Abstract
Legumes have been used to improve soil fertility however, most legume research focuses on crop and not forage legumes. Forage legumes, including Pisum sativum L., increase the nutritional value in pastures and provide high amounts of soil protein and minerals required for plant growth. We investigated the effects of varying soil composition on plant growth, symbiosis establishment, and nutrient acquisition. We also aimed to compare phenolic compound production, since phenolics are reported to play a vital role in plant defense, pollination/dispersal, and symbiosis with quorum-sensing plant growth-promoting bacteria. Using quantitative techniques, we evaluated the effect of nutrient deficiency in plant–microbe symbiosis, nutrition, and carbon costs, as well as the phenolic concentrations in P. sativum. Four distinct regional soils in KwaZulu-Natal (KZN), geographically covering grassland and savannah ecosystems, were used as growth substrates. Plants maintained their root dry weights and growth rates across the four soil types. Low pH, total cations, and high exchange acidity in Bergville soil resulted in decreased total plant dry weights. P. sativum grown in Izingolweni soils relied more on atmospheric N fixed by endophytic/associative bacteria from the genera Cupriavidus, Paenibacillus, Cohnella, and Bacillus, while those grown in Hluhluwe soils relied on soil N. Plant associative microbes might modulate nutrient availability for plant uptake in nutrient poor grassland and savannah ecosystems. P. sativum acclimatized to changes in soil nutrient concentrations and pH in the studied ecosystems by changing N source preferences and phenolic concentrations. The acclimatization of plants is likely modulated by the presence of rhizospheric microorganisms interacting with the plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Sustainable soil management is viewed as a fundamental aspect of sustainable agriculture for supporting the ever-increasing human population and biodiversity (Lal 2008; Nambiar et al. 2001; Ohyama 2017). In Africa, several countries still face accelerating declines in agricultural yield due to unsustainable agricultural practices that are exacerbated by environmental factors, including climate change, rainfall variability, soil quality degradation, and recurrent droughts (Doso Jnr 2014; Lema and Majule 2009). These environmental factors result in detrimental effects on soil fertility and ecosystem functioning, as well as vegetation structure (Doso Jnr 2014; Lema and Majule 2009). Given that the agricultural sector plays a pivotal role in most economies, and the non-renewable nature of soil (Doran and Zeiss 2000), sustainable strategies must be developed to help mitigate negative soil quality and yield potential at the time new crop areas are explored. To achieve this, more research aimed at the identification of plants that can withstand such environmental stress conditions is needed. This enable the establishment of high nutrient pastures for animal feed, and also to improve soil fertility through legume use. African savannahs and grasslands can be used as crop areas; however, their potential for agriculture need to be analyzed in terms of their nutrition and fertility status for plants (Zungu et al. 2020).
Soil is a sink of essential minerals, nutrients, and microbiota that contribute to plant growth (Rutigliano et al. 2004). Soil is also involved in the direct and/or indirect cycling of essential elements such as nitrogen (N), carbon (C), and oxygen (O) (Harper and Pendleton 1993; Nannipieri et al. 2017). Soil fertility and functionality are based on several factors, such as the soil composition, relative acidity (pH), aeration (redox potential), microbial diversity, moisture content, temperature, nutrient availability, and physical characteristics (Husson 2013; Voroney 2007). However, microbial diversity is important due to the ability of some microorganisms to convert insoluble nutrients into plant-available forms (Rengel and Marschner 2005). These microorganisms can either be host-dependent or free-living, such as legume Rhizobia and Aspergillus niger. Rhizobia reduce atmospheric nitrogen (N2) through nitrogenase activity, while Aspergillus niger hydrolyzes phytic acid through phytase activity (Rengel and Marschner 2005). Example of a key plant nutrient that microorganisms recycle is phosphorus (P) and is usually present in a low concentration (≤ 10 µM) in acidic soils (Kunwar et al. 2018).
In South Africa, finding soils of quality for agricultural purposes is a major problem due to the easiness of degradation impelled by drought and poor remediation strategies, which include replenishing soil organic matter (Williams et al. 2004). What is more, most South African soils are regarded as nutrient poor and relatively acidic and tend to be easily degraded (Mandiringana et al. 2005; Roberts et al. 2003). Among the 14 soil groups in South Africa, 12 are distributed across KZN (Fey 2010). This makes KZN, the garden province, suitable for agricultural practices, but nutrient availability is usually the limiting factor in areas including KZN and the Highveld that receive ≥ 600 mm annual rainfall.
Low nutrient availability can negatively affect agricultural productivity (Onwuka et al. 2016), especially if the soils are acidic (Buresh et al. 1997; Haumaier and Zech 1995; Maisels et al. 1994). Legumes have developed adaptations to withstand these soil conditions through the establishment of symbiosis between the host plant and beneficial microorganisms (Ohyama 2017; Sanz-Saez et al. 2017). The limiting nutrients are made available in these symbiotic systems through biological N fixation (BNF) in root nodules and P scavenging hyphae of AM fungi (Lambers et al. 2008). Plants grown in nutrient impoverished soils accumulate phenolic compounds in organelles (Lattanzio, 2013) and they act as a protection mechanism to aid the plant to cope with stress through antioxidant activity (Jaleel et al. 2009). Additionally, phenolics influence the composition of microbes in the rhizosphere, influencing symbiosis establishment, thus optimizing plant nutrient acquisition, stress tolerance, and overall plant physiology (Bi et al., 2007; Bhattacharya et al., 2010; Lattanzio, 2013). However, legume research usually focuses on crop legumes and not forage legumes, especially in nutrient-deficient agricultural systems. Forage legumes, including Pisum sativum (L.), are important in sustainable agricultural systems as they increase the nutritional value in pastures and provide relatively high amounts of soil protein and minerals required for plant growth (McCallum et al. 2000). Thus, the aim of this research was to investigate the effects of four distinct soil types with varying nutrient concentrations and pH levels on plant accumulation of phenolic acids, plant–microbe symbiosis, biomass accumulation, N source preference, and carbon growth costs of Pisum sativum. We hypothesize that P. sativum plants grown under soil nutrient deficit would increase inner mechanisms of protection by modulating their sources of N and the production of phenolic acids.
