Abstract
Knowledge on the mating system of a species is central to understand population dynamics and development. Special attention has been paid to the investigation of monogamous species and evolutionary causes and advantages of this mating system. However, social monogamy does not necessarily imply also genetic monogamy. Given the rarity of genetic monogamy in mammal species and the uncertain conclusions regarding multiple paternity in Eurasian beavers (Castor fiber), here, we undertake a further attempt to clarify the genetic monogamy of Eurasian beavers studying an Austrian beaver population by genotyping of gestating females and their foetuses at 19 microsatellite loci. Microsatellite analysis of mother–offspring groups suggest the occurrence of multiple paternity at a low level: two out of 42 litters (4.8%) were sired by two different males. We discuss the occurrence of extra-pair mating and potential drivers of multiple paternity in the light of beaver biology, population densities, territory characteristics and resulting activity ranges during reproduction period. Especially in the context of increasing beaver population densities in recovering populations and related increase of human-wildlife conflicts, sound knowledge on breeding biology, including species-specific reproduction tactics and their general applicability, is important for population monitoring.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Studying and understanding the mating system of a species is crucial to understand population dynamics and development—also in the context of applied nature conservation. However, the investigation of mating systems in natural, free-living populations is often not feasible as direct observation of mating behaviour and the identification of individuals and/or sexes is simply impossible or extremely time-consuming. Here, genetic techniques have proven excellent tools for studying mating systems and paternity in a broad range of species and have become common practice in wildlife research (Jones and Ardren 2003).
Special attention has been paid to the investigation of monogamous species and evolutionary causes and advantages of this mating system (Jennions and Petrie 2000; Cohas and Allainé 2009; Dobson et al. 2010; Lukas and Clutton-Brock 2013). While in birds, social monogamy is widespread, it is not often observed in mammals (Kleiman 1977; Lukas and Clutton-Brock 2012). Moreover, social monogamy does not necessarily imply also genetic monogamy, as being strictly monogamous is obviously related to certain costs for males (giving up chances of increasing reproductive success by mating with multiple females; Krebs and Davies 2009) and females (less genetic variation among offspring; Yasui 1998; Jennions and Petrie 2000). Hence, genetic studies have revealed that extra-pair copulations and consequently, extra-pair paternity are widespread among socially monogamous animals. In a review Griffith et al. (2002) found that less than 25% of socially monogamous bird species are also genetically monogamous. For mammals, strict genetic monogamy seems to be extremely rare, as it is documented only for a handful of mammalian species (e. g., in oldfield mice Peromyscus polionotus: Foltz 1981, dik-diks Madoqua kirkii: Brotherton et al. 1997, coppery titi monkeys Plecturocebus cupreus: Dolotovskaya et al. 2020). However, the proportion of strict genetic monogamy in mammals is vague, as data sets for socially monogamous living mammals often comprise inadequate sample sizes to exclude the possibility of extra-pair paternity. Hence, in mammalian research there is still a gap of knowledge to better understand mating systems, especially for monogamous living mammals.
The two beaver species, the Eurasian beaver (Castor fiber) and the North American beaver (C. canadensis) were traditionally considered to be socially and genetically monogamous (Busher 2007). Beavers are herbivorous, semi-aquatic, mainly nocturnal rodents, living in family groups consisting of one adult (reproductive) pair, offspring of the current year, and sexually immature subadults from the previous breeding season. Their mating period is strictly seasonally (from January to March/April; Rosell and Campbell-Palmer 2022) with females having two to four oestrus cycles, each with a short conception window of only 12–24 h (Doboszynska and Zurowski 1983, cited by Sun 2003), favouring genetic monogamy. In addition to this short fertility time slot in a season where beaver movements and food resources are naturally limited by low (water) temperatures and early vegetation period, beavers show many behaviours and characteristics being typical for monogamy, e. g. (i) biparental care: both sexes invest the same amount of time and energy rearing the offspring, (ii) strict territoriality and low tolerance to same-sex adults: adults defend territories through aggression and scent marking and both sexes are engaged in territory defence, (iii) long-term pair-bonding, (iv) reduction of sexual dimorphism in morphology, behaviour and space use, (v) mate guarding behaviour (Kleiman 1977; Herr and Rosell 2004; Rosell and Campbell-Palmer 2022). However, even if these factors are unfavourable for polygamous mating, mating systems can show a certain plasticity. There is no guarantee that extra-pair mating does not take place in beavers under particular socioecological circumstances, which reverse the cost–benefit ratio of monogamy for beaver individuals (Sun 2003).
In a study by Crawford et al. (2008b) on the North American beaver, paternity analyses of hunted and trapped individuals revealed that 56% of all litters examined had been sired by two or more males. Previous studies on multiple paternity in the Eurasian beaver led to the following results: while a Russian study reported no evidence of multiple paternity (Syrůčková et al. 2015), in Norway (in a study not yet peer reviewed) Nimje et al. (2019) showed that extra-pair paternity also occurs in Eurasian beavers—at least at a low level: 7% of litter contained a minimum of one extra-pair young. Nimje et al. (2019) indicated that differences in population density and/or small sample sizes (Crawford et al. 2008b: N = 9; Syrůčková et al. 2015: N = 9; Nimje et al. 2019: N = 100) could explain the contradicting results.
