Abstract
Urbanization, the process whereby natural environments are modified for human habitation, is increasing worldwide. Many species are extirpated from urban settings, but others can survive and thrive in this environment. Few studies have compared population densities between urban and natural sites and none have investigated how hurricanes might interact with urbanization to affect animal species population dynamics in cities, which is crucial in evaluating population persistence in urban habitats. We quantified post-hurricane population dynamics between urban and forest populations of the tropical lizard Anolis cristatellus in paired urban and forest sites across the island of Puerto Rico at 4, 11, and 16 months following Hurricane Maria. Though we lacked pre-hurricane population density data, we expected lower than normal densities after the hurricane and a gradual increase through time. We found that urban population density was lower compared to natural areas across all pairs of sites. In forested areas, we found increases in perch density, likely associated with post-disturbance succession, but urban structural habitats remained relatively constant through time, with most hurricane-damaged vegetation being rapidly removed by humans. The two sites in our study that were most heavily impacted by the hurricane initially doubled in population density, and density continued to increase through our sampling period. Our findings suggest that hurricanes and urbanization can interact to shape population dynamics in lizards. Moreover, urban sites experience constant human modifications. As such, understanding population dynamics of urban species will require careful consideration of the effect of human intervention in these habitats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urbanization is an important dimension of global environmental change (Rivkin et al. 2019; Alberti et al. 2020). One consequence of urbanization is the impact that it can have on species composition, diversity, and abundance. For instance, species occurrences are influenced by the availability of suitable habitat in urban spaces, as well as by ecological interactions with other taxa in the urbanized landscape (Aronson et al. 2016; Fournier et al. 2020). As a result, some species can be fully extirpated from urban habitats, while others maintain robust populations (Delgado de la Flor et al. 2017; Pignataro et al. 2020). Others still can increase dramatically in relative abundance within urban areas compared to more natural sites nearby (Jokimäki et al. 2017; Pieniażek et al. 2017). For example, a species of field mouse in Poland maintains larger populations in urban habitats than in nearby forested areas (Pieniażek et al. 2017). On the other hand, Fournier et al. (2020) documented a reduction in species richness due to habitat filtering associated with drier condition and a less specialized diet in urban environments. Similar decreases in species richness have been reported with increasing degrees of urbanization, although this may depend on landscape heterogeneity which can vary as a function of the scale used to quantify the landscape (McKinney 2008). In other cases, urban areas could host population densities as high as non-urban sites, such as is the case for some species of Galapagos finches (De León et al. 2019). Thus, the overall effect of urbanization can be positive for some species and negative for others. Nonetheless, as a general rule the population density and dynamics of urban species tend to differ from natural habitats nearby (McKinney 2008).
Just as the intensity and extent of urbanization is predicted to continue to rise, most global environmental change models predict an increased frequency of catastrophic weather events such as hurricanes (Cochrane and Barber 2009; Knutson et al. 2010; Turner 2010; Holland and Bruyère 2014; Newman 2019). The effect of extreme weather events on urban populations will vary as a function of the interaction between disturbance, urbanization, and the pre- and post-disturbance fitness of urban populations. For example, if urban taxa lack specific habitat-disturbance adaptations they may suffer greater mortality compared to populations that have co-adapted with their disturbance regime (Seidl et al. 2016). An important yet relatively understudied element of urban evolutionary ecology is whether synanthropes (i.e., species that thrive in proximity to humans) in urban environments respond differently to disturbance events, compared to conspecifics in non-urban habitats of the same region.
Hurricanes are known to affect animal populations through the combined effects of the immediate kinetic energy of the storm and the consequent transformation of the structural habitat (Lugo 2000, 2008). For instance, snail density decreased significantly after Hurricane Hugo in 1989 as a result of both direct mortality from the storm, and indirect mortality due to drier conditions on the forest floor resulting from forest canopy damage (Secrest et al. 1996). Post-hurricane mortality can also result from scarcity of food resources, such as in the case of frugivorous bats following both Hurricane Hugo (1989) and Hurricane Georges (1998; Gannon and Willig 1994, 2009). Moreover, populations of some species have experienced rapid growth following a hurricane. For example, certain lepidopterans can increase in abundance in hurricane affected sites, and this is thought to be due to the increased availability of food resources such as young leaves after canopy sheering (Torres 1992). Despite this variety of studies that have investigated the effect of hurricanes on population density in natural environments, virtually nothing is known about patterns of mortality and/or recruitment in urban populations after a hurricane event.
Here, we examine this issue by quantifying changes in population dynamics in urban and forest populations of the tropical lizard Anolis cristatellus following the 2017 category 5 Hurricane Maria in Puerto Rico. We evaluated paired forest and urban populations of Anolis lizards at 4, 11 and 16 months following the hurricane. No previous study had compared the population density of Anolis cristatellus between urban and forested environments. As such, we lacked baseline data to aid our predictions of the population dynamics following this catastrophic storm. We focus our hypothesis on contrasting forest and urban population densities immediately after the hurricane and at 11 and 16 months afterwards. Prior research has established that urban habitats are less structurally complex than nearby forested environments, and large parts of the urban habitat are inhospitable to arboreal lizards (Winchell et al. 2016, 2018a; Avilés-Rodríguez and Kolbe 2019). We hypothesized that reduced vegetation in urban habitats could lead to lower urban population densities, and that populations densities would be lowest 4 months after the hurricane and gradually increase with each sampling date, presumably as lizard populations recovered to their pre-hurricane densities.
