Abstract
Introduction
Bacteremia is a major cause of morbidity and mortality in hospitalized patients. Predictors of mortality are critical for the management and survival of hospitalized patients. The objective of this study was to determine the factors related to blood culture positivity and the risk factors for mortality in patients whose blood cultures were collected.
Methods
A prospective 2-cohort study (derivation with 784 patients and validation with 380 patients) based on the Pitt bacteremia score for all patients undergoing blood culture collection. The score was obtained from multivariate analysis. The Kaplan–Meier survival curve of the cohort derivation and the cohort validation groups was calculated, and the difference was assessed using a log-rank test. Mortality-related factors were older age, extended hospitalization, > 10% of immature cells in the leukogram, lower mean blood pressure, elevated heart rate, elevated WBC count, and elevated respiratory rate. These continuous variables were dichotomized according to their significance level, and a cut-off limit was created.
Results
The area under the ROC curve (AUC) was 0.789. The score was validated in a group of 380 patients who were prospectively evaluated.
Conclusion
Prolonged hospitalization, body temperature, and elevated heart rate were related to positive blood cultures. The Pitt score can be used to assess the risk of death; however it can be individualized according to the epidemiology of each hospital.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacteremia is a major cause of hospital morbidity and mortality. It is usually associated with a severe event and is characterized by extended hospitalizations and higher hospital costs [1]. The severity of bacteremia can be assessed using both objective and subjective data. Objective methods are obviously more reliable and are generally employed in the form of scores. Some scores, such as the Pitt score and the APACHE II that are available today, are based on conventional clinical and laboratory data. This allows for the prediction of mortality in hospitalized patients with or without bacteremia [2, 3]. Usually, these mortality scores are constructed from derivation cohorts and later validated with a new cohort (validation cohort). Bacteremia severity scores can also be targeted to a specific microorganism, as noted in a recent study evaluating a mortality score in patients with carbapenem-resistant Enterobacteriaceae bacteremia [4]. Unfortunately, several clinical and laboratory data were not included in these scores because the study was based on a specific population. The Pitt score uses clinical and laboratory data to predict mortality. However, there are no studies validating this score in Brazil. The Pitt bacteremia scoring system was recently demonstrated to predict mortality more effectively than the APACHE II among intensive care unit (ICU) patients with sepsis. However, this score was not the aim of our study [5].
Blood culture remains the most critical test for the diagnosis of bacteremia. New molecular methods are available, although these are not realistic for most hospitals [6]. A blood culture result may require over 24 h to identify microbial growth. Antibiotics in sepsis patients must not be delayed by such a length of time [7]. In view of this situation, clinical scores that correlate with blood culture positivity are crucial.
The objective of this study was to determine the factors that were related to blood culture positivity and the risk factors for mortality in patients with suspected bacteremia. We also sought to develop a clinical score of culture positivity and mortality based on data from a middle-income country.
Methods
Study design
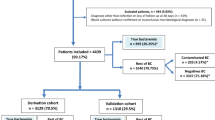
This was a prospective cohort study conducted at a Brazilian university hospital between January 2014 and January 2015 (derivation cohort), followed by a validation period (validation cohort) between February 2015 and January 2016. The study was approved by the Research Ethics Committee (CAAE 42,286,814.6.0000.0103), and all patients signed a consent form to participate in the study. The hospital is in the city of Curitiba, State of Paraná, Southern Brazil. The hospital has 600 beds and 30 adult ICU beds. It is a referral hospital for trauma, burns, and renal transplantation.
Since 2012, the hospital has established that any adult blood sample collected for culture in hospitalized patients outside the ICU required a form to be completed by a physician. This method permitted the blood culture to be performed. The decision to collect a blood sample for culture was determined by the physician. The required form consisted of clinical and laboratory data based on the Pitt bacteremia score [8, 9] (Table 1). In addition, the form contained the justification for the blood culture, previous use of antibiotics, use of antibiotics on the day of the blood sample collection, age, and the length of hospitalization. After completion of the form, the laboratory performed the collection of blood for culture. The routine for blood culture included 10 mL of peripheral blood sample in anaerobe and aerobe bottles using the BACT/ALERT® system (bioMérieux, Durham, NC). Bacteria were identified by phenotypic or automated methods (VITEK 2®, bioMérieux, Durham, NC). Susceptibility tests were performed according to the bacterium species using an automated method (VITEK 2®), disk-diffusion, or E-test (bioMérieux, Durham, NC).