Given the rarity of social and genetic monogamy in mammal species and particularly, the uncertain conclusions regarding multiple paternity in Eurasian beavers, we undertake a further attempt to clarify the genetic monogamy of Eurasian beavers studying an Austrian beaver population.
As in many other countries in Europe, in Austria the Eurasian beaver was exterminated in the 1860s (Sieber and Bauer 2001). During the years 1976 and 1990 within a reintroduction programme 42 beavers (i. e., 27 Castor fiber and 15 Castor canadensis) from Poland, Belarus and Sweden were released in the Austrian Danube watershed (Kollar and Seiter 1990) and the population started to recover (Sieber and Bauer 2001). In 2018 the total population size of beavers in Austria was estimated to be about 7100–7800 individuals (European Commission 2019, FFH report, Art. 17). Although individuals of both beaver species had been released in our study region in the province of Lower Austria, in a previous study by Kropf et al. (2013) only Eurasian beaver individuals were detected—leading to the conclusion that the current beaver population consists of Eurasian beavers only.
Munclinger et al. (2022) showed that while in relict Eurasian beaver populations (e. g., Norway) genetic diversity is very low, in reestablished populations based on multiple translocations from different origins and due to natural expansion, genetic diversity could be restored in many places across Europe and levels of polymorphisms are at least moderate.
Because of the population expansion and increasing population density over the recent years, increasing conflicts with human land use have led to the implementation of a beaver management in the province of Lower Austria (followed by other regions in Austria). The federal beaver management includes prevention and repellent measures and since winter 2006/2007 derogations given by the respective state administration authorities allow also trapping and killing of beavers under controlled conditions where prevention measures cannot be implemented or are ineffective.
Tissue samples of trapped and killed gestating beaver females and their uteri containing the foetuses were used in our study to investigate the genetic mating system in a population of Eurasian beavers in the province of Lower Austria covering regional (sub-)populations still in expansion compared to populations with already filled territories. Specifically, we aim to elucidate the occurrence of multiple paternity (i. e., a pregnant female carries a litter sired by more than one male) by applying established and published polymorphic microsatellite markers to genotype gestating females and their foetuses. Further, we will discuss the occurrence of extra-pair mating in the light of beaver population densities, territory characteristics and resulting activity ranges during the reproduction.
Material and methods
Study area and sample material
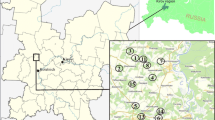
Within the federal beaver management measures of the Austrian province of Lower Austria between 2008 and 2017 tissue samples of trapped and killed gestating beaver females and their uteri containing the foetuses were collected during necropsies and stored deep-frozen for further research. The sample set consists of 190 samples from 42 gravid females and their litters (i. e., up to six offspring per female) stemming from the Danube watershed including northern and southern tributaries in the province of Lower Austria (Fig. 1).
Molecular genetic analyses
Genomic DNA was extracted from tissue samples using the Maxwell® RSC Tissue DNA Kit with a Maxwell® Instrument (Promega, United States of America) following the manufacturer´s protocol. After DNA extraction DNA concentration was measured with a photometer (BioPhotometer D30, Eppendorf, Germany) and standardised for all samples to 20 ng/µl. For paternity analyses the samples were genotyped at all 25 public available microsatellite loci developed and optimised for C. fiber (Frosch et al. 2011; Syrůčková et al. 2015) and for C. canadensis (Crawford et al. 2008a; Pelz-Serrano et al. 2009) except for five loci developed for the North American beaver, which are known to be either monomorphic or not amplifiable in Eurasian beavers (see Tab 1). Further, a sexing marker (SRY gene, Kühn et al. 2002) was used for sex identification. The markers were amplified in four multiplex reactions (set A: Cca18, CF31, CF32, Cca8, CF17, CF30, CF49; set B: Cca13, CF33, CF07, Cca56, Cca92, CF48, sexing marker; set C: CF05, CF44, Cca4, Cca76, CF18; set D: CF06, CF19, CF41, Cca19, Cca62, Cca5, CF21). The reactions contained 1 µl template DNA, 5 µl of QIAGEN Multiplex PCR Kit (Qiagen, Germany), 1 µl of primer mix (2 µM of each primer; forward primer labelled with a fluorescent dye) and 3 µl of nuclease-free water. Amplifications were done using an initial denaturation for 15 min at 95 °C followed by 30 cycles of 30 s at 94 °C, 90 s at annealing temperature (multiplex set A, C, D: 57 °C; multiplex set B: 61 °C), 60 s at 72 °C, and a final extension step for 30 min at 72 °C.
To prevent genotyping errors in the data set and ensure accuracy of scoring for each sample two replicates were analysed.
