Abstract
Heterologous hosts are highly important to detect the expression of biosynthetic gene clusters that are cryptic or poorly expressed in their natural hosts. To investigate whether actinorhodin-overproducer Streptomyces coelicolor ∆ppk mutant strain could be a possible prototype as a heterologous expression host, a cosmid containing most of the elm gene cluster of Streptomyces olivaceus Tü2353 was integrated into chromosomes of both S. coelicolor A3(2) and ∆ppk strains. Interestingly, it was found that the production of tetracyclic polyketide 8-demethyl-tetracenomycin (8-DMTC) by recombinant strains caused significant changes in the morphology of cells. All the pellets and clumps were disentangled and mycelia were fragmented in the recombinant strains. Moreover, they produce neither pigmented antibiotics nor agarase and did not sporulate. By eliminating the elm biosynthesis genes from the cosmid, we showed that the morphological properties of recombinants were caused by the production of 8-DMTC. Extracellular application of 8-DMTC on S. coelicolor wild-type cells caused a similar phenotype with the 8-DMTC-producing recombinant strains. The results of this study may contribute to the understanding of the effect of 8-DMTC in Streptomyces since the morphological changes that we have observed have not been reported before. It is also valuable in that it provides useful information about the use of Streptomyces as hosts for the heterologous expression of 8-DMTC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Streptomyces family takes an important place in medicine, biotechnology, and pharmaceutical area because of their ability to produce highly active secondary metabolites including two-thirds of the known antibiotics, pigments, herbicides, anti-tumor, and anti-inflammatory agents [1]. Secondary metabolite production is a well-timed process that is triggered by differentiation and correlated with growth throughout the complex life cycle of Streptomycetes. In liquid culture, favorable conditions trigger the growth of vegetative mycelium (MI) from the spore and expansion with polar growth unlike most unicellular bacteria [2]. During exponential growth, vegetative hyphae form branches made of compartments that are separated by peptidoglycan cross-walls and also by cross-membranes without peptidoglycan [3]. These extended vegetative hyphae form pellets whose centers are going to suffer from the first round of programmed cell death (PCD) as a next step [4]. Some compartments of vegetative mycelia die off via PCD and then both the viable and dead compartments stay in the same hyphae [5]. Furthermore, alive compartments that survived from the first round of PCD differentiate into multinucleated, secondary metabolite-producing MII mycelia as the second stage [1]. Streptomyces strains produce pellets and clumps in liquid cultures but they generally cannot differentiate into aerial mycelia (late MII) or sporulate in liquid media, although there are exceptions such as Streptomyces venezuelae [6].
On solid media, aerial mycelium (late MII) grows through the air with the help of a hydrophobic protein layer that allows it to grow out of the aqueous environment [7]. Aerial hyphae undergo the second round of the PCD and then the remaining viable multinucleated compartments divide into several uninucleoid segments that will differentiate into spore chains as the third stage. Figure 1 summarizes the life cycle of Streptomyces on solid media. In short, even though the molecular triggers and functions of the PCD mechanism in Streptomycetes have not been illuminated completely, it is crystal clear that these PCD rounds play a key role in the differentiation process of the life cycle and exhibit an undeniable sign of bacterial multicellularity under the name of Streptomycetes mycelium [5].
Studies have shown that the Streptomyces genome has many cryptic biosynthetic gene clusters (BGCs) which have a great possibility to synthesize new drugs and agents. However, these BGCs usually cannot be expressed or detected in their natural hosts under standard fermentation conditions; therefore, they need to be activated by using different methods such as changing cultivation conditions, inducing stress response, and genetic manipulations (expression of BGCs in heterologous hosts, random mutagenesis, regulation of gene expression, ribosomal engineering, using elicitors) [8, 9]. The heterologous expression is one of the most efficient ways among the activation methods of BGCs.
Many groups have preferred Streptomyces as heterologous hosts for the production of specific secondary metabolites, such as Streptomyces albus J1074 for fredericamycin [7], Streptomyces lividans 1326 for capreomycin [10] and viomycin [11], and a deletion mutant of Streptomyces avermitilis (SUKA) for streptomycin production [12]. S. coelicolor is one of the most popular options among heterologous hosts since it has been well-studied to date and its regulatory mechanisms are mostly understood. Furthermore, large gene clusters can be inserted into its genome and the expression of these clusters can be controlled by defined genetic tools (promotors, activators, etc.) [13]. In addition to wild-type S. coelicolor strain, many genetically developed S. coelicolor strains have been constructed in previous studies such as S. coelicolor CH999 for producing polyketides [14], S. coelicolor M512 for the regulation of actinorhodin and prodiginine production [15], S. coelicolor for the erythronolide PKS [14], S. coelicolor 1146 for production of congocidine [16].
