Abstract
We reviewed the alpha taxonomy of the genus Ctenomys Blainville, 1826 in southernmost South America, with emphasis on those nominal forms previously associated with C. magellanicus Bennett, 1836. We integrate distinct lines of evidence, including variation of mtDNA sequences, and the assessment of quantitative and qualitative traits of skins and skulls; when available, karyotypic data was also considered. Phylogenetic analysis of molecular markers shows low levels of divergence among specimens from southern South American mainland and the island of Tierra del Fuego (ca. 0.4%). This evidence plus the results of the multivariate analysis of metric data suggest that the nominal forms C. colburni J. A. Allen, 1903, C. fueginus Philippi, 1880, C. osgoodi J. A. Allen, 1905, C. m. dicki Osgood, 1943, and C. m. obscurus Texera, 1975 are subjective junior synonyms of C. magellanicus. In addition, we reviewed the status of C. fodax Thomas, 1910, a nominal form that have been alternatively considered as a valid species or related to C. magellanicus by previous researchers. Based on quantitative and qualitative morphological traits, we preliminarily regard C. fodax at the species level while citing it for the first time to Chile.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With ca. 69 recognized species, Ctenomys Blainville, 1826 is the one of the two most diverse genera of rodents (the other being Rattus with also 69 species; see details in Mammal Diversity Database 2018). However, our current understanding of the species richness of Ctenomys and on the phylogenetic relationships of its species, are far from complete (Parada et al. 2011; Bidau 2015). For instance, the status of several nominal forms is unclear (e.g., Parada et al. 2012), at the time that new candidate species are often identified (e.g., Caraballo and Rossi 2017) and described (e.g., de Freitas et al. 2012; Gardner et al. 2014).
Based on the analysis of mtDNA sequences of specimens of Ctenomys, Parada et al. (2011) recognized eight species groups and some species without clear phylogenetic relationships within the genus. One of these groups, the magellanicus species group, comprises species from Patagonian and Fueguian open areas and represents the only group of Ctenomys to reach the southern tip of South America. Parada et al. (2011) refers to the magellanicus group the species C. colburni J. A. Allen, 1903, C. coyhaiquensis Kelt and Gallardo, 1994, C. fodax Thomas, 1910, C. haigi Thomas, 1919, C. magellanicus Bennett, 1836, and C. sericeus J. A. Allen, 1903. Of these, C. magellanicus is a widely distributed taxon that occupies open shrubby and grassy habitats in southern Argentina and Chile, including some islands, such as Riesco and Tierra del Fuego, in which constitutes a unique feature in the genus. Additionally, C. magellanicus is the hystricognath species that reaches, by far, the highest southern latitude. As currently understood, C. magellanicus includes in its synonymy the nominal forms dicki Osgood, 1943, fueguinus Philippi, 1880, obscurus Texera, 1975, and osgoodi J. A. Allen, 1905. These forms, depending upon the author and based on differences in skull anatomy and external coloration, have been earlier treated at the species or subspecies level (e.g., Osgood 1943; see details in the generic account of Ctenomys by Bidau 2015). According to Osgood (1943), the synonymy of magellanicus also includes fodax; however, other authors treated this form as a different species (cf. Bidau 2015) or suggested a close relationship of fodax to other species of the magellanicus species group (i.e., C. coyhaiquensis–C. sericeus; see Parada et al. 2011).
Despite recent advances in understanding the diversity of Ctenomys, mostly based on phylogenetic analysis of DNA sequences (e.g., Caraballo and Rossi 2017; Mapelli et al. 2017; Leipnitz et al. 2018), no contemporaneous study has assessed the distinction of the forms associated with C. magellanicus (but see Lizarralde et al. 2001 and Fasanella et al. 2013 for a studies focused on the Fueguian populations). In fact, no detailed morphological study, based in multivariate statistical analyses of large specimen series, is available; the same is true about the lack of geographically broad molecular based analysis of C. magellanicus.
In this study, we addressed the taxonomic status of the tuco-tucos from southernmost Argentina and Chile, with focus on the taxa related to C. magellanicus. We embrace the so-called General Lineage Concept of species (de Queiroz 2007) as we consider it conceptually sound at the time that it is the one used in the majority of current works centered on rodent systematics (D’Elía et al. 2019a). To identify and delimit species lineages we use an integrative approach, analyzing mtDNA sequences and qualitative and quantitative morphological attributes of skins and skulls. Our study is based on the largest sample of individuals of C. magellanicus and associated forms analyzed to date, both in terms of specimen numbers and geographic coverage; it also includes the assessment of some holotypes and topotypic specimens.
Materials and methods
Sampling for the genetic and phylogenetic analyses
We analyzed a fragment of 801 base pairs of the mitochondrial cytochrome-b (cytb) gene of 48 specimens of the C. magellanicus species complex. Specimens were collected at 24 localities and represent all known species of the C. magellanicus species complex. Sampling includes topotypes of the nominal forms C. colburni, C. coyhaiquensis and C. haigi. Sequences of the species C. boliviensis Waterhouse, 1848, C. sociabilis Pearson and Christie, 1985, C. torquatus Lichtenstein, 1830, and C. tucumanus Thomas, 1900, which belong to other species groups of Ctenomys (Parada et al. 2011), were used to conform the outgroup. Some sequences were retrieved from Genbank and others gathered by us from specimens housed in Colección Felix de Azara (CFA, Buenos Aires, Argentina) and Colección de Mamíferos, Universidad Austral de Chile (UACh, Valdivia, Chile). Sequences of the eight specimens from Torres del Paine, were gathered from ear punch samples preserved in alcohol of specimens that were afterwards freed at the capture site.
New sequences were gathered following the protocol outlined by Cañón et al. (2010). The exception to what just noted were the sequences of specimens CFA 11332, CFA 11346, UACH 4232 and UACH 4333 that were gathered from pieces of skin of specimens collected during the decades of 1980–2000. DNA from these samples was extracted following the protocol of Velazco and Patterson (2013) and the cyt b gene was amplified in two fragments using primers MVZ05-oct439R and OCT406F-MVZ16. Amplicons were purified and sequenced by Macrogen Inc., Korea. New sequences were edited with CodonCode and deposited in Genbank. All accession numbers are provided in the “Appendix”.