No-template controls were included throughout the extraction procedure and PCR amplifications to check for possible contaminations. Fragment length analysis was performed on an ABI 3730 XL automated capillary sequencer (Applied Biosystems, United States of America) by a commercial provider (Microsynth, Switzerland). Alleles were scored with the GeneMapper 5.0 software (Applied Biosystems, United States of America).
Basic genetic parameters and genetic variability
Deviations from Hardy–Weinberg equilibrium (HWE) for each locus were tested with Genepop 4.2 (Raymond and Rousset 1995; Rousset 2008) applying the default Markov chain parameters (1000 step dememorization, 100 batches, 1000 iterations per batch), followed by a Bonferroni correction for multiple testing for the significance level (α = 0.05). For population genetic analysis that require high accuracy in genotyping, such as parentage analysis, even rare null alleles can confound results and any loci with strong evidence of null alleles should be excluded. Thus, maternal null alleles were identified through the observation of non-Mendelian segregation of maternal alleles in the progeny array. Further, we used Micro-Checker 2.2.2 (Van Oosterhout et al. 2004) to check our microsatellite data set for presence of null alleles, stuttering, and large allelic dropout.
To gather basic information on genetic variability for the local beaver population, we computed allele frequencies, number of alleles, observed and expected heterozygosity for 42 adult, female beavers using the software GenAlEx 6.5 (Peakall and Smouse 2006, 2012). Further, to estimate the resolution power of our microsatellite data using the software Cervus 3.0.7 (Marshall et al. 1998; Kalinowski et al. 2007) we calculated (1) the probability of identity (PID) and the probability of identity for siblings (PIDsibs), which is a more conservative upper bound for the probability that two individuals share the same genotype (Taberlet and Luikart 1999; Waits et al. 2001), (2) the polymorphism information content (PIC), and (3) the non-exclusion probabilities (NE-1P, NE-2P) referring to the average probability that a given set of loci fail to exclude one or a pair of unrelated candidate parents from parentage of an arbitrary offspring. Here, NE-1P is the average non-exclusion probability for one candidate parent when both parents were unknown, NE-2P means the average non-exclusion probability for one candidate parent given the genotype of a known parent.
Multiple paternity detection
To reveal the power of the loci used for detecting multiple paternity in our data set, we performed simulations using the programme PrDM (Neff and Pitcher 2002). The programme computes the probability of detecting multiple sires depending on (1) the allele frequencies in the adult population, (2) litter sizes, and (3) paternity rates within a litter. Simulations were performed across the range of litter sizes in our study, using allele frequencies estimated from the beaver mothers, and assuming an equal likely paternity of two fathers.
To identify cases of multiple paternity microsatellite profiles of each beaver mother and her offspring allowed a visual reconstruction by hand of the minimum number of paternal genotypes based on Mendelian rules of inheritance. Multiple paternity was inferred if more than two paternal alleles were observed at one or more loci.
In addition to the manual allele counting, based on the microsatellite profiles from the mothers and respective offspring, we estimated the minimum number of sires required to produce the respective set of alleles in each litter using the programme GERUD 2.0 (Jones 2005). Further, all possible multilocus genotypes of fathers were reconstructed by the programme, and the most probable solution was selected. As recommended by Jones (2005), GERUD analysis was conducted with the five microsatellite loci showing the highest variability and lowest non-exclusion probabilities. Here, we used the loci CF31, CF32, CF33, Cca4, Cca62 (Table 2).
To further assess multiple paternity, we used COLONY 2.0.6.8 (Jones and Wang 2010) which infers sibship among offspring and parentage applying a full-pedigree likelihood method. For the COLONY analysis, two marker subsets were used: (i) all 19 loci, (ii) the five loci used in GERUD analysis.
Results
Amplification success and marker suitability
All 190 samples amplified at all loci, leading to an amplification success rate of 100% for both runs. Moreover, both runs showed coherent results after scoring of alleles. The extremely high data quality of this study is an essential prerequisite for the interpretation of the results.
Of the 25 primer pairs initially used in the amplification, two loci (CF41 and CF48) showed ambiguous bands impeding reliable scoring of alleles. Another four loci showed to be monomorphic (CF30, CF49, Cca76, CF21). Thus, these six loci were excluded from further analyses. The remaining 19 loci were polymorphic with allele numbers ranging from 2 to 5 and a mean number of alleles per locus of 3.47. All alleles observed in offspring genotypes were already observed in the adult population, hence, there was no indication of new mutation within the data set. Across all loci, observed and expected heterozygosity for mothers resulted in 0.42 and 0.48, respectively. Neither a large allele dropout, nor stuttering were detected in the data set. However, Micro-Checker software detected the possible presence of null alleles at one locus (Cca19). For the same microsatellite locus deviations from Hardy–Weinberg equilibrium were detected after correcting for multiple testing. As shown below, the omittance of this locus (data set including five loci) did not change the conclusions of the results of paternity analyses. Moreover, a complete concordance of mother–offspring genotypes at all loci indicates Mendelian inheritance of maternal alleles in the progeny array, i. e. no maternal null alleles were identified.