In this study, tetracyclic polyketide 8-demethyl-tetracenomycin (8-DMTC) was heterologously expressed in both S. coelicolor A3(2) wild type and S. coelicolor A3(2) ∆ppk strains and the effect of this agent on the life cycle of Streptomyces was determined. S. coelicolor ∆ppk strain cannot synthesize polyphosphate kinase enzyme (PPK) which catalyzes the biosynthesis of polyphosphate polymer [17]. 8-DMTC inhibits the proliferation of murine L1210 leukemia cells as an anti-tumor agent [18]. It also acts against the Gr+ bacteria by intercalating with their DNA, although the mechanism of the antibacterial activity has not been elucidated in detail yet [18,19,20].
Materials and methods
Microorganisms and growth conditions
The bacteria used in this study are shown in Table 1. Streptomyces strains were grown at 30 °C in R2YE, MS, and TBO media, and E. coli strains were grown at 30 °C or 37 °C in Luria broth and Luria agar. E. coli ET12567/pUZ8002 was used to transfer cosmid DNA to Streptomyces by conjugation. After conjugation, wild-type cells were grown on MS+MgCl2 agar while ∆ppk mutant cells were grown on R2YE plates since they do not grow on MS agar. In order to normalize the inoculation size between strains, we used equal amount of mycelium since recombinant strains carrying cos16F4ie cosmid were not able to sporulate. Specific antibiotics were used to select the cos16F4ie recombinants. Although cos16F4ie carries apramycin, erythromycin, and tetracycline resistance genes, we only used apramycin (50 μg/ml) and erythromycin (50 μg/ml) as selective agents since our experiments showed that tetracycline antibiotic cannot inhibit the growth of wild-type S. coelicolor on agar. Nalidixic acid (25 μg/ml) was used to inhibit E. coli cells after conjugation.
Plasmids, cosmids, and DNA methods
Cosmids and plasmids used in this study are listed in Table 2. cos16F4ie cosmid was a kind gift from Dr. Jose A. Salas and Dr. Carmen Mendez (Universidad de Oviedo, Spain). Cosmid and plasmid isolations, restriction enzyme digestions, and transformation of E. coli were performed according to Sambrook et al. [25]. Primers used in the PCR reactions were prepared according to the elmMII gene (forward primer: 5′TTCCTCGTCCATGACCTCAC3 ′; reverse primer: 5′TCATACGTAGTCGATCTC3′) present on the cos16F4ie cosmid. Chromosomal DNA isolation from Streptomyces cells, transformation of Streptomyces, and intergeneric conjugation with E. coli were carried out according to Kieser et al. [26].
Detection and characterization of 8-DMTC
For the detection of 8-DMTC, high-performance liquid chromatography (HPLC) analysis was optimized and performed according to the study of Heide et al. (2006) [27]. Wild-type, ∆ppk mutant, and their recombinants harboring cos16F4ie cosmid were cultured in 100 ml R2YE at 30 °C, at 180 rpm for 48 h. Then, 5 ml of these pre-cultures was used to inoculate 100 ml fresh R2YE and incubated at 30 °C, at 180 rpm for 5 days. At the end of incubation, 15 ml of each culture was centrifuged and their supernatants were adjusted to pH 3.5 with formic acid and extracted with 5 ml ethyl acetate. C18 Inertsil ODS-3V (4.6 × 250 mm, 5 μm, GL Sciences) column was used to detect the presence of 8-DMTC in the extracts. In the elution program, a linear gradient from 25 to 100% acetonitrile as solvent B in 0.1% phosphoric acid (in water) as solvent A was followed at a flow rate of 1 ml/min for 28 min. UV detection of the peaks was performed at 386 nm [27].
Confocal laser scanning microscopy analysis
To prepare the samples for confocal analysis, the LIVE/DEAD Bac-Light Bacterial Viability Kit (L-7012) was used. One milliliter of the culture was centrifuged at 13,000 rpm for 5 min. After discarding the supernatant, cells were washed with 1 ml dH2O twice to get rid of any medium leftover that might interact with nucleic acid stains. Meanwhile, a dye mix made of 1.5 μl of each fluorescent nucleic acid stain (SYTO 9-green, propidium iodide (PI)-red) in 1 ml dH2O was prepared. The non-permeable nucleic acid stain PI could label only dead cells containing a damaged membrane, while permeable SYTO 9 fluorescent nucleic acid dye could label both live cells containing an intact membrane and dead cells containing a damaged membrane. Since PI has a higher nucleic acid affinity than SYTO 9, it causes a reduction in SYTO 9 stain when it enters the dead cells through damaged membranes. After centrifugation, the pellet was dissolved with 200 μl of the dye mixture and incubated in the dark at least for 10 min. Twenty microliters of the mix was deposited on a clean glass slide and observed under the confocal microscope, at a wavelength 488 and 568 excitation and 530 (green) or 630 (red) emission.
