Abstract
Mountain biodiversity is under unparalleled pressure due to climate change, necessitating in-depth research on high-altitude plant’s microbial associations which are crucial for plant survival under stress conditions. Realizing that high-altitude tree line species of Himalaya are completely unexplored with respect to the microbial association, the present study aimed to elucidate plant growth promoting and secondary metabolite producing potential of culturable endophytic fungi of Himalayan silver birch (Betula utilis D. Don). ITS region sequencing revealed that the fungal isolates belong to Penicillium species, Pezicula radicicola, and Paraconiothyrium archidendri. These endophytes were psychrotolerant in nature with the potential to produce extracellular lytic activities. The endophytes showed plant growth promoting (PGP) traits like phosphorus solubilization and production of siderophore, indole acetic acid (IAA), and ACC deaminase. The fungal extracts also exhibited antagonistic potential against bacterial pathogens. Furthermore, the fungal extracts were found to be a potential source of bioactive compounds including the host-specific compound—betulin. Inoculation with fungal suspension improved seed germination and biomass of soybean and maize crops under net house conditions. In vitro PGP traits of the endophytes, supported by net house experiments, indicated that fungal association may support the growth and survival of the host in extreme cold conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endophytes are defined as the beneficial microorganisms that inhabit the internal tissue without causing any adverse effect to the host. The ubiquitous presence of endophytes in almost all plant species provides which direct benefits to the host. Plants have evolved in a microbial world and their interactions may be supportive in the adaptation of the plant to its environment. Endophytes, including plant growth-promoting bacteria and mycorrhizal fungi, play crucial roles in various ways mainly towards plant productivity and host competitiveness [1]. They improve nutrient mineralization, solubilization, and uptake, act as biocontrol agents, and in turn, provide endurance to biotic and abiotic stresses. The symbiotic relationship can lead to significant alterations in plant physiology, encompassing changes in phytohormonal levels, adjustments to nutrient absorption, and modifications in soil conditions within the rhizosphere [2]. The primary mechanisms contributing to the growth-promoting effects of fungal endophytes on their hosts involve the stimulation of siderophore synthesis [3], enhancement of nitrogen fixation [4], promotion of phosphate solubilization [5], regulate ethylene level through 1-aminocyclopropane-1-carboxylate (ACC) deaminase production [6], impact plant development by producing phytohormones such as indole 3-acetic acid and gibberellins [7], and influence on enzyme synthesis [8]. In addition to their ability to promote plant growth, fungal endophytes are recognized as a valuable source of bioactive pharmaceutical compounds. These endophytes harbor compounds with therapeutic potential, including antimicrobial, anticancer, antioxidant, anti-inflammatory, and antimalarial effects [9]. Furthermore, these endophytes have demonstrated the ability to mimic or generate host-specific bioactive compounds, possibly attributed to horizontal gene transfer [9, 10].
Fungal endophytes are the neglected aspect of plant microbiome, particularly in the context of high-altitude plant species. Plant interacts with the surrounding microbiome following selective approach to sustain in extreme conditions [11] and fungal endophytes can be ideal candidates to mitigate climate-induced stress [12]. However, despite the crucial role of fungal endophytes in plant adaptation and abiotic stress tolerance, so far, very limited efforts have been made to assess microbial association in treeline (the highest elevation at which a single upright tree species found over the landscape) species.
Genera Penicillium has been reported as the dominant genus with > 100 species from different tree species of the Himalayan region [13]. Qadri et al. [14] reported four fungal endophytes from the Abies pindrow, and five endophytic species from Pinus roxburgii including Penicillium species, namely P. oxalicum and P. expansum from Indian Himalayan region (IHR). Penicillium species has also been reported from Taxus wallichiana along with its phosphate solubilization property [15], and from T. fauna [16], Ginkgo biloba [17], and Arnebia euchroma [18] of IHR.
Betula utilis D. Don (English: Himalayan silver birch, Hindi: bhojpatra, Sanskrit: Bhurja; Family: Betulaceae) is a valuable broad leaved tree species of ecological and commercial importance and distributed in sub-alpine zone of the IHR up to 4500 m amsl [19]. It is a pioneer tree species dominating in subalpine forests that form natural treeline in the Himalayan region. The species experiences extreme climatic conditions such as low temperature, heavy rain, and snowfall, and is likely to go through various successions. The species has been reported to exert a suppressive effect on the rhizosphere microbial communities, particularly concerning the inhibition of bacteria and actinobacteria [20, 21]. Pseudomonas putida, a potential plant growth-promoting bacterium, has also been reported from Betula-Rhododendron association [22]. The high extent of microbial colonization including mycorrhizal fungi, dark septate endophytes, and bacterial endophytes in B. utilis roots provided initial lead to isolate and characterize the culturable endophytes for their potential role in plant–microbe interaction [21]. Therefore, the present study aimed to assess the functional traits (i.e., plant growth promoting potential and secondary metabolite production efficiency) of culturable fungal endophytes of the Himalayan silver birch.
Material and methods
Sample collection and isolation of fungal endophytes
The root samples were collected randomly from five different populations of B. utilis and mixed to form a composite sample. The samples were collected in the growing season (altitude 3288; latitude 32°19ʹ44.4ʺ; and longitude 77°13ʹ01.2ʺE) from Kullu district, North-Western Himalaya. The collected root samples were surface sterilized by immersing in 70% ethanol for 3 min followed by in 5% aqueous solution of sodium hypochlorite (2 min) and 70% ethanol (2 min) and finally in 0.1% mercury chloride (1 min) and rinsed with sterile distilled water thrice [23]. After surface sterilization, the root samples were cut longitudinally with sterile surgical blade and transferred aseptically on potato dextrose (PD) agar medium. The last washing water (1 ml) was used as a surface sterilization sterility test following pour plate method and incubated at 25 °C for 2 to 3 weeks. The fungal growth around the internal tissue was purified, transferred to fresh medium, and stored at 4 °C for further study.
Phenotypic characters (morphological, physiological, and biochemical)
The morphological studies of fungal endophytes were done on five different growth media at 25 °C (Supplementary Table S1). The growth characteristics were recorded for vegetative growth, sporulation, pigmentation, and exudation. The microscopic characterization of fungal endophyte was done by slide culture technique [24] with lacto phenol cotton blue staining method. The physiological characterization was done based on their temperature, pH requirements, and salt tolerance in liquid growth medium. The isolates were inoculated in PD broth and incubated at different temperatures (5 to 45 °C), pH (2 to 12), and salt (0.5 to 6%) concentrations; dry mycelium biomass was recorded for optimum growth requirements.