With a probability of identity (PI) of 3.84 × 10–10 and a probability of identity for siblings (PIsibs) of 4.63 × 10–5, our resulting set of 19 microsatellites had enough power to distinguish between closely related beaver individuals. Moderate polymorphism (PIC = 0.42) and low combined non-exclusion probabilities (NE-1P, NE-2P) of 0.06 and 3.00 × 10–3, respectively, further indicate that resolution power of our marker set is suitable for parentage analysis.
PrDM simulations indicated that in the most scenarios evidence of multiple paternity would have been detected with our study design. As expected, detection probability increased with litter size (from 40.2% for three embryos to 87.7% for six embryos).
Multiple paternity
In two of the 42 beaver uteri multiple paternity was detected by both manual allele counting and software packages (GERUD, COLONY) for both data sets (five and 19 loci). All other progeny arrays could be explained with a single, unique paternal genotype by all three methods. For the multiple paternity cases (i. e., litters Z/360/17 and Z/369/17; see Table 2) analyses indicated that a maximum of two fathers contributed to each litter. In litter Z/360/17 one of three pups (F3) was assigned to a second father based on one locus (CF33). Although the pup was homozygous at this locus with an allele matching with its mother, the chance that it is a result of allelic dropout is close to zero, as we rerun each sample and worked with fresh tissue material. In litter Z/369/17 two pups (F3 and F6) out of six foetuses were assigned to a second father, again based on locus CF33. Here, both pups were heterozygous at the respective locus. Electropherograms showing the allelic profile at locus CF33 for all beaver individuals of the respective litters are provided in Online Resource 1 (Fig S1-S11).
Discussion
Based on ecological studies on behaviour and dispersal, the traditional general assumption was that beavers have a socially and genetically monogamous mating system (Busher 2007). However, in addition to former studies by Crawford et al. (2008b) on the North American beaver and by Nimje et al. (2019) on Eurasian beavers, in this study we provide results that further question the former assumption of exclusively, genetically monogamous beavers in Europe. In our notable data set based on 42 uteri of gravid females, microsatellite analyses of mother–offspring groups suggest the occurrence of multiple paternity in Eurasian beavers in Austria. Two out of 42 litters (4.8%) were sired by two different males. This is a low rate compared to Crawford et al. (2008b) for North American beavers (56% rate of multiple paternity; N = 9 litters), but similar to the study by Nimje et al. (2019), who found a multiple paternity rate of 7% for Eurasian beavers in Norway (N = 100 litters).
Unfortunately, our results are based on a single locus (CF33; see Online Resource 1) only and might still be interpreted as monogamy when assuming a genotyping error. We would argue against this, since due to our conservative and thoroughly analytical approach including two independent runs of each sample and comprehensive error checking statistics, we can make it credible that alleles indicating multiple paternity are not the result of incorrectly scored artefacts or other scoring errors. Moreover, mutations could be responsible for falsely assumed additional paternal alleles. However, here we would have detected two independent mutations at the same locus, at the same time at two different localities, which is a theoretically possible, but very unlikely scenario.
The detection probability of multiple paternity in a population may be limited if allelic diversity is low (Sefc and Koblmüller 2009). Here, the maximum number of paternal alleles observed at a locus was three. That means that at least one of the two fathers has an allele common with the mother. Thus, multiple paternity may be underestimated if fathers share too many alleles to be differentiated within the progeny array. In our study observed heterozygosity across all loci was low to moderate, ranging from 0.1 to 0.7 (average Hobs 0.4) and multiple paternity cases in our study are based on one locus only (CF33). Although the levels of heterozygosity and polymorphism is comparable to other populations of beavers in Europe using the same microsatellite loci (Frosch et al. 2014; Syrůčková et al. 2015; Iso-Touru et al. 2020; Fedorca et al. 2021), it is possible that the level of multiple paternity has been underestimated. Furthermore, detection of extra-pair paternity might be even more difficult in cases where females mate with closely related males (e. g., their sons).
The power of detection increases also with litter size and will be further hampered if the putative father is missing in the data set (Neff and Pitcher 2002). However, given the fact that we detected multiple paternity also in a litter of three offspring, shows that our set of microsatellite loci generally had the power to detect multiple paternity cases (also in small litters). To which degree we probably underestimated the rate, remains unknown. However, we used almost all microsatellite loci established and published for the two beaver species (Crawford et al. 2008a; Pelz-Serrano et al. 2009; Frosch et al. 2011; Syrůčková et al. 2015), resulting in a comparatively large initial number (25) of loci, with 19 loci left being suitable for parentage analyses. Although the loci used here show relatively low to moderate levels of polymorphisms, the probabilities of identity and the non-exclusion probabilities were low. This may be due to the sufficiently high number of loci used in this study. Although it is commonly agreed that polymorphism is important to assess paternity from offspring genotypes (Sefc and Koblmüller 2009), the number of loci may have a more significant effect on the power of parentage analysis than expected (Goossens et al. 1998; Weng et al. 2021).