Results
Potential of ∆ppk strain as a host for heterologous expression
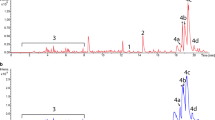
elm gene cluster of Streptomyces olivaceus Tü2353 [18] was used to determine the potential of S. coelicolor ∆ppk strain as a host for heterologous expression. The cos16F4ie cosmid harbors most of not only the elm gene cluster but also ΦC31 integrase and attP for integration into the attB site of the Streptomyces chromosomal DNA and oriT region for conjugal transfer from E. coli. This cosmid was first transferred into methylation deficient E. coli ET12567/pUZ8002 cells, and the presence of cosmid in transformants was confirmed via PCR (Fig. 2a).
Confirmation of E. coli+cos16F4ie transformants with PCR by using elmMII (1053 bp) gene specific primers (a). Comparison of HPLC chromatograms of S. coelicolor A3(2) wild type/wild type + cos16F4ie (b), ∆ppk/∆ppk + cos16F4ie (c), wild type + cos16F4ie/∆ppk + cos16F4ie (d). The red arrow shows 8-DMTC with 4.8-min retention time
Then, one of the E. coli transformants was used to transfer integrative cosmid cos16F4ie into S. coelicolor A3(2) and ∆ppk mutant strains via conjugation. Production of 8-DMTC by the recombinant strains harboring cos16F4ie was seen in HPLC chromatograms (Fig. 2b, c). As we can see in Fig. 2d, ∆ppk + cos16F4ie, and wild type + cos16F4ie produce nearly the same amount of 8-DMTC. The slight increase in 8-DMTC production in ∆ppk + cos16F4ie strain might be negligible. So, it is possible to say that ∆ppk strain was not better than wild strain for heterologous expression of 8-DMTC. Interestingly, while carrying out our experiments, we realized that none of the recombinants produces any actinorhodin in R2YE broth (Fig. 3a, b) or on R2YE agar (Fig. 3c). So we wanted to analyze the recombinant strains in detail by using a confocal microscope.
Confocal analysis of recombinant strains expressing 8-DMTC
For confocal analysis, dead cells containing a damaged membrane were labeled with nucleic acid stain PI (red) and alive cells containing an intact membrane were labeled by SYTO 9 (green). Wild-type cultures show the properties of a classical Streptomyces life cycle (Fig. 4a). After 24-h incubation, the control cultures have already passed through the first programmed cell death round and then differentiated into secondary mycelia as we can see the non-compartmentalized hyphae spreading out of the colony center. The center of the colony is stained red with PI as expected since the death round starts in the center. The rest of the colony is alive at this hour of growth. Through the incubation, the dead cell rate is increasing until 72 h. Then, DNA of the dead segments degraded, and alive cells start to arise again thus increasing the viability of the colonies until 96 h. After that, the dead cell rate increases again so that, at the end of 120-h incubation, most of the culture consists of dead mycelia segments. During this 120-h life cycle, actinorhodin production correlates with secondary mycelia differentiation as expected.
On the other hand, the confocal images of wild type containing cos16F4ie in its genome show a completely different morphology as can be seen in Fig. 4b. The colony structure is totally disrupted in all recombinant strains with cos16F4ie. All mycelia are disentangled and fragmented hence causing a lack of pellet and clump formation in the cultures. Moreover, these short fragments of mycelia cannot be distinguished whether they are primary or secondary since they do not show any specific properties. The morphology of the recombinant culture does not correlate with the life cycle nor the incubation time. Dead and live fragments are randomly present in the cultures, independent from incubation time.
The general morphology of ∆ppk colonies displays the classical life cycle characteristics with an intact colony formation (Fig. 4c). The central area of the colony consists of dead cells, while the outer zone is formed from alive mycelia at 24 and 48 h. The death rate is increasing until 72 h, then decreasing again until 120 h. Alive cells arise from dead segments between 72 and 120 h. The only difference between the morphologies of wild-type and ∆ppk strain is the spread of mycelia around the ∆ppk colonies. When we check the cos16F4ie recombinants of the ∆ppk strain, the morphology is the same as the cos16F4ie harboring wild-type strain (Fig. 4d). They are deficient in colony formation and mycelia continuity. Dismantled mycelia parts consist of dead and alive cells without any correlation with incubation intervals.