2 Methods and Materials
2.1 Soil Collection Sites
Four geographical distinct regions in KwaZulu-Natal province, South Africa, covering grassland and savanna ecosystems were used for soil sampling: Hluhluwe, Northern KZN (28°0′58″S, 30°26′42″E); Izingolweni, Southern KZN (30°43′32 ″S, 30°6′10″E); Bergville, Mountainous KZN (28°34′14″S, 29°4′17″E); Ashburton, Midlands KZN (29°38′55″S, 30°26′42″E). The soil collection sites were largely savanna and grassland. Precipitation is highest in the southwest and declines eastward and northward. Soil properties and vegetation description are as described in (Matiwane et al. 2019 and Zungu et al. 2020). The ratio of concentrations of nitrogen (N) and phosphorus (P) in vegetation has been used as an index of the limitation of biomass production by N and P (Koerselman & Meuleman, 1996; Tessier & Raynal, 2003; Güsewell, 2004). We used this ratio to rank the four soils, resulting in that Hluhluwe was the most fertile soil with a ratio N:P of 25.2; Izingolweni was the second (7.0), followed by Ashburton (6.2) and Bergville (5.8). In each location, five random points were chosen. In each point 10 subpoints were chosen and a whole of 10-cm depth were dug. The 10-cm depth was estimated with a measuring tape. This depth is considered the portion of soil in closer contact with roots and where maximum microbial activity is expected; the collected soil in each point (ten subpoints) was transferred to a bucket and thoroughly mixed with the remaining samples. In total five compound samples were collected per site. A portion of each compound soil sample was stored in sterile plastic bags in a refrigerator at 4ºC until chemical and biological analyses were conducted. In addition, bulk soil (55 kg approximately) was also taken from the compounds samples and use for further experiments (Pérez-Fenández et al. 2019).
2.2 Soil Characteristics and AM Fungi Analysis
Five 50 g soil samples from each site were sent to the KwaZulu-Natal Department of Agriculture and Rural Development’s Analytical Services Unit at Cedara College of Agriculture, South Africa, for total soil nutrient and cation concentrations, cation exchange acidity and pH analysis. Briefly, the Automated Dumas dry combustion method using a LECO CNS 2000 (Leco Corporation, Michigan, USA) was used to measure total soil nitrogen and pH (using a KCl solution). Phosphorus and potassium in the soil samples were measured using atomic absorption method. This involved the extraction of 2.5 ml soil solution with 25 ml ambic-2 solution at pH of 8. The mixture was stirred at 400 rpm for 10 min using a multiple stirrer and filtered using Whatman No.1 paper. Refer to Manson and Roberts (2000) for the detailed methodologies. Arbuscular mycorrhizal spore count analysis was conducted according to Smith and Dickson (1997) at Mycoroot (Pty) Ltd, Rhodes University, South Africa in the five soil samples ranging from 250 to 300 g from each site.
Soil chemical properties are described in Matiwane et al. (2019). To facilitate the study of this results, a summary table is included as Table S1.
2.3 Seed Germination and Plant Growth
P. sativum seed were obtained from AGT foods Africa, Marji Mizuri farm, KZN. Seeds were surface sterilized in bleach solution (30% commercial bleach + 0.02% Triton X-100) for 15 min, washed 10 times with sterile water, and air dried in a sterile laminar flow. Thirty surface sterilized seed (one seed in each 19-cm diameter pot) were planted in the aforementioned four soils from KwaZulu-Natal to guarantee minimum test size. After seedling emergence, only 20 replicates were maintained per soil. To guarantee seedling survival, during the first fortnight after emergence, seedlings were watered daily with ~ 300 ml distilled H2O and every third day from then on. In order to further calculate growth parameters, two harvests were performed at 25 and 55 days after seedling emergence, respectively. The experiment was conducted under ambient conditions in a glasshouse at the Botanical gardens of the University of KwaZulu-Natal, Pietermaritzburg, South Africa. To prevent cross-contamination, pots containing soils from different locations, were places on different benches distant 2.5 m each other. Pots were arranged in a completely randomized block design and they were rearranged on a weekly basis to guarantee homogeneity of growing conditions. The day and night-time temperatures ranged from 30 to 35 °C and 12 to 14 °C, respectively. The daily average humidity was 75% and the irradiance was ca. 35% of full sunlight (415.6 μmol m2 s−1).
2.4 Plant Nutrient Analysis
Five randomly chosen plants per treatment were separated into leaves, stem, and root, and oven-dried at 80 °C until a constant dry weight (DW) was attained. The dry plant material was ground using a tissuelyzer (Qiagen, South Africa) and analyzed for their C, P, and δ15N concentrations as described by Matiwane et al. (2019). δ15N concentration analysis was conducted using a LECO-N analyzer (LECO Corporation, St. Joseph, MI, USA) at the Archaeometry Department, University of Cape Town, South Africa, and C and P concentrations were analyzed using inductively coupled mass spectrometry (ICP-MS) at the Central Analytical Facilities, Stellenbosch University, South Africa. Dry weight of above and below-ground plant parts were used to calculate the root to shoot ratio.
2.5 Growth Calculations
2.5.1 Relative Growth Rate
The Agren and Franklin (2003) method was used to calculate the relative growth rate (RGR).
where W is the dry plant weights accumulated from initial (W1) to final (W2) harvest and t is the time for plant growth.
2.5.2 Specific N Absorption Rate (SNAR)
Plant total N content was used to calculate the specific N absorption rate according to Nielsen et al. (2001).
where L, t, and R represent total N content, duration of plant growth in weeks, and root dry weight, respectively.
2.5.3 Specific N/P Utilization Rates (SNUR/SPUR)
Plant total N/P content was used to calculate the specific N/P utilization rate according to Nielsen et al. (2001).
where W, L, and M represent plant DW, total N, and P content, respectively.
2.5.4 Percentage N Derived from the Atmosphere (%NDFA)
%NDFA was calculated according to Shearer and Kohl (1986) as δ = 1000 (Rsample/Rstandard), where R is the molar ratio of 15 N and 14 N of the samples and standards. Sample preparation and analysis was conducted according to Matiwane et al. (2019) using the same equipment, standards, and facilities.
where β value represents the δ15N natural abundance of the N derived from biological N2 fixation of P. sativum, grown in a N-free culture. The β value of P. sativum for this study was − 2.58%. The total amount of N in the plant derived from N2 fixation (Nfix) was determined by: Nfix = Ndfa × N content.
The δ15Nref is the δ15N from a non-fixing N2 reference plant that in the current study was Briza maxima.
2.5.5 Carbon construction cost (Cw)
Carbon construction cost was calculated using the formula modified by Peng et al. (1993) and further verified by Mortimer et al (2008).
where Cw represents the total carbon construction cost in the tissues (mmolC g−1 dw), C is the total concentration of carbon (mmolC g−1), k is the reduction state of the N substrate (i.e. NH3 = − 3) and N is the total organic nitrogen content of the tissue (Williams et al. 1987; Mortimer et al. 2008).The constant (1/0.89) represents the fraction of the construction cost which provides reductant that is not incorporated into biomass (Williams et al., 1987; Peng et al., 1993; Mortimer et al. 2008) and (6000/180) converts units of g glucose g−1 dw to mmolCg−1 dw.