Genetic and phylogenetic analyses
Sequence alignment was done with Clustal as implemented in MEGA 6 (Tamura et al. 2013). A visual inspection was done to check for the presence of internal stop codons and discard reading frame shifts; no correction was needed. Relationships among cyt b haplotypes were conducted via Bayesian inference (Rannala and Yang 1996) as implemented in MrBayes 3.1 (Ronquist and Huelsenbeck 2003). Two independent runs with 5 heated and 1 cold Markov chains each were implemented. The HKY + G model, selected with jModelTest (Darriba et al. 2012), was used. Model parameters were estimated in MrBayes; base composition and HKY parameters assumed a Dirichlet process prior; all other parameters have uniform interval priors. Runs were run for 20 million generations, with trees sampled every 1000 generations. To check if runs converged on a stable log-likelihood value, we plotted log-likelihood values against generation time. The first 25% of the trees sampled were discarded as burn-in; remaining trees were used to compute a 50% majority rule consensus tree and to obtain posterior probability (PP) values for each clade. Observed percentage of sequence divergence (p-distances) between pairs of haplotypes, local samples and species was calculated with MEGA 6 (Tamura et al. 2013) ignoring sites with missing data.
Studied specimens in the morphological analyses
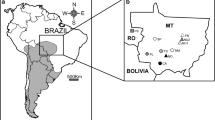
Morphologic analyses were based on 142 adult specimens of Ctenomys from southernmost Argentina and Chile, which are housed in the following museums and mammal collections: Centro Nacional Patagónico (CNP, Chubut, Argentina); Field Museum of Natural History (FMNH, Chicago, U.S.); Fundación de Historia Natural “Félix de Azara” (CFA, Buenos Aires, Argentina); Museo Argentino de Ciencias Naturales “Bernardino Rivadavia” (MACN-Ma, Buenos Aires, Argentina); Colección de Mamíferos, Universidad Austral de Chile (UACh, Valdivia, Chile); U. S. National Museum of Natural History, Smithsonian Institution (USNM, Washington DC, U.S.). Studied specimens and their localities are listed in the Supplementary File 1. Studied specimens were pooled into eight major geographical groups that coincide with recent taxonomic arrangements (e.g., Osgood 1943; Texera 1975; Bidau 2015): C. colburni (including the holotype and part of the type series), C. cf. C. colburni (Estancia El Puma, Santa Cruz), C. fodax (including one topotype), C. m. dicki (including the entire type series), C. m. fueginus, C. m. magellanicus, C. m. obscurus (including three paratypes), and C. m. osgoodi (including the holotype and part of the type series) (Fig. 1). The latter five groups pooled together are referred in the text as C. magellanicus s.l. (see Fig. 1). Geographical groups are representative of the taxa of Ctenomys recognized in southernmost South America (cf. Bidau 2015); as such, we used these groups in a priori classifications in different statistical analyses.
Map of southern South America, depicting the collection localities for Ctenomys specimens studied in this contribution: a specimens of Ctenomys magellanicus used in phylogenetic analysis of DNA sequences (black circles); b specimens of Ctenomys used in morphometric analysis; symbols are as follow (from north to south): crosses = C. fodax; black circles = C. colburni; white circles = C. cf. C. colburni; black squares = C. magellanicus osgoodi; white diamond = C. m. magellanicus; white squares = C. m. fueginus; black triangle = C. m. dicki; black diamond = C. m. obscurus. For reference numbers see Supplementary File 4
Cranial measurements
Sixteen craniodental measurements were recorded from each specimen using a digital caliper to the nearest 0.01 mm following the definitions made by Contreras and Contreras (1984). Measurements are: total length of the skull (TLS); condylo-incisive length (CIL); nasal length (NL); nasal width (NW); rostral width (RW); frontal length (FL); interorbital constriction (IOC); greatest zygomatic breadth (ZB); braincase breadth (BB); bimeatal breadth (BIB); mastoid breadth (MB); infraorbital foramen height (IFH); upper diastema length (DL); palatal length (PL); upper fourth premolar length (PM4L); upper toothrow length (TRL). Only complete skull (N = 135) were measured.
Geographic variation
Patterns of geographic variation among local samples were assessed trough descriptive statistics (i.e., mean, minimum and maximum values, standard deviation) and multivariate analyses, including size-corrected principal component (PCA) and discriminant function analyses (DFA). Principal components (PCs) were extracted from the variance–covariance matrix, after the log10-transformation of the original data (Strauss 2010). To avoid the distortion derived from the effect of size, a size-corrected PCA was performed using variables corrected by the geometric mean (i.e., each species measurement divided by the nth root of the product of values of a species vector of n variables; see Mosimann 1970; Meachen-Samuels and Van Valkenburgh 2009). Discriminant function analyses (DFA) were employed to assess the differences among species and subspecies (Strauss 2010). Multivariate analysis of variance (MANOVA) was performed to test the statistical significance of differences between geographical groups of C. magellanicus s.l. Only those groups with N > 5 were considered; these are C. colburni, C. cf. colburni, C. m. magellanicus, C. m. osgoodi, and C. m. fueginus. Previous researchers documented some variation in quantitative skull characters among sexes within the genus Ctenomys (e.g., Tiranti et al. 2005). However, as both sexes were equally represented on our samples, we pooled them (for a similar procedure, see Kelt and Gallardo 1994). All statistical procedures were performed with PAST ver. 3.21 (Hammer et al. 2001).
Results
Phylogenetic relationships
The Ctenomys magellanicus species group is recovered monophyletic and with high support (PP = 1; Fig. 2). The basal dichotomy of the clade of the Ctenomys magellanicus species group leads to two highly supported clade. One clade (PP = 1) is composed by haplotypes recovered from specimens currently assigned to C. haigi, C. sericeus, C. coyhaiquensis, C. sp. 1 (C. fodax according to Parada et al. 2011; see below), and C. sp. 2. Within this clade, haplotypes of C. haigi do not form a monophyletic group. The haplotype recovered from a topotype of C. haigi (sequence HM777476) is not part of a large clade (PP = 1) formed by the remaining haplotypes recovered from specimens currently allocated to C. haigi. This later clade has a large geographic distribution in northern Patagonia including the general area of the type locality of C. lentulus Thomas, 1919, a nominal form associated with C. haigi. Haplotypes of C. coyhaiquensis form a clade (PP = 0.96) that appears as sister, in a weakly supported relationship (PP = 0.70), to the haplotype of a specimen of C. sp. 1. Haplotypes of C. sericeus form a weakly supported group (PP = 0.80) that is sister (PP = 0.98) to the C. coyhaiquensis–C. sp. 1 clade. Finally, C. sp. 2 (PP = 1) appears sister (PP = 0.98) to the clade C. coyhaiquensis–C. sp. 1–C. sericeus. The other main clade (PP = 1) of the Ctenomys magellanicus species group is composed by haplotypes recovered from specimens assigned to C. magellanicus s.l. and C. colburni. None of these nominal forms appears as monophyletic, rather haplotypes of both taxa are mixed in a relatively shallow (0.4% of average haplotype pairwise distance) and geographically widespread clade; similarly, the genealogy is not structured by mainland vs. Tierra del Fuego (Fig. 2).