Thus far, our findings showed that cos16F4ie recombinants display atypical morphology with the fragmentation of mycelia and lack of pellet formation during incubation from 24 to 120 h. However, we did not examine the cultures with CLSM within the first 24 h. So, our findings do not explain yet whether disentanglement happens after the colonies have been formed within the first 24 h or that the recombinants are not capable of forming colony structure at all. Therefore, confocal analysis of A3(2) wild type and its recombinant strain was performed for 24 h at 6-h intervals. Figure 5a shows wild-type colony morphology during 24-h incubation. In the first image, the colony suffers from the first round of programmed cell death. After the first PCD round, the viable compartments of the primary mycelia come together and transform into the long segmented, multinucleated secondary mycelia. The first image shows a snap of this transition process at the 6th hour. The dead cell rate in the colony is high because of the PCD. Both primary and secondary mycelia exist in the colony at the same hour. At 12 h, the dead cell rate has decreased since the dead parts of the colony have been used as a nutrient and genetic material source for the formation of new ones. There are fewer primary mycelia in the colony at 12 h, most of the colony consists of secondary mycelia. At 18 h, the center of the colony is dead while the outer zone is alive with secondary mycelia. Finally, the colony at 24 h mostly consists of viable secondary mycelia. On the other hand, the confocal images of the recombinant samples do not show any sign of pellet and clump formation (Fig. 5b).
According to all these findings, we can conclude that the recombinant strains exhibit a completely different morphology than S. coelicolor WT and ∆ppk. 8-DMTC-producing recombinant strains do not form colonies, do not exhibit the cell cycle steps, do not produce colored antibiotics (actinorhodin and undecylprodigiosin), and do not have agarase activity (data not shown). They have fragmented mycelia during the all growth cycle. Furthermore, the inoculation of the recombinant cells on the TBO agar shows that these strains cannot sporulate at all on this medium (data not shown).
Construction of cos16F4ie/∆elm
All recombinant strains displayed the same morphological changes in the name of colony structure, lack of pellets, and clumps. We wanted to investigate whether the reason for this altered morphology is the insertion of the cosmid into the genome or the expression of the elm cluster. For this purpose, the elm cluster was deleted from cos16F4ie by cutting it with BamHI restriction enzyme since the elm cluster (24.2 kb) had been cloned into the unique BamHI site in PKC505 as noted earlier. Moreover, the elm cluster has several BamHI restriction sites inside. So that, when we digested cos16F4ie with the BamHI, the elm cluster was digested at many sites and cut out from cos16F4ie while the rest of the cosmid (̴22 kb) was linearized (Fig. 6a). The ̴22-kb band was obtained from agarose gel and then self-ligated. E. coli DH5α cells were transformed with the ligation products. The possible transformants were confirmed with cosmid isolation, and then BamHI digestion (Fig. 6b). Then, E. coli ET12567/pUZ8002 cells were transformed with cos16F4ie/∆elm and again confirmed with BamHI digestion (Fig. 6c). Finally, cos16F4ie/∆elm was transferred to S. coelicolor A3(2) cells via conjugation with E. coli ET12567+cos16F4ie/∆elm.
Confirmation of E. coli DH5α+cos16F4ie/∆elm transformants. BamHI digestion of cos16F4ie (a); cos16F4ie/∆elm (*: ̴22 kb) isolated from DH5α transformants (b). Confirmation of E. coli ET12567/pUZ8002+cos16F4ie/∆elm transformants with BamHI digestion (c). *:cos16F4ie/∆elm ( ̴22 kb), M: Lambda DNA/HindIII Marker
Confocal analysis of S. coelicolor + cos16F4ie/∆elm recombinants
S. coelicolor A3(2) wild type and wild type + cos16F4ie/∆elm cells were analyzed by confocal microscope to examine the effect of elm deletion on the colony morphology of recombinants. For analysis, 24th hour was selected since a whole colony structure with a dead central part and mostly alive secondary hyphae could be seen in wild-type cultures at this hour. As can be seen in Fig. 7a, cos16F4ie/∆elm culture is colored indicating that it produced pigmented antibiotics as wild type did. cos16F4ie/∆elm colonies exhibited the same morphological characteristics as wild-type colonies. The first round of programmed cell death has already occurred since we can see dead central part and alive secondary mycelia (Fig. 7b). More importantly, cos16F4ie/∆elm culture forms pellets, clumps, and wild-type-like colony formation. Its mycelia are not fragmented at all as we can see in Fig. 7c, the area between the separate colonies was clean. Moreover, recombinant cells showed agarase activity on R2YE agar and sporulated on TBO agar (data not shown). These findings prove that the fragmentation of mycelia and lack of colony formation of recombinant cultures were all caused by the expression of the elm cluster in cos16F4ie, not the insertion of the cosmid into the genome.
Treatment of wild-type S. coelicolor A3(2) strain with 8-DMTC-containing supernatant
Our results showed that the expression of the elm cluster causes a deficiency in colony structure and clump-pellet formation in S. coelicolor. We wanted to determine the effect of the supernatant that contains 8-DMTC on S. coelicolor A3(2) wild type. Therefore, S. coelicolor A3(2) wild-type cells were treated by 8-DMTC-containing supernatant of the recombinant (S. coelicolor A3(2) + cos16F4ie) culture at the beginning of fermentation. At one trial, the supernatant of the recombinant strain was filtered to prevent any cell transfer from the recombinant culture. However, there was no difference between the effects of filtered or non-filtered supernatants after 24-h incubation (Fig. 8c, d, f, g).
