Abstract
Background
Vesicoureteral reflux (VUR) is an abnormality frequently seen at a complete duplex system (DS). Operational correction is required and completed after the neonatal period when symptoms occur.
Objective
This study aimed to evaluate the efficacy of Vantris and a need for additional surgery in children with DS VUR in a multicenter study.
Patients and methods
We performed retrospective analysis of prospectively acquired data, from 2009 to 2018, on 172 patients with a mean age of 3 years with VUR into either upper or lower moiety of the DS who underwent endoscopic correction utilizing Vantris at five centers worldwide. All patients were with primary VUR. The median follow-up was 7 years.
Results
Reflux was corrected in 122 patients (70%) after the first injection and 35 patients (20%) after the second injection. 15 patients (9%) failed endoscopic correction and required ureteral reimplantation. 13 patients suffered afebrile urinary tract infection, and 9 patients developed febrile urinary tract infection (UTI). 3 patients required partial nephrectomy of the poorly or non-functioning refluxing moiety following the failure of endoscopic correction.
Conclusion
Our data shows that Vantris injection provides a high reflux resolution in a DS with VUR. Successful VUR resolution might spare some patients associated with VUR poorly functioning moieties further surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ureteric duplication is a prevalent congenital anomaly of the urinary tract. The estimated prevalence of ureteric duplication in the general population is about 1 in 125 [1, 2]. There appears to be an increase in the incidence of ureteric duplication in females compared to males in a ratio of 1.6: 1 [1]. Vesicoureteral reflux (VUR) is the most common associated abnormality found in a duplex system (DS), and is present in 70% of those who present with urinary tract infection (UTI) [3, 4]. Based on Weigert and Meyer’s studies, VUR usually occurs in the lower-pole moiety due to its lateral and proximal displacement within the bladder, which is associated with a shorter intramural ureter resulting in an incompetent ureterovesical junction (UVJ) [5, 6]. Traditionally, when symptoms occur in a DS, surgical correction (open, injection therapy or laparoscopic) is required and is performed after the neonatal period [4]. The endoscopic management of VUR emerged as a first-line treatment for all reflux grades in some centers. As previously published, we have demonstrated the high rate of success of VUR correction with no recurrence using a non-biodegradable tissue-augmenting substance in children with primary VUR [7].
We have hypothesized that endoscopic correction of the primary VUR in DSs is as effective as in single systems. Solving the problem of reflux in poorly functioning moieties has brought on a cessation of febrile UTI; this might spare the need for an unnecessary complicated surgery, such as a partial nephrectomy [8]. In accordance with our hypothesis, we designed a multicenter study aimed to evaluate the efficacy of polyacrylate polyalcohol bulking copolymer (PPC, Vantris, Promedon, Cordoba, Argentina), a non-biodegradable tissue-augmenting substance, as well as examining the need for additional surgery in children with DS VUR.
Patients and methods
We performed retrospective analysis of prospectively acquired data on all patients with ureteral duplication who underwent endoscopic correction of VUR utilizing Vantris injection. A standard protocol for evaluation and endoscopic correction of VUR has been previously published [9, 10]. The protocol was approved by the Ethical Committee of all institutions for the entirety of the study period, with necessary reevaluation and renewal to be done bi-annually. The protocol was similar in all five departments.
Patients
From the year 2009 to 2018, 172 patients (71 boys and 101 girls) with a mean age of 7 years (ranging from 6 months to 13 years) who had VUR into either their upper or lower moiety of the DS underwent endoscopic correction utilizing Vantris, at five different centers. All the patients in our study had primary VUR, while 15 patients (9%) had previous endoscopic treatment utilizing tissue-augmenting substances other than Vantris. Patients with prune belly syndrome, ureterocele, and ectopic ureter were excluded from the study. Symptomatic children who had symptoms of dysfunctional voiding or constipation were given conservative treatment before undergoing an endoscopic procedure. This was performed until the voiding symptoms resolved completely, followed by a preoperative reassessment, including repeated VCUG series. As previously stated, we have adopted in our practice a survey for the evaluation of dysfunctional voiding symptoms score (DVSS) [8]. All patients received antibiotic prophylaxis until VCUG showed spontaneous resolution or definitive cure of the VUR [10].