Extracellular enzymatic activities, namely amylase, lipase, cellulase, xylanase, gelatinase, and laccase, were analyzed using standard procedures as follows. Amylase was tested on starch agar; lipase on tributyrin agar, cellulase, xylanase, and gelatinase were tested using 1% substrate, namely carboxy-methylcellulose (CMC), xylan, and gelatin supplemented in yeast-malt agar medium [25]. Laccase was tested in minimal media [26] with slight modification using syringaldehyde (10 ppm after autoclave) as a substrate. The plates were point inoculated and incubated at 25 °C for 7 days. The indicator reagents used for amylase, xylanase, cellulase, and gelatinase, namely, Gram’s iodine solution, absolute ethyl alcohol, iodine solution, and acidic mercuric chloride, respectively for the confirmation of lytic activity by observing clear zone around the colony [25].
Molecular identification of endophytes
The genomic DNA of the fungal isolates was extracted using standard phenol: chloroform method as described previously [27]. The quantity and quality of DNA were checked using Nanodrop One Spectrophotometer (Thermo Scientific, USA) and then run on 0.8% agarose gel. PCR amplification was performed by targeting the internal transcribed spacer (ITS) region using the universal primer set as ITS 1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS 2 (5′-TCCTCCGCTTATTGATATGC-3′) [28]. PCR conditions included initial denaturation at 94 °C for 5 min, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 1 min, extension at 72 °C for 1 min followed by final polymerization at 72 °C for 10 min and hold at 20 °C [29]. The amplified PCR products were purified using the Rapid Tip kit (Diffinity Genomics) and sequenced using the 3730xl Genetic Analyzer (Applied Biosystems, USA). The obtained raw sequences were curated using DNASTAR Seq-Man Pro (https://www.dnastar.com/t-seqmanpro.aspx) as described in Sharma et al. [30]. The taxonomy was assigned using the NCBI database (https://blast.ncbi.nlm.nih.gov/Blast.cgi) and the ITS gene sequences of the phylogenetically closest neighbors were retrieved. The phylogenetic analyses were carried out using the neighbor-joining method [31] with Kimura two-parameter model [32] in MEGA 11 software [33]. The tree topology was evaluated by bootstrap resampling with 1000 replications [34].
Plant growth promoting (PGP) properties of endophytes
PGP traits that contributed directly like phosphate solubilization [35], indole acetic acid [36], siderophore [37], and 1-aminocyclopropane-1-carboxylate (ACC) deaminase using Dworkin and Foster minimal medium with ACC as a nitrogen source [38] were determined qualitatively and quantitatively following standard procedures. Similarly, the indirect PGP traits, namely ammonia production detected by Nessler’s reagent in peptone water media [39] and HCN production was detected by the modified method of Bakker and Shippers [40]. Quantitative assessments of PGP traits were done in detail at three different temperatures (15, 25, and 35 °C) on weekly basis up to 4 weeks.
Phosphate solubilization, phosphatase, phytase, and organic acid production
The quantitative estimation of phosphate solubilization and produced enzymes (phosphatases and phytase) during the process was measured using UV/Visible spectrophotometer (Amersham Biosciences, Ultrospec 200 pro) in National Botanical Research Institute’s Phosphate growth liquid medium (NBRIP) supplemented with the tricalcium phosphate as substrate [35]. The soluble P was quantified by molybdate blue color method [41] and calculated from standard curve of different P concentrations using KH2PO4. The acid and alkaline phosphatase were measured following the procedure cited in Adhikari and Pandey [15] and phytase was measured using phytase screening medium following the method described by Kerovuo et al. [42].
The organic acids, namely acetic acid (SRL Pvt Ltd India), ascorbic and pyruvic acid (HiMedia Pvt Ltd), gluconic, L-malic, α-ketoglutaric, citric, tartaric, oxalic, and succinic acid (Sigma chemical Co), produced during phosphate solubilization, were quantified using high-performance liquid chromatography (Shimadzu, LC-2030 Plus) equipped with Prominence Diode Array Detector (PDA). The analytical method for quantifying these organic acids involved the use of a mobile phase composed of 1 mM sodium sulfate (pH 2.8), adjusted with 1 mM sulfuric acid. The flow rate was set at 0.5 ml/min, and the injection volume was 20 µl. The column temperature was maintained at 25 °C, and the total analysis time was 19 min. Separation was achieved using a reverse phase C18 column (Shimadzu-pack solar, 5 μm, 4.6 × 250 mm). Before analysis, the enzyme extracts including blank (sterilized DDW) were passed through 0.2-μm Whatman membrane filter. The quantification of organic acid was done at 210 nm except ascorbic acid (254 nm) by comparing the retention time and absorption wavelength spectra profiles of each organic acid standard.
Estimation of indole acetic acid (IAA), siderophore, and 1-aminocyclopropane carboxylate (ACC) deaminase
IAA quantification was performed in Tryptone soya broths (Himedia Lab Pvt Ltd), both with and without tryptophan (L-trp), using a spectrophotometer as described by Ahmad et al. [36]. Further to confirm the results, HPLC–PDA analysis was conducted. The mobile phase for IAA determination consisted of a mixture of water (A) and methanol (B) in a 76:24 ratio, with a flow rate of 1 ml/min, resulting in a total run time of 25 min. The linearity of the standard curves was determined based on the correlation coefficient for IAA and tryptophan. Identification and quantification of the compounds were performed by comparing the retention time and absorption wavelength spectra profiles of IAA and tryptophan.
Siderophore production was detected by using Chrome azurol S (CAS) assay as described by Schwyn and Neilands [37] with slight modification (with respect to extract volume). Different chemical nature of siderophore like hydroxamate type was examined by tetrazolium test, catecholates type by Arnow’s test [43], and both hydroxamate and catechol type was examined by FeCl3 test [44].
ACC deaminase activity was measured by the method of Honma and Shimomura [45]. The enzyme activity was measured by the amount of a-ketobutyrate produced by the catalytic activity of ACC deaminase that cleaves ACC into α-ketobutyrate and ammonia. The α-ketobutyrate (µM) produced was determined by comparing the absorbance at 540 nm of a sample to a standard curve of a-ketobutyrate [46].
Fungal inoculation-plant assay
The fungal endophytes possessing PGP potential were tested for their effect on two agriculturally important crops, maize (Zea mays) and black soybean (Glycine max). The seeds of the test crops were washed with tap water followed by surface sterilization by soaking in 4% sodium hypochlorite for 2 min, 70% ethanol for 1 min, and then washed with sterile distilled water four to five times. Fungal pure cultures were grown in PD broth at 25 °C for 7 days. Surface disinfected seeds were immersed in fungal suspension at room temperature for 12 h. For control, seeds were treated in nutrient broth without microbial inoculum.