Effect of supernatant that contains 8-DMTC on S. coelicolor A3(2) morphological differentiation in R2YE broth. Macroscopic view of actinorhodin (blue color) production of control and 8-DMTC-treated culture at 24-h (a); CLSM analysis of S. coelicolor A3(2) (b, e); treated with filtered supernatant containing 8-DMTC (c, f) and treated with non-filtered supernatant containing 8-DMTC (d, g). White arrows indicate the compartments of primary mycelia
Firstly, the color of the cultures in the flask was checked for actinorhodin production (Fig. 8a). The control wild-type sample was dark blue as a sign of high colored antibiotic production. However, 8-DMTC-treated samples were slightly colored which means the 8-DMTC compound decreased the production levels of pigmented antibiotics. It is known that the production of actinorhodin by secondary mycelial cells is directly proportional to the pellet structure and even the size. As explained in detail below, it is possible that as 8-DMTC slows down the growth rate and prevents pellet formation, antibiotic production has also decreased.
The detailed confocal analysis showed that the wild-type sample grows well and forms many colonies (Fig. 8b). The mycelia of these colonies are secondary as we expected to see at the 24th hour. The long compartments are signified as secondary mycelia. There are very few disconnected mycelia between colonies (Fig. 8e). On the other hand, the samples treated with supernatant with 8-DMTC exhibit a different profile. There are fewer colonies in the sample, and the mycelia of these colonies are primary mycelia at the 24th hour instead of secondary mycelia. The primary mycelia can be easily differentiated from the secondary mycelia since the primary mycelia compartmentalized into several short segments with cross-membranes in addition to peptidoglycan cell walls (Fig. 8f, g) that is atypical for Streptomyces growth because normally primary mycelia are detectable only in the first 10 h [3]. That means the 8-DMTC application decreases the growth rate of wild-type culture so that the treated culture could not reach the secondary mycelia phase at 24 h. Moreover, in the space between colonies, there are many disentangled and fragmented mycelia parts unlike the wild-type sample (Fig. 8f, g). 8-DMTC treatment dismantled the mycelia and disrupted the colony structure and pellet formation. In short, extracellular application of 8-DMTC caused the same morphological results as in the case of recombinant strains that express it.
Discussion
In the scope of this study, we wanted to evaluate the potential of actinorhodin-overproducer S. coelicolor ∆ppk strain [17] as a heterologous host for the production of an anti-tumor and antibacterial agent 8-DMTC [18,19,20]. S. coelicolor A3(2) wild type and ∆ppk cells were transformed with cos16F4ie which carries most of the elm gene cluster encoding 8-DMTC. Production of 8-DMTC by recombinant strains was detected by using HPLC. The 8-DMTC peak with 4.8-min retention time was present only in the chromatograms of recombinant of both S. coelicolor A3(2) wild-type and ∆ppk strains. There was a small difference between the production levels of 8-DMTC of wild type + cos16F4ie and ∆ppk + cos16F4ie. ∆ppk recombinant strain produced slightly more 8-DMTC than wild-type recombinant strain. However, this difference was not significant enough to announce ∆ppk strain as a heterologous host at least for this metabolite.
During the fermentation process of wild type, ∆ppk, and their cos16F4ie recombinants for HPLC analysis, we have noticed some abnormalities in the appearance of the recombinants’ culture in R2YE broth and also on R2YE agar. Normally, the Streptomyces wild-type and ∆ppk strains produce blue-pigmented actinorhodin and red-pigmented undecylprodigiosin antibiotics. However, the cos16F4ie recombinants did not produce any colored antibiotics.
The cos16F4ie recombinants of S. coelicolor A3(2) wild type and ∆ppk were analyzed with confocal laser scanning microscopy (CLSM) to understand whether any changes were present in the morphology of the recombinants’ colony structure. Colonies of A3(2) wild-type and ∆ppk strains were intact without fragmentation and most parts of the colonies were alive within the first 48 h. The death ratio was increasing until 72 h. Between 72 and 96 h, alive segments were growing again. Then, most of the segments died up to 120 h. The confocal images of the cos16F4ie harboring recombinants of both A3(2) wild-type and ∆ppk strains showed that the colony structure was totally disrupted, with no correlation between the incubation time and the alive-death cell ratio. Dead and viable fragments of mycelia were randomly spread around the culture.
Confocal analysis of A3(2) wild type and its recombinant strain was performed for 24 h at 6-h intervals to determine whether disentanglement happens after the colonies have been formed within the first 24 h or that the recombinants are not capable of forming colony structure at all. Confocal images showed that the recombinant strain never exhibited a healthy colony formation, pellets, and clumps.