Majority rule consensus tree obtained from the Bayesian analysis of 48 cytochrome-b gene sequences of the Ctenomys magellanicus species group (sensu Parada et al. 2011) and using sequences of C. boliviensis, C. sociabilis, C. torquatus, and C. tucumanus as the outgroup. Numbers indicate posterior probability values of adjacent nodes. Terminal designations are the museum catalog and GenBank accession numbers, respectively. Locality data are provided in “Appendix”. Sequences from specimens identified in GenBank as C. colburni are indicated with “col” following accession numbers; similarly, the sequence of the specimen identified in GenBank as C. fodax is indicated with “fod” after the accession number
Qualitative morphological variation
Two main morphotypes were identified among the studied samples. One encompasses those specimens referred to C. colburni and C. magellanicus s.l., which despite some relatively large variation in skull size (see Supplementary File 2), are not distinguishable by a single qualitative trait (Figs. 3, 4, 5). Most samples from Tierra del Fuego and southwestern mainland South America have strongly built skulls, with well developed postorbital processes on frontals and narrow fronto-temporal sutures (Figs. 3, 4). Within this southern morph are included the holotypes and type series of C. m. dicki, and C. robustus (= C. osgoodi), and several samples of C. m. fueguinus, C. m. magellanicus, and C. m. obscurus (Figs. 3, 4). Among samples here referred to C. colburni, those specimens from the type locality and adjoining areas (e.g., Estancia La Cantera, Bajo Caracoles, see Fig. 1) are characterized by relatively less massive skulls, with less developed postorbital processes and broad fronto-temporal sutures, while the individuals from Estancia El Puma presented an intermediate size and skull massiveness between topotypical C. colburni and C. magellanicus s.l. (Fig. 5). The external coloration was variable between samples and even within a same population (Figs. 6, 7). Individuals representing C. colburni are characterized by an overall yellowish-brown dorsal coloration and buffy venters (Fig. 7). Among the remaining samples, the dorsum varies from yellowish brown to pale grizzled grayish buff, more or less saturated with fulvous or yellowish (e.g., C. m. fueginus, C. m. osgoodi, C. m. magellanicus), to smoke gray (e.g., C. m. dicki), while the venters varies from buffy (e.g., C. m. fueginus, C. m. magellanicus) to cinnamon (e.g., C. m. osgoodi) or blackish brown (e.g., C. m. dicki) (Fig. 6). Despite some variation in external size, samples of C. colburni have a similar overall coloration than those samples here referred to C. m. magellanicus or C. m. fueginus (cf. Figs. 6, 7).
The second main morphotype was found in the samples from Río Ñireguao, Aysén, Chile, and Lago Blanco, Chubut, Argentina, which is here referred to C. fodax (see below) and were remarkable homogeneous in its skull features (Fig. 8). This morph differs from C. magellanicus s.l. by its relatively larger skull, its conspicuously broader nasals, and its tympanic bullae less visible from above (Figs. 8, 9; Supplementary File 2). One qualitative trait that helps to easily distinguish between both species is that in C. fodax the naso-frontal suture reaches the level of the premaxillary–frontal suture, while in C. colburni and C. magellanicus s.l., the premaxillary–frontal suture noticeably surpasses the naso-frontal suture (Fig. 9). Externally, the fur of C. fodax is pale cinnamon to isabella, but averaging more cinnamon than in individuals of magellanicus s.l. (Fig. 6).
Selected differences in the cranial anatomy of Ctenomys magellanicus (left) and C. fodax (right). The figure portrays characteristic contrasts between both taxa, including, in C. magellanicus, (1) narrower nasals (n), (2) naso-frontal suture surpassing the level of the premaxillary (pm)-frontal (f) suture; (3) broader interparietals, and (4) tympanic bullae well visible from above. Individuals are not in scale to facilitate comparisons
Quantitative morphological variation
Craniodental measurements, including mean, standard deviation (SD), and range, are summarized in Supplementary File 2. PCA performed with a sample of 135 adult specimens and 16 craniodental measurements revealed that all variables were positively correlated with the 1st principal component (PC1 56.95% of the total variance), suggesting that it correspond mostly to a size vector (Table 1; Fig. 10). The multivariate space of C. fodax along the first two PCs do not overlap with that of C. colburni and C. magellanicus s.l. Meanwhile, a north to south gradient in size and shape could be recognized between these two latter nominal forms. Topotypical samples of C. colburni grouped mostly towards positive values along the PC1, while those from the geographically intermediate Estancia El Puma occupy an intermediate position between them and those from the southwestern mainland and Tierra del Fuego, which grouped towards negative values. There is a high superimposition of those samples labeled as C. m. dicki, C. m. fueginus, C. m. magellanicus, C. m. obscurus, and C. m. osgoodi, suggesting low levels of quantitative morphological differentiation, both in size and shape (Fig. 10). Samples mostly overlap along the third PC (Supplementary File 3).
Individual scores of adult specimens of Ctenomys (N = 106) for: a Principal components 1 and 2; b Canonical variates 1 and 2, extracted from 8 taxonomical group discriminant function analysis; symbols are as follow: crosses (and shadow area) = C. fodax; black circles = C. colburni; white circles = C. cf. C. colburni; black squares = C. magellanicus osgoodi; white diamond = C. m. magellanicus; black triangle = C. m. dicki; white squares = C. m. fueginus; black diamond = C. m. obscurus
The DFA shows that the defined groups segregate in four main areas of the morphospace defined by the 1st and 2nd discriminant functions, which summarize 75.56% of the total variance (Table 1; Fig. 10). One part of the morphospace is occupied by those samples referred to C. fodax, the second and the third by those animals labeled as C. colburni from the type locality and Estancia El Puma, respectively, and the fourth by C. magellanicus s.l. The last three groups overlapped moderately towards the center of the multivariate space (Fig. 10). Finally, there is a marked superposition of the multivariate space of the different subspecific samples currently recognized within C. magellanicus s.l., with a moderate differentiation along the second axis between osgoodi and the remaining nominal forms (Fig. 10). There is no sample segregation along the 3rd discriminant function (Supplementary File 3). The classification matrix determined by the DFA is presented in the Table 2.