The pots (size, 9-inch dia) containing soil:sand (3:1) mixture were air-dried, sieved with a 2-mm sieve, and autoclaved twice on alternative days for 1 h. The nutrient characteristics of soil-sand mixture is estimated as follows: pH = 8.6 ± 0.2, moisture content = 17.09 ± 0.7%, organic carbon = 3.2 ± 0.6%, available N = 74.7 ± 3.8 ppm, available P = 0.0011 ± 0.0001 ppm, available K = 27.5 ± 0.63 ppm. The inoculated and uninoculated (control) seeds of test crops were sown in the pots. Following seed germination, the plantlets were inoculated with each fungal suspension. The plants were grown in a greenhouse and irrigated with tap water on regular basis. After 50 days, the plants were uprooted, roots and shoots were separated, and root length, roots and shoots fresh weight and then dry weights (oven-dried at 60 °C for 72 h) were measured.
Extract preparation and antimicrobial activity of fungal endophytes
To prepare the extracts, fungal strains were individually cultured in liquid PD medium for a duration of 14 days at 25 °C. After this incubation, the crude fermentation broth underwent a comprehensive blending process, followed by centrifugation at 4000 rpm for 5 min. The resulting supernatant was extracted three times using an equal volume of ethyl acetate, and the pooled organic phase was then evaporated under reduced pressure. The resultant crude extracts were subsequently dissolved in methanol and stored at a temperature of 4 °C for further experimentation, i.e., antimicrobial assays and GC–MS analysis. The antimicrobial potential of fungal endophytes was assessed following the method described by Fauda et al. [25] with slight modification. The antimicrobial potential of the fungal extract against the bacterial strains, namely Bacillus subtilis (NRRLB-30408), Priestia megaterium (previously known as Bacillus megaterium: MCC 3124), Escherichia coli, and Serratia marcescens (MTCC 4822), was estimated by disc diffusion method described by Dasila and Singh [47].
Detection of bioactive compounds in fungal extract by GC–MS analysis
The GC–MS analysis was conducted at Advanced Instrumentation Research Facility (AIRF) JNU, Delhi. Extracts (1 ml), filtered with a 0.22-micron filter, were used in the GC–MS analysis. The GC–MS analysis was performed using Shimadzu GCMS QP-2010 plus equipment. The column operating conditions included the oven temperature program from 0 to 80 °C at 4 °C/min with a 2-min hold time and from 80 to 290 °C at 10 °C/min with a 17-min hold time, and the final temperature was kept for 20 min. The injector temperature was kept at 260 °C, pressure 81.9 kPa, linear velocity 40.5 cm/s, injected sample volume was 0.3 μl, column flow 1.21 ml min−1, purge flow 3.0 ml min−1, total flow 16.3 ml min−1, split ratio: 10.0 scan mass range of m/z 40–650, interface line temperature 270 °C, and ion source temperature 220 °C. The peak area based on retention time was expressed as percentage composition of the crude extract. Compounds were identified and characterized by comparing mass spectra from the NIST 14 (National Institute of Standards and Technology, USA) and WILEY 8 libraries.
Statistical analysis
Microsoft Excel was used for the calculation of mean and standard error value. SPSS-16 was used for ANOVA with post hoc Duncan’s multiple range test (DMRT) to compare the means and measuring the significant difference between different PGP activities under different temperatures.
Results
Identification and characterization of fungal endophytes
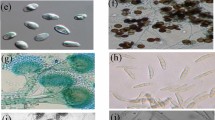
Four fungal endophytes, namely GBPI_beF1, GBPI_beF2, GBPI_beF4, and GBPI_beF5, were isolated from the birch roots (Supplementary Fig. S1A). The morphological characteristics including features like color, vegetative growth, sporulation, pigmentation, and exudation were assessed using various growth media (Supplementary Table S1). Each endophyte produced watery exudates (guttation) only in mycological agar medium (Supplementary Fig. S1B). Specifically, GBPI_beF4 produced pigment when grown in both Mycological and V8 juice agar medium (Supplementary Fig. S1C). Potato dextrose and mycological agar were found to be the best medium for the vegetative growth of all the fungal endophytes. Consequently, PD agar was selected for microscopic analysis, i.e., hyphae septation, sterigmata, conidia, size, and number of conidiophores (Table 1).
Each of the isolated endophytes exhibited psychrotolerant characteristics, demonstrating the ability to thrive at temperature as low as 5 °C. However, their pH requirement for optimal growth varied, spanning from acidic to alkaline conditions. Additionally, the fungal isolates exhibited a notable tolerance to salt indicating halotolerant nature (Table 1). Furthermore, these isolated fungal endophytes exhibited the potential to produce various extracellular enzymes, including amylase, cellulase, xylanase, gelatinase, and laccase lytic activities (Table 1).
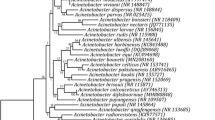
The results of ITS sequencing depicted the fungal isolates belonging to the phylum Ascomycota and were categorized into the families Aspergillaceae, Dermateaceae, and Didymosphaeriaceae. These isolates were further identified as belonging to the genera Penicillium (GBPI_beF1 and GBPI_beF2), Pezicula (GBPI_beF4), and Paraconiothyrium (GBPI_beF5). The phylogenetic analyses of the ITS sequences revealed that isolates GBPI beF1 and GBPI beF2 have highest homology with Penicillium sp. CBS 139.45 T, GBPI beF4 with Pezicula radicicola CBS:640.94 T, and GBPI beF5 with Paraconiothyrium archidendri CBS 168.77 T (Fig. 1).
PGP characters of the fungal endophytes
The direct and indirect PGP activities of fungal endophytes were evaluated. All fungal endophytes were able to produce ammonia while GBPI_beF4 was able to produce hydrogen cyanide only (Table 1 and Supplementary Fig. S2).
Phosphate solubilization, phosphatase, and phytase production
Temperature and incubation period significantly (p < 0.05) affected the P-solubilization, phosphatase, phytase, and organic acid production (Supplementary Table S2). The maximum solubility exhibited by GBPI_beF1 (71.97 ± 0.54 µg/ml) at 35 °C in 4th week, GBPI_beF2 (58.46 ± 1.61 µg/ml) at 15 °C in 2nd week, GBPI_beF4 (95.47 ± 1.41 µg/ml) at 25 °C in 2nd week, and GBPI_beF5 (65.32 ± 0.99 µg/ml) at 15 °C in 3rd week (Supplementary Table S2). Fungal endophytes showed higher acid phosphatase activity as compared to alkaline phosphatase activity and value ranged from 6.59 ± 0.47 to 160 ± 4.40 µM/ml. A decrease in pH was also observed during P-solubilization (Supplementary Table S2). Similarly, fungal endophytes produced phytase in a liquid medium supplemented with two different substrates, namely sodium (Na) and calcium (Ca) phytate. Phytase was produced maximum in medium supplemented with Ca phytate (117.42 ± 4.36 µM/ml by GBPI_beF1 at 15 °C in 3rd week) as compared to Na phytase, i.e., 68.13 ± 0.65 µM/ml by GBPI_beF4 at 35 °C in 4th week (Supplementary Table S2).
