Abstract
In this study, we describe the acoustic structure of ultrasonic alarm calls of Mongolian gerbils Meriones unguiculatus in the wild and verify these calls as belonging to Mongolian gerbils by comparison of their acoustic parameters with alarm calls recorded in captivity. Both in captivity and in the wild, the alarm calls of Mongolian gerbils represented prolonged calls with an average duration of 118 ms and a flat contour and an average maximum fundamental frequency of 26.84 kHz. We found that alarm calls of captive Mongolian gerbils were shorter and higher in fundamental frequency and followed in a quicker succession than in the wild. Although the dataset size is not sufficient to determine significant acoustic variation between the populations, we discuss the potential reasons of the acoustic differences between the ultrasonic alarm calls produced in the wild and in captivity in our study and between the alarm calls reported in literature for different captive populations. We propose a method for non-invasive estimation of occupancy of the burrows by Mongolian gerbils in fragmented colonies at very low population density, by presence of the ultrasonic alarm calls.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alarm calls occur across mammalian taxa: in primates (Seyfarth et al. 1980; Fichtel and Kappeler 2002; Zuberbühler 2009; Leyn et al. 2023), hyraxes (Fourie 1977), carnivores (Manser 2001; Leuchtenberger et al. 2016; Kern et al. 2017), lagomorphs (pikas) (Conner 1985; Volodin et al. 2018, 2021a), artiodactyls, including camelids and giraffes (Kiley 1972; Minami and Kawamichi 1992; Volodin et al. 2017; Volodina et al. 2018), and in rodents (Hare 1998; Randall and Rogovin 2002; Randall et al. 2005; Blumstein 2007; Matrosova et al. 2011; Loughry et al. 2019). Most rodents produce their calls in both ultrasonic and sonic frequency ranges (Zaytseva et al. 2020; Dymskaya et al. 2022; Fernández-Vargas et al. 2022). However, alarm calls of most of the rodents studied are produced in the sound range audible to humans at frequencies below 20 kHz. The use of ultrasonic (above 20 kHz) alarm calls was reported for four species of rodents: for Norway rats Rattus norvegicus (Blanchard et al. 1991; Brudzynski and Holland 2005; Litvin et al. 2007; Inagaki and Ushida 2021), Richardson’s ground squirrels Spermophilus richardsonii (Wilson and Hare 2004, 2006), speckled ground squirrels S. suslicus (Matrosova et al. 2012) and Mongolian gerbils Meriones unguiculatus (Ter-Mikaelian et al. 2012).
Many species of rodents living in arid habitats use podophony (also termed foot-thumping or foot-drumming) as an alarm signal, often additionally to the alarm call (review: Randall 2001). Gerbils may use podophony in case of danger or at elevated arousal, alone or simultaneously with alarm calling. Podophony was reported for fat sand rats Psammomys obesus (Daly and Daly 1975a; Bridelance 1986), fat-tailed gerbils Pachyuromys duprasi (Bridelance 1989), great gerbils Phombomys opimus (Randall et al. 2000) and many species of the genus Meriones (Daly and Daly 1975b; Bridelance and Paillette 1985; Bridelance 1989; Randall 2001), including the Mongolian gerbil (Agren et al. 1989). The great gerbil combines podophony with audible (sonic) alarm calls (Randall et al. 2000) and Mongolian gerbils combine podophony with ultrasonic alarm calls (Ter-Mikaelian et al. 2012). For other species of gerbils for which podophony was reported, the alarm calls were not reported.
The Mongolian gerbil was extensively studied regarding vocalization, primarily as an animal model for human epilepsy (e.g., Kumar et al. 2006). So far, all studies of acoustic communication in Mongolian gerbils are made in captivity (De Ghett 1974; Broom et al. 1977; Holman 1980, 1981; Holman and Hutchison 1985; Holman and Seale 1991; Holman et al. 1995; Nishiyama et al. 2011; Kobayasi and Riquimaroux 2012; Ter-Mikaelian et al. 2012; Kozhevnikova et al. 2021; Peterson et al. 2023; Silberstein et al. 2023; Volodin et al. 2023). Pups of the Mongolian gerbil produce isolation calls purely sonic, purely ultrasonic and combining sound and ultrasound (Kozhevnikova et al. 2021; Silberstein et al. 2023). Adult Mongolian gerbils also produce both sonic and ultrasonic calls of different types, used in different contexts (Holman 1980; Kobayasi and Riquimaroux 2012; Ter-Mikaelian et al. 2012; Volodin et al. 2023).
The alarm calls of Mongolian gerbils were commonly produced in series separated by intervals with a median duration of 893 ms (Ter-Mikaelian et al. 2012). The series of ultrasonic alarm calls, recorded in semi-natural conditions in a large outdoor enclosure, were often accompanied by rhythmic podophony and evoked fleeing and hiding responses of conspecifics (Ter-Mikaelian et al. 2012). The alarm calls of Mongolian gerbils differed from other ultrasonic calls with their longer duration, on average 116 ms (up to 260 ms) and constant fundamental frequency, on average 23.3 kHz, and by context (Ter-Mikaelian et al. 2012). Other ultrasonic calls (contact and mating) had duration from 19 to 57 ms and fundamental frequency from 30 to 49 kHz (Holman 1980; Kobayasi and Riquimaroux 2012; Ter-Mikaelian et al. 2012; Peterson et al. 2023; Volodin et al. 2023). This is the range of frequencies, in which adult and subadult Mongolian gerbils have a good hearing sensitivity, although the most acute hearing of this species is in the audible frequency range (Ryan 1976; Overstreet et al. 2003).
Mongolian gerbils are small rodents with low sexual dimorphism in body mass (males: average 73.8 g ± 12.4 SD, females: average 72.8 g ± 15.2 SD, Volodin et al. 2023). In natural habitats in Central Asia, Mongolian gerbils are territorial diurnal social rodents with seasonal breeding, living in underground burrows in extended family groups (Agren et al. 1989; Scheibler et al. 2006; review: Gromov 2022). This species is subjected to density waves typical for rodent populations, with outbreaks and depressions (Wang and Zhong 2006; Tian et al. 2015; Liu and Deng 2022).