Methods
Focal species
We studied the species Anolis cristatellus, a relatively small (50–70 mm adult snout-to-vent length) arboreal lizard, that is found at high densities in a variety of different types of habitats throughout Puerto Rico (Williams 1972), including urban environments (Winchell et al. 2016, 2018a). Caribbean Anolis species, including A. cristatellus, can have very high population densities in natural environments. For example, A. sagrei, a Cuban and Bahamian species, has measured densities ranging from 200 up to 9870 individuals per hectare (Schoener and Schoener 1980). Likewise, A. cristatellus, the focal taxon of this study, has estimated densities from around 1000 up to 7200 lizards per hectare (Lazell 1991; Rodda et al. 2001). Other Anolis congeners can occur in densities up to 32,867 lizards per hectare (Hite et al. 2008). Previous research on A. cristatellus has compared urban and forest populations with respect to behavior (Avilés-Rodríguez and Kolbe 2019), morphology (Winchell et al. 2016; Falvey et al. 2020), habitat use (Winchell et al. 2018a), and locomotor performance (Winchell et al. 2018b), but not population density.
Several congeners of A. cristatellus have been studied in the context of hurricanes (Reagan 1991; Spiller et al. 1998; Schoener et al. 2001, 2004). This body of work shows, among other things, that hurricanes can result in demographic changes due to varying levels of mortality, including total extirpation on very small islands (Schoener et al. 2004). Additionally, recent work suggests adaptive phenotypic shifts in response to hurricanes in anoles (Donihue et al. 2018, 2020), but the direction of these shifts vary as a function of habitat, including urbanization (Avilés-Rodríguez et al. 2021). Prior work on the population biology of Anolis following hurricanes has focused on species on small islands and in forested or otherwise ‘natural’ settings, rather than in urban areas.
Sampling
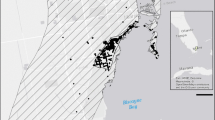
In September of 2017 the island of Puerto was affected by the strong winds and rainfall associated with two category 5 hurricanes. First, Hurricane Irma passed just north of Puerto Rico but nonetheless caused significant damage on the island. Subsequently, on September 20, 2017, Hurricane Maria made landfall in the southeast, near the municipality of Yabucoa, and then traversed the island eventually exiting via the northwest coast of the island near the municipality of Arecibo, about 8 h later (Fig. 1). Hurricane Maria was the strongest storm to make landfall in Puerto Rico since 1928 (Hurricane San Felipe II; Pasch et al. 2017).
Location of survey sites in Puerto Rico. Municipalities have been colored based on the average maximum gust sustained during the passage of Hurricane Maria based on data collected by the Pacific Disaster Center. The red line shows the passage over land of the hurricane and the photos show the sites at 4 months after Hurricane Maria. Note that at this time tree canopies were still damaged by the hurricane and downed tree branches and trunks commonly occurred at all sites. Letters correspond to paired urban and forest sites in the municipalities of to San Juan (A), Aguadilla (B) and Mayagüez (C) (Color figure online)
We sampled three pairs of urban and forest habitats across the island of Puerto Rico. We chose sites to match areas where we had conducted prior research on Anolis cristatellus in urban areas. As such, our sites were not chosen based on their geographical relationship to the hurricane path. Nonetheless, because our sites covered a large range of the island, by happenstance these localities experienced varying degrees of winds due to their position with respect to the hurricane’s trajectory across the island (Fig. 1). In particular, the municipality of San Juan was closest to the entry point of the hurricane and experienced the strongest winds. Aguadilla and Mayagüez experienced the weakest wind intensity due to their location in the west region of the island and thus farthest from the hurricane’s path.
Another important consideration of site selection is that we were unable to select a random plot within urban environment to sample. This is due to a high level of landscape heterogeneity in urban environments (Cadenasso et al. 2007) which includes areas that are inhospitable to lizards and inaccessible to researchers. We address this limitation in the Discussion.
Field data collection
We sampled each of our six sites (three urban and three forested) during three time periods following Hurricane Maria’s passage over Puerto Rico: 4, 11, and 16 months after the event. We used a three-sample capture-mark-recapture protocol in plots measuring 100 m\(^{2}\) (0.01 ha) in area. One observer (KAR) sampled each plot for 3 h over three consecutive days, for a total of 9 h of sampling effort per site during each sampling period. On the first day of each sampling period, we randomized whether the urban or forest site would be visited in the morning. Subsequently, we alternated visiting the urban or the forest sites in the morning vs. the afternoon of each sampling day.
We captured lizards by hand or by lassoing, using a dental floss slip noose on a collapsible pole. We numbered each captured lizard using a non-toxic marker, measured the body size (snout-to-vent length: SVL) with a ruler, and marked it using a single injectable plastic elastomer bead. We employed redundant marking (non-toxic marker and injectable bead) to avoid counting the same individual multiple times due to skin shedding, which could cause the non-toxic mark to be lost. We re-marked any recaptured lizard with evidence of loss of its non-toxic skin marking, and matched it to our previously captured animals using SVL measurements and sex. Since each plot was sampled over three consecutive days, mark loss via shedding was relatively rare.
Although we collected data on all species of Anolis lizards encountered in our plots, we primarily estimated population density for A. cristatellus since it was the most abundant species across all sites. In addition, the other species that we encountered in our plots tend to be more specialized in the habitat they typically used and thus were not uniformly present in the full extent of our sampling area. Nonetheless, we document the total number of other anole species captured during our study (Fig. S1). Given that the density of A. cristatellus could be affected by niche overlap with other Anolis ecomorphs following the hurricane (Reagan 1991), we repeated our analyses by estimating the density of all Anolis lizards (regardless of species) captured at each site.