The organic acid concentration varied with incubation period and temperature (Supplementary Table S3). Gluconic, α-ketoglutaric, pyruvic, and acetic acid are more frequent and dominant organic acids produced during P-solubilization. However, citric acid was not detected in any fungal extract during solubilization process (Supplementary Table S3).
Indole acetic acid (IAA), siderophore, and 1-aminocyclopropane-1-carboxylate (ACC) deaminase production
The fungal endophytes were able to produce IAA in absence of L-trp; however, IAA synthesis was significantly increased in tryptophan-dependent medium. The weekly quantification of IAA confirms its maximum production in 2nd week of incubation (Fig. 2). IAA production by GBPI_beF1, GBPI_beF2, GBPI_beF4, and GBPI_beF5 ranged from 1.03 ± 0.10 to 31.5 ± 2.04 µg/ml, 1.2 ± 0.09 to 26.6 ± 0.14 µg/ml, 0.1 ± 0.01 to 18.5 ± 0.13 µg/ml, and 0.30 ± 0.02 to 20.2 ± 0.13 µg/ml, respectively. Synthesis of IAA was also confirmed by HPLC–PDA analysis. The absence of L-trp in fungal extract confirmed the complete utilization of substrate for IAA synthesis (Supplementary Fig. S3A–C). CAS assay showed maximum halo zone formation at 25 °C, except in the case of GBPI_beF4. Different chemical nature, i.e., catecholates and hydroxamate of siderophore were also exhibited by fungal endophytes (Supplementary Table S4). Quantitative results of siderophore production showed significant difference (p < 0.05) at different temperatures in liquid CAS assay. Among studied isolates, GBPI_be1 produced the highest siderophore production unit (91%) at 15 °C followed by GBPI_be2 (90%) at 25 °C (Fig. 3). Similarly, ACC deaminase activity also varied significantly (p ≤ 0.001) and GBPI_beF1 exhibited maximum (3.07 ± 0.14 µM/ml) activity while the minimum was recorded by GBPI_beF4 (0.35 ± 0.004 µM/ml) (Table 2).
Effect of fungal endophyte inoculation on test crops
Each fungal endophytic inoculum was found to exhibit significantly positive effect (p < 0.05) on the germination and growth parameters (root length and root and shoot biomass) of soybean and maize (Table 3 and Fig. 4) crops as compared to un-inoculated under net house conditions. GBPI_beF4 inoculum enhanced the seed germination of soybean from 58% (un-inoculated seeds) to 92%, whereas, GBPI_beF2 followed by GBPI_beF4 were recorded to enhance the percent seed germination of maize up to 100 and 95%, respectively.
Antibacterial activity of endophytic fungal extract
Each endophytic fungal extract exhibited significant (p < 0.05) inhibitory potential against tested bacterial pathogens except for E. coli (Fig. 5). The fungal extract exhibited high inhibitory potential against Gram-positive bacteria, i.e., P. megaterium and B. subtilis compared to Gram-negative bacteria, i.e., S. marcescens and E. coli (Table 4). GBPI_beF1 showed the highest inhibition activity against B. subtilis (12 ± 0.6 mm) followed by P. megaterium (11 ± 0.6 mm), S. marcescens (6 ± 0.6 mm), and E. coli (5 ± 0.3 mm). GBPI_beF2 exhibited inhibition in order to P. megaterium (13 ± 0.9 mm), B. subtilis (10 ± 0.7 mm), S. marcescens (6 ± 0.6 mm), and E. coli (6 ± 0.3 mm). GBPI_be4 showed maximum inhibition against B. subtilis (10 ± 0.01 mm) followed by S. marcescens (8 ± 0.3 mm), P. megaterium (7 ± 0.6 mm), and E. coli (5 ± 0.3 mm). GBPI_be5 showed antibacterial activity in order of B. subtilis (8 ± 0.9 mm) > P. megaterium (7 ± 0.6 mm) > S. marcescens (7 ± 0.3 mm) > E. coli (0.3 ± 0.1 mm).
Bioactive compounds in fungal extracts (GC–MS analysis)
To detect bioactive compounds, the 14-day fermented cultures of each fungal endophyte were extracted using ethyl acetate. The analysis of the endophytic fungal extracts revealed the presence of bioactive compounds belonging to various metabolic classes, such as long fatty acid esters, alcohols, ketones, terpenes, and steroids (Supplementary Table S5–S8). The compound with the highest peak area was identified as 1-nonadecene (18%) in the extract of GBPI_beF1, acetophenone (61.06%) in GBPI_beF2, hydroperoxide, 1-methyl-1-phenylethyl (53.42%) in GBPI_beF4, and acetophenone (67.39%) in GBPI_beF5. Additionally, the analysis confirmed the presence of a host signature compound, betulin, in fungal extract (GBPI_beF5).
Discussion
The representative fungal endophytes from Himalayan silver birch roots are two Penicillium species, Pezicula radicicola and Paraconiothyrium archidendri. The present study indicated the limited number of endophytic fungal isolates potentially attributed to the unculturable nature of the colonizing fungi. Microbial species, Penicillium genus stands out as one of the largest fungus group fungi, composed of over 300 well-organized species [48]. Penicillium species are ubiquitous due to their minimal nutritional requirements, adaptability, and versality in different environmental conditions [49]. Penicillium as endophytes has been documented in diverse plant species [50] which have potential to protect the host against biotic stress and plant growth-promoting activities [51]. Notably, Penicillium has also been reported as the most well-known fungal genera for the exploration of numerous bioactive compounds [50]. Interestingly, the other two fungal endophytes, namely Pezicula radicicola and Paraconiothyrium archidendri, are being reported for the first time as endophytes from silver birch growing in the alpine region of IHR. The fungal isolate, P. radicicola, was observed with the production of melanin like dark brown pigment on Mycological and V8 juice medium. It shows the importance of using various media in isolation of culturable microbes. Ingredients like yeast extract, lactose, and tyrosine are known to express melanin pigment production [52]. Melanin is known for its environmental stress resistant nature [52] and the associated virulence factors [53]. Melanin content in the dark brown color pigment produced by P. radicicola requires further investigation. Furthermore, the fungal endophytes showed potential to produce watery exudates in mycological agar medium, commonly known as guttation. Guttation is a common phenomenon that depends on nutrient composition in growth medium, culture conditions, and incubation temperature. These exudate droplets are reported as a rich source of secondary metabolites and proteins with mycotoxin, antiviral, herbicidal, and insecticidal properties [54]. Properties like the production of pigments and exudates are likely to be adaptive strategies against biotic stresses (pathogens and metabolites) and abiotic stresses like temperature, pH, moisture content, and nutrient availability [55, 56].