2.6 Bacterial isolation, colony PCR, and identification
Plant harvest was conducted sinking the pots in water until the soil was lose and roots easy to extract, this way, root biomass was little or not damaged and nodules, when formed, remained attached to the roots. Only P. sativum grown in Izingolweni soil formed root nodules. All nodules were counted and removed individually from each plant. Nodules were rinsed with dH2O and immediately surface sterilized using 70% (v/v) ethanol for 30 s before submerging them in 3.5% (v/v) sodium hypochlorite solution for 3 min. Thereafter, the nodules were thoroughly rinsed 10 times with dH2O and stored at 4 ºC. Bacterial isolation and identification was conducted according to Matiwane et al. (2019), which is the protocol usually applied in our laboratory for bacterial amplification and identification., Nodule bacteria were isolated by crushing the root nodules in 15% (v/v) glycerol and culturing the suspension on yeast mannitol agar (YMA) and incubated at 28 °C under micro-aerobic conditions. Single colonies/cultures were obtained by repeated streaking and culturing at 72-h incubation periods under the same conditions as above. No slow-growing bacteria were observed after 15-day growth in Petri dishes. The 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and the 1492R (5′-GGTTACCTTGTTACGACTT-3′) primers were used for colony PCR. Each 25 μl PCR reaction contained 12.5 μl Emerald AMP master mix, 0.25 μl of the respective primers, 11 μl of sterile milliQ water, and 1 μl of diluted bacterial pure colony. DNA amplification was performed on BioRad Mini Opticon thermal cycler (BioRad, South Africa) using the following protocol: initial denaturation for 5 min at 94 °C, 30 cycles of denaturation for 30 s at 94 °C, annealing for 30 s at 55 °C, and elongation for 2 min at 72 °C, followed by a final elongation step of 10 min at 72 °C. The acquired amplicons were viewed on a 1% agarose gel and were confirmed to be of the expected size (1500 base pairs). The PCR products were sent for sequencing at the Central Analytical Facilities at Stellenbosch University, South Africa. The resulting sequences were edited and subjected to BLASTN searches for identification (National Center for Biotechnology Information, NCBI (https//www.ncbi.nlm.nih.gov) to compare them to all of the other bacterial 16S rRNA sequences already in the database.
2.7 Quantification of phenolic acids
Ten plants per treatment were divided into roots and shoots and pulverized in liquid nitrogen using a mortar and pestle and freeze-dried. The samples were immersed in a canister containing liquid nitrogen and immediately sent by courier to the Laboratory of Growth Regulators, Centre of the Region Haná for Biotechnological and Agricultural Research, Faculty of Science, Palacký University & Institute of Experimental Botany AS CR, Šlechtitelů, Czech Republic, which were prepared for analyses on the arrival day, only one and a half days after harvest. Details of the quantification were as described by Gruz et al. (2008). Briefly, plant materials (approximately 25 mg) were extracted with 80% (v/v) methanol containing internal standards of salicylic (3,4,5,6-2H4) and 4-hydroxybenzoic (2,3,5,6-2H4) acids at the concentration of 10–4 mol/L. After extraction, phenolic acid in the plant materials were determined using ultra-high-performance liquid chromatography (UHPLC)-tandem mass spectrometry. The extracted phenolic acids were protocatechuic, 4-hydroxibenzoic, caffeic, vanillic, syringic, 4-coumaric, sinapic, ferulic, and salicylic, all of them described as inducer of plant–microbe symbioses in some selected species (Dixon 2001; Mandal et al. 2009; Boudet, 2007), including P. sativum (Vaughan and Ord, 1990).
2.8 Statistical analysis
IBM SPSS Statistics 25 software was used for all data analysis. The relationship between pH and biomass production and fertility and biomass production was calculated by means of a simple regression analysis. Analysis of variance (ANOVA) statistical models were used to compare means of all the plant variables obtained in the four different soils. Values were considered significant when p ≤ 0.05. Where the ANOVA showed significant differences between treatments, a Bonferroni’s post hoc test was used to separate the means. Where two-way ANOVA showed significant effects at 95% (P < 0:05), Fisher’s least significant differences (Lyman, 1998) were determined using one-way ANOVA for all replicates showing some germination. Normality and homoscedasticity of data were tested by means of David’s and Cochran’s tests.
3 Results
3.1 Seedling Emergence and Growth Kinetics
Seedling emergence was first observed at day 4 after planting, and the last seedling emergence happened at day seven (Fig.1). Hluhluwe and Ashburton had the highest and similar seedling emergence after four (91%) and seven days (100%) of planting (Table 1). Hluhluwe plants had the highest total plant biomass while the Bergville soil-grown plants had the least biomass (Table 2). Plants grown in Hluhluwe and Ashburton soils had significantly higher above-ground biomass and lower root-shoot values (Table 2). Izingolweni and Bergville plants produced the significantly lowest shoot biomass, and had the significantly greatest root-shoot ratio (1.39 and 5.52, respectively) (Table 2). Bergville plants also had the lowest above-ground biomass and highest root biomass (Table 2). Izingolweni plants had significantly lower biomass than Hluhluwe and Ashburton ones and had the second greatest root:shoot ratio. There was a clear positive correlation between pH and total plant biomass production (r2 = 0.8723) (Fig. 2).
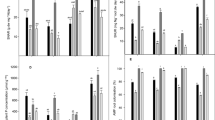
Pisum sativum total plant nitrogen (N) concentration (A), N derived from the atmosphere (B), percentage N derived from atmosphere (C), and N derived from the soil (D) after a 55-day growth period in four soils of KwaZulu-Natal. Values are means ± SE, n. Bars with the same letter do not differ significantly (p ≤ 0.05)
No significant difference was observed in total plant N concentration in plants grown in Ashburton and Hluhluwe soils (Fig. 3A). While plants grown in Izingolweni soils and Bergville had significantly lower total plant N concentration (Fig. 3A). Izingolweni plants relied more on N2 fixation (~ 55%) than Ashburton (~ 43%), then Bergville (~ 31%) and Hluhluwe plants (~ 5%) (Fig. 3C). Ashburton and Izingolweni plants showed significantly higher concentrations of N derived from the atmosphere than plants grown in the other two soils (Fig. 3B). Hluhluwe plants had the highest soil-derived N, followed by Ashburton, Bergville, and Izingolweni (Fig. 3D). Hluhluwe and Ashburton soil-grown plants had the highest soil N absorption rates, while Bergville plants had the least N absorption rate (Table 2). However, Ashburton and Hluhluwe plants had the least specific N utilization rate, in comparison with Izingolweni and Bergville plants which had the highest specific N utilization rate (Table 2).