Beavers in our study area originated from reintroductions from various source populations and hence, express higher levels of genetic diversity compared to relict beaver populations (Senn et al. 2014; Munclinger et al. 2022). Nevertheless, the recent beaver population in Austria (comprising 7,100 to 7,800 individuals, European Commission 2019, FFH report, Art. 17) is mainly based on only 27 Castor fiber founder individuals released in the Austrian Danube watershed within a reintroduction programme from 1976 to 1990 (Kollar and Seiter 1990; Sieber and Bauer 2001). Consequently, inbreeding (at least for the first years after reintroductions), and genetic bottlenecks may have occurred, leading to limited genetic variation reflected in our data set. In terms of maternally inherited mitochondrial haplotype diversity of the control region (d-loop) five haplotypes have been yet reported from our study region (Kropf et al. 2013; Attili et al. 2023), which is an expectable number considering the limited number of founder individuals originating from various source populations.
A study by Syrůčková et al. (2015) on Eurasian beavers in Russia provided no evidence for the presence of multiple paternity. Based on genetic polymorphisms and exclusion probabilities similar to our study, the authors claimed the suitability of the applied marker set. But they admitted that their results should be treated with caution due to low sample size (N = 9).
However, as beavers exhibit numerous characteristics and behaviours associated with monogamy, that do not necessarily facilitate or even impede extra-pair mating, both, genetic monogamy and multiple paternity at low rates are conclusive results for studies on Eurasian beaver mating tactics. Under certain socioecological conditions presence or absence of multiple paternity and its frequency and prevalence may vary between populations, between conspecific individuals, or even in the same individual (Sun 2003). According to Jennions and Petrie (2000), there are two critical determinants of extra-pair paternity: (i) the availability of additional mates at the time the female is receptive, and (ii) the capacity of social males to control the accessibility of females to potential extra-pair males.
Generally, the strict seasonality of mating period and short duration of the female oestrus (Doboszynska and Zurowski 1983 cited by Sun 2003; Rosell and Campbell-Palmer 2022) enables efficient mate guarding to prevent extra-pair mating. Since in beavers the males contribute to the rearing of the young just as much as the females, i. e., biparental care, it makes sense for the males to guard their females intensively to ensure their paternity. Moreover, beavers are strictly territorial with both sexes spending the same amount of time defending their territory (Herr and Rosell 2004) and show low tolerance to same-sex adults. Thus, to find additional mates, the own territory must be left, which bears the risk of being attacked by same-sex territory owners. Also, predation risk is increased as beaver individuals searching for mating opportunities in foreign territories do not have a permanent lodge or shelters. And as mating period takes place in winter, especially in higher latitudes, low air and water temperatures, potential ice and snow cover limit beaver movements, because they are highly energy-consuming. In addition, as their plant-based diet is of low nutritional value, beavers spend a considerable amount of time to forage (Sun 2003). Also, construction works, i. e., building dams and burrows, are time and energy intensive, and Sun (2003) consequently assumes that these energy and time constraints prevent beavers from leaving their territories aiming for extra-pair copulations.
Nevertheless, mating systems appear to be flexible if social and/or ecological conditions change in space and time reversing the cost–benefit ratio of monogamy. Consequently, individuals of a species that is considered monogamous may adapt their reproductive strategies to the prevailing social and ecological conditions (Sun 2003). Hence, as availability of additional mates increases with population density and making mate guarding for male beavers more difficult, deviations from a monogamous mating system may be more likely under high population densities (e. g., Goossens et al. 1998; Streatfeild et al. 2011; Shimozuru et al. 2019; Batsuren et al. 2022).
Furthermore, the location of a territory of a beaver couple in a river basin may influence mating opportunities. Territories in upper reaches of streams, for example, may be less frequented by transient individuals (sexually mature dispersing individuals without a territory) and therefore lower mating opportunities. Low densities and/or isolated/remote sites would therefore lower the probability of encountering additional mates. Another factor which may affect the probability of alternative mating opportunities, could be territory size in combination with food resource quality and food distribution patterns. Patrolling larger territories means that beaver individuals need more time to travel longer distances within their territory boundaries to cover their dietary needs. This makes guarding of the pair bond partner more difficult and may increase again the probability of encountering additional mates. Hence, population density of sexually mature individuals and territory site characteristics may be assumed to be major drivers for the occurrence and frequency of multiple paternity in this monogamous species.
In our study the two cases of multiple paternity (litters Z/360/17 and Z/369/17) were found at sites, which were neither remote territories nor regions with still expanding numbers at the time of sampling (Fig. 1). Unfortunately, we do not have exact counts for beaver densities in the respective areas at the time of sampling, preventing a comparison with other studies providing information on beaver densities and occurrences of multiple paternity (Syrůčková et al. (2015): 0.25 colonies per km stream, 0.4 colonies per square km; Crawford et al. (2008b): 0.4 colonies per km stream, 3.3 colonies per square km; Nimje et al. (2019): 0.35 colonies per square km). However, according to local landowners reporting increasing conflicts due to beaver activities, it can be assumed that both sites (as well as neighbouring territories) were already recolonized by the beaver for several (at least 5) years (pers. observation) and well-established territories existed.