The low temperature tolerance potential upto 5 °C indicates the psychrotolerant nature of isolated fungal endophytes. The optimal growth temperature for isolated fungal endophytes was recorded at 25 °C. Wang et al. [57] reviewed the living strategies of cold-adapted fungi as saprobes, host mutualists (symbionts), endophytes, parasites, and pathogens to perform ecological functions and adaptation mechanisms. Moghaddam and Soltani [58] recognized the importance of low temperature tolerance in synthesizing cold active secondary metabolites and their contribution to ameliorating cold stress. Li et al. [59] identified 46 taxa of cold adapted fungal endophytes from five dominant plant species, namely Quercus pannosa, Q. spinosa, and three Rhododendron spp. from 4000- to 4300-m altitude of Baima Snow Mountain, Southwest China. Fungal genera belonging to Ascomycetes, Basidiomycetes, and Oomycetes reported for release of antifreeze proteins and support the mycelial growth at extreme cold temperature by controlling the freezing rate of extracellular environment [60]. Wide pH tolerance potential of microbial endophytes of silver birch, from acidophilic to alkaliphilic, is contradictory to the acidic habitat of the host. Dhakar and Pandey [61] reported the adaptation of extremophilic microorganisms to wide pH range and described it as a cope up mechanism against the changing environmental conditions by expression/regulation of the specific genes. Salinity is one of the major challenging abiotic stresses that harshly affect the physiological and metabolic processes of plants. It is responsible for the reduction in seedling growth, photosynthetic activity, ion toxicity, and decrease in protein synthesis rate and lipid metabolism [62]. Endophytes proliferate successively inside the host tissue even at the high salt concentration [63]. Therefore, the salt tolerance potential of root associated microbial endophytes of silver birch may support the species to cope up in extreme climate of the Himalayan region. The fungal endophytes exhibited extracellular enzyme activities like amylase, cellulase, xylanase, gelatinase, and laccase. Enzymes play a significant role in various functional mechanisms in the ecosystem. Lytic activities regulate the organic compound degradation, nutrient acquisition, and elicitation of host defense mechanisms against phytopathogens. In accordance to our results, Toghueo et al. [64] reported cellulase, amylase, and lipase production potential of Penicillium and Paraconiothyrium species on solid media.
Plant growth promoting activities of fungal endophytes
The fungal endophytes support host sustenance in harsh climatic conditions directly via P-solubilization, IAA, and siderophore production and indirectly via ammonia, HCN productivity, ACC deaminase (lowering the ethylene stress), and by producing lytic enzyme activities. Microbial secreted ammonia fulfills nitrogen demand of the host and triggers the defense system against pathogens [51]. The birch fungal endophytes have potential to produce ammonia which may contribute in increase of plant biomass by promoting root and shoot elongation. HCN, a volatile secondary metabolite, suppresses the growth of fungal, insects, termite nematode pathogens, and support plant growth [65]. All the fungal endophytes solubilized tri-calcium phosphate and phytate in liquid medium. Temperature and incubation time showed significant effect on P-solubilization and production of organic acids, phosphatases, and phytase. The significant decrease in pH in each fungal extract indicated the organic acid liberation during P-solubilization. Gluconic and α-ketoglutaric acids were the most frequent organic acids detected in Penicillium species extract while pyruvic acid followed by α-ketoglutaric and gluconic acids were the most frequent organic acids detected in P. radicicola and P. archidendri during P-solubilization. Besides, fungal endophytes produced high acid phosphatase and phytase with lesser extent of alkaline phosphatase and exhibited high potential to solubilize calcium phytate as compared to sodium phytate which may contribute in P- deficit and Ca-rich soil of Himalayan region. Reports on these lines from Himalayan region are limited. Endophytic Penicillium species isolated from T. wallichiana reported for organic acid production and enzymes responsible for P- solubilization under influence of a range of temperature [15]. Temperature has been considered as one of the most critical factors to control various biological processes.
The fungal endophytes exhibited ability to produce IAA in both the culture medium supplemented with tryptophan (0.1%) and without tryptophan (control). Growth hormone like auxins stimulates fungal growth via the germination of spores and growth of hyphae along with cellular elongation. Root-associated microbes are studied for mitigating various stress responses inducing systemic stress tolerance and regulate the hormonal and nutritional balance in plants [66]. While P. radicicola is likely to be the first report for its potential PGP activities including IAA production, IAA production by P. archidendri is comparable with the P. hawaiiense isolated from the leaf sample of wild orchid Vanda cristata from central Nepal [67].
Catecholate and hydroxamate types of siderophores are the most frequent groups involved to bind with iron [68]. Fungal endophytes, under present study, showed the potential to produce different types of siderophores including hydroxamates and catecholates. The high siderophore production abilities of B. utilis endophytes might be due to iron-limiting conditions in high altitude [69]. The siderophore affinity towards other minerals including cobalt, manganese, molybdenum, and nickel has been recognized. This variable affinity of siderophores towards other mineral elements provides competitive benefits to the endophytes over the pathogens/non-producers colonizing or inhabiting in the same ecological niche such as rhizosphere [70]. This aspect of endophytic PGP microbes, associated with high-altitude medicinal plants, has been highlighted by several research groups [3, 18]. Several studies reported that siderophore producing endophytes reduce chlorotic symptoms, increased chlorophyll a and b content, and enhance growth under stress conditions (nickel stress) in inoculated over non-inoculated plants [71, 72].
Production of aminocyclopropane-1-carboxylate by the fungal endophytes may also contribute to reduce cold stress conditions in the plants growing in subalpine zone of Himalayan region. ACC is an immediate precursor of ethylene in plants; therefore, the enzyme, i.e., ACC deaminase, catalyzes ACC to ammonia and α-ketobutyrate [73] resulting growth promotion by lowering ethylene level in the plants [74]. ACCD stimulates plants ACC efflux, decreases the ACC along with ethylene concentration in roots, and thus, enhances the root growth and development [74].
The cumulative effect of fungal PGP potential was successfully demonstrated on two test crops, viz. black soyabean and maize under net house conditions; Pezicula radicicola showed the highest positive effect on growth parameters of black soyabean whereas Penicillium species was more effective in case of maize. These observations support that studied endophytes improve germination and plant growth and may recognize as an environmentally friendly option for possible application in increasing the yield of agricultural crops in hilly regions.