Inclusion and exclusion criteria
The two endpoints analyzed in this study were blood culture positivity and global mortality (hospital mortality). A blood culture was considered positive and included in the study when the same agent was identified in two separate blood cultures, the patient was an adult (> 18 years), and the form was properly completed. False positives due to contamination (e.g., coagulase-negative Staphylococci) were minimized when only patients with suggestive clinical findings, plus two separate blood cultures were included, as previously described. Patients with two positive blood cultures with two different species of coagulase-negative Staphylococci were considered to be contaminated. Each patient was analyzed only once, and patients with two episodes of bacteremia were excluded from the analysis.
Statistical analysis and calculation
Statistical analysis was performed using SPSS 23.0. Continuous data are expressed as means with standard deviation or median, and categorical variables are expressed as percentages. The use of median and mean was defined according to the normality test (Kolmogorov–Smirnov test). A Chi-square test, Fisher’s exact test, and Student’s t test were applied according to the type and quantity of variables. For variables with p < 0.05, a multivariate forward binary regression model was used to evaluate independent factors related to blood culture positivity as well as to mortality. Continuous variables were split before inclusion in the model, using the best cut-off according to the lowest p value. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated to determine the strength of the association. The area under the ROC curve (AUC) was calculated to quantify the discriminative ability of the score. A value of 0.5 denoted random predictions, and a value of 1.0 denoted perfect predictions.
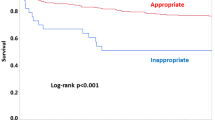
After a statistical analysis of the original data, the score was validated using a prospective group that included 380 patients. For this, a Kaplan–Meier survival curve for the derivation cohort and the validation cohort was constructed, and the difference was assessed using a log-rank test.
Results
Of the 1,048 blood cultures evaluated in the derivation cohort, 784 forms were complete and were from adult patients (> 18 years). Among these, 155 had positive blood cultures (20%), with a predominance of Staphylococcus spp. (44%). Among the cultures positive for Staphylococcus, 23.5% were S. aureus. The frequency of the bacteria identified in the blood cultures are described in Table 2.
The use of antibiotics at the time of blood collection for culture (n = 407, 52%) was a factor associated with negative blood cultures (OR, 0.58; 95% CI, 0.41–0.83; p = 0.003). Recent use of antibiotics (in the past 10 days), except the last 24 h (n = 337, 43%), was not related to blood culture positivity (OR, 0.87; 95% CI, 0.60–1.26; p = 0.47). A positive blood culture did not contribute to patient mortality (odds ratio [OR], 0.82; 95% confidence interval [CI], 0.57–1.16; p = 0.27). The continuous variables are listed in Table 3. Factors related to a positive blood culture were a prolonged hospitalization (with a mean of 15.5 Â days and a median of 8.5 Â days), body temperature (with a mean of 37.4 ºC), and heart rate (with a mean of 101.9 bpm). Considering that only two variables were associated with positive blood cultures (length of hospitalization and mean temperature), it was not possible to develop a blood culture positivity score.
Of the 784 patients included in the analysis, 335 died. The mortality-related factors are listed in Table 4. Significant risk factors were advanced age, an extended hospitalization, > 10% immature cells in the leukogram, lower mean blood pressure, elevated heart rate, elevated WBC count, and elevated respiratory rate. The use of antibiotics in the past 10 days (OR 0.58; 95% CI 0.43–0.79, p < 0.001), as well as the use of antibiotics on the day of collection (OR 0.36; 95% CI 0.27–0.48, p < 0.001) were associated with lower mortality, regardless of the bacterium susceptibility profile, the prescribed antibiotic, or the route of administration.