As shown in Alpine marmots (Marmota marmota), climate can affect frequency of multiple paternity (Bichet et al. 2016). With increasing frequency of mild winters due to climate change in combination with high population densities, winter movements of beavers increase, and consequently, the likelihood of encountering additional mates during mating season as well (Crawford et al. 2008b). Thus, multiple paternity in beavers may be seen as consequence of global warming, specifically as an alternative mating tactic according to changes in environmental conditions. This circumstance may also explain the lack of multiple paternity in the study of Syrůčková et al. (2015). During mating period in Kirov region large parts of the landscape is usually covered by ice and snow, preventing beavers from visiting foreign territories.
Another factor which we would like to discuss here in context with mating behaviour is the disturbance of social structures and alteration of territory boundaries due to dam removal, trapping and shooting beaver individuals, as it takes place in our study area since winter 2006/2007. It is conceivable that such management measures (e. g., the removal of one of the reproducing beaver couple in a territory) may lead to instable conditions in the social system, and therefore altering reproduction tactics.
Conclusion
Although based on a single microsatellite locus only, our study suggests the occurrence of multiple paternity in Eurasian beavers at a low rate. Our rate of 4.8% and the previously reported rate (7%; Nimje et al. 2019) of multiple paternity for Eurasian beavers were quite similar. However, while the indications of multiple paternity become more, the causes and frequency of this mating behaviour remain still unknown. Here, we discussed potential drivers of multiple paternity (namely, population density, location, size and quality of a territory, increase of activity ranges during reproduction time due to mild winters, disturbance of social structures due to hunting of beavers), but further investigations are needed to identify the influential factors under which social and genetic monogamy is favoured, and confirm whether multiple paternity is uniform in this species or whether its mating system is characterised by higher plasticity. Especially in the context of increasing beaver population densities and related increase of human-wildlife conflicts, knowledge on species-specific reproduction tactics and their plasticity is important for population monitoring (e. g., reproduction success, genetic variation) in the course of nature conservation and wildlife management.
To prevent underestimation of multiple paternity rates in future studies and/or validate results of present studies, additional marker systems (e. g., SNPs, Senn et al. 2014) and genomic approaches should be applied.
data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Attili L, Pizzarelli A, Viviano A et al (2023) First molecular evidence on the puzzling origin of beavers in central Italy. Hystrix, Ital J Mammal 34:139–142. https://doi.org/10.4404/hystrix-00623-2023
Batsuren E, Zhang X, Song M et al (2022) Density-dependent changes of mating system and family structure in Brandt’s voles (Lasiopodomys brandtii). Ecol Evol 12:e9199. https://doi.org/10.1002/ece3.9199
Bichet C, Allainé D, Sauzet S, Cohas A (2016) Faithful or not: Direct and indirect effects of climate on extra-pair paternities in a population of alpine marmots. Proc R Soc B: Biol Sci 283:1845. https://doi.org/10.1098/rspb.2016.2240
Brotherton PNM, Pemberton JM, Komers PE, Malarky G (1997) Genetic and behavioural evidence of monogamy in a mammal, Kirk’s dik-dik (Madoqua kirkii). Proc R Soc B: Biol Sci 264:675–681. https://doi.org/10.1098/rspb.1997.0096
Busher PE (2007) Social organization and monogamy in the beaver. In: Woolf JO, Sherman PW (eds) Rodent Societies. University of Chicago Press, Chicago, pp 280–290
Cohas A, Allainé D (2009) Social structure influences extra-pair paternity in socially monogamous mammals. Biol Lett 5:313–316. https://doi.org/10.1098/rsbl.2008.0760
European Commission (2019) FFH report, Art. 17, Period 2013–2018. https://nature-art17.eionet.europa.eu/article17/species/report/?period=5&group=&country=. Accessed 21 January 2024
Crawford J, Liu Z, Nelson T et al (2008a) Isolation and characterization of microsatellite loci in the beaver (Castor canadensis). Mol Ecol Resour 8:616–618. https://doi.org/10.1111/j.1471-8286.2007.02016.x
Crawford JC, Liu Z, Nelson TA et al (2008b) Microsatellite analysis of mating and kinship in beavers (Castor canadensis). J Mammal 89:575–581. https://doi.org/10.1644/07-MAMM-A-251R1.1
Dobson FS, Way BM, Baudoin C (2010) Spatial dynamics and the evolution of social monogamy in mammals. Behav Ecol 21:747–752. https://doi.org/10.1093/beheco/arq048
