Abstract
The immobilization of soil cadmium (Cd) by biochar and modified biochar is an eco-friendly and cost-effective strategy. In the current study, the effect of raw biochar (BC) and iron-modified biochar (Fe-BC) derived from common reed on the fractionation and mobility of Cd was evaluated, as was its effect on soil microbial activity in contaminated calcareous soil. Treatments involved a combination of two factors: type of biochar (CK: Control, BC, and Fe-BC) and soil Cd concentration (0, 15, and 30 mg kg−1). Treatments were applied to the soil and incubated for 90 days. The application of both biochars increased soil pH and soil organic carbon content (16.6–48.0%), microbial biomass carbon (40.5–75.1%), basal respiration (16.6–48.0%), substrate-induced respiration (12.4–41.9), and dehydrogenase activity (25.5–102.1%), while it reduced diethylene-triamine pentaacetic acid (DTPA)-extractable Cd (22.1–39.5%). The addition biochars, particularly Fe-BC, prominently decreased the concentration of exchangeable and carbonate fractions and increased the concentration of Fe-MnOx, as well as the organic and residual fractions of Cd in the soil. Moreover, relative to the control treatment, the incorporation of raw and Fe-modified biochar into 30 mg kg−1 Cd-spiked soil significantly decreased the Cd mobility factor (MF) value by 14.5 and 21.8%, respectively. Fe-modified biochar had a more significant impact than raw biochar on the immobilization of Cd in the soil, and its improved soil microbial activity to a greater extent. Overall, the findings indicate that Fe-modified biochar derived from common reed can immobilize Cd and improve soil microbial attributes in contaminated calcareous soil. Therefore, it can be used as an eco-friendly amendment for restoring Cd-contaminated calcareous soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The contamination of soils with potentially toxic elements (PTEs) has become a global concern due to its harmful effects on soil biota and plant and human health (Karimi et al. 2018; Khodaverdiloo et al. 2020). Cadmium (Cd) is a highly toxic mobile PTE that is carcinogenic to all living organisms (Karimi et al. 2018; Khodaverdiloo et al. 2020). Anthropogenic activities, such as applying agrochemicals, smelting, mining, and irrigating wastewater irrigation, have accelerated Cd contamination in soils (Kamran et al. 2019; Rajendran et al. 2019). Cadmium is a non-biodegradable PTE and is highly persistent in soils. Cadmium has no biological role, and a high concentration of this element in soil is toxic to soil microorganisms. Toxic levels of Cd have adverse effects on microbial activities via several mechanisms, including by displacing essential nutrients, inhibiting enzyme synthesis, damaging cell membranes, and creating oxidative stress (Xu et al. 2018). Therefore, Cd-contaminated soils must be remediated if the associated environmental risks are to be restricted (Tan et al. 2020).
In situ immobilization is a known efficacious and environmentally friendly method for reducing the availability and mobility of PTEs in contaminated soil via the application of proper amendments such as biochar (Chen et al. 2020; Tu et al. 2020). Biochar is a carbon-rich material produced from pyrolysis of biomass under anaerobic or oxygen-limited conditions (IBI 2015; Wang et al. 2020). Biochar has attracted the attention of researchers interested in the immobilization of PTEs in contaminated soils due to its favorable sorption properties such as its microporous structure, high carbon content, active functional groups, and large specific surface areas (SSAs) (Wang and Wang 2019; Tu et al. 2020). Moreover, biochar can affect PTE availability in soil by changing specific attributes of the soil, such as pH and organic matter (OM) content. Generally, the efficacy of biochar in the immobilization of PTEs in soil depends on its physicochemical traits (Wang et al. 2020; Lyu et al. 2020; Wan et al. 2020).
The effects of biochar on soil biological attributes are often neglected in PTE-contaminated soil. Eco-friendly amendments should not only decrease the mobility of PTEs in contaminated soil, but they should also improve the soil biological status (Tang et al. 2020b). Biochar can modulate microbial activities in PTE-contaminated soils by improving the soil physical and chemical attributes, supplementing soil labile carbon and nutrients, and reducing the toxicity of PTEs in the soil (Xu et al. 2018).