For the independent variables with p < 0.05, the multivariate model was applied, where the constants used in Eq. (1) were obtained, allowing the creation of a mortality score.
Below is the equation for the \(E\) score:
where I is the age, Ti is the hospitalization time, Pa is the mean blood pressure, Fc is the heart rate, and L is the number of WBCs.
These continuous variables were dichotomized according to their significance level, creating a cut-off limit. The dichotomies resulted in the following mortality-related factors: age > 60 years, length of hospitalization > 5 days, leukocytosis > 12,000/mm3, heart rate > 100 bpm, and mean blood pressure < 81 mmHg with Eq. (1) Subsequently, the scores for each variable were obtained (Table 5). The mortality attributed to each score ranged from 15 to 90%, with 90% for scores > 25 points, as described in Table 6.
To evaluate the efficacy of the score for predicting mortality related to the significant factors mentioned above, the area under the ROC curve (AUC) was calculated to be 0.789 (Fig. 1). The score was validated in a group of 380 patients who were prospectively evaluated. In this group all factors had a p value < 0.001 in the multivariate analysis, except for the length of hospitalization. The survival curves between the validation and derivation groups were similar (Fig. 2).
Discussion
According to our findings, factors related to positive blood cultures were prolonged hospitalization time, body temperature, and heart rate. Patients with suspected bacteremia with age > 60 years, time of hospitalization > 5 days, leukocytosis > 12.000 cells/mm3, heart rate > 100 bpm, and mean blood pressure < 81 mmHg were at increased risk of death and had an elevated Pitt score. When considering the mortality score from our data, age > 60 years was the most important factor related to a poor prognosis.
One of the methods to assess the severity of patients with bacteremia is by using tools that provide scores. The Pitt score reflects the severity of patients with bacteremia. The parameters necessary to calculate the score are vital signs, mental status, requirement for mechanical ventilation, and recent cardiac arrest. According to the literature, Pitt scores higher than 4 are considered indicative of severe bacteremia and suggest an increase in the relative risk of death [10]. Nevertheless, even in non-bacteremic patients, the traditional Pitt score and/or quick Pitt score (qPitt) has been validated [11]. Thus, the Pitt score remains a useful tool for predicting mortality in septic patients independent of blood culture results.
Compared with the Pitt score, the Acute Physiology and Chronic Health Evaluation II (APACHE II) has an inferior ability to estimate the sensitivity and specificity that predicts mortality among ICU patients with sepsis. The APACHE II is a general ICU score and is not specific to septic/bacteremic patients [5]. The variables used in the Pitt score were similar to our score. However, when the factors are pooled, various weights are presented, which may refine the analysis. This newly adapted score could be validated, thus demonstrating a strong correlation between the groups in the survival curve.
The results of this study justify changes to the blood culture collection form. Laboratory and clinical data must be adapted to the circumstances of the institution. It is possible that this is also necessary for other hospitals. Various factors must be considered including the patient setting, the microbiological profile, and the issues related to the proper management of sepsis. These factors can vary significantly between institutions.
Despite the internal validation, this mortality score cannot be extrapolated to other institutions without external validation or multicenter studies. Another limitation has previously been described, which is to attribute mortality only to baseline conditions. This may be modifiable variables with appropriate management, such as hypotension. Although it was not the objective of this study, it is worth noting that the rate of positivity of blood cultures in this study is superior to that of the literature. This is most likely due to the presence of the specific method of our study, encouraging the physician to collect blood culture only in specific indications, avoiding samples in patients with mild infection and low pre-test probability. The advantage is a lower cost; however there may be cases of bacteremia that are not diagnosed. Silva et al. evaluated 4,214 blood cultures and found that 93.4% were negative [12]. Our study demonstrated the predominance of bacteremia caused by Staphylococcus spp., with 22% being S. aureus. This profile is similar to that of several hospitals in various countries [13]. Although a gram-negative bacilli such as E. coli may predominate in other regions [14], a study conducted in another developing country showed the same profile as our study, with a predominance of Staphylococcus spp. [15]. Unfortunately, in our institution we do not used the “incubation time to detection” in the automated system to distinguish coagulase-negative staphylococcal contamination from infection. This approach could increase the percentage of coagulase-negative Staphylococcus bacteremia in our sample. The time-to-positivity is an useful adjunctive test to determine the clinical significance of isolation of coagulase-negative from a blood culture [16].