Antibacterial activity and bioactive compounds of culturable endophytic fungal extract
Fungal endophytes are being reported as a vital source of novel bioactive compounds including phenols, flavonoids, alkaloids, peptides, and steroids possessing applications in pharmaceutical and agricultural industries [10]. The disc diffusion results of the fungal extracts confirmed the antimicrobial potential of the fungal endophytes against Gram + ve as well as Gram –ve test bacteria, inhibition being higher in case of Gram + ve bacteria. Similarly, fungal endophytes, isolated from many high-altitude tree species including Eucommia ulmoides [75], Pinus canerensis [76], Juniperus procera [77], Pinus roxburghii [78], and Taxus wallichiana [79], were reported for antimicrobial activities. The GC–MS analysis showed that vast range of bioactive compounds of fungal extracts belonged to various metabolic classes including long fatty acid esters, alcohols, ketones, terpenes, and steroids. Most of the identified compounds are reported for various biological properties including antibacterial, anticancer, antidiabetic, antidiarrheal, antifouling, antifungal, anthelmintic, anti-inflammatory, antimutagenic, antioxidant, antiproliferative, antisepsis, antituberculosis, insecticidal, immunomodulatory, and antiviral. Detection of host specific dominant compound, i.e., betulin is the remarkable finding of this study. It is a pentacyclic triterpenoid compound that has been reported for various pharmaceutical applications like anti-HIV, anti-inflammatory, anticancer, antibacterial, anti-malarial, anti-inflammatory, antihelminthic, antinociceptive, and anti-HSV-1 [80]. The first report on microbial endophyte regarding in vitro secondary metabolites production was the discovery of Paclitaxel (taxol) from endophytic fungus Taxomyces andreanae which was isolated from host species Taxus brevifolia [81]. Similarly endophytic fungi isolated from Sinopodophyllum hexandrum, Diphylleia sinensis, and Dysosma veitchii were reported for the potential to produce host specific compound podophyllotoxin [82]. Earlier, microbe-based successful production of secondary metabolites like quercetin and ginkgolide B having anti-inflammatory and antiallergic potential from Aspergillus species and Fusarium oxysporum endophytes, isolated from Ginkgo biloba, has been reported [83]. Host specific-bioactive compound production by microbial endophytes might be owing to genetic material exchange between host and endophytes because of direct contact, long-term coexistence, and same ecological and climatic inhabitation conditions [84].
Conclusion
To the best of our knowledge, this study represents the inaugural investigation into the PGP and secondary metabolite production properties of culturable endophytic fungal associated with root tissues of B. utilis commonly known as Himalayan silver birch. The observed PGP traits exhibited by these fungal endophytes suggest the potential for developing bioformulations aimed at cultivating robust seedlings of B. utilis in nursery conditions. This development could integrate into biodiversity management strategies, with a particular focus on the conservation of this ecologically and economically significant species within sub-alpine region of Himalaya. Furthermore, these endophytes may find applicability in the inoculation of agricultural and forest species to hilly regions.
The production of the host-specific compound, i.e., betulin from the fungal extract of Paraconiothyrium archidendri, represents a noteworthy and remarkable outcome of this study. The fungus could serve as an alternative source of betulin, presenting an additional avenue for the conservation of B. utilis, which is categorized as critically endangered Himalayan species. To fully harness the potential of this bioactive compound, it will be imperative to optimize the growth conditions for fungal betulin production.
Data availability
The accession numbers MZ613188.1, MN327637.1, MN327638.1, and MN327639.1 correspond to isolates GBPI beF1, GBPI beF2, GBPI beF4, GBPI beF5 respectively and the sequencing data is accessible on NCBI.
References
Vimal SR, Singh JS, Arora NK et al (2017) Soil-plant-microbe interactions in stressed agriculture management: a review. Pedosphere 27:177–192
Vincent D, Rafqi M, Job D (2020) The multiple facets of plant–fungal interactions revealed through plant and fungal secretomics. Front Plant Sci 10:1626
Prathyusha P, Rajitha SAB, Ashokvardhan T (2015) Antimicrobial and siderophore activity of the endophytic fungus Acremonium sclerotigenum inhabiting Terminalia bellerica Roxb. Int J Pharm Sci Rev Res 30:84–87
Yang B, Wang XM, Ma HY et al (2015) Fungal endophyte Phomopsis liquidambari affects nitrogen transformation processes and related microorganisms in the rice rhizosphere. Front Microbiol 6:1–15
Bader AN, Salerno GL, Covacevich F et al (2020) Native Trichoderma harzianum strains from Argentina produce indole-3 acetic acid and phosphorus solubilization, promote growth and control wilt disease on tomato (Solanum lycopersicum L.). J King Saud Univ Sci 32:867–873
Zhang S, Gan Y, Xu B (2019) Mechanisms of the IAA and ACC-deaminase producing strain of Trichoderma longibrachiatum T6 in enhancing wheat seedling tolerance to NaCl stress. BMC Plant Biol 19:1–18
Chanclud E, Morel JB (2016) Plant hormones: a fungal point of view. Mol Plant Pathol 17:1289–1297
Mishra R, Kushveer JS, Revanthbabu P et al (2019) Endophytic fungi and their enzymatic potential. In: Singh BP (ed) Advances in endophytic fungal research: present status and future challenges. Springer Intesrnational Publishing, Cham, pp 283–337
Jia M, Chen L, Xin HL et al (2016) A friendly relationship between endophytic fungi and medicinal plants: a systematic review. Front Microbiol 7:906
Strobel G, Daisy B, Castillo U et al (2004) Natural products from endophytic microorganisms. J Nat Prod 67:257–268
Rodriguez R, Durán P (2020) Natural holobiome engineering by using native extreme microbiome to counteract the climate change effects. Front Bioeng Biotechnol 8:568
Suryanarayanan TS, Shanker RU (2021) Can fungal endophytes fast-track plant adaptations to climate change? Fungal Ecol 50:101039
Rashmi M, Kushveer JS, Sarma VV (2019) A worldwide list of endophytic fungi with notes on ecology and diversity. Mycosphere 10:798–1079