Although biochar can immobilize PTEs in contaminated soil, its PTE immobilization efficiency is limited by its lack of adsorption sites. Therefore, a suitable method for improving biochar’s adsorption capacity needs to be developed (Zhang et al. 2020; Wang et al. 2020; Lyu et al. 2020). Qiao et al. (2018) reported that the combined amendment of biochar and zero-valent iron (ZVI) had great potential for reducing Cd availability in contaminated paddy soil over single biochar or ZVI amendments. Chemical modifications present a promising method for improving the physicochemical traits of biochar, such as its porous structure, specific surface areas, and the surface functional groups, which can increase its capability for the stabilization of PTEs in contaminated soils (Wang et al. 2020; Zhang et al. 2020). Among the currently known chemical modification approaches, saturating the biomass with metal ions (such as FeCl2, MgCl2, and ZnCl2) has been highlighted as an exceptional method for PTE stabilization in contaminated environments (Yu et al. 2020; Feng et al. 2020). Metal ion modification can enhance the SSA of biochar and can increase the number of oxygen-containing functional groups of biochar. These effects, in turn, enhance the interactions between the biochar and PTEs in the soil via several mechanisms, such as the formation of surface complexes, precipitation, ion exchange, and cation bonding (Li et al. 2017; Chen et al. 2020). Previous researches shows that the biomass pretreatment of FeCl3 solutions improves the stabilization performance of PTE adsorption (Feng et al. 2018; Xu et al. 2020). Pan et al. (2019) application of Fe-modified biochar successfully decreased Cd availability in a contaminated paddy soil.
Determinations of PTEs chemical fractions in soil can be considered when investigating the immobilization performance of biochar (Boostani et al. 2019; Wang et al. 2020). It is widely confirmed that distribution of PTEs chemical fractions affects their mobility. The conversion of PTEs from labile fractions (exchangeable and carbonate bounded) to less-labile fractions (Fe-Mn oxide bounded, organically bounded, and residual) leads to PTE immobilization (Boostani et al. 2019; Palansooriya et al. 2020; Wang et al. 2020). Previous studies have used different biochar amendments to stabilize Cd-contaminated soils (Ali et al. 2020; Xu et al. 2020). For example, Xu et al. (2020) reported that corn straw biochar reduced the availability of Cd in soil by transforming Cd into a state of lower availability. Ali et al. (2020) found that the labile fractions of Cd in smelter-contaminated soil decreased after the addition of apricot shell– and apple tree–derived biochar; meanwhile, the soil recalcitrant fractions and microbial activity increased. Qiao et al. (2019) reported that the ZVI-modified biochar decreased Cd availability in an alkaline paddy soil by enhancing the transformation of available Cd fractions into less available fractions.
Changes in Cd mobility induced by biochar can affect the microbial traits of soil (Tang et al. 2020b). However, to the best of our knowledge, there is little information available about the effect of Fe-modified biochar on the microbial attributes of contaminated calcareous soils with low OM content with varying levels of Cd. Moreover, the performance of Fe-modified common reed biochar on the stabilization of Cd in contaminated calcareous soils has not yet been studied. Thus, this study was designed to investigate the potential of raw and Fe-modified biochar to immobilize Cd and its impact on the microbial activity of contaminated calcareous soil. The results provide new insights into the development of biochar amendments for the sustainable remediation of Cd-contaminated calcareous soils.
We hypothesized that modifying common reed biochar by adding Fe would enhance its Cd stabilization capability in the soil. We also proposed that adding raw and Fe-modified biochars derived from common reed would promote the immobilization of Cd and soil microbial attributes while exhibiting a clear dependence on the biochar’s traits and changes in Cd mobility and distribution.
2 Materials and Methods
The experimental soil (classified as Typic Haplocalcids) was obtained from the surface layer (0–20 cm) of an experimental farm situated in Ahvaz, Khuzestan province, SW Iran. Twenty samples from different plots were collected and mixed thoroughly to obtain a composite sample. Detailed information about the study area, soil sampling process, and analysis is given in Karimi et al. (2019). The soil sample was air-dried and sieved (< 2 mm). Afterward, specific characteristics of the soil were measured using standard laboratory methods as described in Carter and Gregorich (2008).