The antibiotic production is closely related to pellet and clump formation in Streptomycetes. Previous studies showed that pellet formation is essential for antibiotic production in S. coelicolor [1, 8]. So, the reason for the lack of ability to produce pigmented antibiotics might be the lack of pellet and clump formation of the cos16F4ie recombinants.
Although unlikely, another reason for the cease in antibiotic production could be the presence of a partial IclR family regulator gene (GenBank: CAP12614.1) that lies in the elm cluster. Generally, IclR family regulators are known to be transcriptional repressors that bind to the specific sequences of promotors or operators [28]. A study based on DoxR protein which belongs to IclR family regulators has shown that DoxR expression in S. coelicolor inhibited the expression of pigmented actinorhodin or undecylprodigiosin antibiotics. Moreover, the transcriptional analysis has demonstrated that there was no expression of actII-ORF4, the regulator of actinorhodin biosynthetic gene cluster, and less expression of redD, the regulator of undecylprodigiosin biosynthetic gene cluster, in DoxR-expressing S. coelicolor [29]. Furthermore, another group has targeted an IclR family regulator NdgRyo, to reveal new secondary metabolites in Streptomyces youssoufiensis. The removal of repression by the deletion of ndgRyo has led to the discovery of a new fatty acid amide [30]. Considering these findings, the partial IclR family regulator located in the elm cluster may play a repressing role in the production of actinorhodin and undecylprodigiosin in S. coelicolor A3(2) wild type+cos16F4ie and ∆ppk+cos16F4ie strains. Further study is needed to prove this possibility.
cos16F4ie is an integrative cosmid; the ΦC31 integrase enzyme is responsible for the integration of the cosmid into the attB site of the S. coelicolor genome [31]. The reason for the strange developmental pattern of the recombinant strains could be the disruption of the genome caused by the integration of the cosmid itself or it could be the expression of 8-DMTC compound encoded by elm cluster. To answer this question, we have deleted the whole elm cluster including partial IclR family regulator gene from cos16F4ie, and then transformed S. coelicolor A3(2) wild-type cells with this cos16F4ie/∆elm construct. At first sight, S. coelicolor wild type + cos16F4ie/∆elm cells produced pigmented antibiotics after 24-h incubation in R2YE broth. Moreover, they showed agarase activity on R2YE agar and sporulate on TBO agar. Confocal analysis showed that S. coelicolor wild type + cos16F4ie/∆elm strain could form colonies similar to wild-type ones. At 24 h, most of the colony was alive, only the center was suffering from PCD. The only change in features of cos16F4ie/∆elm recombinant from wild type was the delayed growth. Normally, wild-type colonies have already passed the first PCD round by 24 h, though there were few primary mycelia present in the colony, most of them have transformed to secondary mycelia. However, S. coelicolor wild type + cos16F4ie/∆elm colonies contained more primary mycelia than secondary ones. That means the growth was slower than wild-type colonies. Nonetheless, S. coelicolor wild type + cos16F4ie/∆elm showed quite similarities with S. coelicolor wild-type colonies. Thereby, we concluded that the deficiency in colony morphology and production of pigmented antibiotics was not caused by the insertion of the cosmid but the expression of elm cluster, thereby the production of 8-DMTC.
We have also searched whether the extracellular addition of 8-DMTC causes deformation of Streptomyces colonies as we observed in our cos16F4ie recombinant strains. So that, S. coelicolor wild-type colonies were treated with the supernatant of S. coelicolor wild type + cos16F4ie culture which contained 8-DMTC. We have performed the treatment with both filtered and non-filtered supernatant to avoid any transfer of cell or fragmented mycelia from the recombinant culture. After 24 h of the treatment, we have observed that the pigmented antibiotic production was lower in 8-DMTC-treated wild-type culture than that in the untreated ones. When the colony structure of 8-DMTC-treated wild-type culture was checked under confocal microscopy, we found that it had fewer colonies than wild-type culture. These few colonies were formed from primary mycelia indicating that the growth was delayed. Furthermore, the area between the colonies was full of fragmented and disentangled mycelia. The CLSM analysis proved that 8-DMTC causes fragmentation of mycelia and disentanglement of colonies, thereby causing a lack of pellet formation. We could not explain why recombinants suffer from the anti-microbial effect of 8-DMTC since they contain a resistance gene in the elm gene cluster. Further research is needed to determine if the resistance gene is expressed or not in these recombinant strains.
To the best of our knowledge, the morphological changes that we have observed in Streptomyces that expresses 8-DMTC have not been reported before. Moreover, the results of this study provide useful information about the use of Streptomyces as hosts for the heterologous expression of this tetracyclic polyketide.