There is only one study reporting podophony of Mongolian gerbils in the alarm context in natural conditions (Agren et al. 1989). Data on vocalization of Mongolian gerbils in nature are absent. Currently, inexpensive portative ultrasonic recorders enabling collecting the ultrasonic calls of rodents in the wild, enable the recording of calls from burrow entrances out of sight of the callers (Volodin et al. 2022). However, such recordings should be verified as belonging to the particular species by comparison of the acoustic structure of the calls with referential calls recorded in captivity from animals of known species (Volodin et al. 2022).
The aim of this study was to describe the acoustic structure of ultrasonic alarm calls of Mongolian gerbils in the wild and to verify these calls as belonging to Mongolian gerbils by comparison with those recorded in captivity from known callers. Based on these results, we propose a method for non-invasive estimation of occupancy of the burrows by Mongolian gerbils in fragmented colonies at very low population density, by the presence of ultrasonic alarm calls produced toward humans.
Materials and methods
Acoustic recording in captivity
In captivity, ultrasonic alarm calls were recorded from Mongolian gerbils kept in the laboratory colony of Moscow Zoo (Moscow, Russia) from November 2020 to January 2021. The colony originated in 2009 from 11 wild individuals from natural habitats of Tuva, Russia. The alarm calls were recorded from 9 family groups, including in total 41 individuals, from 4 to 7 individuals per group. The groups consisted of adult founders (one male and one female in 7 groups and one male and two sisters in 2 groups) and their 1–5 subadult offspring of 3 months of age or older.
The animals of each group were kept together in plastic-and-wire-mesh cages measuring 50 × 30 × 20 cm with bedding of sawdust and hay, various shelters, wooden hiding boxes without a bottom and tree branches as enrichment. They received custom-made small desert rodent chow with insect and mineral supplements, fruits and vegetables were provided ad libitum as a source of water. The animals were kept and recorded under a natural light regime at room temperature (22–24 °C).
In captivity, each recording session (one per group, 9 sessions in total) was conducted from the focal group home cage. Before recording, the group home cage was transferred to a separate room with a window, where no other animals were present and the electric lamps and power equipment switched off to avoid ultrasonic noise pollution. The recordings were conducted during daytime. Animals produced ultrasonic contact calls and sometimes ultrasonic alarm calls in response to removal of some hides out of the cage and moving the remaining hiding boxes from one location to another within the cage. These manipulations imitated the routine cage cleaning and forced the animals to move in the cage and to contact each other. The acoustic recording started when the first hide was removed from the home cage. The duration of each recording session lasted from 20 to 40 min. At the end of the recording session, all hiding boxes were returned to their original location within a cage and the cage was returned to the colony room.
The recordings were made at collective basis from all animals of the focal group. Individual callers could not be discriminated during recordings aside from the cases when callers (young individuals from 6 family groups aged between 3 and 6 months) produced podophony over the walls of wooden boxes or the floor of the plastic cage simultaneously with the alarm calls. At the remaining 3 groups, the age of the callers could not be noticed. In 7 out of 9 groups, the animal vocalized from a gap between the wooden box wall and the cage wall; in 2 groups, the animal vocalized from inside the wooden box. The gap was approximately of the same width as animal’s body, thus promoting the caller some perceived safety.
In captivity, for recordings in the ultrasonic range of frequencies (sampling rate 256 kHz, 16-bit resolution), we used an Echo Meter Touch 2 PRO (Wildlife Acoustics, Inc., Maynard, MA USA) attached to a smartphone. The researcher could track the ultrasonic calls of the callers visualized as spectrograms in real time on a compatible smartphone display. The microphone was placed at a distance of 30–40 cm above the tested animals. In total, in captivity, we made 248 min of acoustic recordings with the Echo Meter. Audio track of each recording was recorded as a wav-file and then uploaded to PC.
Acoustic recording in the wild
Ultrasonic vocalizations of wild-living individually unidentified Mongolian gerbils were recorded in Dauria (Transbaikalia, Russia, 50.004°N, 115.721°E), along the wide isthmus between the lakes Zun-Torey and Barun-Torey, around the Daursky State Nature Biosphere Reserve International biological station «Kordon Utochi», from 21 June to 07 July 2021. The area is a grassy steppe undulating at elevations of 600–1100 m (Kirilyuk et al. 2013; Obyazov et al. 2021; Volodin et al. 2021a). Potential terrestrial predators of Mongolian gerbils are small carnivores, the corsac Vulpes corsac and the red fox V. vulpes (Murdoch et al. 2010).
In midsummer 2021, when data were collected, the population density of Mongolian gerbils and all other common species of rodents and lagomorphs (primarily Brandt’s voles Lasiopodomys brandtii, narrow-headed voles Lasiopodomys gregalis and Daurian pikas Ochotona daurica) was very low. The reason could be that, in May 2021, during the start of breeding of local species of rodents and pikas, the holes were flooded after atypically strong snowfall followed with snow melting (VEK, unpublished personal observation). As a result, in June we found a large number of excavated holes with fresh emissions, which appeared inhabited, but live animals were only rarely spotted. In semi-arid climate, the burrow systems can exist for many years, and Mongolian gerbils regularly use burrows of other species (pikas and voles) and vice versa (Gromov 2022). The low population numbers of rodents and pikas was indirectly confirmed by the lack of loud series of audible alarm calls from Daurian pikas (Volodin et al. 2021a) and Brandt’s voles (Nikolskii and Sukhanova 1992; Rutovskaya 2012). For comparison, in summer 2019, at the same locality during a population number outbreak of the Daurian pikas and Brandt’s voles, a walking observer could regularly hear up to 3–4 animals mobbing him/her with audible alarm calls (over 10 times per hour, IAV and EVV, unpublished personal observations). In contrast, in 2021 during 17 diurnal walks we registered only 20 audible series of alarm calls from Daurian pikas and observed visually only 3 individuals.
As in captivity, for recording ultrasonic calls in the wild (sampling rate 256 kHz, 16-bit resolution), we used an Echo Meter Touch 2 PRO attached to a smartphone. The recordings were made during daylight hours. A few aggregations of burrow entrances of Mongolian gerbils were found on a plot of steppe 700 m × 300 m located in immediate vicinity (north-east) to the biological station «Kordon Utochi». In total, on this plot, we found 10 aggregations, with minimal distance between aggregations of 100 m. These 10 aggregations represented points of acoustic recordings.