It was observed that patients taking antibiotics and those who took antibiotics in the past 10 days exhibited a significant reduction in overall mortality when compared to patients who did not take antibiotics. Antibiotic therapy not only reduces mortality by treating the initial infection, but also reduces the occurrence of new infections in the respiratory tract. Consequently, this reduces global mortality [17]. However, the antimicrobial approach must be used with caution and must be culture-driven in order to combat the emergence of bacterial resistance, improve clinical outcomes, and control costs [18]. Although the previous use of antibiotics in the past 10 days prior to collection proved to be relevant for reducing mortality, it did not influence the results of the blood cultures. Patients receiving antibiotics on the day of collection had a lower blood culture positivity rate.
Blood cultures should preferably be collected prior to the initiation of antibiotic therapy to avoid interference with bacterial growth and consequent false negatives [19]. However, the dilution of antimicrobials in the culture medium may result in insufficient concentrations to inhibit bacterial growth. Thus, this allows cultures to be collected even when antibiotics have previously been employed [19]. A positive blood culture was associated with prolonged hospitalization, fever, and tachycardia. Previsdomini et al. demonstrated greater positivity in patients with longer hospitalizations and temperatures higher than 38.5 °C [20].
The mortality score presented in this study was beneficial, and we believe that its’ implementation is important for continuous surveillance and greater validation of the results. The application of this score can be associated with cost minimization, avoidance of unnecessary blood cultures, adverse events due to blood collection, and unwarranted treatment of positive cultures without clinical significance. Sogaard et al. evaluated patients with bacteremia, stratified by age group over 30 days, and found that patients over 65 years were at higher risk of death [21]. This is very similar to our study. It is worth mentioning that five of the factors related to mortality in our study correspond to the criteria of systemic inflammatory response syndrome. We emphasize that age > 60 years represents the highest scoring factor followed by tachycardia. It is also possible to establish a relationship between the new mortality score offered in the present study with the local hospital situation using the Pitt score.
In conclusion, this study failed to present a score to predict blood culture positivity. However, the mortality score was beneficial. A prospective study is under development at another institution to provide external validation of the presented score considering our results.
Data availability
Data are available on demand.
Change history
16 January 2022
ORCiD for Dr. Raquel Zanella Bertoldo was removed.
References
Brooks D, Smith A, Young D, Fulton R, Booth MG (2016) Mortality in intensive care: the impact of bacteremia and the utility of systemic inflammatory response syndrome. Am J Infect Control 44(11):1291–1295
Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) APACHE II: a severity of disease classification system. Crit Care Med 13(10):818–829
Hilf M, Yu VL, Sharp J, Zuravleff JJ, Korvick JA, Muder RR (1989) Antibiotic therapy for Pseudomonas aeruginosa bacteremia: outcome correlations in a prospective study of 200 patients. Am J Med 87(5):540–546
Gutierrez-Gutierrez B, Salamanca E, de Cueto M, Hsueh PR, Viale P, Pano-Pardo JR et al (2016) A predictive model of mortality in patients with bloodstream infections due to carbapenemase-producing enterobacteriaceae. Mayo Clin Proc 91(10):1362–1371
Rhee JY, Kwon KT, Ki HK, Shin SY, Jung DS, Chung DR et al (2009) Scoring systems for prediction of mortality in patients with intensive care unit-acquired sepsis: a comparison of the Pitt bacteremia score and the acute physiology and chronic health evaluation iI scoring systems. Shock 31(2):146–150