Finally, we quantified perch density for each site by tallying the substrates available as perches for lizards within each plot.
Statistical analyses
We performed all statistical analyses using R 3.6.1 (R Core team 2019). We first estimated population size of Anolis cristatellus using the R package Rcapture (Baillargeon and Rivest 2007), and selected the abundance estimator that best fit our data based on the Akaike Information Criterion (AIC). Subsequently, we compared paired differences within sites by contrasting the estimated abundances and standard errors using paired t-tests. We adjusted our P-values for multiple testing using the False Discovery Rate method (Benjamini and Hochberg 1995). To verify the effect of other anole congeners we repeated these analyzes using all anole species observed within our sites and verified whether including these observations led to a different result compared to a model including only A. cristatellus.
We evaluated changes in lizard body size (SVL) by first log-transforming all of our size data. Then we modeled the change in size as a function of sampling period after the hurricane, habitat (i.e, forest or urban), and sex. Because we did not sex juvenile lizards, we grouped them with females in our analyses. We also analyzed perch counts with a general linear mixed effect model using Poisson-distributed error, with sampling period and habitat as fixed effects, and municipality as a random effect.
Results
Population density estimates
Population density of Anolis cristatellus lizards was high for all sites and time periods (Fig. 2). Among all population density estimates, the lowest estimate obtained was 2150/ha (± 220) for our San Juan urban site, 4 months after Hurricane Maria. By contrast, the highest estimated population density (10,210/ha ± 860) was obtained for the San Juan forest site, 16 months after the hurricane. In general, for all municipalities and time periods, forested environments had higher overall lizard density compared to urban sites nearby (Kruskal–Walis H test: \(\chi ^2\) = 8.75, df =1, P = 0.003; Fig. 1).
Changes in population density
We observed the lowest population density for any site or context 4 months after the hurricane for the urban population in San Juan (all pairwise t-tests; P < 0.001). By 11 months post Hurricane Maria, this site had nearly doubled in population density (\(\Delta _{[11mo.-4mo.]} = 3340\)/ha \(\pm\) 123.3; P < 0.001). Finally, by 16 months after the hurricane the urban San Juan population increased by approximately 30% (\(\Delta _{[16mo.-11mo.]} = 1450\)/ha \(\pm\) 167.3; P < 0.01). We found a similar trend of population size increase with time after the hurricane in the San Juan forest population. Specifically, this population nearly doubled at 11 months after the hurricane (\(\Delta _{[11mo.-4mo.]} = 4420\)/ha ± 151.100; P < 0.01). Subsequently, San Juan forest population density increased by a further small amount by 16 months after the hurricane (\(\Delta _{[16mo.-11mo.]} = 470\)/ha \(\pm\) 167.942; P \(=\) 0.008).
By contrast, we did not observe a significant increase in population density in successive periods after the hurricane in the other municipalities of our sampling. In Aguadilla, we found that forest populations decreased with time following the hurricane (\(\Delta _{[11mo.-4mo.]} = -2140/{\rm ha}\) ± 242.928; P < 0.01; \(\Delta _{[16mo.-11mo.]} = -340/{\rm ha}\) \(\pm\) 266.333; P = 0.012). Urban populations in Aguadilla similarly decreased following the hurricane (\(\Delta _{[11mo.-4mo.]} = -4490/{\rm ha}\) ± 251.419; P < 0.01; \(\Delta _{[16mo.-11mo.]} = -160/{\rm ha}\) ± 57.581; P \(=\) 0.013).
Forest populations in Mayagüez first experienced a large decrease and then a smaller decrease in population density (\(\Delta _{[11mo.-4mo.]} = -1,410/{\rm ha}\) \(\pm\) 293.470; P < 0.01; \(\Delta _{[16mo.-11mo.]} = -460/{\rm ha}\) \(\pm\) 73.969; P < 0.01). By contrast, urban Mayagüez populations first decreased (\(\Delta _{[11mo.-4mo.]} = -1,420/{\rm ha}\) ± 59.106; P < 0.001) and subsequently increased (\(\Delta _{[11mo.-4mo.]} = 2,220/{\rm ha}\) ± 51.240; P < 0.01), resulting in a final population density that was larger than that sampled at 4 months after the hurricane (\(\Delta _{[16mo.-4mo.]} = 800/{\rm ha}\) \(\pm\) 56.824, P < 0.01).
Snout vent lengths of Anolis cristatellus across sites. Colors denote habitat with green for forest and blue for urban population. Means for each given are shown for each site and sampling period with lines colored according to habitat. Facet labels correspond to months after Hurricane Maria (Color figure online)
Changes in population body size distribution
Mean body size did not differ significantly as a function of sampling period after the hurricane (Fig. 3; \(F_{1/709}=0.001\); P \(= 0.972\)), but was significantly different by sex, habitat, and municipality. Males were significantly larger than females and juveniles (ANOVA, \(F_{1/709}=801.238\); P \(< 0.001\)). Urban lizards were significantly larger than forest lizards (\(F_{1/709}=5.622\); P \(= 0.018\)), but populations in San Juan were more similarly sized to forest populations, with San Juan forest lizards having the largest mean body size overall (\(\beta =\) 6.200 ± 0.162; Table 1). Comparing across municipalities, lizard mean body size was largest in San Juan and smallest in Mayagüez (\(F_{2/709}=64.423\); P \(< 0.001\)).