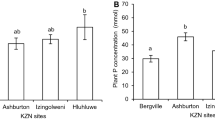
Ashburton plants accumulated significantly higher concentration of P relative to plants from the other soils (Fig. 4A). Izingolweni and Hluhluwe soil-grown plants had the second and third least total plant P concentration, respectively which were also statistically different (Fig. 4A). No significant differences were detected in the carbon construction costs (Table 2) and specific P utilization rates (Fig. 4B) of plants grown in the four different soils.
3.2 Endophytic Bacteria Identification
Nodules only developed in Izingolweni plants, amplification and comparison of the 16S ribosomal RNA gene partial sequence revealed the presence of 17 strains in the root nodules belonging to five genera, Paenibacillus, Cupriavidus, Ralstonia, Saccharibacillus, and Cohnella (Table 3).
3.3 Phenolic Acids Concentration
The concentration of phenolic acids was higher in shoots than in the roots, with the exception of salicyclic and vanillic acid (Table 4). The only phenolic acid concentrations that showed significant differences were caffeic, vanillic, and sinapic acids in the shoots (Table 4). Shoot of the Izingolweni plants showed significantly higher concentrations of caffeic and vanillic acids than plants grown in the other soils, while Hluhluwe soil-grown plant shoots had the highest concentration of sinapic acid (Table 4). Izingolweni and Bergville soil-grown plants had relatively high concentrations of most phenolic acids, with the exception of salicylic acid in which Bergville soil-grown plants had the least concentration (Table 4). Furthermore, Bergville soil-grown plants had the highest concentration of 4-coumaric acid (Table 4). As a whole, Izingolweni plants accumulated significantly the greatest or the second greatest amounts of all the analyzed phenolic acids. Bergville plants were the second in accumulation of phenolic acids. In average, Ashburton plants accumulated the less amounts of phenolic acids.
4 Discussion
Pisum sativum plants successfully established in four soils from KZN savannah and grassland ecosystems by modulating their growth kinetics, plant nutrient assimilation and utilization rates and accumulating different concentrations of phenolic acids. The plants regulated their phenolic acid concentrations to adjust themselves to the soil nutrient concentration as the greatest amounts of phenolic acids accumulated in plants, either shoot or roots, grown in the soils with lower pH and fertility. This study also focused on hydroxicinnamic acids (i.e., ferrulic acid, sinapic acid, caffeic acid, and 4-coumaric acid) and hydrobenzoic acids (salicylic acid, vanillic acid, protocatechuic acid, syringic acid, and 4-hydrozybenzoic acid). The aforementioned allelochemicals, in conjunction with other phenolics influence plant performance and tolerance to both biotic and abiotic stress (Bhattacharya et al. 2010) (Table S2).
In our study, abiotic stress is present in the form of acidic soils and reduced fertility all of which hamper agriculture (Mantri et al. 2012). In fact, our results show a clear positive correlation between both, soil fertility and pH and biomass production. Plants of P. sativum are negatively affected by low levels of pH in the soil as demonstrated in other studies (Wu and Hendershot 2010). To combat abiotic stress the plants produce and accumulate different defense metabolites in different plant organs (Parvaiz and Satyawati 2008; Li et al. 2010; Ramakrisha and Ravishankar, 2011; Lattanzio, 2013). Several studies report the production of salicylic acid to ensure the endurance of plants (Jones and Bangl, 2006). Bergville and Izingolweni soils were the ones with the lowest pH. The lower fertility was attributed to Bergville soils and that of Izingolweni, although slightly higher did not statistically differ from that in Bergville. Plants grown in these two soils accumulated the highest concentration of all the studied phenolic compounds and were the smallest ones in terms of dry biomass production. In consequences, the presence of these compounds have a protective role for plant persistence under drought, severe nutrient deficiency or even exposure to ultra-violet (UV) radiation (Demkura et al. 2010; Lattanzio, 2013).
Plants from Izingolweni soil were the only ones that produced nodules. This can be explained by the dynamics of N and P, as well as pH, as well as by the presence of phenolic compounds. Legumes produce and release phenolic acids from emerging roots during seed germination and seedling growth (Hassan et al. 2019). These phenolic compounds regulate nod gene expression by the symbiont (Rhizobium) and so modify the legume-rhizobial symbiosis. The host root secretes phenolic compounds that act as signaling molecule during expression of various symbiotic plasmid encoded nod (nodulation) genes (Mandal et al. 2010). The chemo-attractant and gene-inducing properties of phenolics like p-hydroxybenzoic, caffeic, ferulic, protocatechuic, sinapic or syringic acids (Table 4) selectively influence the composition of microbes in the rhizosphere, thus optimizing plant nutrient acquisition, symbiosis establishment, stress tolerance, and overall plant physiology (Bi et al. 2007; Bhattacharya et al. 2010; Lattanzio, 2013). It is possible that the nodulation of P. sativum plants in Izingolweni is related to the amount of N in that soil, together with the presence of the aforementioned phenolic acids. Several studies have demonstrated that when P and N are low or absent legumes have to acquire N through symbiotic N2 fixation (Lü et al. 2013; Magadlela et al. 2016), as in our study with P. sativum growing in Izingolweni soil. In fact, plants in these soils were more reliant on atmospheric N (Fig. 3). BNF is a very expensive process in terms of photosynthates; when P and N are sufficient in the soil, plants are more likely to use these nutrients rather than investing in BNF. The higher amount of N in Hluhluwe made the plants rely on soil N as demonstrated by the greater accumulation on mineral N in plants from this soil, thus avoiding the expensive process of nodule formation and biological nitrogen fixation (Chapin 1991; Robert et al. 2014). Legumes need a given amount of N in the growing media to trigger nodule formation. Low levels of N would hamper nodulation in plants from Bergville and Ashburton.
Plants with decreased K concentrations experience its negative effects at the level of photosynthates, nutrients and H2O movement (Prajapati and Modi 2012). Also, K acts as a co-factor in enzyme activation, therefore, limited K affects the rate of photosynthesis and production of proteins and energy (Gajdanowicz et al. 2011). Koksal et al. (1988) reported that K deficiency resulted in increased concentration of abscisic acid (ABA), a growth inhibitor phytohormone, consequently inhibiting shoot growth. This means that Izingolweni soil-grown plants would have decreased above-ground biomass or shoot length, nutrient concentrations and growth kinetics. However, the impact of K deficiency may have been reduced in these plants by the establishment of symbiosis with endophytic microorganisms. Although Bergville soil had significantly higher concentrations of K than Izingolweni, plants also invested more in roots that in shoots, possibly due to the reduced fertility, as it also happens in plants from Ashburton. Different species from the genera Bacillus, Pseudomonas/Cupriavidus, Paenibacillus and Burkholderia possess K solubilizing capabilities which help by increasing exchangeable K in soils for plant uptake (Meena et al. 2016; Sheng et al. 2008; Syed and Patel 2014). Furthermore, members of Paenibacillus including P. polymyxa are efficient N2 fixers (free-living and/or symbiotic) and increase available P for plant through solubilization and mineralization thus aiding the plants in their growth (Puri et al. 2016; Villadas et al. 2019; Wang et al. 2012). While members of Bacillus (including B. cereus and B. thuringiensis) promote root growth, nodulation and N2 fixation in co-inoculation experiments (John Bullied et al. 2002; Mishra et al. 2009) others like Ralstonia (Ralstonia taiwanensis) and Cohnella fix N2 in Mimosa roots (Chen et al. 2003; Wang et al. 2015). These may have resulted in Izingolweni soil-grown plants increased reliance in atmosphere derived N.