Two researchers (IAV or EVV) visited each point 1–5 times (on average, 2.7 times ± 1.5 SD) and conducted one recording session per visit. The researcher stood or slowly moved over the aggregation of burrow entrances with the Echo Meter, pointing the recorder first at one burrow hole and then to others. Each recording session lasted from 10 to 30 min, in total, 27 recording sessions were conducted. During 3 recording sessions a researcher could see the Mongolian gerbil at a colony surface (on three different points of recordings); during other sessions, the animals were out of sight.
In contrast to recordings in captivity, the researcher could not visually track call spectrograms in live-mode because the screen of the smartphone reflected in the sun and thus recorded the calls blindly. The distance from the microphone to the nearest burrow entrance was about 1 m. So, the calls could potentially be recorded from the entrance to which the microphone was directed or from other entrances, located at distances over 1 m and not oriented towards the microphone. Podophony was not be heard or recorded in the wild. In total, we obtained 198 min of ultrasonic recordings in the wild.
Acoustic analysis
Spectrograms of the wav-files were visually inspected using Avisoft SASLab Pro software (Avisoft Bioacoustics, Berlin, Germany). Two researchers (IAV and AVK) independently inspected all files recorded in captivity and in the wild in the spectrogram window of Avisoft. All acoustic files recorded during the 9 experimental sessions in captivity contained ultrasonic calls. However, among the acoustic files recorded in the wild, the ultrasonic calls were only found in 17 of 27 recording sessions from all 10 points of recordings, corresponding to the 10 burrow aggregations, 1 – 4 (on average, 1.7 ± 1.1 SD) recording sessions per point. The remaining 10 recording sessions in the wild did not contain any ultrasonic calls.
From the acoustic files, following Ter-Mikaelian et al. (2012), we defined the ultrasonic alarm calls of Mongolian gerbils as prolonged calls with constant fundamental frequency (f0) ranging from 20 to 26 kHz and, as a rule, produced in series. Acoustic files made in captivity contained, in addition to the ultrasonic alarm calls, also large amounts of ultrasonic contact calls, which were shorter in duration, higher in f0, lower in intensity, and displayed an upward contour of frequency modulation (Holman 1980; Kobayasi and Riquimaroux 2012; Ter-Mikaelian et al. 2012; Volodin et al. 2023). Acoustic files made in the wild contained only the ultrasonic alarm calls.
Only the ultrasonic alarm calls were included in the spectrographic analysis. From the recordings made in captivity, we selected up to 20 ultrasonic alarm calls per recording session, attempting to distribute them evenly across the available sample of recorded calls. Similarly, from the recordings made in the wild, we selected up to 20 ultrasonic alarm calls per recording session. If less than 20 ultrasonic alarm calls per recording session were available, we included all available calls of appropriate quality in the analysis. When alarm calls were consecutive in a series, we avoided including the neighboring calls within the series in the analysis to reduce pseudo-replication. In total, from 9 recording sessions made in captivity, we selected 88 alarm calls, from 3 to 20 calls (on average, 9.8 calls ± 7.8 SD). In total from 17 recording sessions made in the wild, we selected 111 alarm calls, from 1 to 20 alarm calls per recording session (on average, 6.5 calls ± 6.4 SD).
Acoustic parameters of alarm calls were measured with Avisoft and automatically exported to Microsoft Excel (Microsoft Corp., Redmond, WA, USA). Since the visual inspection of spectrograms showed that the minimum f0 of the calls always exceeded 10 kHz, we applied a high-pass filter at 10 kHz to remove low-frequency noise. We measured the alarm calls with the following settings, providing 250 Hz frequency resolution and 0.5 ms time resolution: sampling frequency 256 kHz, Hamming window, FFT (Fast Fourier Transform) length 1024 points, frame 50%, and overlap 87.5%.
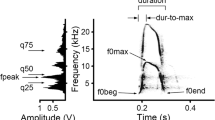
For each alarm call we measured, in the spectrogram window of Avisoft, the duration with the standard marker cursor and the fundamental frequency parameters: the maximum f0 (f0max), the minimum f0 (f0min), the f0 at the onset of a call (f0beg), and the f0 at the end of a call (f0end) with the reticule cursor (Fig. 1). The depth of frequency modulation (df0) was calculated as the difference between f0max and f0min. In addition, we measured, in the power spectrum of Avisoft, the frequency of maximum amplitude (fpeak) from the call mean power spectrum (Fig. 1). We did not measure the power quartiles, as many calls were emitted during strong background noise. For alarm calls produced in series, we measured the inter-call interval, from the end of the preceding call to the start of the next call. We noted in the calls the occurrence of a nonlinear vocal phenomenon (subharmonics), identified by presence of intermediate frequency bands of 1/2 of f0 between harmonics (Wilden et al. 1998; Yurlova et al. 2020; Dymskaya et al. 2022; Rutovskaya et al. 2024).
Measured acoustic parameters for the ultrasonic alarm calls of captive and wild Mongolian gerbils Meriones ungiuculatus. Spectrogram (right) and the mean power spectrum of the call (left). Designations: duration call duration, f0max the maximum fundamental frequency, f0min the minimum fundamental frequency, f0beg the fundamental frequency at the onset of a call, f0end the fundamental frequency at the end of a call, fpeak the frequency of maximum amplitude within a call. The spectrogram was created at 125 kHz sampling frequency, FFT length 1024, Hamming window, frame 50%, overlap 93.75%
Statistical analyses
Statistical analyses were conducted with STATISTICA, v. 8.0 (StatSoft, Tulsa, OK, USA). Means are given as mean ± SD, all tests were two-tailed, and differences were considered significant whenever p < 0.05. Distributions of 18 measured parameter values of 24 distributions did not depart from normality (Kolmogorov–Smirnov test, p > 0.05), so we could apply parametric tests (Dillon and Goldstein 1984). For 4 acoustic parameters (f0max, fpeak, df0 and inter-call interval) the assumption of homogeneity of variance was not met (Levene's test, p < 0.05), so we log-transformed the values of these parameters.
We used a nested design of ANOVA with recording session identity (ID) nested within captive/wild factor (with captive/wild factor included as fixed factor and recording session ID included as random factor) to compare variability of acoustic parameters between captive and wild conditions. Since the results of the ANOVA did not differ for log-transformed and non-transformed data, we further used the non-transformed data for all acoustic parameters.