The MANOVA shown an overall significant inter-group variation (λ = 0.07080, df = 64, 467.2, p < 0.001). Posterior pairwise comparisons, using Bonferroni corrected p values, showed that C. m. magellanicus does not differ from any of the other taxonomical groups (i.e., C. colburni, C. cf. colburni, C. m. fueguinus, and C. osgoodi); meanwhile, C. m. fueginus does not differ from C. m. osgoodi.
Discussion
Taxonomy
Studies aimed to clarify the taxonomic status of the populations and nominal forms of the genus Ctenomys are challenged by the remarkable morphological homogeneity, both external and cranial, that exists among specimens of different species, presumably, as a result of the constrains imposed by their fossorial mode of life (Bidau 2015). However, there is considerable diversity in body size that usually allows the identification of species trough multivariate statistical procedures of linear measurements or geometric morphometrics of the skull (e.g., Tiranti et al. 2005; D’Anatro and D’Elía 2011; Fornel et al. 2018). Species recognition, and sometimes also species delimitation, have also benefited from cytogenetic evidence (e.g., Freitas and Lessa 1984; Freitas 2006) and, more recently, by the analysis of DNA sequences (e.g., Parada et al. 2011, 2012; Caraballo and Rossi 2017), as well as the integration of distinct sources of evidence (e.g., Freitas et al. 2012).
Based on phylogenetics analysis of DNA sequences, plus qualitative and quantitative morphological traits, we provisionally recognize two species among the nominal forms associated to C. magellanicus: C. fodax and C. magellanicus (including C. colburni, C. m. dicki, C. m.fueginus, C. m. magellanicus, C. m. obscurus, and C. m. osgoodi). Synonyms, distributions, and general remarks are summarized below:
Ctenomys fodax Thomas, 1910
Ctenomys fodax Thomas, 1910:243; type locality: “Valle del Lago Blanco, Cordillera region of Southern Chubut, Patagonia (about 46° S., 71° W.)”; restricted to “Estancia Valle Huemules (45° 57′ S, 71° 31′ W, Río Senguerr, Chubut),” Argentina, by Pardiñas et al. (2007).
Ctenomys talarum fodax: Rusconi, 1928:243
Ctenomys magellanicus osgoodi: Osgood, 1943:120
Distribution: southwestern Chubut province, Argentina, and adjoining areas of south-central Chile. Specimens for Río Ñireguao, Aysen, represents the first Chilean record of the species (Fig. 1).
Remarks: Reig et al. (1992) described a cytotype of 2n = 28, FN = 42 for C. fodax that almost do not differ from the karyotype currently assigned to C. coyhaiquensis (cf. Gallardo 1991; Kelt and Gallardo 1994). In addition, a mtDNA sequence gathered from a specimen identified as C. fodax by Parada et al. (2011); see also Londoño-Gaviria et al. (2019) from Lago Blanco, Chubut, Argentina, appears in our analysis as sister to C. coyhaiquensis. The sequenced specimen is a small-sized animal (TSL < 43 mm) with a brownish coloration, as is usual in C. coyhaiquensis and C. sericeus (cf. Vincon 2004). On the contrary, the holotype of C. fodax corresponds to a much larger animal (TSL = 57.7 mm), morphologically closer to C. magellanicus s.l, and with an overall cinnamon coloration (cf. Figs. 6, 8). Based on these findings, we suggest that at least two different species of Ctenomys inhabit southwestern Chubut province and adjoining areas of Chile, one small belonging to the C. coyhaiquensis-C. sericeus complex (to which belong the karyotyped specimen reported by Reig et al. (1992) and the sequenced specimen analyzed by Parada et al. 2011) and another large for which the name fodax is available. We refer the small form as C. sp 1 pending additional studies that would clarify if C. coyhaiquensis occurs in Argentina (see also the mention to this species in Argentina by Saba and De Lamo 1994) or if C. sp. 1 corresponds to another species.
Based on the examination of one topotypic specimen (FMNH 18191) from Lago Blanco, Chubut, Osgood (1943) included C. fodax into the synonymy of C. m. osgoodi. This author also referred to C. m. osgoodi three individuals from Río Ñireguao, Aysén, Chile (FMNH 23232-23234). However, is important to note that Osgood (1943) did not review the type series of C. osgoodi or other topotypical specimens of this taxon. Our examination of these same four specimens at the FMNH (i.e., FMNH 18191, 23232–23234), the type series of C. osgoodi, and photographs of the holotype of C. fodax, allows us to conclude that C. fodax differs from C. osgoodi and other forms of C. magellanicus in several cranial traits that were firstly reported by Thomas (1910) as nearly constant between these two nominal forms (e.g., broadness of nasals, posterior projection of premaxillary bones; see the results section above and Fig. 9). With the data at hand, and based on qualitative and quantitative morphological traits, we hypothesize that C. fodax represents a distinct species of the genus Ctenomys. Our taxonomic hypothesis should be further tested with the analysis of more specimens as well as genetic data.
Ctenomys magellanicus Bennett, 1836
Ctenomys magellanicus Bennett, 1836:190; type locality: “Port Gregory, near eastern end of north side of Straits of Magellan, Chile,” Bahía San Gregorio, Magallanes y Antártica Chilena, Chile (cf. Allen 1905; Osgood 1943).
Ctenomys fueginus Philippi, 1880:276; type locality “östlichen Insel,” eastern island or Isla Grande, Tierra del Fuego, Magallanes y Antártica Chilena, Chile (Osgood 1943).
Ctenomys neglectus Nehring, 1900:535; type locality “Patagonien.”
Ctenomys colburni J. A. Allen, 1903:188; type locality: “Arroyo Ayke, in the basalt canyons, 50 miles southeast of Lake Buenos Ayres, Patagonia,” restricted to “río Ecker en Ea. Casa de Piedra, ca. 10 km al SSW de su confluencia con el río Pinturas (47.12° S, 70.86° W, 700 m; Carta Topográfica IGN 4772-24, 1947, “Río Pinturas”, escala 1:100,000),” Santa Cruz, Argentina, by Christie and Pardiñas (2016).
Ctenomys robustus J. A. Allen, 1903:185; type locality: “Rio Chico de Santa Cruz, near the Cordilleras,” restricted to “río Tucu Tucu, ca. 8 km aguas abajo desde su nacimiento (48.47º S, 71.87º W, departamento Río Chico, Santa Cruz, Argentina),” Santa Cruz, Argentina, by Pardiñas (2013). Preoccupied by Ctenomys robustus Philippi (1896).