Diagnostic workup and indications for surgery
Diagnosis of VUR was obtained with voiding cystourethrogram (VCUG) scans, which demonstrated the reflux grade before and after surgery, or during conservative treatment, according to the International Classification System (International Reflux Study Committee). Renal scintigraphy, which was performed 2 h after injection of 99m technetium 2,3 dimercapto-succinic acid (DMSA), was used to assess renal scarring. Fractional left and right renal activity was calculated for each kidney, as well as separately for each moiety after background correction. A kidney uptake of 45% to 55% of total renal activity was considered normal. Relative renal function (RRF) of 30–45% was considered moderate, and renal function of less than 30% was considered poor. Following the calculation of total renal activity, the contribution of each renal moiety to overall renal function was assessed. In our series, the DMSA scan was performed on 72 (42%) patients, at least six months after the last febrile UTI, and revealed scarring in 32 (15%) refluxing renal units (RRU). DMSA demonstrated 28 (13%) RRU with normal renal function, 24 (11%) with moderate renal function, and the remaining 21 (10%) with poor renal function. All patients with ultrasound (US) findings such as thinning of the renal parenchyma or suspicion of renal scaring underwent obligatory DMSA. In most cases, the indications for endoscopic correction were persistent high-grade (grade IV–V) VUR or breakthrough infections while on antibiotic prophylaxis. However, in 4 (2%) patients, reflux correction was performed according to the guardians’ request. Grade I VUR was treated only in children with contralateral high-grade VUR.
Subureteric injection of bulking material technique
The technique of endoscopic correction used was a distinctive technique for the DS, as shown in the video [Online Resource 1]. A needle was introduced under the bladder mucosa, 2–3 mm below the lower ureteric orifice at the 6 o’clock position. The entire length of the needle (8 mm) was advanced behind the two ureteric orifices, and the implant was then placed submucosally without needle withdrawal [11]. All children’s families gave full written informed consent regarding the procedure and tissue-augmenting substance used for the reflux correction as part of our routine protocol.
Follow-up
According to our standard practice, a renal US scan was performed one month after injection to identify hydronephrosis; also, obligatory VCUG was performed 3 months after endoscopic correction. Reflux was considered cured if VCUG did not demonstrate VUR of any grade. Furthermore, we performed renal US and VCUG at 1 and 3 years after the surgery after the time of injection as part of our prospective follow-ups and as part of the prospective evaluation of the Vantris efficacy in patients with VUR. 14 (7.6%) patients underwent repeat DMSA or mercaptuacetyltriglycerine (MAG 3) renal scans due to slowing or complete kidney growth arrest found in an annual US scan. The median follow-up time was 7 years (range 4 months to 11 years).
If renal US showed a new onset of hydronephrosis, we repeated the US scan, and when indicated—performed a dynamic renal scan with MAG 3 or magnetic resonance urography (MRU) to confirm possible UVJ obstruction. Following the diagnosis of UVJ obstruction and the decision of performing ureteric reimplantation, patients underwent ureteral reimplantation following one of the existing techniques (Cohen, Polittano-Leadbetter, or recently laparoscopic robotic-assisted extravesical reimplantation) according to the surgeons’ preferences [12].
Results
The demographic data and indication for surgery are presented in Table 1. In brief, reflux was diagnosed following evaluation of antenatal hydronephrosis in 34 patients (20%), and reflux in the remaining 138 patients (80%) was diagnosed due to breakthrough infections. VUR into upper moiety was unilateral in 15 patients (9%), VUR into lower moiety was unilateral in 126 patients (73%), ipsilateral VUR to both moieties was found in 5 patients (3%), and bilateral VUR into lower moiety was found in 31 patients (20%); comprising 208 RRU. Notably, patients with reflux into unilateral upper moiety and ipsilateral reflux to both moieties were considered as unique anatomy variant and not ectopic ureter or incomplete DS, respectively.
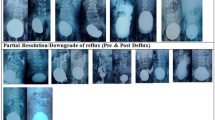
VUR was Grade I in 4 RRU, Grade II in 25, Grade III in 94, Grade IV in 63, and Grade V in 22. Reflux was corrected in 122 patients (70%) after the first injection and in 35 patients (22%) after the second injection.
Of 15 patients, with previously failed other than Vantris tissue-augmenting substance injection, 13 demonstrated reflux cessation after the first injection, while 2 required second attempt to correct VUR. Fifteen patients (9%) failed the second endoscopic correction and required ureteric reimplantation. An average of 0.6 ± 0.4 cc (mean ± SD) of Vantris was injected per ureter. 13 patients (7.5%) suffered afebrile UTI once, and 9 patients (5%) developed febrile UTI once. 80 patients (46%) underwent VCUG one year after the surgery, and 31 patients underwent VCUG 3 years after the surgery. None of the patients demonstrated VUR recurrence. 4 (2%) patients demonstrated a reduction of greater than 5% in relative function of the kidney without new scarring of the refluxing moiety, and 3 (2%) required partial nephrectomy of either poorly or non-functioning refluxing moiety following failure of the endoscopic correction (all three had Grade IV–V VUR). None of the patients developed UVJ obstruction following injection.