References
Manteca Á, Yagüe P (2018) Streptomyces differentiation in liquid cultures as a trigger of secondary metabolism. Antibiotics 7(2):41. https://doi.org/10.3390/antibiotics7020041
Barka EA, Vatsa P, Sanchez L, Gaveau-Vaillant N, Jacquard C, Klenk H-P, Clément C, Ouhdouch Y, van Wezel GP (2016) Taxonomy, physiology, and natural products of Actinobacteria. Microbiol Mol Biol Rev 80(1):1–43. https://doi.org/10.1128/MMBR.00019-15
Yagüe P, Willemse J, Koning RI, Rioseras B, López-García MT, Gonzalez-Quinonez N, Lopez-Iglesias C, Shliaha PV, Rogowska-Wrzesinska A, Koster AJ (2016) Subcompartmentalization by cross-membranes during early growth of Streptomyces hyphae. Nat Commun 7(1):1–11. https://doi.org/10.1038/ncomms12467
Manteca A, Alvarez R, Salazar N, Yagüe P, Sanchez J (2008) Mycelium differentiation and antibiotic production in submerged cultures of Streptomyces coelicolor. Appl Environ Microbiol 74(12):3877–3886. https://doi.org/10.1128/AEM.02715-07
Manteca A, Claessen D, Lopez-Iglesias C, Sanchez J (2007) Aerial hyphae in surface cultures of Streptomyces lividans and Streptomyces coelicolor originate from viable segments surviving an early programmed cell death event. FEMS Microbiol Lett 274(1):118–125. https://doi.org/10.1111/j.1574-6968.2007.00825.x
Schlimpert S, Flärdh K, Buttner M (2016) Fluorescence Time-lapse imaging of the complete S venezuelae. https://doi.org/10.3791/53863
Wendt-Pienkowski E, Huang Y, Zhang J, Li B, Jiang H, Kwon H, Hutchinson CR, Shen B (2005) Cloning, sequencing, analysis, and heterologous expression of the fredericamycin biosynthetic gene cluster from Streptomyces g riseus. J Am Chem Soc 127(47):16442–16452. https://doi.org/10.1021/ja054376u
Manteca A, Yagüe P (2019) Streptomyces as a source of antimicrobials: novel approaches to activate cryptic secondary metabolite pathways. Antimicrobials, Antibiotic Resistance, Antibiofilm Strategies and Activity Methods, In. https://doi.org/10.5772/intechopen.81812
Jonsbu E, McIntyre M, Nielsen J (2002) The influence of carbon sources and morphology on nystatin production by Streptomyces noursei. J Biotechnol 95(2):133–144. https://doi.org/10.1016/S0168-1656(02)00003-2
Felnagle EA, Rondon MR, Berti AD, Crosby HA, Thomas MG (2007) Identification of the biosynthetic gene cluster and an additional gene for resistance to the antituberculosis drug capreomycin. Appl Environ Microbiol 73(13):4162–4170. https://doi.org/10.1128/AEM.00485-07
Barkei JJ, Kevany BM, Felnagle EA, Thomas MG (2009) Investigations into viomycin biosynthesis by using heterologous production in Streptomyces lividans. Chembiochem 10(2):366–376. https://doi.org/10.1002/cbic.200800646
Komatsu M, Uchiyama T, Ōmura S, Cane DE, Ikeda H (2010) Genome-minimized Streptomyces host for the heterologous expression of secondary metabolism. Proc Natl Acad Sci 107(6):2646–2651. https://doi.org/10.1073/pnas.0914833107
Gomez-Escribano JP, Bibb MJ (2014) Heterologous expression of natural product biosynthetic gene clusters in Streptomyces coelicolor: from genome mining to manipulation of biosynthetic pathways. J Ind Microbiol Biotechnol 41(2):425–431. https://doi.org/10.1007/s10295-013-1348-5
McDaniel R, Ebert-Khosla S, Hopwood DA, Khosla C (1993) Engineered biosynthesis of novel polyketides. Science 262(5139):1546–1550. https://doi.org/10.1126/science.8248802
Floriano B, Bibb M (1996) afsR is a pleiotropic but conditionally required regulatory gene for antibiotic production in Streptomyces coelicolor A3 (2). Mol Microbiol 21(2):385–396. https://doi.org/10.1046/j.1365-2958.1996.6491364.x
Gomez-Escribano JP, Bibb MJ (2011) Engineering Streptomyces coelicolor for heterologous expression of secondary metabolite gene clusters. Microb Biotechnol 4(2):207–215. https://doi.org/10.1111/j.1751-7915.2010.00219.x
Camci İY, Doruk T, Avican Ü, Gedik ST (2012) Deletion of polyphosphate kinase gene (ppk) has a stimulatory effect on actinorhodin production by Streptomyces coelicolor A3 (2). Turk J Biol 36(4):373–380. https://doi.org/10.3906/biy-1110-19
Rohr J, Zeeck A (1990) Structure-activity relationships of elloramycin and tetracenomycin C. J Antibiot 43(9):1169–1178. https://doi.org/10.7164/antibiotics.43.1169