Ctenomys osgoodi J. A. Allen, 1905:191.
Ctenomys magellanicus dicki Osgood, 1943:123; type locality: “Estancia Ponsonby, east end of Riesco Island, Magallanes, Chile,” Isla de Riesco, Magallanes y Antártica Chilena, Chile.
Ctenomys magellanicus obscurus Texera, 1975:163; type locality: “Estancia Lago Escondido, 20 km S de la Sección Rio Grande, cerca de Lago Blanco, Tierra Del Fuego, Magallanes y Antártica Chilena, Chile, ca. 500 m.”
Distribution: southernmost Argentina and Chile; from central-western Santa Cruz province (ca. 48° S) in Argentina and central Aysen (ca. 47° S) in Chile to Tierra del Fuego (Argentina and Chile) and some adjoining islands (e.g., Riesco, Chile) (Fig. 1). Additional records referred in the literature as C. colburni from western Río Negro and northeastern Santa Cruz province (cf. Bidau 2015) need to be confirmed.
Remarks: Populations from Tierra del Fuego referred to C. m. fueginus are chromosomally polytypic, with reported diploid numbers of 34, 36 and 38 (Reig and Kiblisky 1968; Lizarralde et al. 2001). Gallardo (1979) reported a 2n = 34, FN = 68 for specimens of C. m. magellanicus from La Cumbre, cordillera Baguales, provincia de Última Esperanza. This same author (Gallardo 1991) documented a 2n = 34, FN = 64 for specimens (UACH 4232, UACH 4233) collected near the type locality of C. colburni; these specimens were sequenced in this study and their haplotypes fall in the shallow clade of C. magellanicus (Fig. 2). Specimens from Bajo Caracoles and Estancia El Puma, Santa Cruz, have a 2n = 34, FN = 67 (Moreno et al. 2000).
Even when our samples are insufficient to evaluate the geographic differentiation within C. magellanicus and/or to address some nomenclatural questions to their proper depth (e.g., the distinction of the subspecies) we can advance some general considerations. With the data at hand, the dispersion of specimens along the first PC (Fig. 10) suggest a moderate north to south trend in cranial size and shape, with individuals with proportionally larger nasals and diastema to the south (dicki, fueginus, obscurus, osgoodi) and those with proportionally broader braincases and larger frontals to the north (colburni). MANOVA analysis shows that samples from the extremes of the distribution (i.e., colburni and cf. colburni vs. fueginus) significatively differ, but those from the center (i.e., magellanicus) do not differ from any other of the analyzed samples; these results reinforce the existence of a north to south pattern of morphologic variation. Differences in body size among Ctenomys could be influenced by primary productivity, food quality, and resource abundance (Medina et al. 2007). A larger sampling of specimens of C. magellanicus from distinct populations across its distributional range is needed before testing for an association between body size and environmental variables of primary productivity. In addition, Fornel et al. (2018) suggested that different types of soil hardness could play a role in biomechanical constraints and diversification in skull morphology of Ctenomys; for these authors, smaller skulls are related to hard soils, while larger skulls correspond to species that inhabit in soft soils. Wherever the case, additional studies (including ecological, behavioral, and environmental data) are needed to identify the causes of such large variation in skull size and shape among C. magellanicus.
Haplotypes retrieved from specimens of C. colburni are nested in a large and shallow clade form by haplotypes of specimens of C. magellanicus (Fig. 2). This genealogical pattern may constitute a case of difference between gene and species trees (Pamilo and Nei 1988), a well known pattern (e.g., Jayat et al. 2019; see also D’Elía et al. 2019a) that may emerge due to distinct evolutionary process, including incomplete lineage sorting (e.g., Pagès et al. 2013) and introgression (e.g., Patton and Smith 1994). However, the fact that haplotypes of C. colburni and C. magellanicus are very similar (i.e., only 0.4% of observed divergence) forming a shallow genealogy together with the lack of qualitative morphological differences between them, indicate that in this case the gene tree matches the species tree. As such, we consider C. colburni as a synonym of C. magellanicus; this hypothesis should be further tested with the analyses of nuclear loci.
The mitochondrial genealogy of C. magellanicus is not geographically structured (e.g., Fueguian haplotypes are not sister to those from the mainland; Fig. 2). Importantly, there are some large geographical gaps between the studied samples, which need to be filled before advancing a formal subspecific classification. Based both on its geographic isolation and darker coloration, Osgood (1943) strongly defended the taxonomic distinction of C. m. dicki. While it is possible that this form, endemic to Riesco Island, Chile, could represent a distinct subspecies, additional samples and evidence (e.g., genetic) are required to evaluate with accuracy its distinction. Variation in coat color is relatively structured geographically (Figs. 6, 7); besides those blackish individuals form Riesco Island, most specimens from northern Santa Cruz (colburni), southern Chile (magellanicus) and Tierra del Fuego (fueginus) are almost similar in its external coloration, having yellowish brown to pale grizzled grayish buff dorsa and buffy venters (Figs. 6, 7). Animals from west-central Santa Cruz province, Argentina (osgoodi) have a yellowish-brown dorsal coloration, although with a much more cinnamon tinge at the venter (Figs. 6, 7). Texera (1975) recognized a southern Fueguian population as C. m. obscurus, based on its overall darker coloration; however, individuals darker than typical fueginus are usually present in other populations of this island (e.g., eastern Tierra del Fuego). The same is true for those samples referred to osgoodi, in which lighter and darker individuals coexist at a same locality (i.e., the topotypical series, see Allen 1903).
Conservation
Ctenomys magellanicus is currently listed as Least Concern by the IUCN (Bidau 2019). However, it was listed as Vulnerable [VU A2acd] in the 2012 Argentinean National Red List (Bidau et al. 2012), and as Vulnerable [VU A2c; B2ab(iii)] (subspecies fueginus, magellanicus, obscurus, and osgoodi) or even Extinct (for the subspecies dicki) in the Chilean country-level red list assessment (http://www.mma.gob.cl/). Based on historical reports, Osgood (1943) called the attention about the scarcity of C. magellanicus through its former distributional range, suggesting its regional extirpation over most of this original distribution due to human activities such as the sheep rising. According to Osgood (1943), large packs of sheep, as those that characterized the southern portion of Patagonia during most of the XX century, were responsible of the death of large numbers of this rodent trough trampling. In addition, C. magellanicus was largely pursued by the ranchers of Tierra del Fuego, which used traps and steel barbed rollers to kill large numbers of these animals (Massoia and Chebez 1993). The situation of C. m. dicki is perhaps even worst, due to the possibility of its extinction from Riesco Island. Judging for the available information, and pending of an adequate assessment, a global consideration as a Near Threatened or Vulnerable for this species appears to be justified.