Dolotovskaya S, Roos C, Heymann EW (2020) Genetic monogamy and mate choice in a pair-living primate. Sci Rep 10:20328. https://doi.org/10.1038/s41598-020-77132-9
Fedorca A, Ciocirlan E, Pasca C et al (2021) Genetic structure of Eurasian beaver in Romania: insights after two decades from the reintroduction. Eur J Wildl Res 67:104. https://doi.org/10.1007/s10344-021-01546-7
Foltz DW (1981) Genetic evidence for long-term monogamy in a small rodent, Peromyscus polionotus. Am Nat 117:665–675
Frosch C, Haase P, Nowak C (2011) First set of microsatellite markers for genetic characterization of the Eurasian beaver (Castor fiber) based on tissue and hair samples. Eur J Wildl Res 57:679–682. https://doi.org/10.1007/s10344-010-0486-6
Frosch C, Kraus RHS, Angst C et al (2014) The genetic legacy of multiple beaver reintroductions in central Europe. PLoS ONE 9:e97619. https://doi.org/10.1371/journal.pone.0097619
Goossens B, Graziani L, Waits LP et al (1998) Extra-pair paternity in the monogamous Alpine marmot revealed by nuclear DNA microsatellite analysis. Behav Ecol Sociobiol 43:281–288
Griffith SC, Owens IPF, Thuman KA (2002) Extra pair paternity in birds: A review of interspecific variation and adaptive function. Mol Ecol 11:2195–2212
Herr J, Rosell F (2004) Use of space and movement patterns in monogamous adult Eurasian beavers (Castor fiber). J Zool 262:257–264. https://doi.org/10.1017/S0952836903004606
Iso-Touru T, Huitu O, Tapio M et al (2020) Low genetic polymorphism in the re-introduced Eurasian beaver (Castor fiber) population in Finland: implications for conservation. Mamm Res 65:331–338. https://doi.org/10.1007/s13364-020-00487-x
Jennions MD, Petrie M (2000) Why do females mate multiply? A review of the genetic benefits. Biol Rev 75:21–64. https://doi.org/10.1111/j.1469-185X.1999.tb00040.x
Jones AG (2005) GERUD 2.0: A computer program for the reconstruction of parental genotypes from half-sib progeny arrays with known or unknown parents. Mol Ecol Notes 5:708–711. https://doi.org/10.1111/j.1471-8286.2005.01029.x
Jones AG, Ardren WR (2003) Methods of parentage analysis in natural populations. Mol Ecol 12:2511–2523. https://doi.org/10.1046/j.1365-294X.2003.01928.x
Jones OR, Wang J (2010) COLONY: A program for parentage and sibship inference from multilocus genotype data. Mol Ecol Resour 10:551–555. https://doi.org/10.1111/j.1755-0998.2009.02787.x
Kalinowski ST, Taper ML, Marshall TC (2007) Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol Ecol 16:1099–1106. https://doi.org/10.1111/j.1365-294X.2007.03089.x
Kleiman DG (1977) Monogamy in mammals. Q Rev of Biol 52:39–69
Kollar HP, Seiter M (1990) Biber in den Donau-Auen östlich von Wien. Eine erfolgreiche Wiederansiedlung. Umwelt Schriftenreihe für Ökologie und Ethologie 14:1–75
Krebs JR, Davies NB (2009) Behavioural ecology: an evolutionary approach. John Wiley and Sons, Oxford
Kropf M, Hölzler G, Parz-Gollner R (2013) Genetic evidence on the origin of the current beaver (Castor fiber) population in Lower Austria. Sumar List 137:591–596
Kühn R, Schwab G, Schröder W, Rottmann OJ (2002) Molecular sex diagnosis in Castoridae. Zoo Biol 21:305–308. https://doi.org/10.1002/zoo.10018
Lukas D (2013) Clutton-Brock TH (2013) The evolution of social monogamy in mammals. Science 341:526–530. https://doi.org/10.1126/science.1238677
Lukas D, Clutton-Brock T (2012) Cooperative breeding and monogamy in mammalian societies. Proc R Soc B: Biol Sci 279:2151–2156. https://doi.org/10.1098/rspb.2011.2468
Marshall TC, Slate J, Kruuk LEB, Pemberton JM (1998) Statistical confidence for likelihood-based paternity inference in natural populations. Mol Ecol 7:639–655. https://doi.org/10.1046/j.1365-294x.1998.00374.x
Munclinger P, Syrucková A, Náhlovsky J, Durka W, Saveljev AP, Rosell F, Stubbe A, Stubbe M, Ulevicius A, Samiya R, Yanuta G, Vorel A (2022) Recovery in the melting pot: complex origins and restored diversity in newly established Eurasian beaver (Rodentia: Castoridae) populations. Biol J Linn Soc 135:793–811. https://doi.org/10.1093/biolinnean/blac003
Neff BD, Pitcher TE (2002) Assessing the statistical power of genetic analyses to detect multiple mating in fishes. J Fish Biol 61:739–750. https://doi.org/10.1006/jfbi.2002.2101
Nimje PS, Tinnesand HV, Buesching C et al (2019) Almost faithful: SNP markers reveal low levels of extra-pair paternity in the Eurasian beavers. PeerJ Prepr. https://doi.org/10.7287/peerj.preprints.27866v1
Peakall R, PeterE S (2006) Genalex 6: genetic analysis in excel. population genetic software for teaching and research. Mol Ecol Notes 6:288–295. https://doi.org/10.1111/j.1471-8286.2005.01155.x
Peakall R, Smouse PE (2012) Genalex 6.5: genetic analysis in excel. population genetic software for teaching and research - an update. Bioinformatics 28:2537–2539. https://doi.org/10.1093/bioinformatics/bts460