Structural habitat changes and anole community shifts
We found that perch density within our plots varied both as a function of time since the hurricane, and of habitat type (general mixed effect model, hurricane: \(\chi = 161.11\), d.f. = 2, P \(< 0.001\); habitat: \(\chi = 396.58\), d.f. = 1, P \(< 0.001\), Table 2). Specifically, we found that forest sites had a greater abundance of perches and that the number of perches increased over our sampling period. Secondary succession led to nearly one hundred new plants within most of the forest localities (Fig. 4); however, not all the new saplings that colonized the plot at 11 months were still present 16 months after the storm. By contrast, urban sites did not experience a dramatic change in the quantity of available lizard perches through time (Fig. 4).
Including our focal taxon, A. cristatellus, we observed a total of six Anolis lizard species during our sampling efforts. Anolis cristatellus was the most abundant Anolis lizard species at all sampling periods and sites (Figure S1). The next most abundant species that we found in our plots was A. stratulus, a tree trunk and crown specialist. After the first sampling period (i.e., 11 and 16 months after the storm) we identified an established population of the grass-bush specialist A. krugi within the Mayagüez forest site (population estimate \(= 9.6 \pm 1.9\) and \(13.3 \pm 5.7\), respectively), that was not present during our 4 month post-hurricane sampling period. (We did not extrapolate this population to individuals per hectare, as this species was only present in a small fraction of the plot.) The other three species that we encountered were extremely rare (less than five captures in total). Anolis gundlachi (a trunk-ground specialist that occurs in closed forest habitats) and A. cuvieri (a crown giant) were only observed at the Mayagüez forest site. The final species, A. pulchellus (a grass bush specialist), was only observed at the urban site in San Juan. Unsurprisingly, given the much higher relative abundance of A. cristatellus compared to co-occurring congeners, population density estimates across all Anolis species in the community closely recapitulated our findings with A. cristatellus. Specifically, we found an increase in population densities in San Juan after the hurricane and lower population densities in urban sites (Figure S3).
Discussion
Projected increases in the frequency and severity of environmental disturbance under most scenarios of global environmental change (Knutson et al. 2010; Mendelsohn et al. 2012) represent a major challenge for species persistence (Johnstone et al. 2016; Seidl et al. 2016). How other types of anthropogenic pressures, such as urbanization, interact with more frequent or more intense disturbances to affect population dynamics remains understudied.
Following Hurricane Maria, a major category 5 storm that cut a path of destruction across the island of Puerto Rico in 2017, we contrasted the population density of A. cristatellus in paired urban and forest sites. We hypothesized that reduced vegetation in urban habitats could lead to lower urban population densities, and that densities would be lowest 4 months after the hurricane and gradually increase, presumably as lizard populations recovered to their pre-hurricane densities. Indeed, all urban sites had lower population densities compared to forested sites nearby. Additionally, the two sites which were most severely impacted by the hurricane also exhibited the most consistent increases in estimated population density through time. Specifically, estimated population density in our forest and urban localities of San Juan nearly doubled at 11 months, and then increased again at 16 months, after the hurricane (Fig. 2). For the remaining four sites in our study, between 4 and 11 months after the hurricane estimated population density decreased irrespective of the habitat type. Subsequently, between 11 and 16 months after the hurricane, estimated population density decreased slightly, except for at our urban site in the municipality of Mayagüez where estimated population density nearly doubled.
Post-hurricane population dynamics between natural and urban habitats
An important motivation of this study was to evaluate whether urban populations differed in density compared to conspecific forest populations nearby. This has been an important, but unanswered part of our understanding of the success of Anolis cristatellus in urban environments (Winchell et al. 2016, 2018a). We found that, irrespective of our sampling period (i.e., 4, 11, and 16 months after the hurricane), estimated urban population densities were invariably almost 50% smaller than forest population densities nearby (Fig. 2). Furthermore, urban habitats tended to have fewer perches suitable for arboreal lizards, and the number of perches remained fairly stable through time (Fig. 4). By contrast, forest habitats experienced a dramatic increase in the number of perches due to secondary succession after the hurricane.
Three significant considerations substantially limit our ability to draw any conclusive generalities about the differences in population density between urban and forested sites in A. cristatellus.
First, for logistical reasons, neither our urban nor our forest sites were selected randomly with respect to available habitat for A. cristatellus in urban and forested areas. Unfortunately, this was unavoidable principally because of limited access to private property in urban environments. For instance, a randomly chosen study plot within nearly any urban area of Puerto Rico would include spaces (e.g., private dwellings and patios) off-limits to an investigator. Likewise, natural sites within or adjacent to urbanization in Puerto Rico are highly fragmented. Nonetheless, we do not think that our non-random site selection is likely to have downwardly biased our estimated population density of urban areas in A. cristatellus. To the contrary, a truly random urban plot would have included, with high probability, areas (such major roads and highways) largely inhospitable to both anoles and investigators; whereas our non-random sites (mostly within or adjacent to small urban parks) did not contain any habitat strictly unsuitable or off-limits to lizards.