Final remarks Our molecular based analysis (see also Parada et al. 2011) suggests that C. haigi, as currently delimited, encompasses two main lineages that would correspond to distinct species (Fig. 2). Currently, in the synonymy of C. haigi is the nominal form lentulus Thomas 1919, which sometimes has been regarded as subspecies of C. haigi (e.g., Woods and Kilpatrick 2005) or as a full synonym of it (Bidau 2015). One of the main clades of C. haigi s.l., in its large distributional area, covers the type locality of lentulus; as such, we could tentatively refer to this widespread lineage of species level as C. lentulus. However, we note that both lineages of C. haigi s.l., together with the clade C. sericeus-C. coyhaiquensis-C. sp. 1-C. sp. 2, fall in trichotomy, whose one of its possible resolution is a monophyletic C. haigi s.l. In addition, both main lineages of C. haigi s.l. are closely distributed to each other; one is known only from the type locality of C. haigi (i.e., El Maitén, Chubut), while the other, the one that covers the general area of the type locality of C. lentulus, is registered from 16 km away of the former. As such, we prefer to not make taxonomical changes and not elevate lentulus to the species level. To solve this question, additional evidence, including the detailed inspection of the holotypes of both nominal forms and new sequences (e.g., from topotypes of C. lentulus) are needed. In addition, our results suggest that C. sericeus is a widely distributed species, closely related to the morphologically similar C. coyhaiquensis and C. sp. 1. The distinction of these three forms should be further evaluated, as may in fact represent a single species. Similarly, the taxonomic suggestions advanced here need to be further tested with the integral analysis of more specimens. We call the attention that large areas of Patagonia, both in Argentina and Chile, have not been sampled, at the time that distinct cytotypes found in the Patagonian Atlantic coast, have not been characterized in terms of their morphology and genetic variation. Therefore, an extensive field program (see comments on Chilean collecting regulations in D’Elía et al. 2019b) together with an integrative museum based work should be undertaken as a way to gain a correct picture of the species richness of Patagonian Ctenomys and their distribution.
References
Allen JA (1903) Descriptions of new rodents from Southern Patagonia, with a note on the genus Euneomys Coues, and an addendum to article IV, on Siberian mammals. Bull Am Mus Nat Hist 19:185–196
Allen JA (1905) Volume III. Zoölogy. Part I. Mammalia of Southern Patagonia. pp 1–210 + 29 láms. In: Scott WB (ed) Reports of the Princeton University Expeditions to Patagonia, 1896–1899. J. B. Hatcher in charge. The University, Stuttgart, Princeton
Bidau C (2015) Family Ctenomyidae Lesson, 1842. In: Patton JL, Pardiñas UFJ, D’Elía G (eds) Mammals of South America, vol. 2: rodents. University of Chicago Press, Chicago, pp 818–877
Bidau CJ (2019) Ctenomys magellanicus. The IUCN Red List of Threatened Species 2019: e.T5812A22193726. http://dx.doi.org/10.2305/IUCN.UK.2019-1.RLTS.T5812A22193726.en
Bidau C, Lessa EP, Ojeda RA (2012) Familia Ctenomyidae. In: Ojeda RA, Chillo V, Díaz Isenrath G (eds) Libro Rojo. Mamíferos amenazados de La Argentina. Sociedad Argentina para el Estudio de los Mamíferos, Mendoza, pp 177–187
Cañón C, D’Elía G, Pardiñas UFJ, Lessa EP (2010) Phylogeography of Loxodontomys micropus with comments on the alpha taxonomy of Loxodontomys (Cricetidae: Sigmodontinae). J Mamm 91:1449–1458
Caraballo DA, Rossi MS (2017) Integrative lineage delimitation in rodents of the Ctenomys Corrientes group. Mammalia 82:35–47
Contreras JR, Contreras AN (1984) Craneología y craneometría del género Ctenomys. II: Craneometría. Hist Nat 4:245–248
Christie MI, Pardiñas UFJ (2016) Localidades típicas de micromamíferos en Patagonia: el viaje de Hatcher a la meseta del lago Buenos Aires, Santa Cruz, Argentina. Mastozool Neotrop 23:533–541
D’Anatro A, D’Elía G (2011) Incongruent patterns of morphological, molecular, and karyotipic variation among populations of Ctenomys pearsoni Lessa and Langguth, 1983 (Rodentia, Ctenomyidae). Mamm Biol 76:36–40
D’Elía G, Fabre P-H, Lessa EP (2019a) Rodent systematics in an age of discovery: recent advances and prospects. J Mamm 100:852–871
D’Elía G, Jaksic F, Bacigalupe LD, Bozinovic F, Canto JL, Correa C, Fontúrbel FE, Lisón F, Méndez MA, Nespolo R, Opazo JC, Palma E, Rau JR, Rodríguez SM, Rodríguez-Serrano E, Sabat P, Vásquez RA, Victoriano P (2019b) Sugerencias para mejorar la regulación chilena de manipulación de vertebrados terrestres en poblaciones naturales en el contexto de investigaciones científicas. Gayana 83:63–67
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9(8):772. https://doi.org/10.1038/nmeth.2109
de Queiroz K (2007) Species concepts and species delimitation. Syst Biol 56:879–886
Fasanella M, Bruno C, Cardoso Y, Lizarralde M (2013) Historical demography and spatial genetic structure of the subterranean rodent Ctenomys magellanicus in Tierra del Fuego (Argentina). Zool J Linn Soc 169:697–710
Fornel R, Cordeiro-Estrela P, Freitas TRO (2018) Skull shape and size variation within and between mendocinus and torquatus groups in the genus Ctenomys (Rodentia: Ctenomyidae) in chromosomal polymorphism context. Genet Mol Biol 41:263–272
Freitas TRO (2006) Cytogenetics status of four Ctenomys species in the south of Brazil. Genetica 126:227–235
Freitas TRO, Lessa EP (1984) Cytogenetics and morphology of Ctenomys torquatus (Rodentia-Octodontidae). J Mamm 65:637–642