Discussion
Based on the autopsy series, the estimated prevalence of ureteric duplication affects about 1% of the population. The incidence of VUR is higher in patients with complete ureteral duplication [4]. There is a notable controversy regarding the management of VUR in a single system in general and the management of VUR in DS in particular. Prospective randomized trials comparing three management arms (I, endoscopic correction; II, antibiotic prophylaxis; III, surveillance without antibiotic prophylaxis) in 203 children aged 1–2 years with grade III/IV reflux, showed that endoscopic treatment had the highest resolution rate, 71%, compared with 39% and 47% for treatment arms II and III, respectively, at two years of follow-up [13]. Furthermore, the American Urological Association (AUA) guidelines from 2010, which were amended in 2017, for the management and screening of primary VUR in children, recommended that patients be given continuous antibiotic prophylaxis with a febrile breakthrough UTI, and that they be considered for endoscopic injection of bulking agents or open surgical ureteral reimplantation for intervention with curative purpose [14]. Endoscopic management of VUR has thus become the standard treatment in some centers when symptoms, such as breakthrough infections, additional renal scarring, or worsening reflux occurs. The effectiveness of endoscopic treatment of VUR is achieved by creating a prominent physical bulge in the bladder wall below the ureteric orifice, thereby increasing the submucosal length of the ureter. Secondly, this also creates a fixation point for the ureter. The endoscopic correction technique used in this study is a distinctive technique for the DS, which tends to have an anomaly of the ureteric entrance into the bladder. The needle is introduced under a non-refluxing upper ureter, hence in most cases of the low-moiety VUR, the needle is inserted under the lower orifice of the ureter (more distal and median). The entire needle length (8 mm) is passed behind the ureteric orifices, and with no needle withdrawal, the implant is placed submucosally. In our opinion, placing the implant under both ureters increases the fixation point for the ureters within the bladder, in a sense following the principle of common sheath ureteral reimplantation. Our results further prove that, only 15 children (9%) failed the second endoscopic correction and were required to undergo reimplantation or partial nephrectomy due to non-functioning pole. All failed cases had Grade IV or V reflux.
More than 80% of studied patients here presented with febrile UTI upon endoscopic treatment. Following successful correction, only 9 children (5%) went on to develop febrile UTI. A decrease in acute pyelonephritis incidence might hypothetically lead to a decrease in renal parenchymal damage and renal scarring, and our data further supports that. None of the children with cured VUR showed new renal scarring, and only 4 (2%) children showed an overall decrease in relative renal function during follow-up. These children with poorly functioning moieties were free of UTIs on the follow-ups and were hence spared a major surgery. According to the claim, poorly functioning renal moieties left in-situ following successful endoscopic surgery do not cause additional morbidity and do not require partial nephrectomy in most cases [15].
Although we have never questioned the Vantris durability, we cannot disregard the previously reported higher incidence of UVJ obstruction in some series that used Vantris for VUR correction [16,17,18]. This UVJ obstruction incidence led us to reevaluate our indications for surgery and reevaluate our experience to identify the patients most likely to develop obstruction and, therefore, cannot undergo endoscopic correction [19, 20]. Furthermore, we know that patients who developed febrile UTI or who failed endoscopic correction and needed open surgery have not developed new hydronephrosis or progressed past the previous one, so we can be sure that they did not develop UVJ obstruction.
Nevertheless, this study is not without limitations. First, the endoscopic correction of VUR was conducted by a large number of surgeons with various levels of expertise in endoscopic reflux correction. Second, the study protocol was slightly different in some centers in preoperative evaluation and postoperative follow-up. For example, several hospitals did not use preoperative DMSA scanning as a routine diagnostic workup because it is not freely available and is not widely accepted. Furthermore, there was variability among the centers about performing VCUG examination in each subject 1 and 3 years after endoscopic correction; therefore, we cannot comment on the radiological recurrence of VUR. However, our previous publication demonstrated no recurrence after utilizing Vantris in the long-term follow-up [21]. Additionally, we are unable to comment on the volume of tissue-augmenting substance required for successful correction of VUR. Our department is currently conducting the study, and we hope to be able to publish our results soon. Moreover, all participating centers monitored all patients prospectively and could record all incidences of UTIs or other adverse effects. As of yet, we have done ureteral reimplantation in the DS in just a few patients who were previously injected Vantris. Therefore, we do not have enough data to comment on any specific challenges in these patients and whether it will be challenging to perform ureteral reimplantation in patients following VUR correction failure in DS with Vantris. These questions are to be addressed in future studies.
Conclusion
Our data show that Vantris injection provides a high level of reflux resolution in the DS VUR. The majority of patients with corrected VUR did not require further surgery and could be spared partial nephrectomy, even in poorly functioning moieties.