Qadri M, Johri S, Shah BA et al (2013) Identification and bioactive potential of endophytic fungi isolated from selected plants of the Western Himalayas. Springerplus 2:1–14
Adhikari P, Pandey A (2019) Phosphate solubilization potential of endophytic fungi isolated from Taxus wallichiana Zucc roots. Rhizosphere 9:2–9
Fatima N, Kondratyuk TP, Park EJ et al (2016) Endophytic fungi associated with Taxus fuana (West Himalayan Yew) of Pakistan: potential bio-resources for cancer chemopreventive agents. Pharm Biol 54:2547–2554
Yuan Z, Tian Y, He F et al (2019) Endophytes from Ginkgo biloba and their secondary metabolites. Chin Med 14:1–40
Jain R, Bhardwaj P, Pandey SS et al (2021) Arnebia euchroma, a plant species of cold desert in the Himalayas, harbors beneficial cultivable endophytes in roots and leaves. Front Microbiol 12:1–16
Shaw K, Roy S, Wilson B (2014) Betula utilis. The IUCN red list of threatened species 2014:e.T194535A2346136. https://doi.org/10.2305/IUCN.UK.2014-3.RLTS.T194535A2346136.en
Pandey A, Palni LMS (2007) The rhizosphere effect in trees of the Indian Central Himalaya with special reference to altitude. Appl Eco Env Res 5:93–102
Dasila K, Pandey A, Samant SS et al (2020) Endophytes associated with Himalayan silver birch (Betula utilis D. Don) roots in relation to season and soil parameters. Appl Soil Ecol 149:103513
Pandey A, Trivedi P, Kumar B et al (2006) Characterization of a phosphate solubilizing and antagonistic strain of Pseudomonas putida (B0) isolated from a sub-alpine location in the Indian Central Himalaya. Curr Microbiol 53:102–107
Dos Santos RMG, Rodrigues-Fo E, Rocha WC et al (2003) Endophytic fungi from Melia azedarach. World J Microbiol Biotechnol 19:767–770
Woo PC, Ngan AH, Chui HK et al (2010) Agar block smear preparation: a novel method of slide preparation for preservation of native fungal structures for microscopic examination and long-term storage. J Clin Microbiol 48:3053–3061
Fauda AH, Hassan SE, Eid AM et al (2015) Biotechnological applications of fungal endophytes associated with medicinal plant Asclepias sinaica (Bioss). Ann Agric Sci 60:95–104
Dhakar K, Pandey A (2013) Laccase production from a temperature and pH tolerant fungal strain of Trametes hirsuta (MTCC 11397). Enzyme Res 2013:9
Jani K, Bandal J, Rale V et al (2019) Antimicrobial resistance pattern of microorganisms isolated and identified from Godavari River across the mass gathering event. J Biosci 44:1–6
White TJ, Bruns T, Lee SJWT et al (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc: a guide to methods and applications 18:315–322
Kajale SC, Sonawane MS, Sharma R et al (2015) Leptoxyphium kurandae – new record of insect gut associated sooty mold fungus from India. Mycosphere 6:133–138
Sharma A, Jani K, Feng GD et al (2018) Subsaxibacter sediminis sp. nov., isolated from arctic glacial sediment and emended description of the genus Subsaxibacter. Int J Syst Evol Microbiol 68:1678–1682
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Kimura M (1980) A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Tamura K, Stecher G, Kumar S (2021) MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol 38:3022–3027
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Nautiyal CS (1999) An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol Lett 170:265–270
Ahmad F, Ahmad I, Khan MS (2005) Indole acetic acid production by the indigenous isolates of Azotobacter and fluorescent Pseudomonas in the presence and absence of tryptophan. Turk J Biol 29:29–34
Schwyn A, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56
Penrose DM, Glick BR (2003) Methods for isolating and characterizing ACC deaminase containing plant growth promoting rhizobacteria. Physiol Plant 118:10–15
Singh P, Kumar V, Agrawal S (2014) Evaluation of phytase producing bacteria for their plant growth promoting activities. Int J Microbiol 2014:7
Bakker AW, Shippers B (1887) Microbial cyanide production in the rhizosphere in relation to potato yield reduction and Pseudomonas spp. mediated plant growth stimulation. Soil Biol Biochem 19:451–457
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters, Analytica. Chimica Acta 27:31–36
Kerovuo J, Lauraeus M, Nurminen P, Kalkkinen N, Apajalahti J (1998) Isolation, characterization, molecular gene cloning, and sequencing of a novel phytase from Bacillus subtilis. Appl Environ Microbiol 64(6):2079–2085
Arnow LE (1937) Colorimetric determination of the components of 3, 4-dihydroxyphenylalanine–tyrosine mixtures. J Biol Chem 118:531–537
Neilands JB (1981) Microbial iron transport compounds (siderophores) as chelating agents. In: Anderson WF, Badman DG (eds) Martell E. Development of iron chelators for clinical use, Elsevier Amsterdam, pp 13–31
Honma M, Shimomura T (1978) Metabolism of 1-aminocyclopropane-1-carboxylic acid. Agric Biol Chem 42:1825–1831
Jasim B, John Jimtha C, Jyothis M et al (2013) Plant growth promoting potential of endophytic bacteria isolated from Piper nigrum. Plant Growth Regul 71:1–11
Dasila K, Singh M (2022) Bioactive compounds and biological activities of Elaegnus latifolia L.: an underutilized fruit of North-East Himalaya, India. S Afr J Bot 45:177–185
Kirk PM, Cannon PF, Minter DW et al (2008) Dictionary of the fungi Wallingford. CABI, UK, p 335
Dhakar K, Sharma A, Pandey A (2014) Cold, pH and salt tolerant Penicillium spp. inhabit the high-altitude soils in Himalaya, India. World J Microbiol Biotechnol 30:1315–1324
Nicoletti R, Fiorentino A, Scognamiglio M (2014) Endophytism of Penicillium species in woody plants. Open Mycol J 8:1–26
Hassan SED (2017) (2017) Plant growth-promoting activities for bacterial and fungal endophytes isolated from medicinal plant of Teucrium polium L. J Adv Res 8:687–695
Pombeiro-Sponchiado SR, Sousa GS, Andrade JC et al (2017) Production of melanin pigment by fungi and its biotechnological applications. Melanin 1(4):47–75
Perez-Cuesta U, Aparicio-Fernandez L, Guruceaga X et al (2020) Melanin and pyomelanin in Aspergillus fumigatus: from its genetics to host interaction. Int Microbiol 23:55–63
Krain A, Siupka P (2021) Fungal guttation, a source of bioactive compounds, and its ecological role- a review. Biomolecules 11(9):1270
Farrar K, Bryant D, Cope-Selby N (2014) Understanding and engineering beneficial plant–microbe interactions: plant growth promotion in energy crops. Plant Biotechnol J 12:1193–1206