Results
Both in captivity and in the wild, the alarm calls of Mongolian gerbils were prolonged calls with an average duration of 118 ms ± 60 SD ranging from 20 to 273 ms, with a flat contour of f0 band lying in the ultrasonic range of frequencies from 22 to 28 kHz (Table 1, Fig. 2). Both in captivity and in the wild, the alarm calls could be produced either singly or in series. In captivity, 46.8% (52 of 111) of the analyzed alarm calls were produced in series; in the wild, 36.4% (32 of 88) of the analyzed alarm calls were produced in series. While in captivity, 90.9% (80 of 88) of the alarm calls were combined with podophony, in the wild we could not track whether the alarm calls were combined with podophony or not. In captivity, subharmonics were noted in 3 alarm calls recorded within a single recording session. In the wild, not a single call contained subharmonics.
Spectrogram illustrating acoustic differences between ultrasonic alarm calls of Mongolian gerbils recorded in captivity and in the wild. The panels Captive 1 and Captive 2 display the calls recorded at two different captive family groups. The panels Wild 1 and Wild 2 display the calls recorded at two different wild colonies. The calls of captive gerbils (panels Captive 1 and Captive 2) are accompanied with vertical strikes indicating podophony. In each panel, the intervals between calls and/or strikes of podophony are original (unmodified). The spectrogram was created at 125 kHz sampling frequency, FFT length 1024, Hamming window, frame 50%, overlap 87.5%. The audio file of these calls is available as a Supplementary material
Two-way ANOVA showed that in captivity, the alarm calls had a significantly shorter duration, significantly lower f0max, f0beg and fpeak and followed with significantly shorter inter-call intervals (Table 1, Fig. 2). At the same time, f0min, f0end and df0 did not differ significantly between the alarm calls recorded in captivity and in the wild (Table 1). The df0 comprised 10.7% of f0max in captivity and 12.8% of f0max in the wild (Table 1).
Discussion
The ultrasonic alarm calls of Mongolian gerbils, recorded in this study under laboratory conditions from the groups consisting of adult male–female pairs with their subadult offspring were similar in the acoustic structure with alarm calls described previously from captive breeding groups of Mongolian gerbils (Ter-Mikaelian et al. 2012; Peterson et al. 2023). Compared with our data, in semi-captive conditions, the ultrasonic alarm calls were identified by a brief ascending frequency modulation turning into a brief constant frequency portion at 23.3 kHz ± 1.7 SD followed by a downward frequency shift covering a total range of 2.8 kHz ± 2.0 SD (Ter-Mikaelian et al. 2012). These alarm calls had a duration from 20 to 260 ms (116 ms ± 69 SD) (Ter-Mikaelian et al. 2012). At the same time, the study by Kobayasi and Riquimaroux (2012) did not mention the ultrasonic alarm calls in the vocal repertoire of Mongolian gerbils. As Kobayasi and Riquimaroux (2012) classified the calls primarily based on contour shapes of the fundamental frequency, the alarm calls were probably mixed with long contact calls into one call type with intermediate acoustic characteristics. To our knowledge, Mongolian gerbils produce only the ultrasonic alarm calls, as not a single study mentions the presence of sonic (human-audible) alarm calls in this species.
We found that ultrasonic alarm calls of captive Mongolian gerbils were shorter, lower in fundamental frequency and followed with shorter intervals compared to alarm calls recorded in the wild (Table 1). We propose that such differences in the acoustic structure of alarm calls are related to different levels of perceiving danger and respective increase of alertness of a caller (Briefer 2012). In captivity, the source of danger (human person) was in immediate vicinity (less than 1 m) from the animal, which was restricted in the ability to escape. In the wild, gerbils produced their alarm calls from their burrows, being in relatively safe conditions and at larger distance from the source of danger. The second potential reason for acoustic differences between the alarms from captive and wild conditions may be due to the rapid adaptive physiological changes which are prominent even after a few generations of Mongolian gerbils bred in captivity (Blottner et al. 2000; Blottner and Stuermer 2006). The third potential reason for the found differences can be related to interpopulation variability, because captive animals used in our study originated from Tuva population, whereas in the wild, the alarm calls were recorded from Dauria population. For wild rodents, the interpopulation variation is known for sonic alarm calls of ground squirrels (Matrosova et al. 2016, 2019) and for male songs of rodents from the genus Scotinomys (Campbell et al. 2010). In captivity, the interpopulation variation was reported for soft chirps of naked mole-rats Heterocephalus glaber (Barker et al. 2021). The fourth potential reason for the acoustic differences could be related to the age of the alarm callers. In captivity, the alarm calls were primarily recorded from young individuals, whereas in the wild, the alarm calls were most probably recorded from mature adults, because after the snowfall at the start of breeding in May, the number of surviving pups could be very small.
Sexual behaviour is related to high arousal of a male (Ågmo 2011; Wiemer et al. 2023), what may predetermine call similarity between the alarm and sexual contexts. Consistently, ultrasonic calls reminiscent of the acoustic structure the ultrasonic alarm calls (with average duration of 145 ms and f0 of 26 kHz) were reported at sexual behaviour in pairs of Mongolian gerbils (Holman 1980, 1981; Holman and Hutchison 1985; Holman and Seale 1991). Male gerbils emitted these calls within 3-min after ejaculation but sometimes also before ejaculation (Holman 1980). Castration of males prevented both sexual behaviour and emission of these calls; in contrast, implantation of testosterone to females provoked production of such calls by females (Holman 1981).
Previously, the use of audible alarm calls by rodents immediately after copulation was described in male Pallas’s squirrels Callosciurus erythraeus and Belding’s ground squirrels Urocitellus beldingi (Tamura 1995; Manno et al. 2007). In addition to rodents, polygynous male ruminants insert alarm calls in their sequences of rutting vocalizations, e.g., male topi antelope Damaliscus lunatus (Bro-Jørgensen and Pangle 2010) and impala Aepyceros melampus (Volodin et al. 2021b). Among passerine birds, male superb lyra-birds Menura novaehollandiae use the alarm calls in vocal sequences at sexual context during the mating (Crisologo et al. 2023). Also, male Japanese bush warblers Cettia diphone use the same song when exposed to stuffed predators or stuffed conspecific females (Hamao 2024). Further study is necessary to evaluate whether the calls produced by male Mongolian gerbils at sexual behaviour are indeed indistinguishable by their acoustic characteristics from those of the ultrasonic alarms of this species.