Decker H, Rohr J, Motamedi H, Zähner H, Hutchinson C (1995) Identification of Streptomyces olivaceus Tü 2353 genes involved in the production of the polyketide elloramycin. Gene 166(1):121–126. https://doi.org/10.1016/0378-1119(95)00573-7
Drautz H, Reuschenbach P, Zähner H, Rohr J, Zeeck A (1985) Metabolic products of microorganisms. 225 Elloramycin, a new anthracycline-like antibiotic from Streptomyces olivaceus. J Antibiot 38(10):1291–1301. https://doi.org/10.7164/antibiotics.38.1291
Hanahan D (1983) Studies on transformation of Escherichia coli with plasmids. J Mol Biol 166(4):557–580. https://doi.org/10.1016/s0022-2836(83)80284-8
Hopwood DA (1999) Forty years of genetics with Streptomyces: from in vivo through in vitro to in silico. Microbiology 145(9):2183–2202. https://doi.org/10.1099/00221287-145-9-2183
Paget MS, Chamberlin L, Atrih A, Foster SJ, Buttner MJ (1999) Evidence that the extracytoplasmic function sigma factor ςE is required for normal cell wall structure in Streptomyces coelicolor A3 (2). J Bacteriol 181(1):204–211. https://doi.org/10.1128/JB.181.1.204-211.1999
Díaz M, Sevillano L, Rico S, Lombo F, Braña AF, Salas JA, Mendez C, Santamaría RI (2013) High level of antibiotic production in a double polyphosphate kinase and phosphate-binding protein mutant of Streptomyces lividans. FEMS Microbiol Lett 342(2):123–129. https://doi.org/10.1111/1574-6968.12098
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, vol Ed. 2. Cold spring harbor laboratory press, New York
Kieser T, Bibb MJ, Buttner MJ, Chater KF, Hopwood DA (2000) Practical streptomyces genetics, vol 291. John Innes Foundation, Norwich
Freitag A, Méndez C, Salas JA, Kammerer B, Li S-M, Heide L (2006) Metabolic engineering of the heterologous production of clorobiocin derivatives and elloramycin in Streptomyces coelicolor M512. Metab Eng 8(6):653–661. https://doi.org/10.1016/j.ymben.2006.07.003
Tropel D, Van Der Meer JR (2004) Bacterial transcriptional regulators for degradation pathways of aromatic compounds. Microbiol Mol Biol Rev 68(3):474–500. https://doi.org/10.1128/MMBR.68.3.474-500.2004
Chaudhary AK, Singh B, Maharjan S, Jha AK, Kim B-G, Sohng JK (2014) Switching antibiotics production on and off in actinomycetes by an IclR family transcriptional regulator from Streptomyces peucetius ATCC 27952. J Microbiol Biotechnol 24(8):1065–1072. https://doi.org/10.4014/jmb.1403.03026
Hou J, Liu J, Yang L, Liu Z, Li H, Che Q, Zhu T, Li D, Li W (2019) Discovery of an unusual fatty acid amide from the ndgRyo gene mutant of marine-derived Streptomyces youssoufiensis. Marine Drugs 17(1):12. https://doi.org/10.3390/md17010012
Combes P, Till R, Bee S, Smith MC (2002) The Streptomyces genome contains multiple pseudo-attB sites for the φC31-encoded site-specific recombination system. J Bacteriol 184(20):5746–5752. https://doi.org/10.1128/JB.184.20.5746-5752.2002
Acknowledgements
We are grateful to Dr. Jose A. Salas and Dr. Angel Manteca for their critical reading of the manuscript and valuable comments and suggestions. We would like to thank Dr. Jose A. Salas and Dr. Carmen Mendez (Universidad de Oviedo, Spain) for providing us cos16F4ie.
Funding
This work was supported by a research grant (BAP-2019-A101-07) from Gebze Technical University, Turkey.
Author information
Authors and Affiliations
Contributions
In order to recognize the authors’ participation, we highlight each individual contribution: S.T. designed the study and supervised the experiments, B.C. and Z.D. contributed in confocal analysis of S. coelicolor and all recombinant strains expressing 8-DMTC, and B.C. made also contribution in the construction of cos16F4ie/∆elm and treatment of wild-type S. coelicolor A3(2) strain with 8-DMTC-containing supernatant. All these authors have substantial contributions to the final manuscript and approved this submission. All authors are aware of the order of authorship and that no further change in authorship will be performed after submission, except those previously authorized by the editor-in-chief.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Lucy Seldin
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cinar, B., Demir, Z. & Tunca, S. Heterologous expression of 8-demethyl-tetracenomycin (8-dmtc) affected Streptomyces coelicolor life cycle. Braz J Microbiol 52, 1107–1118 (2021). https://doi.org/10.1007/s42770-021-00499-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-021-00499-y