Plants grown in Bergville soil had low above-ground dry weight, but had significantly high below-ground DW. This may be due to the significantly low pH of Bergville soils. In our study, the plants responded positively to the increase of pH and fertility in terms of increased germination and biomass production. Low soil pH decreases the base saturation, nutrient availability, total cations and increase exchange acidity in soils (Stiles 2004). This limits the availability of essential nutrients and co-factors required for growth and development and it has been demonstrated that plants of P. sativum under low pH show significantly reduced growth (Wu and Hendershot, 2010). Under slightly acidic conditions, phosphate available for plants is sequestered into insoluble cation bound phosphate making it unavailable for plant assimilation (Giesler et al. 2002). Additionally, low pH alters the soil microflora composition, diversity, abundance and viability (Zhalnina et al. 2015) hence, reduces plant growth-promoting microorganisms in the soil (Siciliano et al. 2014; Zhalnina et al. 2015). As a response, plants can increase their root systems under stressful conditions to prevent senescence among other negative effects of low pH (Franco et al. 2011; Huang and Fry 1998). This agrees with our observations in Bergville soil-grown plants with increased root dry weights thereby increasing root surface and favoring the contact with plant growth-promoting microorganisms and the acquisition of soil nutrients. When exuded through the roots, the accumulated phenolics in Bergville plants have the ability to increase bioavailability and mobility of soil nutrients, hence improving nutrient assimilation (Bhattacharya et al., 2010). (Chapin 1991; Robert et al. 2014). Regulation of metabolites, including phenolic acids, play a role in plant stress response through antioxidant activity (Jaleel et al. 2009). Plants grown in Izingolweni and Bergville soil accumulated the greatest concentrations of all the studied phenolic acids. Ghasemzadeh et al. (2010a, b) reported increases in antioxidant activity and the concentrations of phenolic acid in Zingiber officinale Roscoe under high CO2 levels. This corresponds with the findings of the present study where the concentrations of phenolic acids increased due to acidity and nutrient deficiency in the soil (Ghasemzadeh et al. 2010a, b) thus protecting the plants under the strongest stress induced in this study. This corroborates our initial hypothesis as plants modulate both their mechanisms of nutrient acquisition and inner protection mechanisms by modulating the production of phenolic acids in plant parts. When nutrients are sufficient, plant growth is only dependent on mineral nutrition. However, under limited nutrients and reduced pH, plants both, shift the use of nutrients from mineral nutrition to biological N fixation and protect themselves accumulating protective phenolic compounds. Very limited nutrients that hamper the initiation of nodulation, drive plants to modify their anatomy devoting more photosynthates to roots and also accumulating phenolic compounds (Rivas-San Vicente and Plasencia 2011).
Due to the growing needs of agricultural land in South Africa and the environmental challenges faced by natural and agricultural systems, there is a need for eco-friendly agricultural practices, and to ensure the future demand for food and fodder, exploitation of sustainable solutions is very much necessary. This brings us to the attention of leguminous plants that are able to accumulate phenolic compounds with diverse beneficial roles as crop yield enhancers, protectors against varied environmental stresses and inducers of nodulation. Here, we have explored the capabilities of P. sativum and results point towards that the diversity of phenolic compounds they can produce make of this species a great candidate for various fields of agriculture.
5 Conclusions
Pisum sativum were able to grow in soils with varying levels of nutrition that resulted in different amounts of plant biomass production and changes in the concentrations of phenolic acid in plant tissues. Plant production is maintained at the expenses of both soil and atmospheric N and due to the inner regulation of metabolites that confer the plant resistance to stressful conditions. This makes this species an ideal candidate to be used as forage in agriculture as it would need little fertilization to grow in soils with low levels of fertility and pH.
Data Availability
Experimental raw data will be available on request.
References
Ågren GI, Franklin O (2003) Root: shoot ratios, optimization and nitrogen productivity. Ann Bot 92:795–800
Aguilar JMM, Ashby AM, Richards AJM, Loake GJ, Watson MD, Shaw CH (1998) Chemotaxis of Rhizobium leguminosarum biovar phaseoli towards flavonoid inducers of the symbiotic nodulation genes. J Gen Microbiol 134:2741–2746
Behihun, T., Tolosa, S., Tadele, M. and Kebede, F. 2017. Effect of biochar application on growth of garden pea (Pisum sativum L.) in acidic soils of Blue Woreda Gedeo zone southern Ethiopia. Intn. J. of Agron. Article ID 6827323, 8 pages https://doi.org/10.1155/2017/6827323
Bhattacharya A, Sood P, Citovsky V (2010) The roles of plant phenolics in defence and communication during Agrobacterium and Rhizobium infection. Molec Plant Pathol 11:705–719
Bi HH, Song YY, Zeng RS (2007) Biochemical and molecular responses of host plants to mycorrhizal infection and their roles in plant defence. Allelopathy J 20:15
Boudet AM (2007) Evolution and current status of research in phenolic compounds. Phytochemistry 68:2722–2735
Buresh RJ, Smithson PC, Hellums DT (1997) Building soil phosphorus capital in Africa. In: Buresh, R.J., Sanchez, P.A., and Calhoun, F. (eds.), Replenishing soil fertility in Africa. SSSA/ASA, Madison, WI, pp. 111–149. https://doi.org/10.2136/sssaspecpub51.c6
Chapin FS (1991) Integrated responses of plants to stress. BioSci 41:29–36. https://doi.org/10.2307/1311538