Second, we did not estimate population density in any of our sites prior to Hurricane Maria. Unfortunately, no previous study (to our knowledge) had ever estimated population density of A. cristatellus in any urbanized site, let alone in the localities of our research. Instead, we surmised that population density, if affected by the storm (either positively or negatively), would gradually re-equilibrate to a pre-storm level. However, because we lacked pre-hurricane data, we cannot rule out other factors such as seasonal changes in reproduction and rainfall. Other research on Anolis lizards has found that higher densities during the dry season were in part associated with recruitment from wet season reproduction (Sexton et al. 1963; Fleming and Hooker 1975). In contrast, during the wet season population densities were lower, presumably as a consequence of increased territorial behavior coinciding with courtship and reproduction (Sexton et al. 1963; Fleming and Hooker 1975). Moreover, prior research has shown that over the course of 5 years green anole (Anolis carolinensis) populations fluctuated substantially both within and between seasons (Lailvaux 2020). In our data, urban and forest populations sampled in San Juan, which experienced the strongest hurricane winds, increased in estimated density irrespective of sampling season (Fig. 2), whereas at other sites population densities both increased and decreased from period to period after the storm. This might be because San Juan was also the site that experienced the highest kinetic wind energy during Hurricane Maria; however, we cannot rule out stochasticity or alternative deterministic factors.
Third, our methodological approach was designed to target lizards perching only up to 2.5 m from the ground level. Thus, our capture rates for the tree canopy specialists observed within our sites (crown and trunk-crown ecomorphs in Figure S1), may not reflect the density of these species that are typically found at each site. This limits our ability to address how the densities of Anolis congeners could influence the density of A. cristatellus at our localities. Nonetheless, we suspect that this effect is likely to be quite small, due to the fact that other Anolis species represent only a relatively small portion of our capture data (137 congeners compared to 753 A. cristatellus). Indeed, when including these density data in our analyses, we found a broadly similar pattern of increases in population density in San Juan after the hurricane, and lower population density in urban sites (Figure S3). In addition, even if this pattern is affected by both hurricane induced mortality and niche overlap with other species, our subsequent sampling periods showed both increases in the abundance of A. cristatellus and decreases in the presences of A. stratulus. We interpret these changes as the gradual recovery of tree canopy (decreasing niche overlap) and increases of the population densities of A. cristatellus (either due to reproduction and/or recolonization) following the hurricane. To further test the dynamics of niche overlap and mortality following a hurricane would require exhaustive sampling at each site of the habitat (e.g., searching tree canopies) and of individual anole lizards (e.g., not bound by a standardized sampling effort).
Temporal changes in population mean body size
In addition to merely affecting population density overall, hurricane-induced mortality could vary by sex and life stage. For instance, after Hurricane Iris in 2001, howler monkey social groups had relatively fewer males and juveniles when compared to their pre-hurricane proportions (Pavelka et al. 2007).
In Anolis, male lizards often perch at higher elevations compared to females (Stamps et al. 1997; Butler et al. 2000). This could influence sex and size-specific mortality associated to the hurricane. Because our study lacks pre-hurricane data, we evaluated changes in the overall distribution of size throughout the recovery period to see if the composition of size varied across our different sampling periods.
In general, we did not detect a significant change in body size in the months after the hurricane. We did observed a pattern of smaller body sizes in San Juan forest corresponding to an increased sampling of juveniles at this site (Figure S2). This increase in the proportion of smaller bodied lizards is temporally concurrent with the peak of the reproductive season for this species (Otero et al. 2015). We also noted remarkably few adult male lizards within our plots in the Mayagüez forest (Figure S2) immediately after the hurricane. However, it is important to note that this site was affected by landslides caused by heavy rainfall during the Hurricane (Bessette-Kirton et al. 2020), which may have contributed to increase adult mortality at the plot.
Post-hurricane structural habitat and Anolis community changes
Among the most dramatic effects of hurricanes on forest ecosystems are structural changes immediately following the hurricane and throughout the post-storm recovery (Brokaw and Grear 1991; Fernandez and Fetcher 1991; Lugo et al. 2000). Secondary succession of new plant species and refoliation of the canopy often occur rapidly in the Caribbean (Lugo et al. 2000). Thus, demographic responses to hurricanes are largely a function of both immediate and long-term habitat changes, they and can be either positive or negative (Lugo 2008; Sergio et al. 2018).
Our results are consistent with both of these assertions. First, we showed species composition changing according to both canopy defoliation and also due to secondary succession. We found that after the disturbance there were higher abundances of the trunk-crown specialist (A. stratulus, Figure S1). This might indicate that the habitat use of this species shifted downward as a result of canopy loss. A similar pattern has been observed between ground and crown specialists following a hurricane, with niche space diverging again after canopy refoliation (Reagan 1991). Second, we found that nearly 1 year after the hurricane all forest sites dramatically increased in perch densities (Fig. 4). Most of the newly established vegetation was either young saplings or shrubby vegetation (Fig. 4). Subsequently, we found a population of the grass bush anole specialist, A. krugi, at one of our forest sites (Figure S1), where none had been present in prior surveys.
An important contrast between forest and urban habitats is the lack of structural habitat change in urban habitats and overall lower perch density at all sampling periods (Fig. 4). Previous research has documented high male-male aggression in non-urban populations of A. cristatellus with relatively few males occupying the same tree (Philibosian 1975) and higher aggression toward lizard models in urban populations of A. carolinensis (McMillan and Irschick 2010). Perhaps lower urban perch density can partly account for the smaller population densities in urban sites observed here (Fig. 2). Additionally, constant human modification of urban sites may have limited recruitment of new plant species following the hurricane. Consequently, urban spaces could sustain nearly constant structural habitats in contrast to the more dynamic habitat features of post-hurricane forests [summarized in Zimmerman et al. (2021)].
Conclusions
Our present contribution is, to our knowledge, the first study to measure population densities of a synanthropic tropical lizard between urban and forest sites. It is also the first research contrasting population dynamics of a lizard following a major hurricane between forest and urban environments. We hypothesized that population density might have decreased due to mortality during the storm event and would increase and re-equilibrate around pre-hurricane levels. We found patterns of population density fluctuations that only partly fit our a priori expectation. Interestingly, in some sites we measured small but significant decreases in population density with increasing time after the hurricane. This likely reflect seasonal fluctuations in population densities of anoles (Lailvaux 2020), rather than an outcome resulting as a consequence of the storm.