Freitas TRO, Fernandes FA, Fornel R, Roratto PA (2012) An endemic new species of tuco-tuco, genus Ctenomys (Rodentia: Ctenomyidae), with a restricted geographic distribution in southern Brazil. J Mamm 93:1355–1367
Gallardo M (1979) Las especies chilenas de Ctenomys (Rodentia, Octodontidae). I. Estabilidad cariotípica. Arch Biol Med Exp 12:71–81
Gallardo M (1991) Karyotypic evolution in Ctenomys (Rodentia, Ctenomyidae). J Mamm 72:11–21
Gardner SL, Salazar Bravo J, Cook JA (2014) New species of Ctenomys Blainville 1826 (Rodentia: Ctenomyidae) from the lowlands and central valleys of Bolivia. Special Public. Mus Texas Tech Univ 62:1–34
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron 4:9. http://palaeo-electronica.org/2001_1/past/issue1_01.htm
Jayat JP, Ortiz PE, Ojeda AA, Novillo A, Teta P, D’Elía G, Ojeda RA (2019) Quantitative morphological characters of the skull suggest that Akodon oenos (Rodentia, Cricetidae, Sigmodontinae) is not a junior synonym of A. spegazzinii. Mammalia. https://doi.org/10.1515/mammalia-2019-0043
Kelt DA, Gallardo MH (1994) A new species of tuco-tuco, genus Ctenomys (Rodentia: Ctenomyidae) from Patagonian Chile. J. Mamm 75:338–348
Leipnitz LT, Fornel R, Ribas LEJ, Kubiak BB, Galiano D, de Freitas TRO (2018) Lineages of tuco-tucos (Ctenomyidae: Rodentia) from midwest and northern Brazil: late irradiations of subterranean rodents towards the Amazon Forest. J Mamm Evol. https://doi.org/10.1007/s10914-018-9450-0
Lizarralde MS, Deferrari GA, Alvarez SE, Escobar JM (2001) Diferenciación evolutiva en Ctenomys magellanicus: variación morfológica, alozímica y consideraciones biogegráficas de 2 formas cromosómicas. Interciencia 26:13–17
Londoño-Gaviria M, Teta P, Ríos SD, Patterson BD (2019) Redescription and phylogenetic position of Ctenomys dorsalis Thomas 1900, an enigmatic tuco tuco (Rodentia, Ctenomyidae) from the Paraguayan Chaco. Mammalia 83:227–236
Mammal Diversity Database. 2018. https://mammaldiversity.org/
Mapelli FJ, Mora MS, Lancia JP, Gómez Fernández MJ, Mirol PM, Kittlein MJ (2017) Evolution and phylogenetic relationships in subterranean rodents of the Ctenomys mendocinus species complex: effects of Late Quaternary landscape changes of Central Argentina. Mamm Biol 87:130–142
Massoia E, Chebez JC (1993) Mamíferos silvestres del archipiélago fueguino. Literature of Latin America, Buenos Aires
Meachen-Samuels J, Van Valkenburgh B (2009) Craniodental indicators of prey size preference in the Felidae. Biol J Linn Soc 96:784–799
Medina AI, Martí DA, Bidau CJ (2007) Subterranean rodents of the genus Ctenomys (Caviomorpha, Ctenomyidae) follow the converse to Bergmann’s rule. J Biogeogr 34:1439–1454
Moreno AC, Davies YE, Merani MS, Contreras JR (2000) Aportes citogenéticos para el género Ctenomys (Rodentia, Ctenomyidae) en el norte de la Patagonia Argentina. Libro de Resúmenes de las XV Jornadas Argentinas de Mastozoología, p 85
Mosimann JE (1970) Size allometry: size and shape variables with characterizations of the lognormal and generalized gamma distributions. J Am Stat Assoc 65:930–945
Osgood WH (1943) The mammals of Chile. Field Mus Nat Hist Zool Ser 30:1–268
Pagès M, Bazin E, Galan M, Chaval Y, Claude J, Herbreteau V, Michaux J, Piry S, Morand S, Cosson JF (2013) Cytonuclear discordance among Southeast Asian black rats (Rattus rattus complex). Mol Ecol 22:1019–1034
Pamilo P, Nei M (1988) Relationships between gene trees and species trees. Mol Biol Evol 5:568–583
Parada A, D’Elía G, Bidau CJ, Lessa EP (2011) Species groups and the evolutionary diversification of tuco-tucos, genus Ctenomys (Rodentia: Ctenomyidae). J. Mamm. 92:671–682
Parada A, Ojeda AA, Tabeni MS, D’Elia G (2012) The population of Ctenomys from the Ñacuñán Biosphere Reserve (Mendoza, Argentina) belongs to Ctenomys mendocinus Philippi, 1869 (Rodentia, Ctenomyidae): molecular and karyotypic evidence. Zootaxa 3402:61–68
Pardiñas UFJ (2013) Localidades típicas de micromamíferos en Patagonia: el viaje de J. Hatcher en las nacientes del Río Chico, Santa Cruz, Argentina. Mastozool Neotrop 20:413–420
Pardiñas UFJ, Teta P, D’Elía G, Cirignoli S, Ortiz PE (2007) Resolution of some problematic type localities for sigmodontine rodents (Cricetidae, Sigmodontinae). In: Kelt DA, Lessa EP, Salazar-Bravo J, Patton JL (eds) The quintessential naturalist: Honoring the life and legacy of Oliver P. Pearson, vol 134. Univ. California Publ. Zool., pp v-xii + 1–981, 391–416
Patton JL, Smith MF (1994) Paraphyly, polyphyly, and the nature of species boundaries in pocket gophers (genus Thomomys). Syst Biol 43:11–26
Rannala B, Yang ZH (1996) Probability distribution of molecular evolutionary trees: a new method of phylogenetic inference. J Mol Evol 43:304–311
Reig OA, Kiblisky P (1968) Chromosomes in four species of rodents of the genus Ctenomys (Rodentia, Octodontidae) from Argentina. Experientia 24:274–276
Reig OA, Massarini AI, Ortells MO, Barros MA, Tiranti SI, Dyzenchauz FJ (1992) New karyotypes and C-banding patterns of the subterranean rodents of the genus Ctenomys (Caviomorpha, Octodontidae) from Argentina. Mammalia 56:603–623
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Saba SL, De Lamo DA (1994) Dynamic responses of mammals to the eruption of volcán Hudson. Mastozool Neotrop 1:113–122
Strauss RE (2010) Discriminating groups of organisms. In: Ashraf E (ed) Morphometrics for nonmorphometricians. Lecture notes in earth sciences, vol 124. Springer, pp 73–91
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Texera WA (1975) Descripción de una nueva subespecie de Ctenomys magellanicus (Mammalia; Rodentia; Ctenomyidae) de Tierra del Fuego. Magallanes, pp 163–167