Martinez RM, Wolk DM (2016) Bloodstream infections. Microbiol Spectr 4(4):1–34
Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S et al (2006) Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 34(6):1589–1596
Chow JW, Fine MJ, Shlaes DM, Quinn JP, Hooper DC, Johnson MP et al (1991) Enterobacter bacteremia: clinical features and emergence of antibiotic resistance during therapy. Ann Intern Med 115(8):585–590
Chow JW, Yu VL (1999) Combination antibiotic therapy versus monotherapy for gram-negative bacteraemia: a commentary. Int J Antimicrob Agents 11(1):7–12
Forsblom E, Aittoniemi J, Ruotsalainen E, Helmijoki V, Huttunen R, Jylhava J et al (2014) High cell-free DNA predicts fatal outcome among Staphylococcus aureus bacteraemia patients with intensive care unit treatment. PLoS One. 9(2):e87741
Henderson H, Luterbach CL, Cober E, Richter SS, Salata RA, Kalayjian RC et al (2020) The Pitt bacteremia score predicts mortality in non-bacteremic infections. Clin Infect Dis. 70(9):1826–1833
Silva CS, Sena KX, Chiapetta AA, Queiroz MM, VIlar MC, Coutinho HM (2006) Incidência Bacteriana em Hemoculturas. NewsLab 77:12
Martin GS, Mannino DM, Eaton S, Moss M (2003) The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 348(16):1546–1554
Buetti N, Marschall J, Atkinson A, Kronenberg A, Swiss Centre for Antibiotic R (2016) National bloodstream infection surveillance in Switzerland 2008–2014: different patterns and trends for university and community hospitals. Infect Control Hosp Epidemiol 37(9):1060–7
Wasihun AG, Wlekidan LN, Gebremariam SA, Dejene TA, Welderufael AL, Haile TD et al (2015) Bacteriological profile and antimicrobial susceptibility patterns of blood culture isolates among febrile patients in Mekelle Hospital. Northern Ethiopia Springerplus 4:314
Haimi-Cohen Y, Shafinoori S, Tucci V, Rubin LG (2003) Use of incubation time to detection in BACTEC 9240 to distinguish coagulase-negative staphylococcal contamination from infection in pediatric blood cultures. Pediatr Infect Dis J 22(11):968–974
Liberati A, D’Amico R, Pifferi S, Torri V, Brazzi L, Parmelli E (2009) Antibiotic prophylaxis to reduce respiratory tract infections and mortality in adults receiving intensive care. Cochrane Database Syst Rev. 4:CD000022
Lawrence KL, Kollef MH (2009) Antimicrobial stewardship in the intensive care unit: advances and obstacles. Am J Respir Crit Care Med 179(6):434–438
Schermer CR, Sanchez DP, Qualls CR, Demarest GB, Albrecht RM, Fry DE (2002) Blood culturing practices in a trauma intensive care unit: does concurrent antibiotic use make a difference? J Trauma 52(3):463–468
Previsdomini M, Gini M, Cerutti B, Dolina M, Perren A (2012) Predictors of positive blood cultures in critically ill patients: a retrospective evaluation. Croat Med J 53(1):30–39
Sogaard M, Schonheyder HC, Riis A, Sorensen HT, Norgaard M (2008) Short-term mortality in relation to age and comorbidity in older adults with community-acquired bacteremia: a population-based cohort study. J Am Geriatr Soc 56(9):1593–1600
Author information
Authors and Affiliations
Contributions
FFT, conceptualization and final review; JPT, data analysis and draft manuscript; MBB, data evaluation and draft; RZB, data evaluation and draft; JC, database organization and analysis; VTR, draft review.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the local ethical committee.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Afonso Luis Barth
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tuon, F.F., Telles, J.P., Cieslinski, J. et al. Development and validation of a risk score for predicting positivity of blood cultures and mortality in patients with bacteremia and fungemia. Braz J Microbiol 52, 1865–1871 (2021). https://doi.org/10.1007/s42770-021-00581-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-021-00581-5