Our measured population densities are comparable with what other studies have reported for Anolis lizards: ranging from around 0.2 to 1 lizard per \(\mathrm{m}^2\) (Schoener and Schoener 1980). Moreover, urban populations occupied habitats with fewer perches, and showed lower and more narrowly distributed population densities between sites and over time (0.16–0.64 lizards per \(\mathrm{m}^2\)). By contrast, forest populations densities were larger overall (ranging from 0.43 to 1.02 lizards per \(\mathrm{m}^2\)) and showed higher variation between sites and across the sampling periods. As such, we hypothesize that less dense urban populations could be at greater risk of extirpation following large scale mortality events. Furthermore, if the fragmented nature of the urban landscape limits migration and gene flow among sites, urban populations may be at even greater risk of extinction due to inbreeding depression (Benson et al. 2016). Although our ability to fully characterize the effect of the hurricane is limited by a lack of pre-hurricane population data, our findings indicate that both natural and anthropogenic disturbances can shape population dynamics in urban environments. Thus, given current rates of urbanization, further research in needed to understand how climatic disturbances and constant human modification of these habitats affect the persistence of populations in urban environments.
References
Alberti M, Palkovacs EP, Roches SD, Meester LD, Brans KI, Govaert L, Grimm NB, Harris NC, Hendry AP, Schell CJ, Szulkin M, Munshi-South J, Urban MC, Verrelli BC (2020) The complexity of urban eco-evolutionary dynamics. Bioscience 70(9):772–793
Aronson MF, Nilon CH, Lepczyk CA, Parker TS, Warren PS, Cilliers SS, Goddard MA, Hahs AK, Herzog C, Katti M, La Sorte FA, Williams NS, Zipperer W (2016) Hierarchical filters determine community assembly of urban species pools. Ecology 97(11):2952–2963
Avilés-Rodríguez KJ, Winchell KM, De León LF, Revell LJ (2021) Phenotypic response to a major hurricane in Anolis lizards in urban and forest habitats. Biol J Linn Soc 133(3):880–895
Avilés-Rodríguez KJ, Kolbe JJ (2019) Escape in the city: urbanization alters the escape behavior of Anolis lizards. Urban Ecosyst 22(4):733–742
Baillargeon S, Rivest L-P (2007) Rcapture: loglinear models for capture–recapture. J Stat Softw 19(5):1–31
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B (Methodol) 57(1):289–300
Benson JF, Mahoney PJ, Sikich JA, Serieys LE, Pollinger JP, Ernest HB, Riley SP (2016) Interactions between demography, genetics, and landscape connectivity increase extinction probability for a small population of large carnivores in a major metropolitan area. Proc R Soc B Biol Sci 283:20160957
Bessette-Kirton EK, Coe JA, Schulz WH, Cerovski-Darriau C, Einbund MM (2020) Mobility characteristics of debris slides and flows triggered by Hurricane Maria in Puerto Rico. Landslides 17(12):2795–2809
Brokaw N, Grear J (1991) Forest structure before and after Hurricane Hugo at three elevations in the Luquillo Mountains, Puerto Rico. Biotropica 23(4):386–392
Butler MA, Schoener TW, Losos JB (2000) The relationship between sexual size dimorphism and habitat use in Greater Antillean Anolis lizards the relationship between sexual size dimorphism and habitat use in Greater Antillean Anolis lizards. Evolution 54(1):259–272
Cadenasso ML, Pickett STA, Schwarz K (2007) Spatial heterogeneity in urban ecosystems: conceptualizing land cover and a framework for classification. Front Ecol Environ 5(2):80–88
Chao A (1987) Estimating the population size for capture-recapture data with unequal catchability. Biometrics 43(4):783-791
Cochrane MA, Barber CP (2009) Climate change, human land use and future fires in the Amazon. Glob Change Biol 15(3):601–612
Darroch JN, Fienberg SE, Glonek GFV, Junker BW (1993) A three-sample multiple-recapture approach to census population estimation with heterogeneous catchability. J Am Stat Assoc 88(423):1137–1148
De León LF, Sharpe DM, Gotanda KM, Raeymaekers JA, Chaves JA, Hendry AP, Podos J (2019) Urbanization erodes niche segregation in Darwin’s finches. Evol Appl 12(7):1329–1343
Delgado de la Flor YA, Burkman CE, Eldredge TK, Gardiner MM (2017) Patch and landscape-scale variables influence the taxonomic and functional composition of beetles in urban greenspaces. Ecosphere 8(11):e02007. https://doi.org/10.1002/ecs2.2007
Donihue CM, Herrel A, Fabre AC, Kamath A, Geneva AJ, Schoener TW, Kolbe JJ, Losos JB (2018) Hurricane-induced selection on the morphology of an island lizard. Nature 560(7716):88–91
Donihue CM, Kowaleski AM, Losos JB, Algar AC, Baeckens S, Buchkowski RW, Fabre AC, Frank HK, Geneva AJ, Reynolds RG, Stroud JT, Velasco JA, Kolbe JJ, Mahler DL, Herrel A (2020) Hurricane effects on neotropical lizards span geographic and phylogenetic scales. Proc Natl Acad Sci USA 117(19):10429–10434