References
Kaplan WE, Nasrallah P, King LR (1978) Reflux in complete duplication in children. J Urol 120:220–222
Nation EF (1944) Duplication of the kidney and ureter a statistical study of 230 new cases. J Urol 51:456–654
Privett JT, Jeans WD, Roylance J (1976) The incidence and importance of renal duplication. Clin Radiol 27:521–530
Whitten SM, Wilcox DT (2001) Duplex systems. Prenat Diagn 21:952–957
Weigert VC (1877) Über einige Bildungsfehler der Ureteren. Virchows Arch Pathol Anat Physiol Klin Med 70:490–501
Meyer R (1946) Normal and abnormal development of the ureter in the human embryo—a mechanistic consideration. Anat Rec 96:355–371
Kocherov S, Ulman I, Nikolaev S et al (2014) Multicenter survey of endoscopic treatment of vesicoureteral reflux using polyacrylate-polyalcohol bulking copolymer (Vantris). J Ped Urol 84:689–693
Chertin B, Arafeh WA, Zeldin A et al (2011) Preliminary data on endoscopic treatment of vesicoureteric reflux with polyacrylate surgical outcome following single injection. J Ped Urol 7:654–656
Capozza N, Lais A, Nappo S et al (2004) The role of endoscopic treatment of vesicoureteral reflux: a 17-year experience. J Urol 172:1626–1628
Chertin B, De Caluwe D, Puri P (2003) Endoscopic treatment of primary grades IV and V vesicoureteral reflux in children with subureteral injection of polytetrafluoroethylene. J Urol 169:1847–1849
Farkas A, Moriel EZ, Lupa S (1990) Endoscopic correction of vesicoureteral reflux: our experience with 115 ureters. J Urol 2(Pt 2):534–536
Rappaport YH, Kord E, Noh PH et al (2020) Minimally invasive dismembered extravesical cross-trigonal ureteral reimplantation for obstructed megaureter: a multi-institutional study comparing robotic and laparoscopic approaches. J Ped Urol 149:211–215
Holmdahl G, Brandstrom P, Lackgren G et al (2010) The Swedish reflux trial in children, II: vesicoureteral reflux outcome. J Urol 184:280–285
American Urological Association, Inc. (2010) American Urological Association pediatric vesicoureteral reflux clinical guidelines panel. Management and screening of primary vesicoureteral reflux in children: AUA guideline. American Urological Association, Inc., Baltimore
Chertin B, Rabinowitz R, Pollack A et al (2005) Does prenatal diagnosis influence the morbidity associated with left in situ nonfunctioning or poorly functioning renal moiety after endoscopic puncture of ureterocele? J Urol 173:1349–1352
Alizadeh F, Mazdak H, Khorrami MH et al (2013) Postoperative ureteral obstruction after endoscopic treatment of vesicoureteral reflux with polyacrylate polyalcohol copolymer (Vantris). J Pediatr Urol 9:488–492
Sencan A, Yıldırım H, Ozkan KU (2014) Late ureteral obstruction after endoscopic treatment of vesicoureteral reflux with polyacrylate polyalcohol copolymer. J Urol 84:1188–1191
Warchoł W, Krzemień G, Szmigielska A et al (2017) Endoscopic correction of vesicoureteral reflux in children using polyacrylatepolyalcohol copolymer (Vantris): 5-years of prospective follow-up. Cent European J Urol 70:314–319
Chertin B, Mele E, Kocherov S et al (2018) What are the predictive factors leading to ureteral obstruction following endoscopic correction of VUR in the pediatric population? J Ped Urol 14:538.e1–538.e7
Kocherov S, Nikolaev S, Gaber G et al (2020) Incidence of UVJ obstruction during long-term follow-up after endoscopic correction of VUR utilizing polyacrylate polyalcohol copolymer (PPC). J Ped Endosc Surg 2:183–187
Chertin B, Abu Arafeh W, Zeldin A et al (2013) Endoscopic correction of VUR using vantris as a new non-biodegradable tissue augmenting substance: three years of prospective follow-up. J Urol 82:201–204
Acknowledgements
All senior authors (Tatiana Sklyarova, Ibrahim Ulman, Girolamo Mattioli, Nicola Capozza and Boris Chertin) equally contributed to this study.
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design, material preparation, data collection and interpretation, drafting of the manuscript, and critical revision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors have no conflicts of interest to declare that they are relevant to this article’s content.
Ethics approval
The protocol was approved by the Ethical Committee of all institutions for the entirety of the study period, with necessary reevaluation and renewal to be done bi-annually. The protocol was similar in five departments.
Consent
Informed consent was obtained from legal guardians.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (MP4 1495 KB)
Rights and permissions
About this article
Cite this article
Cohen, S., Kocherov, S., Jaber, J. et al. Multicenter survey of endoscopic treatment of vesicoureteral reflux utilizing polyacrylate-polyalcohol-bulking copolymer (Vantris) in patients with duplex systems. J Ped Endosc Surg 3, 205–209 (2021). https://doi.org/10.1007/s42804-021-00105-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42804-021-00105-w