The method of recording ultrasonic alarm calls from burrows is advantageous, because it is non-invasive, fast, inexpensive and does not require animal captures. This method enabled us to estimate the occupancy of natural colonies of Mongolian gerbils at very low population densities. Potentially, it is also applicable for monitoring dispersion of Mongolian gerbils after population depression. At high population density, the animals can be easily observed visually or trapped, whereas at depressions during population waves, limited animals are rarely visible at colony surface. We could only visually observe 3 individual gerbils during this study, but we could nevertheless record the ultrasonic alarm calls 17 times.
One limitation of this method is in fact that the absence of calls does not mean per se the absence of animals. At the same time, the detection of the alarm calls would certainly mean that the animals are present at this place. Furthermore, the use of inexpensive portable ultrasonic recorders enables to expand research of acoustic behaviour in the wild to those species of rodents and other small mammals which produce ultrasonic calls. However, the limitation for expanding this method to a broader number of species is the need of verifying the recordings from the wild with recordings of ultrasonic calls from the given species in captivity (Volodin et al. 2022).
Data availability
Audio file with ultrasonic alarm calls is included in this published article as supplementary file. Additional raw data will be available from the corresponding author on reasonable request.
References
Ågmo A (2011) On the intricate relationship between sexual motivation and arousal. Horm Behav 59:681–688. https://doi.org/10.1016/j.yhbeh.2010.08.013
Agren G, Zhou Q, Zhong W (1989) Ecology and social behaviour of Mongolian gerbils, Meriones unguiculatus, at Xilinhot, Inner Mongolia, China. Anim Behav 37:11–27. https://doi.org/10.1016/0003-3472(89)90002-X
Barker AJ, Veviurko G, Bennett NC, Hart DW, Mograby L, Lewin GR (2021) Cultural transmission of vocal dialect in the naked mole-rat. Science 371:503–507. https://doi.org/10.1126/science.abc6588
Blanchard RJ, Blanchard DC, Agullana R, Weiss SM (1991) Twenty-two kHz alarm cries to presentation of a predator, by laboratory rats living in visible burrow systems. Physiol Behav 50:967–972. https://doi.org/10.1016/0031-9384(91)90423-l
Blottner S, Stuermer IW (2006) Reproduction of wild Mongolian gerbils bred in the laboratory with respect to generation and season: II. Spermatogenic activity and testicular testosterone concentration. Anim Sci 82:389–395. https://doi.org/10.1079/ASC200639
Blottner S, Franz C, Rohleder M, Zinke O, Stuermer IW (2000) Higher testicular activity in laboratory gerbils compared to wild Mongolian gerbils (Meriones unguiculatus). J Zool Lond 250:461–466. https://doi.org/10.1111/j.1469-7998.2000.tb00789.x
Blumstein DT (2007) The evolution of alarm communication in rodents: structure, function, and the puzzle of apparently altruistic calling. In: Wolff JO, Sherman PW (eds) Rodent societies: an ecological and evolutionary perspective. University of Chicago Press, Chicago, pp 317–327. https://doi.org/10.7208/9780226905389-029
Bridelance P (1986) Les podophones de Psammomys obesus en milieu naturel: comparaison avec les podophones de Meriones (Gerbillidae, Rodentia). Mammalia 50:145–152. https://doi.org/10.1515/mamm.1986.50.2.145
Bridelance P (1989) Communication acoustique entгe individus adultes des genres Meriones, Pachyuromys, Psammomys (Geгbillidae, Rodentia). I. Analyse des repertoires. Mammalia 53:3–17. https://doi.org/10.1515/mamm.1989.53.1.3
Bridelance P, Paillette M (1985) Un système original de communication sonore chez des rongeurs désertiques : la podophonie chez quatre espèces de Meriones (Rongeurs, Gerbillidés). Mammalia 49:161–172. https://doi.org/10.1515/mamm.1985.49.2.161
Briefer EF (2012) Vocal expression of emotions in mammals: mechanisms of production and evidence. J Zool Lond 288:1–20. https://doi.org/10.1111/j.1469-7998.2012.00920.x
Bro-Jørgensen J, Pangle WM (2010) Male topi antelopes alarm snort deceptively to retain females for mating. Am Nat 176:E33–E39. https://doi.org/10.1086/653078
Broom DM, Elwood RW, Lakin J, Willy SJ, Pretlove AJ (1977) Developmental changes in several parameters of ultrasonic calling by young Mongolian gerbils (Meriones unguiculatus). J Zool Lond 183:281–290. https://doi.org/10.1111/j.1469-7998.1977.tb04187.x
Brudzynski SM, Holland G (2005) Acoustic characteristics of air puff-induced 22-kHz alarm calls in direct recordings. Neurosci Biobehav Rev 29:1169–1180. https://doi.org/10.1016/j.neubiorev.2005.04.007
Campbell P, Pasch B, Pino JL, Crino OL, Phillips M, Phelps SM (2010) Geographic variation in the songs of Neotropical singing mice: testing the relative importance of drift and local adaptation. Evolution 64:1955–1972. https://doi.org/10.1111/j.1558-5646.2010.00962.x
Conner DA (1985) The function of the pika short call in individual recognition. Z Tierpsychol 67:131–143. https://doi.org/10.1111/j.1439-0310.1985.tb01382.x
Crisologo TL, Dzielski SA, Purcell JR, Webster MS, Welbergen JA, Dalziell AH (2023) Selective alarm call mimicry in the sexual display of the male superb lyrebird (Menura novaehollandiae). Evol Ecol 37:245–266. https://doi.org/10.1007/s10682-022-10200-w
Daly M, Daly S (1975a) Behavior of Psammomys obesus (Rodenth: Gerbillinae) in the Algerian Sahara. Z Tierpsychol 37:298–321. https://doi.org/10.1111/j.1439-0310.1975.tb00882.x