Falvey CC, Avilés-Rodríguez KJ, Hagey TTJ, Winchell KKM, Aviles-Rodriguez K, Hagey TTJ, Winchell KKM (2020) The finer points of urban adaptation: intraspecific variation in lizard claw morphology. Biol J Linn Soc 131(2):304–318
Fernandez DS, Fetcher N (1991) Changes in light availability following Hurricane Hugo in a subtropical montane forest in Puerto-Rico. Biotropica 23(234a):393–399
Fleming TH, Hooker RS (1975) Anolis cupreus: the response of a lizard to tropical seasonality. Ecology 56(6):1243–1261
Fournier B, Frey D, Moretti M (2020) The origin of urban communities: from the regional species pool to community assemblages in city. J Biogeogr 47(3):615–629
Gannon MR, Willig MR (1994) The effects of Hurricane Hugo on Bats of the Luquillo experimental forest of Puerto Rico. Biotropica 26(3):320–331
Gannon MR, Willig MR (2009) Island in the storm: disturbance ecology of plant-visiting bats in the hurricane-prone island of Puerto Rico. In: Fleming TH, Racey PA (eds) Island bats: evolution, ecology, and conservation, chapter 10. University of Chicago Press, Chicago, pp 281–301
Hite JL, Gómez CAR, Larimer SC, Díaz-lameiro AM, Powell R (2008) Anoles of St. Vincent (Squamata: Polychrotidae): population densities and structural habitat use. Carib J Sci 44(1):102–115
Holland G, Bruyère CL (2014) Recent intense hurricane response to global climate change. Clim Dyn 42(3–4):617–627
Johnstone JF, Allen CD, Franklin JF, Frelich LE, Harvey BJ, Higuera PE, Mack MC, Meentemeyer RK, Metz MR, Perry GL, Schoennagel T, Turner MG (2016) Changing disturbance regimes, ecological memory, and forest resilience. Front Ecol Environ 14(7):369–378
Jokimäki J, Selonen V, Lehikoinen A, Kaisanlahti-Jokimäki ML (2017) The role of urban habitats in the abundance of red squirrels (Sciurus vulgaris, L.) in Finland. Urban For Urban Green 27:100–108
Kishore N, Marqués D, Mahmud A, Kiang MV, Rodriguez I, Fuller A, Ebner P, Sorensen C, Racy F, Lemery J, Maas L, Leaning J, Irizarry RA, Balsari S, Buckee CO (2018) Mortality in Puerto Rico after Hurricane Maria. N Engl J Med 379(2):162–170
Knutson TR, McBride JLL, Chan J, Emanuel K, Holland G, Landsea C, Held I, Kossin JP, Srivastava AK, Sugi M (2010) Tropical cyclones and climate change. Nat Geosci 3(3):157–163
Lailvaux SP (2020) It’s not easy being green: behavior, morphology, and population structure in urban and natural populations of green anole (Anolis carolinensis) lizards. Front Ecol Evol 8:315
Lazell JD (1991) The herpetofauna of Guana Island: diversity, abundance, rarity, and conservation. Status y distribución de los reptiles y anfibios de la region de Puerto Rico. Publicación Cientıfico Misceláneo 1:28–33
Lugo AE (2000) Effects and outcomes of Caribbean hurricanes in a climate change scenario. Sci Total Environ 262:243–251
Lugo AE (2008) Visible and invisible effects of hurricanes on forest ecosystems: an international review. Austral Ecol 33(4):368–398
Lugo AE, Rogers CS, Nixon SW (2000) Hurricanes, coral reefs and rainforests: resistance, ruin and recovery in the Caribbean. AMBIO J Hum Environ 29(2):106–114
McKinney ML (2008) Effects of urbanization on species richness: a review of plants and animals. Urban Ecosyst 11(2):161–176
McMillan DM, Irschick DJ (2010) Experimental test of predation and competition pressures on the green anole (Anolis carolinensis) in varying structural habitats. J Herpetol 44(2):272–278
Mendelsohn R, Emanuel K, Chonabayashi S, Bakkensen L (2012) The impact of climate change on global tropical cyclone damage. Nat Clim Change 2(3):205–209
Newman EA (2019) Disturbance ecology in the anthropocene. Front Ecol Evol 7(147):1–6
Otero LM, Huey RB, Gorman GC (2015) A few meters matter: local habitats drive reproductive cycles in a tropical lizard. Am Nat 186(3):E72–E80
Pasch RJ, Penny AB, Berg R (2017) Hurricane Maria (AL152017). Technical report April, National Hurricane Center Tropical Cyclone Report
Pavelka MS, McGoogan KC, Steffens TS (2007) Population size and characteristics of Alouatta pigra before and after a major hurricane. Int J Primatol 28(4):919–929
Philibosian R (1975) Territorial behavior and population regulation in the lizards, Anolis acutus and A. cristatellus. Copeia 3:428–444
Pieniażek A, Sokół M, Kozakiewicz M (2017) Ecological characteristics of two closely related rodent species in urban environment-permanent inhabitant vs newcomer. Nat Resour 08(02):69–80
Pignataro T, Bressan P, Santos AL, Cornelissen T (2020) Urban gradients alter the diversity, specific composition and guild distribution in tropical butterfly communities. Urban Ecosyst 23(4):723–730
R Core team (2019) R: a language and environment for statistical computing. R foundation for statistical computing. R: a language and environment for statistical computing. R Foundation for Statistical Computing , Vienna, Austria. ISBN:3-900051-07-0. http://www.R-project.org/
Reagan DP (1991) The response of Anolis lizards to hurricane-induced habitat changes in a Puerto Rican rain forest. Biotropica 23(4):468–474