Thomas O (1910) A collection of mammals from eastern Buenos Ayres, with descriptions of related new mammals from other localities. Ann Mag Nat Hist Ser 8(5):239–247
Tiranti SI, Dyzenchauz FJ, Hasson ER, Massarini AI (2005) Evolutionary and systematic relationships among tuco-tucos of the Ctenomys pundti complex (Rodentia: Octodontidae): a cytogenetic and morphological approach. Mammalia 69:69–80
Velazco PM, Patterson BD (2013) Diversification of the yellow-shouldered bats, genus Sturnira (Chiroptera, Phyllostomidae) in the New World tropics. Mol Phylogenet Evol 68:683–698
Vincon SG (2004) Sistematica y distribución del género Ctenomys (Rodentia, Octodontidae) en los alrededores de Esquel (Chubut, Argentina). Unpublished thesis. Facultad de Ciencias Naturales, Universidad Nacional de la Patagonia San Juan Bosco, Chubut
Woods CA, Kilpatrick CW (2005) Infraorder Hystricognathi. In: Wilson DE, Reeder DM (eds) Mammal species of the World. A taxonomic and geographic reference, 3rd edn. The John Hopkins University Press, Baltimore, pp 1538–1600
Acknowledgements
We are grateful to the curators and staff of the following collections: Sergio Bogan (CFA), Ulyses F. J. Pardiñas (CNP), Bruce Patterson (FMNH), Sergio Lucero (MACN), Fredy Mondaca (UACH), and Darrin Lunde (USNM). Yolanda Davies (MACN) provided us valuable information about some specimens now housed at CFA. Cristián Saucedo from Vida Silvestre de Tompkins Conservation granted access to samples from Chacabuco. Mauricio Soto-Gamboa allowed access to samples from Torres del Paine. Kattina Zavala and Alex Gonzalez provided technical support. The American Society of Mammalogists contributed to PT with funds through its “Oliver P. Pearson Award” to travel across the United States. Noé de La Sancha and Family provided support during the stay of one of the authors (PT) in Chicago. Financial support was partially provided by FONDECYT grants 1180366 (GD) and 1160627 (JCO).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Eva Bärmann.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Appendix
List of specimens of Ctenomys included in genetic based analyses. Species allocation follows the taxonomic scheme here proposed (see text). For each specimen of the Ctenomys magellanicus species group we provide locality information, catalog number, and Genbank accession number. Sequences gathered here are indicated with an * next to Genbank accession numbers.
Ctenomys coyhaiquensis Chile: Región de Aysen, Provincia General Carrera, Chile Chico (FMNH 134264/AF119112, FMNH 134296/AF119113, FMNH 134300/AF071753, topotypes).
Ctenomys haigi Argentina: Provincia del Chubut, Departamento Cushamen, El Maitén, 42°3′ S 71° 10′ W (SV62/HM777476; topotype), Departamento Cushamen, Laguna Nahuelquir, Estancia El Maitén (MNT018/KU659607, SM01/KU659602, SM02/KU659603, SM03/KU659604, SM04/KU659605, SM05_KU659606), Departamento Telsen, Talagapa (CNP 1269/HM777505); Provincia del Neuquén, Departamento Los Lagos, Cueva Traful (HA2C222/GU433041, HA2AC61/GU433042, HAC201/GU433043, HA3C83/GU433044, HA1AC266/GU433045, HA4C62/GU433046); Provincia de Rio Negro, Departamento Bariloche, near Hipodromo, 13 km WNW Bariloche (MVZ 166421/AF007063), Departamento Bariloche, Bariloche (MHNG1276071/KU659608), Departamento Pilcaniyeu, 13.5 km E Estación Perito Moreno (MVZ 184878/AF422920), Departamento Pilcaniyeu, Estancia San Ramón (H047_KY013599); Departamento Valcheta, Cerro Corona (CNP 3610/HM777506).
Ctenomys magellanicus Argentina: Provincia de Santa Cruz, Departamento Lago Buenos Aires, Estancia La Cantera (UACH 4232/MN176514*, UACH4233/MN176515*), Departamento Lago Buenos Aires, Río Ecker, 500 m aguas abajo casco Ea. Casa de Piedra (CNP 3613/HM777474), Río Chico, Estancia El Puma (CFA 11332/MN176516*, CFA11346/MN176517*). Provincia de Tierra del Fuego, Departamento de de Río Grande, no locality neither voucher specimen especified (DQ333326, DQ333327), Departamento de de Río Grande, Estancia Sara (CNP 3594/HM777479); Chile: Región de Aysen, Provincia de Capitán Prat, Parque Patagonia (UACH 8087/MN176518*, UACH 8088/MN176519*); Región de Magallanes, Provincia de Tierra del Fuego, Tres Arroyos (I308/AF370690); Provincia de Última Esperanza, Parque Nacional Torres del Paine, Laguna Amarga (UACH 8089/MN176520*, UACH 8090/MN176521*, UACH 8091/MN176522*, UACH 8092/MN176523*, UACH 8093/MN176524*, UACH 8094/MN176525*, UACH 8095/MN176526*, UACH 8096/MN176527*).
Ctenomys sericeus Argentina: Provincia de Santa Cruz, Departamento Corpen Aike, La Porteña, Río Lista (SV45/HM777496), Departamento Deseado, Cerro del Paso (CNP 3605/HM777502), Departamento Deseado, La Paloma (CNP 3604/HM777501), Departamento Río Chico, Cerro Ventana (CNP 3615/HM777500).
Ctenomys sp. 1. Argentina: Provincia de Chubut, Departamento Río Senguer, Lago Blanco (SV52/HM777475).
Ctenomys sp. 2 Argentina: Provincia de Chubut, Departamento Paso de Indios, Pichiñan (CNP 1437/HM777503), Departamento Languiñeo, Estancia Quichaura (CNP 1043/HM777504).
OutgroupCtenomys torquatus (CA743/AF119111); Ctenomys tucumanus, (C04670/HM777499); Ctenomys boliviensis (NK15726/AF007038); Ctenomys sociabilis (EAL545/HM777495).
Rights and permissions
About this article
Cite this article
Teta, P., D’Elía, G. & Opazo, J.C. Integrative taxonomy of the southernmost tucu-tucus in the world: differentiation of the nominal forms associated with Ctenomys magellanicus Bennett, 1836 (Rodentia, Hystricomorpha, Ctenomyidae). Mamm Biol 100, 125–139 (2020). https://doi.org/10.1007/s42991-020-00015-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42991-020-00015-z