Chen WM, James EK, Prescott AR, Kierans M, Sprent JI (2003) Nodulation of Mimosa spp. by the β-proteobacterium Ralstonia taiwanensis. Molec Plant-Microbe Interact 16:1051–1061. https://doi.org/10.1094/MPMI.2003.16.12.1051
Demkura PV, Abdala G, Baldwin IT, Ballaré CL (2010) Jasmonate-dependent and-independent pathways mediate specific effects of solar ultraviolet B radiation on leaf phenolics and antiherbivore defence. Plant Physiol 152:1084–1095
Dixon RA (2001) Natural products and plant disease resistance. Nature 411:843–847
Doran JW, Zeiss MR (2000) Soil health and sustainability: managing the biotic component of soil quality. App Soil Ecol 15:3–11. https://doi.org/10.1016/S0929-1393(00)00067-6
Doso Jnr S (2014) Land degradation and agriculture in the Sahel of Africa: causes, impacts and recommendations. J Agric Sci App 3:67–73. https://doi.org/10.14511/jasa.2014.030303
Fey MV (2010) A short guide to the soils of South Africa, their distribution, properties, classification, genesis, use and environmental significance. In: Soils of South Africa, Cambridge University press, pp. 11–53. Editor: Karoline Hanks
Franco JA, Bañón S, Vicente MJ, Miralles J, Martínez-Sánchez JJ (2011) Root development in horticultural plants grown under abiotic stress conditions–a review. J Hort Sci Biotech 86:543–556. https://doi.org/10.1080/14620316.2011.11512802
Gajdanowicz P, Michard E, Sandmann M, Rocha M, Corrêa LGG, Ramírez-Aguilar SJ, Gómez-Porras JL, González W, Thibaud JB, Van Dongen JT, Dreyer I (2011) Potassium (K+) gradients serve as a mobile energy source in plant vascular tissues. Proc Nat Acad Sci 108:864–869. https://doi.org/10.1073/pnas.1009777108
Ghasemzadeh A, Jaafar HZ, Rahmat A (2010) Elevated carbon dioxide increases contents of flavonoids and phenolic compounds, and antioxidant activities in Malaysian young ginger (Zingiber officinale Roscoe.) varieties. Molecules 15:7907–7922. https://doi.org/10.3390/molecules15117907
Ghasemzadeh A, Jaafar HZ, Rahmat A, Wahab PEM, Halim MRA (2010) Effect of different light intensities on total phenolics and flavonoids synthesis and anti-oxidant activities in young ginger varieties (Zingiber officinale Roscoe). Int J Molec Sci 11:3885–3897. https://doi.org/10.3390/ijms11103885
Giesler R, Petersson T, Högberg P (2002) Phosphorus limitation in boreal forests: effects of aluminum and iron accumulation in the humus layer. Ecosystems 5:300–314. https://doi.org/10.1007/s10021-001-0073-5
Gruz J, Novák O, Strnad M (2008) Rapid analysis of phenolic acids in beverages by UPLC–MS/MS. Food Chem 111:789–794. https://doi.org/10.1016/j.foodchem.2008.05.014
Güsewell S (2004) N: P ratios in terrestrial plants: variation and functional significance. New Phytol 164:243–266
Harper KT, Pendleton RL (1993) Cyanobacteria and cyanolichens: can they enhance availability of essential minerals for higher plants? The Great Basin Naturalist 53:59–72. https://www.jstor.org/stable/41712758
Hassan MK, McInroy JA, Kloepper JW (2019) The interactions of rhizodeposits with plant growth-promoting rhizobacteria in the rhizosphere: a review. Agriculture 9:142
Haumaier L, Zech W (1995) Black carbon—possible source of highly aromatic components of soil humic acids. Organic Geochem 23:191–196. https://doi.org/10.1016/0146-6380(95)00003-W
Huang B, Fry JD (1998) Root anatomical, physiological, and morphological responses to drought stress for tall fescue cultivars. Crop Sci 38:1017–1022. https://doi.org/10.2135/cropsci1998.0011183X003800040022x
Husson O (2013) Redox potential (Eh) and pH as drivers of soil/plant/microorganism systems: a transdisciplinary overview pointing to integrative opportunities for agronomy. Plant Soil 362:389–417. https://doi.org/10.1007/s11104-012-1429-7
Jaleel CA, Riadh K, Gopi R, Manivannan P, Ines J, Al-Juburi HJ, Chang-Xing Z, Hong-Bo S, Panneerselvam R (2009) Antioxidant defense responses: physiological plasticity in higher plants under abiotic constraints. Acta Physiol Plant 31:427–436. https://doi.org/10.1007/s11738-009-0275-6
John Bullied W, Buss TJ, Kevin Vessey J (2002) Bacillus cereus UW85 inoculation effects on growth, nodulation and N accumulation in grain legumes: field studies. Can J Plant Sci 82:291–298. https://doi.org/10.4141/P01-048
Jones JDG, Dangl JL (2006) The plant immune system. Nature 444:323–329. https://doi.org/10.1038/nature05286
Khang DT, Dung TN, Elzaawely AA, Xuan TD (2016) Phenolic profiles and antioxidant activity of germinated legumes. Foods 5:27. https://doi.org/10.3390/foods5020027
Koerselman W, Meuleman AFM (1996) The vegetation N: P ratio: a new tool to detect the nature of nutrient limitation. J App Ecol 33:1441–1450
Koksal G, Gurkan E, Kucukcezzar R (1988) Relationship of potassium deficiency and abscisic acid metabolism in Soyabean plants. J Plant Nutri 11:517–523. https://doi.org/10.1080/01904168809363819
Kunwar VS, Lamichhane J, Gauchan DP (2018) Strategies to improve phosphorus availability in a sustainable agricultural system. Int J Inn Sci Res Tech 3:323–331
Lal R (2008) Soils and sustainable agriculture. Rev Agr Sust Develop 28:57–64. https://doi.org/10.1051/agro:2007025
Lambers H, Raven JA, Shaver GR, Smith SE (2008) Plant nutrient-acquisition strategies change with soil age. Trends Ecol Evo 23:95–103. https://doi.org/10.1016/j.tree.2007.10.008
Lattanzio V (2013) Phenolic Compounds: Introduction 50. Nat Prod pp 1543–1580
Lema MA, Majule AE (2009) Impacts of climate change, variability and adaptation strategies on agriculture in semi-arid areas of Tanzania: The case of Manyoni District in Singida Region, Tanzania. Afr J Environ Sci Tech 3:206–218. https://doi.org/10.1016/j.tree.2007.10.008
Li ZH, Wang Q, Ruan X, Pan CD, Jiang DA (2010) Phenolics and plant allelopathy. Molecules 15:8933–8952. https://doi.org/10.3390/molecules15128933
Lü XT, Reed S, Yu Q, He NP, Wang ZW, Han XG (2013) Convergent responses of nitrogen and phosphorus resorption to nitrogen inputs in a semiarid grassland. Glob Change Biol 19:2775–2784