Daly M, Daly S (1975b) Socio-ecology of Saharan gerbils, especially Meriones libycus. Mammalia 39:289–311. https://doi.org/10.1515/mamm.1975.39.2.289
De Ghett VJ (1974) Developmental changes in the rate of ultrasonic vocalization in the Mongolian gerbil. Dev Psychobiol 7:261–272. https://doi.org/10.1002/dev.420070311
Dillon WR, Goldstein M (1984) Multivariate analysis: Methods and applications. Wiley, New York
Dymskaya MM, Volodin IA, Smorkatcheva AV, Vasilieva NA, Volodina EV (2022) Audible, but not ultrasonic, calls reflect surface-dwelling or subterranean specialization in pup and adult Brandt’s and mandarin voles. Behav Ecol Sociobiol 76:106. https://doi.org/10.1007/s00265-022-03213-6
Fernández-Vargas M, Riede T, Pasch B (2022) Mechanisms and constraints underlying acoustic variation in rodents. Anim Behav 184:135–147. https://doi.org/10.1016/j.anbehav.2021.07.011
Fichtel C, Kappeler PM (2002) Anti-predator behavior of group-living Malagasy primates: mixed evidence for a referential alarm call system. Behav Ecol Sociobiol 51:262–275. https://doi.org/10.1007/s00265-001-0436-0
Fourie PB (1977) Acoustic communication in the rock hyrax, Procavia capensis. Z Tierpsychol 44:194–219. https://doi.org/10.1111/j.1439-0310.1977.tb00993.x
Gromov VS (2022) Ecology and social behaviour of the Mongolian gerbil: a generalised review. Behaviour 159:403–441. https://doi.org/10.1163/1568539X-bja10128
Hamao S (2024) A vocalization in male Japanese bush warblers in response to both predators and conspecific females. Ethology. https://doi.org/10.1111/eth.13422
Hare JF (1998) Juvenile Richardson’s ground squirrels, Spermophilus richarrdsonii, discriminate among individual alarm callers. Anim Behav 55:451–460. https://doi.org/10.1006/anbe.1997.0613
Holman SD (1980) Sexually dimorphic, ultrasonic vocalizations of Mongolian gerbils. Behav Neural Biol 28:183–192. https://doi.org/10.1016/S0163-1047(80)91535-6
Holman SD (1981) Neonatal androgenic influences on masculine ultrasonic vocalizations of Mongolian gerbils. Physiol Behav 26:583–586. https://doi.org/10.1016/0031-9384(81)90128-1
Holman SD, Hutchison JB (1985) Effects of intracranial androgen on the development of masculine ultrasonic vocalizations in the Mongolian gerbils (Meriones unguiculatus). J Endocrinol 107:355–363. https://doi.org/10.1677/joe.0.1070355
Holman SD, Seale WT (1991) Ontogeny of sexually dimorphic ultrasonic vocalizations in Mongolian gerbils. Dev Psychobiol 24:103–115. https://doi.org/10.1002/dev.420240204
Holman SD, Seale WT, Hutchison JB (1995) Ultrasonic vocalizations in immature gerbils: Emission rate and structural changes after neonatal exposure to androgen. Physiol Behav 57:451–460. https://doi.org/10.1016/0031-9384(94)00237-Y
Inagaki H, Ushida T (2021) The effect of playback of 22-kHz and 50-kHz ultrasonic vocalizations on rat behaviors assessed with a modified open-field test. Physiol Behav 229:113251. https://doi.org/10.1016/j.physbeh.2020.113251
Kern JM, Laker PR, Radford AN (2017) Contextual variation in the alarm call responses of dwarf mongooses, Helogale parvula. Anim Behav 127:43–51. https://doi.org/10.1016/j.anbehav.2017.03.002
Kiley M (1972) The vocalizations of ungulates, their causation and function. Z Tierpsychol 31:171–222. https://doi.org/10.1111/j.1439-0310.1972.tb01764.x
Kirilyuk O, Kirilyuk V, Maksakovsky N, Kobyakova S, Goroshko O, Tkachuk T, Butorin A, Namkhai A, Urtnasan N, Tseveenmyadag N, Oyungerel B, Dashdorj K, Knapp HD, Golde A (2013) Landscapes of Dauria. ANNIE, Moscow
Kobayasi KI, Riquimaroux H (2012) Classification of vocalizations in the Mongolian gerbil, Meriones unguiculatus. J Acoust Soc Am 131:1622–1631. https://doi.org/10.1121/1.3672693
Kozhevnikova JD, Volodin IA, Zaytseva AS, Ilchenko OG, Volodina EV (2021) Pup ultrasonic isolation calls of six gerbil species and the relationship between acoustic traits and body size. R Soc Open Sci 8:201558. https://doi.org/10.1098/rsos.201558
Kumar SS, Wen X, Yang Y, Buckmaster PS (2006) GABAA receptor-mediated IPSCs and alpha1 subunit expression are not reduced in the substantia Nigra pars reticulata of gerbils with inherited epilepsy. J Neurophysiol 95:2446–2455. https://doi.org/10.1152/jn.01173.2005
Leuchtenberger C, Almeida SB, Andriolo A, Crawshaw PG (2016) Jaguar mobbing by giant otter groups. Acta Ethol 19:143–146. https://doi.org/10.1007/s10211-016-0233-4
Leyn J, Thiriau C, Crockford C, Zuberbühler K (2023) Comprehension of own and other species’ alarm calls in sooty mangabey vocal development. Behav Ecol Sociobiol 77:56. https://doi.org/10.1007/s00265-023-03318-6
Litvin Y, Blanchard DC, Blanchard RJ (2007) Rat 22 kHz ultrasonic vocalizations as alarm cries. Behav Brain Res 182:166–172. https://doi.org/10.1016/j.bbr.2006.11.038
Liu W, Deng K (2022) Population dynamics of wild Mongolian gerbils: quadratic temperature effects on survival and density-dependent effects on recruitment. Diversity 14:586. https://doi.org/10.3390/d14080586
Loughry WJ, Oeser M, Hoogland J (2019) Alarm calls of the same individual vary during a response to the same predator in Gunnison’s prairie dogs. Can J Zool 97:1092–1100. https://doi.org/10.1139/cjz-2019-0064
Manno TG, Nesterova AP, Debarbieri LM, Kennedy SE, Wright KS, Dobson FS (2007) Why do male Columbian ground squirrels give a mating call? Anim Behav 74:1319–1327. https://doi.org/10.1016/j.anbehav.2007.02.033