Pelz-Serrano K, Munguia-Vega A, Piaggio AJ et al (2009) Development of nine new microsatellite loci for the American beaver, Castor canadensis (Rodentia: Castoridae), and cross-species amplification in the European beaver, Castor fiber. Mol Ecol Resour 9:551–554. https://doi.org/10.1111/j.1755-0998.2008.02364.x
Raymond M, Rousset F (1995) GENEPOP (Version 1.2): Population genetics software for exact tests and ecumenicism. J Hered 86:248–249. https://doi.org/10.1093/oxfordjournals.jhered.a111573
Rosell F, Campbell-Palmer R (2022) Beavers – Ecology, behaviour, conservation and management. Oxford University Press
Rousset F (2008) GENEPOP’007: A complete re-implementation of the GENEPOP software for Windows and Linux. Mol Ecol Resour 8:103–106. https://doi.org/10.1111/j.1471-8286.2007.01931.x
Sefc KM, Koblmüller S (2009) Assessing parent numbers from offspring genotypes: the importance of marker polymorphism. J Hered 100:197–205. https://doi.org/10.1093/jhered/esn095
Senn H, Ogden R, Frosch C et al (2014) Nuclear and mitochondrial genetic structure in the Eurasian beaver (Castor fiber) - implications for future reintroductions. Evol Appl 7:645–662. https://doi.org/10.1111/eva.12162
Shimozuru M, Shirane Y, Tsuruga H et al (2019) Incidence of multiple paternity and inbreeding in high-density brown bear populations on the shiretoko Peninsula, Hokkaido, Japan. J Hered 110:321–331. https://doi.org/10.1093/jhered/esz002
Sieber J, Bauer K (2001) Europäischer und Kanadischer Biber. In: Spitzenberger F (ed) Grüne Reihe des BMLFUW, vol 13. Austria, Vienna, pp 366–374
Streatfeild CA, Mabry KE, Keane B et al (2011) Intraspecific variability in the social and genetic mating systems of prairie voles, Microtus ochrogaster. Anim Behav 82:1387–1398. https://doi.org/10.1016/j.anbehav.2011.09.023
Sun L (2003) Monogamy correlates, socioecological factors, and mating systems in beavers. In: Reichard U, Boesch C (eds) Monogamy: Mating strategies and partnerships in birds, humans and other mammals. Cambridge University Press, pp 138–146
Syrůčková A, Saveljev AP, Frosch C et al (2015) Genetic relationships within colonies suggest genetic monogamy in the Eurasian beaver (Castor fiber). Mamm Res 60:139–147. https://doi.org/10.1007/s13364-015-0219-z
Taberlet P, Luikart G (1999) Non-invasive genetic sampling and individual identification. Biol J Linn Soc 68:41–55. https://doi.org/10.1111/j.1095-8312.1999.tb01157.x
Van Oosterhout C, Hutchinson WF, Wills DPM, Shipley P (2004) Micro-Checker: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes 4:535–538. https://doi.org/10.1111/j.1471-8286.2004.00684.x
Waits LP, Luikart G, Taberlet P (2001) Estimating the probability of identity among genotypes in natural populations: cautions and guidelines. Mol Ecol 10:249–256. https://doi.org/10.1046/j.1365-294X.2001.01185.x
Weng Z, Yang Y, Wang X et al (2021) Parentage analysis in giant grouper (Epinephelus lanceolatus) using microsatellite and SNP markers from genotyping-by-sequencing data. Genes (Basel) 12:1042. https://doi.org/10.3390/genes12071042
Yasui Y (1998) The genetic benefits of female multiple mating reconsidered. Trends Ecol Evol 13:246–250. https://doi.org/10.1016/s0169-5347(98)01383-4
Acknowledgements
We are grateful to the team of the Central Research Laboratories at the Natural History Museum Vienna for any support in the DNA laboratory, with special thanks to Elisabeth Haring for her advice in design and conception of molecular analyses. We also would like to thank all colleagues involved for their awesome engagement during field work and data collection, especially Gerald Hölzler. Furthermore, we thank the team of the pathology lab of the Research Institute of Wildlife Ecology (University of Veterinary Medicine Vienna) for their good collaboration and essential expertise in beaver necropsies. We also would like to thank the reviewers for their thoughtful comments and efforts towards improving our manuscript.
Funding
Open access funding provided by University of Natural Resources and Life Sciences Vienna (BOKU). No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by MS, MC, and RP-G. The first draft of the manuscript was written by MS and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interests. The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
All authors have read, understood, and have complied as applicable with the statement on "Ethical responsibilities of Authors" as found in the Instructions for Authors.
Additional information
Handling editor: Heiko G. Rödel.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sittenthaler, M., Chai, M., Kruckenhauser, L. et al. Truly monogamous? Investigating multiple paternity in Eurasian beavers (Castor fiber) in a reestablished population in Austria. Mamm Biol (2024). https://doi.org/10.1007/s42991-024-00450-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42991-024-00450-2