Lyman OR (1998) An introduction to statistical methods and data analysis, 4th edn. Duxbury Press, United State, pp 12–19
Magadlela A, Pérez-Fernández MA, Kleinert A, Dreyer LL, Valentine AJ (2016) Source of inorganic N affects the cost of growth in a legume tree species (Virgilia divaricata) from the Mediterraneantype Fynbos ecosystem. J Plant Ecol 9:752–761
Maisels F, Gautier-Hion A, Gautier JP (1994) Diets of two sympatric colobines in Zaire: more evidence on seed-eating in forests on poor soils. Int J Primatol 15:681. https://doi.org/10.1007/BF02737427
Mandal SM, Mandal M, Das AK, Pati BR, Ghosh AK (2009) Stimulation of indoleacetic acid production in a Rhizobium isolate of Vigna mungo by root nodule phenolic acids. Arch Microbiol 191:389–393
Mandal SM, Chakraborty D, Dey S (2010) Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Signal Behav 5:359–368. https://doi.org/10.4161/psb.5.4.10871
Mandiringana OT, Mnkeni PNS, Mkile Z, Van Averbeke W, Van Ranst E, Verplancke H (2005) Mineralogy and fertility status of selected soils of the Eastern Cape Province, South Africa. Comm Soil Sci Plant Analysis 36:2431–2446
Mantri N, Patade V, Penna S, Ford R, Pang E (2012) Abiotic stress responses in plants: Present and future. Abiotic Stress Responses in Plants. Springer, New York, NY, USA, pp 1–19
Matiwane SE, Aremu AO, Valentine AJ, Magadlela A (2019) Nutritional status of KwaZulu-Natal soils affects microbe symbiosis, nitrogen utilization and growth of Vigna radiata (L.) R. Walczak s Afri J Bot 126:115–120. https://doi.org/10.1016/j.sajb.2019.06.007
McCallum MH, Peoples MB, Connor DJ (2000) Contributions of nitrogen by field pea (Pisum sativum L.) in a continuous cropping sequence compared with a lucerne (Medicago sativa L.)-based pasture ley in the Victorian Wimmera. Austral J Agric Res 51:13–22. https://doi.org/10.1071/AR99023
Meena VS, Bahadur I, Maurya BR, Kumar A, Meena RK, Meena SK, Verma JP (2016) Potassium-solubilizing microorganism in evergreen agriculture: an overview. In Potassium solubilizing microorganisms for sustainable agriculture, Springer, New Delhi. pp. 1–20. Editors: Vijay Singh Meena, Bihari Ram Maurya, Jay Prakash Verma and Ram Swaroop Meena. https://doi.org/10.1007/978-81-322-2776-2
Mishra PK, Mishra S, Selvakumar G, Kundu S, Shankar Gupta H (2009) Enhanced soybean (Glycine max L.) plant growth and nodulation by Bradyrhizobium japonicum-SB1 in presence of Bacillus thuringiensis-KR1. Acta Agric. Scan Section B-Soil and Plant Sci 59:189–196. https://doi.org/10.1080/09064710802040558
Moran JF, Klucas RV, Grayer RJ, Abian J, Becana M (1997) Complexes of iron with phenolic compounds from soybean nodules and other legume tissues: prooxidant and antioxidant properties. Free Radic Biol Med 22:861–870
Mortimer PE, Pérez-Fernández MA, Valentine AJ (2008) The role of arbuscular mycorrhizal colonization in the carbon and nutrient economy of the tripartite symbiosis with nodulated Phaseolus vulgaris. Soil Biol Biochem 40:1019–1027
Nambiar KKM, Gupta AP, Fu Q, Li S (2001) Biophysical, chemical and socio-economic indicators for assessing agricultural sustainability in the Chinese coastal zone. Agri Ecos Environ 8:209–214. https://doi.org/10.1016/S0167-8809(01)00279-1
Nannipieri P, Ascher J, Ceccherini M, Landi L, Pietramellara G, Renella G (2017) Microbial diversity and soil functions. Europ J Soil Sci 54:655–670. https://doi.org/10.1111/ejss.4_12398
Nielsen KL, Eshel A, Lynch JP (2001) The effect of phosphorus availability on the carbon economy of contrasting common bean (Phaseolus vulgaris L.) genotypes. J Exp Bot 52:329–339
Ohyama T (2017) The Role of Legume-rhizobium symbiosis in sustainable agriculture. In Legume Nitrogen Fixation in Soils with Low Phosphorus Availability. Springer, Cham. pp. 1–20. Editors: Saad Sulieman and Lam-Son Phan Tran.
Onwuka MI, Ozurumba UV, Nkwocha OS (2016) Changes in soil pH and exchangeable acidity of selected parent materials as influenced by amendments in South East of Nigeria. J Geosci Environ Protec 4:80. https://doi.org/10.4236/gep.2016.45008
Parvaiz A, Satyawati S (2008) Salt stress and phyto-biochemical responses of plants—a review. Plant, Soil Environ 54:89–99. https://doi.org/10.17221/2774-PSE
Peng S, Eissenstat DM, Graham JH, Williams K, Hodge NC (1993) Growth depression in mycorrhizal citrus at high-phosphorous supply. Plant Physiol 101:1063–1071
Pérez-Fernández MA, Elliot CP, Valentine A, Oyola JA (2019) Seed provenance determines germination responses of Rumex crispus (L.) under water stress and nutrient availability. J Plant Ecol 12:949–961
Pietta P, Minoggio M, Bramati L (2003) Plant polyphenols Structure, occurrence and bioactivity. Studies Natural Products Chem Elsevier 28:257–312
Prajapati K, Modi HA (2012) The importance of potassium in plant growth–a review. Indian J Plant Sci 1:177–186
Puri A, Padda KP, Chanway CP (2016) Evidence of nitrogen fixation and growth promotion in canola (Brassica napus L.) by an endophytic diazotroph Paenibacillus polymyxa P2b–2R. Biol Fert Soils 52:119–125. https://doi.org/10.1007/s00374-015-1051-y
Ramakrishna A, Ravishankar GA (2011) Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav 6:1720–1731. https://doi.org/10.4161/psb.6.11.17613
Rengel Z, Marschner P (2005) Nutrient availability and management in the rhizosphere: exploiting genotypic differences. New Phytol 168:305–312. https://doi.org/10.1111/j.1469-8137.2005.01558.x
Rivas-San Vicente M, Plasencia J (2011) Salicylic acid beyond defence: its role in plant growth and development. J Exp Bot 62:3321–3338. https://doi.org/10.1093/jxb/err031
Robert CA, Ferrieri RA, Schirmer S, Babst BA, Schueller MJ, Machado RA, Arce CC, Hibbard BE, Gershenzon J, Turlings TC, Erb M (2014) Induced carbon reallocation and compensatory growth as root herbivore tolerance mechanisms. Plant, Cell & Environ 37:2613–2622. https://doi.org/10.1111/pce.12359