Manser MB (2001) The acoustic structure of suricates’ alarm calls varies with predator type and the level of response urgency. Proc R Soc B 268:2315–2324. https://doi.org/10.1098/rspb.2001.1773
Matrosova VA, Blumstein DT, Volodin IA, Volodina EV (2011) The potential to encode sex, age, and individual identity in the alarm calls of three species of Marmotinae. Naturwissenschaften 98:181–192. https://doi.org/10.1007/s00114-010-0757-9
Matrosova VA, Schneiderová I, Volodin IA, Volodina EV (2012) Species-specific and shared features in vocal repertoires of three Eurasian ground squirrels (genus Spermophilus). Acta Theriol 57:65–78. https://doi.org/10.1007/s13364-011-0046-9
Matrosova VA, Rusin MY, Volodina EV, Proyavka SV, Savinetskaya LE, Shekarova ON, Rashevska HV, Volodin IA (2016) Genetic and alarm call diversity across scattered populations of speckled ground squirrels (Spermophilus suslicus). Mamm Biol 81:255–265. https://doi.org/10.1016/j.mambio.2016.01.001
Matrosova VA, Ivanova AD, Volodina EV, Volodin IA, Alexandrov DY, Sibiryakova OV, Ermakov OA (2019) Phylogenetic relationship and variation of alarm call traits of populations of red-cheeked ground squirrels (Spermophilus erythrogenys sensu lato) suggest taxonomic delineation. Integr Zool 14:341–353. https://doi.org/10.1111/1749-4877.12383
Minami M, Kawamichi T (1992) Vocal repertoires and classification of the sika deer Cervus nippon. J Mamm Soc Japan 17:71–94. https://doi.org/10.11238/jmammsocjapan.17.71
Murdoch JD, Munkhzul T, Buyandelger S, Reading RP, Sillero-Zubiri C (2010) Seasonal food habits of corsac and red foxes in Mongolia and the potential for competition. Mamm Biol 75:36–44. https://doi.org/10.1016/j.mambio.2008.12.003
Nikolskii AA, Sukhanova MV (1992) Situation dependent variations of a call emitted by great gerbil, Rhombomys opimus and by Brandt’s vole, Microtus brandtii, retreating to burrows. Zool Zh 71(12):125–132 (in Russian)
Nishiyama K, Kobayasi KI, Riquimaroux H (2011) Vocalization control in Mongolian gerbils (Meriones unguiculatus) during locomotion behavior. J Acoust Soc Am 130:4148–4157. https://doi.org/10.1121/1.3651815
Obyazov VA, Kirilyuk VE, Kirilyuk AV (2021) Torey lakes as an indicator of moisture long-term changes in Southeastern Transbaikalia and Northeastern Mongolia. Hydrosphere Hazard Process Phenom 3:204–232. https://doi.org/10.34753/HS.2021.3.3.204. (in Russian)
Overstreet EH, Richter CP, Temchin AN, Cheatham MA, Ruggero MA (2003) High-frequency sensitivity of the mature gerbil cochlea and its development. Audiol Neurotol 8:19–27. https://doi.org/10.1159/000067892
Peterson RE, Choudhri A, Mitelut C, Tanelus A, Capo-Battaglia A, Williams AH, Schneider DM, Sanes DH (2023) Unsupervised discovery of family specific vocal usage in the Mongolian gerbil. Elife 12:RP89892. https://doi.org/10.7554/eLife.89892.1
Randall JA (2001) Evolution and function of drumming as communication in mammals. Am Zool 41:1143–1156. https://doi.org/10.1093/icb/41.5.1143
Randall JA, Rogovin KA (2002) Variation in and meaning of alarm calls in a social desert rodent Rhombomys opimus. Ethology 108:513–527. https://doi.org/10.1046/j.1439-0310.2002.00797.x
Randall JA, Rogovin KA, Shier DM (2000) Antipredator behavior of a social desert rodent: footdrumming and alarm calling in the great gerbil, Rhombomys opiums. Behav Ecol Sociobiol 48:110–118. https://doi.org/10.1007/s002650000199
Randall JA, McCowan B, Collins KC, Hooper SL, Rogovin K (2005) Alarm signals of the great gerbil: acoustic variation by predator context, sex, age, individual, and family group. J Acoust Soc Am 118:2706–2714. https://doi.org/10.1121/1.2031973
Rutovskaya MV (2012) The sound signals of Brandt’s vole (Lasiopodomys brandti). Sens Systems 26:31–38 ([In Russian])
Rutovskaya MV, Volodin IA, Feoktistova NY, Surov AV, Gureeva AV, Volodina EV (2024) Acoustic complexity of pup isolation calls in Mongolian hamsters: 3-frequency phenomena and chaos. Curr Zool 70:zoad036. https://doi.org/10.1093/cz/zoad036
Ryan A (1976) Hearing sensitivity of the Mongolian gerbil, Meriones unguiculatis. J Acoust Soc Am 59:1222–1226. https://doi.org/10.1121/1.380961
Scheibler E, Liu W, Weinandy R, Gattermann R (2006) Burrow systems of the Mongolian gerbil (Meriones unguiculatus Milne Edwards, 1867). Mamm Biol 71:178–182. https://doi.org/10.1016/j.mambio.2005.11.007
Seyfarth RM, Cheney DL, Marler P (1980) Vervet monkey alarm calls: semantic communication in a free-ranging primate. Anim Behav 28:1070–1094. https://doi.org/10.1016/S0003-3472(80)80097-2
Silberstein Y, Felmy F, Scheumann M (2023) Encoding of arousal and physical characteristics in audible and ultrasonic vocalizations of Mongolian gerbil pups testing common rules for mammals. Animals 13:2553. https://doi.org/10.3390/ani13162553
Tamura N (1995) Postcopulatory mate guarding by vocalization in the Formosan squirrel. Behav Ecol Sociobiol 36:377–386. https://doi.org/10.1007/BF00177333
Ter-Mikaelian M, Yapa WP, Rübsamen R (2012) Vocal behavior of the Mongolian gerbil in a seminatural enclosure. Behaviour 149:461–492. https://doi.org/10.1163/156853912X639778
Tian HY, Yu PB, Luis AD, Bi P, Cazelles B, Laine M, Huang SQ, Ma CF, Zhou S, Wei J, Li S, Lu XL, Qu JH, Dong JH, Tong SL, Wang JJ, Grenfell B, Xu B (2015) Changes in rodent abundance and weather conditions potentially drive hemorrhagic fever with renal syndrome outbreaks in Xi’an, China, 2005–2012. PLoS Negl Trop Dis 9:e0003530. https://doi.org/10.1371/journal.pntd.0003530