Rivest L-P, Baillargeon S (2007) Applications and extensions of Chao’s moment estimator for the size of a closed population. Biometrics 63(4):999–1006
Rivkin LR, Santangelo JS, Alberti M, Aronson MF, de Keyzer CW, Diamond SE, Fortin MJ, Frazee LJ, Gorton AJ, Hendry AP, Liu Y, Losos JB, MacIvor JS, Martin RA, McDonnell MJ, Miles LS, Munshi-South J, Ness RW, Newman AE, Stothart MR, Theodorou P, Thompson KA, Verrelli BC, Whitehead A, Winchell KM, Johnson MT (2019) A roadmap for urban evolutionary ecology. Evol Appl 12(3):384–398
Rodda GH, Perry G, Rondeau RJ, Lazell J (2001) The densest terrestrial vertebrate. J Trop Ecol 17(2):331–338
Santos-Burgoa C, Sandberg J, Suárez E, Goldman-Hawes A, Zeger S, Garcia-Meza A, Pérez CM, Estrada-Merly N, Colón-Ramos U, Nazario CM, Andrade E, Roess A, Goldman L (2018) Differential and persistent risk of excess mortality from Hurricane Maria in Puerto Rico: a time-series analysis. Lancet Planet Health 2(11):e478–e488
Schoener TW, Schoener A (1980) Densities, sex ratios, and population structure in four species of Bahamian Anolis lizards. J Anim Ecol 49(1):19–53
Schoener TW, Spiller DA, Losos JB (2001) Natural restoration of the species-area relation for a lizard after a hurricane. Science 294(5546):1525–1528
Schoener TW, Spiller DA, Losos JB (2004) Variable ecological effects of hurricanes: the importance of seasonal timing for survival of lizards on Bahamian islands. Proc Natl Acad Sci USA 101(1):177–181
Secrest MF, Willig MR, Peppers LL (1996) The legacy of disturbance on habitat associations of terrestrial snails in the Luquillo Experimental Forest, Puerto Rico. Biotropica 28(4):502–514
Seidl R, Spies TA, Peterson DL, Stephens SL, Hicke JA (2016) Searching for resilience: addressing the impacts of changing disturbance regimes on forest ecosystem services. J Appl Ecol 53(1):120–129
Sergio F, Blas J, Hiraldo F (2018) Animal responses to natural disturbance and climate extremes: a review. Glob Planet Change 161:28–40
Sexton OJ, Heatwole HF, Meseth EH (1963) Seasonal population changes in the lizard, Anolis limifrons, in Panama. Am Midl Nat 69(2):482–491
Spiller DA, Losos JB, Schoener TW (1998) Impact of a catastrophic hurricane on island populations. Science 281(5377):695–697
Stamps JA, Losos JB, Andrews RM (1997) A comparative study of population density and sexual size dimorphism in lizards. Am Nat 149(1):64–90
Torres J (1992) Lepidoptera outbreaks in response to successional changes after the passage of hurricane hugo in Puerto Rico. J Trop Ecol 8(3):285–298
Turner MG (2010) Disturbance and landscape dynamics in a changing world. Ecology 91:2833–2849
Williams EE (1972) The origin of faunas. Evolution of lizard congeners in a complex island fauna: a trial analysis. In: Evolutionary biology. Springer, New York, NY, 47–89
Winchell KM, Reynolds RG, Prado-Irwin SR, Puente-Rolón AR, Revell LJ (2016) Phenotypic shifts in urban areas in the tropical lizard Anolis cristatellus. Evol Int J Org Evol 70(5):1009–1022
Winchell KM, Carlen EJ, Puente-Rolón AR, Revell LJ (2018) Divergent habitat use of two urban lizard species. Ecol Evol 8:25–35
Winchell KM, Maayan I, Fredette JR, Revell LJ (2018) Linking locomotor performance to morphological shifts in urban lizards. Proc R Soc Biol 285:20180229
Zimmerman JK, Wood TE, González G, Ramirez A, Silver WL, Uriarte M, Willig MR, Waide RB, Lugo AE (2021) Disturbance and resilience in the Luquillo experimental forest. Biol Conserv 253:108891
Acknowledgements
Nearly 3000 people are now thought to have lost their lives on the island of Puerto Rico due to Hurricane Maria or in the aftermath (Kishore et al. 2018; Santos-Burgoa et al. 2018). We recognize the devastating effects this hurricane had on the island of Puerto Rico and its people. We are extremely grateful to the support we received from Alberto Puente Rolón, Sondra Vega Castillo and Fernando Bird Picó who aided us with the logistic of field work during a time when many parts of the island were without electrical power. We are also grateful to the fieldwork assistance of Cleo Falvey. Lastly, we are thankful to the Department of Natural Resources in Puerto Rico who provided access to forested sites at a time when they were all closed to the public. This study was conducted under permits from the Puerto Rico Departamento de Recursos Naturales y Ambientales (DRNA, 2018, R-VS-PVS15-SJ-00685-28022018). Animal procedures were approved by institutional animal care and use committees (IACUC) at the University of Massachusetts Boston IACUC 2012001.
Funding
Funding was provided by Division of Environmental Biology (1354044).
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Avilés-Rodríguez, K.J., De León, L.F. & Revell, L.J. Population density of the tropical lizard Anolis cristatellus in urban and forested habitats after a major hurricane. Trop Ecol 64, 122–132 (2023). https://doi.org/10.1007/s42965-022-00251-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42965-022-00251-z