Jhala YK, Panpatte DG, Adetunji CO et al (2020) Management of biotic and abiotic stress affecting agricultural productivity using beneficial microorganisms isolated from higher altitude agro-ecosystems: a remedy for sustainable agriculture. In: Goel R, Soni R, Suyal DH (eds) Microbiological advancements for higher altitude agro-ecosystems & sustainability. Springer, Singapore, pp 113–134
Wang M, Tian J, Xiang M et al (2017) Living strategy of cold-adapted fungi with the reference to several representative species. Mycology 8:178–188
Moghaddam MSH, Soltani J (2014) Psychrophilic endophytic fungi with biological activity inhabit Cupressaceae plant family. Symbiosis 63:79–86
Li HY, Li DW, He CM et al (2012) Diversity and heavy metal tolerance of endophytic fungi from six dominant plant species in a Pb–Zn mine wasteland in China. Fungal Ecol 5:309–315
Hoshino T, Xiao N, Tkachenko OB (2009) Cold adaptation in the phytopathogenic fungi causing snow molds. Mycoscience 50:26–38
Dhakar K, Pandey A (2016) Wide pH range tolerance in extremophiles: towards understanding an important phenomenon for future biotechnology. Appl Microbiol Biotechnol 100:2499–2510
Gupta A, Singh SK, Singh MK et al (2019) Plant growth-promoting rhizobacteria and their functional role in salinity stress management. In: Singh P, Kumar A, Borthakur A (eds). Abatement of environmental pollutants: trends and strategies, Elsevier, Cambridge: MA, USA, 151–60
Bal HB, Nayak L, Das S et al (2013) Isolation of ACC deaminase producing PGPR from rice rhizosphere and evaluating their plant growth promoting activity under salt stress. Plant Soil 366:93–105
Toghueo RMK, Zabalgogeazcoa I, de Aldana BV et al (2017) Enzymatic activity of endophytic fungi from the medicinal plants Terminalia catappa, Terminalia mantaly and Cananga odorata. S Afr J Bot 109:146–153
Choudhary DK, Prakash A, John BN (2007) Induced systemic resistance (ISR) in plants: mechanism of action. Indian J Microbiol 47(4):289–297
Etemadi M, Gutjahr C, Couzigou JM et al (2014) Auxin perception is required for arbuscule development in arbuscular mycorrhizal symbiosis. Plant Physiol 166:281–292
Chand K, Shah S, Sharma J et al (2020) Isolation, characterization, and plant growth-promoting activities of endophytic fungi from a wild orchid Vanda cristata. Plant Signal Behav 15:1744294
Yadav AN (2021) Beneficial plant-microbe interactions for agricultural sustainability. J Appl Biol Biotechnol 9:1–4
Saeed S, Barozai MYK, Ahmad A et al (2014) Impact of altitude on soil physical and chemical properties in SraGhurgai (Takatu mountain range) Quetta, Balochistan. Int J Sci Eng Res 5:730–735
Goswami D, Thakker JN, Dhandhukia PN (2016) Portraying mechanics of plant growth promoting rhizobacteria (PGPR): a review. Cogent Food Agric 2:1127500
Sharma A, Johri B, Sharma A et al (2003) Plant growth-promoting bacterium Pseudomonas sp. strain GRP3 influences iron acquisition in mung bean (Vigna radiata L. Wilzeck). Soil Biol Biochem 35:887–894
Sessitsch A, Kuffner M, Kidd P et al (2013) The role of plant-associated bacteria in the mobilization and phytoextraction of trace elements in contaminated soils. Soil Biol Biochem 60:182–194
Glick BR, Todorovic B, Czarny J et al (2007) Promotion of plant growth by bacterial ACC deaminase. Crit Rev Plant Sci 26:227–242
Hardoim P, Van-Overbeek L, Van-Elsas J (2008) Properties of bacterial endophytes and their proposed role in plant growth. Trends Microbiol 16:463–471
Chen X, Sang X, Li S et al (2010) Studies on a chlorogenic acid-producing endophytic fungi isolated from Eucommia ulmoides Oliver. J Ind Microbiol Biotechnol 37:447–454
Thalavaipandian B, Ramesh V, Arivudainambi USE et al (2012) A novel endophytic fungus Pestalotiopsis sp. inhabiting Pinus caneriensis with antibacterial and antifungal potential. Int J Adv Life Sci 2:1–7
Gherbawy YA, Elhariry HM (2016) Endophytic fungi associated with high-altitude Juniperus trees and their antimicrobial activities. Plant Biosyst 150:131–140
Bhardwaj A, Sharma D, Jadon N et al (2015) Antimicrobial and phytochemical screening of endophytic fungi isolated from spikes of Pinus roxburghii. Arch Clin Microbiol 6:1–9
Gauchan DP, Kandel P, Tuladhar A et al (2020) Evaluation of antimicrobial, antioxidant and cytotoxic properties of bioactive compounds produced from endophytic fungi of Himalayan yew (Taxuswallichiana) in Nepal. F1000Research 9:379
Moghaddam MG, Ahmad FBH, Samzadeh-Kermani A (2012) Biological activity of betulinic acid: a review. Pharmacol Pharm 3:1–5
Stierle A, Strobel G, Stierle D (1993) Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew. Science 260:214–216
Yang X, Guo S, Zhang L et al (2003) Select of producing podophyllotoxin endophytic fungi from podophyllin plant. Nat Prod Res Dev 12:419–422
Cui Y, Yi D, Bai X et al (2012) Ginkgolide B produced endophytic fungus (Fusarium oxysporum) isolated from Ginkgo biloba. Fitoterapia 83:913–920
Wang Y, Dai CC (2011) Endophytes: a potential resource for biosynthesis, biotransformation, and biodegradation. Ann Microbiol 61:207–215
Acknowledgements
Director, G. B. Pant National Institute of Himalayan Environment, India, is acknowledged for providing the facilities. Advance Instrumentation Research Facility (AIRF)- Jawaharlal Nehru University (JNU), India, is acknowledged for extending the GC/MS facilities. Sequencing was performed at Centre for Excellence, National Centre for Microbial Resource, NCCS, Pune.
Funding
The work was supported by the National Mission on Himalayan Studies (NMHS Reference No.: GBPNI/NMHS- 2018/NSMW/SG29).
Author information
Authors and Affiliations
Contributions
KD: conceptualization, methodology, experimentation, analysis, writing; AP: conceptualization, investigation, supervision, validation, review, editing; AS: molecular identification and phylogeny; MS: review; SSS: plant identification and sample collection; MS: review; MS: supervision, review, editing.
Corresponding authors
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors have approved the final version submitted.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Responsible Editor: Luiz Henrique Rosa
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dasila, K., Pandey, A., Sharma, A. et al. Endophytic fungi from Himalayan silver birch as potential source of plant growth enhancement and secondary metabolite production. Braz J Microbiol 55, 557–570 (2024). https://doi.org/10.1007/s42770-024-01259-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-024-01259-4