Roberts VG, Adey S, Manson AD (2003) An investigation into soil fertility in two resource-poor farming communities in KwaZulu-Natal (South Africa). South Afri J Plant Soil 20:146–151. https://doi.org/10.1080/02571862.2003.10634924
Rutigliano FA, D’Ascoli R, De Santo AV (2004) Soil microbial metabolism and nutrient status in a Mediterranean area as affected by plant cover. Soil Biol Biochem 36:1719–1729. https://doi.org/10.1016/j.soilbio.2004.04.029
Sanz-Saez A, Morales F, Arrese-Igor C, Aranjuelo I (2017) P Deficiency: a major limiting factor for rhizobial symbiosis. In: Legume Nitrogen Fixation in Soils with Low Phosphorus Availability. Springer, Cham. pp. 21–39. Editors: Saad Sulieman and Lam-Son Phan Tran. https://doi.org/10.1007/978-3-319-55729-8
Seneviratne G, Jayasinghearachchi HS (2003) Phenolic acids: possible agents of modifying N2-fixing symbiosis through rhizobial alteration? Plant Soil 252:385–395
Shearer G, Kohl DH (1986) N2-fixation in field settings: estimations based on natural 15N abundance. Funct Plant Biol 13:699–756
Sheng XF, Zhao F, He LY, Qiu G, Chen L (2008) Isolation and characterization of silicate mineral-solubilizing Bacillus globisporus Q12 from the surfaces of weathered feldspar. Can J Microbiol 54:1064–1068. https://doi.org/10.1139/W08-089
Siciliano SD, Palmer AS, Winsley T, Lamb E, Bissett A, Brown MV, van Dorst J, Ji M, Ferrari BC, Grogan P, Chu H (2014) Soil fertility is associated with fungal and bacterial richness, whereas pH is associated with community composition in polar soil microbial communities. Soil Biol Bioch 78:10–20. https://doi.org/10.1016/j.soilbio.2014.07.005
Stiles WC (2004) Soil analysis and interpretation. NY Fruit Quart 12:28–30
Syed BA, Patel B (2014) Investigation and correlation of soil biotic and abiotic factors affecting agricultural productivity in semi-arid regions of North Gujarat, India. Int J Res Stud Biosci 2:18–29
Tessier JT, Raynal DJ (2003) Use of nitrogen to phosphorus ratios in plant tissue as an indicator of nutrient limitation and nitrogen saturation. J Appl Ecol 40:523–534
Thiruvengadam M, Baskar V, Kim SH, Chung IM (2016) Effects of abscisic acid, jasmonic acid and salicylic acid on the content of phytochemicals and their gene expression profiles and biological activity in turnip (Brassica rapa ssp. rapa). Plant Growth Regul 80:377–390. https://doi.org/10.1007/s10725-016-0178-7
Vaughan D, Ord B (1990) Influence of phenolic acids on morphological changes in roots of Pisum sativum. J Sci Food Agric 52:289–299
Villadas PJ, Díaz-Díaz S, Rodríguez-Rodríguez A, Arco-Aguilar MD, Fernández-González AJ, Pérez-Yépez J, Arbelo C, González-Mancebo JM, Fernández-López M, León-Barrios M (2019) The Soil Microbiome of the Laurel Forest in Garajonay National Park (La Gomera, Canary Islands): comparing unburned and burned habitats after a wildfire. Forests 10:1051–1067. https://doi.org/10.3390/f10121051
Voroney RP (2007) The soil habitat. In Soil Microbiology, Ecology and Biochemistry (3rd Ed). Academic Press. pp. 25–49. Editor: Eldor A. Paul. https://doi.org/10.1016/B978-0-08-047514-1.50006-8
Wang Y, Shi Y, Li B, Shan C, Ibrahim M, Jabeen A, Xie G, Sun G (2012) Phosphate solubilization of Paenibacillus polymyxa and Paenibacillus macerans from mycorrhizal and non-mycorrhizal cucumber plants. African J Microbiol Research 6:4567–4573. https://doi.org/10.5897/AJMR12.261
Wang LY, Wang TS, Chen SF (2015) Cohnella capsici sp. nov., a novel nitrogen-fixing species isolated from Capsicum annuum rhizosphere soil, and emended description of Cohnella plantaginis. Antonie Van Leeuwenhoek 107:133–139. https://doi.org/10.1007/s10482-014-0310-5
Williams K, Percival F, Merino J, Mooney HA (1987) Estimation of tissue construction cost from heat of combustion and organic nitrogen content. Plant Cell Environ 10:725–734
Williams J, Bordas V, Gascoigne H (2004) Conserving land and water for society: global challenges and perspectives. In: Conserving soil and water for society: Sharing solutions 13th ISCO, 2004. Paper no. 101. ISCO, Brisbane, Queensland (pp. 1–16).
Wu Y, Hendershot W (2010) The effect of calcium and pH on nickel accumulation in and rhizotoxicity to pea (Pisum sativum L.) root-empirical relationship and modelling. Environm Pollu 158:1850–1856
Zawoznik MS, Garrido LM, del Pero Martinez MA, Tomaro ML (2000) Effect of vanillin on growth and symbiotic ability of Bradyrhizobium sp. (Arachis) strain. Proc Int Plant Growth-promoting Rhizobacteria.
Zhalnina K, Dias R, de Quadros PD, Davis-Richardson A, Camargo FA, Clark IM, McGrath SP, Hirsch PR, Triplett EW (2015) Soil pH determines microbial diversity and composition in the park grass experiment. Microbial Ecol 69:395–406. https://doi.org/10.1007/s00248-014-0530-2
Zungu NS, Egbewale SO, Olaniran AO, Pérez-Fernández MA, Magadlela A (2020) Soil nutrition, microbial composition and associated soil enzyme activities in KwaZulu-Natal grasslands and savannah ecosystems soils. App Soil Ecol 155:103663
Acknowledgements
The technical assistance rendered by Lucie Slobodianová is greatly appreciated. We acknowledge the Central Analytical Facilities at Stellenbosch University and the Archeometry Department at the University of Cape Town for their research facilities. The authors would like to thank the National Research Foundation (NRF), South Africa for funding this work (grant no. UID 113576
Funding
The authors would like to thank the National Research Foundation (NRF), South Africa for funding this work (grant no. UID 113576). We also appreciate the financial support from the Czech Science Foundation (no. 17-06613S) and ERDF project “Development of pre-applied research in nanotechnology and biotechnology (No. CZ.02.1.01/0.0/0.0/17_048/0007323).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Magadlela, A., Zungu, N.S., Khoza, T. et al. Metabolic Self-regulation of Pisum sativum L. Under Varying Soil Fertility in South Africa. J Soil Sci Plant Nutr 23, 177–189 (2023). https://doi.org/10.1007/s42729-022-00930-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-022-00930-9