Volodin IA, Volodina EV, Frey R, Gogoleva SS, Palko IV, Rozhnov VV (2017) Acoustic structure of alarm calls in Indian sambar (Rusa unicolor) and Indian muntjac (Muntiacus vaginalis) in South Vietnam. Dokl Biol Sci 474:110–113. https://doi.org/10.1134/S0012496617030061
Volodin IA, Matrosova VA, Frey R, Kozhevnikova JD, Isaeva IL, Volodina EV (2018) Altai pika (Ochotona alpina) alarm calls: individual acoustic variation and the phenomenon of call-synchronous ear folding behavior. Sci Nat 105:40. https://doi.org/10.1007/s00114-018-1567-8
Volodin IA, Volodina EV, Frey R (2021a) Rutting vocal display in male impala (Aepyceros melampus) and overlap with alarm context. Front Zool 18:2. https://doi.org/10.1186/s12983-020-00383-9
Volodin IA, Volodina EV, Frey R, Karaseva KD, Kirilyuk VE (2021b) Daurian pika (Ochotona dauurica) alarm calls: individual acoustic variation in a lagomorph with audible through ultrasonic vocalizations. J Mammal 102:947–959. https://doi.org/10.1093/jmammal/gyab048
Volodin IA, Dymskaya MM, Smorkatcheva AV, Volodina EV (2022) Ultrasound from underground: cryptic communication in subterranean wild-living and captive northern mole voles (Ellobius talpinus). Bioacoustics 31:414–434. https://doi.org/10.1080/09524622.2021.1960191
Volodin IA, Kozhevnikova JD, Ilchenko OG, Sapozhnikova SR, Volodina EV (2023) Cross-fostering effects on ultrasonic calls in two gerbil species. Russ J Theriol 22:16–23. https://doi.org/10.15298/rusjtheriol.22.1.02
Volodina EV, Volodin IA, Chelysheva EV, Frey R (2018) Hiss and snort call types of wild-living giraffes Giraffa camelopardalis: acoustic structure and context. BMC Res Notes 11:12. https://doi.org/10.1186/s13104-017-3103-x
Wang G, Zhong W (2006) Mongolian gerbils and Daurian pikas responded differently to changes in precipitation in the Inner Mongolian grasslands. J Arid Environ 66:648–656. https://doi.org/10.1016/j.jaridenv
Wiemer J, Kurstak S, Sellmann F, Lindner K (2023) Sexual stimuli cause behavioral disinhibition in both men and women, but even more so in men. Arch Sex Behav 52:1445–1460. https://doi.org/10.1007/s10508-022-02514-1
Wilden I, Herzel H, Peters G, Tembrock G (1998) Subharmonics, biphonation, and deterministic chaos in mammal vocalization. Bioacoustics 9:171–196. https://doi.org/10.1080/09524622.1998.9753394
Wilson DR, Hare JF (2004) Ground squirrel uses ultrasonic alarms. Nature 430:523. https://doi.org/10.1038/430523a
Wilson DR, Hare JF (2006) The adaptive utility of Richardson’s ground squirrel (Spermophilus richardsonii) short-range ultrasonic alarm signals. Can J Zool 84:1322–1330. https://doi.org/10.1139/Z06-120
Yurlova DD, Volodin IA, Ilchenko OG, Volodina EV (2020) Rapid development of mature vocal patterns of ultrasonic calls in a fast-growing rodent, the yellow steppe lemming (Eolagurus luteus). PLoS ONE 15:e0228892. https://doi.org/10.1371/journal.pone.0228892
Zaytseva AS, Volodin IA, Ilchenko OG, Volodina EV (2020) Audible calls and their ontogenetic relationship with ultrasonic vocalization in a rodent with a wide vocal range, the fat-tailed gerbil (Pachyuromys duprasi). Behav Process 180:104241. https://doi.org/10.1016/j.beproc.2020.104241
Zuberbühler K (2009) Survivor signals: the biology and psychology of animal alarm calling. Adv Study Behav 40:277–322. https://doi.org/10.1016/S0065-3454(09)40008-1
Acknowledgements
We thank S.R. Sapozhnikova for her help and support during data collection. We thank Daursky State Nature Biosphere Reserve for help and support. We thank two anonymous reviewers for valuable comments thoroughly contributed to the improvements of the first version of manuscript. The study was conducted according to the ASAB/ABS ‘Guidelines for the ethical treatment of nonhuman animals in behavioural research and teaching’ (Animal Behaviour, 2023, 195, I-XI). Acoustic recording in captivity was the part of the research program of the Small Mammals Department of Moscow Zoo. One author is zoo staff member, so no special permission was required to work with laboratory animals in Moscow Zoo. The recordings were aimed for evaluating potential ultrasonic noise pollution in small mammal expositions and accompanied routine zoo management (inspection of breeding activity and animal health before regular cage cleaning); no additional actions specially for the needs of this research was applied. All recordings in the wild were purely observational.
Funding
This study was not supported by any foundation.
Author information
Authors and Affiliations
Contributions
IAV and EVV contributed to the study conception and designed the methodology; IAV, VEK, OGI and EVV collected the data in captivity and in the wild; AVK performed data analyses and designed the figures. All authors contributed critically to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Handling editor: Sabine Begall.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
42991_2024_416_MOESM1_ESM.wav
Audio file (sampling frequency 256 kHz) of ultrasonic alarm calls of Mongolian gerbils recorded in captivity from two different captive family groups accompanied by podophony, and in the wild from two different wild colonies
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Volodin, I.A., Klenova, A.V., Kirilyuk, V.E. et al. Ultrasonic alarm call of Mongolian gerbils (Meriones ungiuculatus) in the wild and in captivity: a potential tool for detecting inhabited colonies during population depression. Mamm Biol 104, 407–416 (2024). https://doi.org/10.1007/s42991-024-00416-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42991-024-00416-4