
Stanley JG, Allen SK, Hidu H (1981) Polyploidy induced in the American oyster, Crassostrea virginica, with cytochalasin B. Aquaculture 23:1–10
Tan A, Hwai S, Teh CP, Chang GO, Yasin Z (2017) Tetraploid induction in tropical oysters, Crassostrea belcheri (Sowerby) and Crassostrea iredalei (Faustino). Aquac Res 48:1406–1412
Verdugo CA, RamiRez JL, Allen S, Ibarra AM (2000) Triploid catarina scallop (Argopecten ventricosus Sowerby II, 1842): growth, gametogenesis, and suppression of functional hermaphroditism. Aquaculture 186:13–32
Wadsworth P, Wilson AE, Walton WC (2019) A meta-analysis of growth rate in diploid and triploid oysters. Aquaculture 499:9–16
Wu XW (2019) Study on triploidy induction and mechanisms of growth advantages and fertility profiles of triploids in Crassostrea sikamea. PhD thesis. University of Chinese Academy of Sciences.
Wu X, Zhang Y, Xiao S, Qin Y, Ma H, Yu Z (2019) Comparative studies of the growth, survival, and reproduction of diploid and triploid Kumamoto oyster, Crassostrea sikamea. J World Aquacult Soc 50:866–877
Yang H, Guo X (2006a) Tetraploid induction by inhibiting mitosis I with heat shock, cold Shock, and nocodazole in the Hard Clam Mercenaria mercenaria (Linnaeus, 1758). Mar Biotechnol 8:501
Yang H, Guo X (2006b) Polyploid induction by heat shock-induced meiosis and mitosis inhibition in the dwarf surfclam, Mulinia lateralis Say. Aquaculture 252:171–182
Yang H, Zhang F, Guo X (2000) Triploid and tetraploid Zhikong Scallop, Chlamys farreri Jones et Preston, produced by inhibiting Polar Body I. Mar Biotechnol 2:466–475
Yang H, Guo X, Scarpa J (2019) Tetraploid induction and establishment of breeding stocks for all-triploid seed production. EDIS 2019:FA215
Acknowledgements
This research was supported by the National Science Foundation of China (32002387); the Chinese Ministry of Science and Technology through the National Key Research and Development Program of China (2018YFC1406505; 2018YFD0901400; 2020YFD0901102); Key Special Project for Introduced Talents Team of Southern Marine Science and Engineering Guangdong Laboratory (Guangzhou) (GML2019ZD0404); the Innovation Academy of South China Sea Ecology and Environmental Engineering, Chinese Academy of Sciences (ISEE2018PY01, ISEE2018ZD02, ISEE2018PY03); the China Agriculture Research System Project (CARS-49), and the Science and Technology Planning Project of Guangdong Province, China (2017B030314052).
Author information
Authors and Affiliations
Contributions
YQ, ZY, and YZ designed experiments. YQ carried out all of the experiments with the help of ZN, XL, JL, HM, YZ and RM. YQ analyzed the data and wrote the paper. ZY and YZ critically revised the manuscript and approved the final version to be published.
Corresponding authors
Ethics declarations
Conflict of interest
The author declares that there is no conflict of interest.
Animal and human rights statement
This study was conducted in accordance with the Institutional Animal Care and Use Committee of South China Sea Institute of Oceanology, Chinese Academy of Sciences, and it does not contain any studies with human participants.
Additional information
Edited by Xin Yu.
Rights and permissions
About this article
Cite this article
Qin, Y., Noor, Z., Li, X. et al. Tetraploid induction of Crassostrea hongkongensis and C. sikamea by inhibiting the polar body 1 release in diploid fertilized eggs. Mar Life Sci Technol 3, 463–473 (2021). https://doi.org/10.1007/s42995-021-00107-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42995-021-00107-w