The raw common reed was collected, air-dried, and passed through a 2-mm sieve (Singh et al. 2017). The prepared common reed biomass was soaked in a 0.5 M FeCl2 solution. The methods described by Singh et al. (2017) were followed when measuring the biochar’s traits.
An incubation study involving nine treatments and three replications was performed following a randomized complete design in a factorial arrangement. Cadmium-contaminated soil samples were thoroughly mixed with the biochars in plastic jars. The samples were kept in an incubator for 90 days. At the end of the incubation period, all soil samples were air-dried, thoroughly mixed, and passed through a 2-mm sieve. The soil traits were measured using the same methods as those described for the initial soil. The chemical fractions of Cd in the soil were determined using the sequential extraction method of Tessier et al. (1979).
2.1 Soil Spiking with Cd
The prepared soil was spiked with salt of cadmium nitrate Cd(NO3)2 at concentration of 15 (Cd15) and 30 (Cd30) mg Cd kg−1 dry soil. Control soil (Cd0), without addition of Cd, was also used for comparison. The soil was thoroughly mixed in plastic containers. The spiked soil was placed into plastic containers in three replicates for each treatment. The Cd-spiked soil was mixed completely and incubated at 25 °C under periodic wetting-drying moisture conditions (without drainage) for 7 months to achieve equilibrium distribution and homogenization of Cd in the soil (Karimi et al. 2017a, b; Karimi et al. 2018). After incubation period, the Cd-spiked soil was air-dried and prepared for future study.
2.2 Biochar Preparation and Characterization
The raw common reed was collected, air-dried, and passed through a 2-mm sieve (Singh et al. 2017). The prepared common reed biomass was soaked in 0.5 M FeCl2 solution with the biomass/FeCl2 ratio of 1:5 for 2 days then filtered and dried at 105 °C for 4 h under anoxic conditions (Feng et al. 2020). The raw and Fe-soaked common reed biomass were then pyrolyzed at 500 °C for 3 h with a heating rate of 5 °C min−1 in an electrical furnace under anaerobic conditions. The N2 flow was used to maintained anaerobic condition (Khajavi-Shojaei et al. 2020). The raw (un-modified) biochar and modified biochar were named BC and Fe-BC respectively.
The methods described by Singh et al. (2017) were used to measure biochars traits. The specific surface area (SSA) was measured by the BET (Brunauer-Emmett-Teller) method (Micromeritics Gemini 2380, US), using N2 as the adsorbate gas. The total elemental composition (C, H, and N) of prepared biochars was determined by elemental analyzer (Vario EL III, Germany). The scanning electron microscope (SEM) (Leo 1455 VP, UK) imaging analyses were used to evaluate the surfaces and structure of biochar samples. The surface functional groups of the biochar samples were analyzed using a Fourier-transform infrared spectrometer (FTIR) (Spectrum Perkin Elmer GX, USA).
2.3 Incubation Test and Analysis
An incubation study was performed based on randomized complete design, as a factorial arrangement, with nine treatments and three replications. Treatments were the combination of two factors: (1) soil Cd concentration at the rate of 0, 15, and 30 mg kg−1 dry soil, and (2) biochar types including control treatment (CK: without the biochar addition), BC, and Fe-BC (applied at 2% w/w). Cadmium-contaminated soil samples (500 g) were thoroughly mixed with the biochars in plastic jars. Afterwards, samples were kept in an incubator for 90 days at moisture of 70% of the field water holding capacity and temperature of 25 °C.
At the end of incubation period, soil samples were air-dried, thoroughly mixed, and passed through a 2-mm sieve. The soil pH was measured in a 1:1 soil/deionized water ratio. The soil OC and available Cd concentration were measured using the same methods described above for the initial soil. Chemical fractions of Cd in the soil were determined using sequential extraction method of Tessier et al. (1979), which separated the soil Cd into five fractions: (1) exchangeable (Exch), (2) carbonates bounded (Car), (3) iron–manganese oxides bounded (Fe-MnOx), (4) organic matter bounded (OM), and (5) residual (Res) (Table SI). For evaluating the potential of biochar treatments in immobilization of Cd mobility at different levels of Cd contamination, the mobility factor (MF) of Cd in the soil was calculated as follows (Kabala and Singh 2001).
For evaluating the influence of biochar amendments on microbial activity, soil basal respiration (BR) by Anderson method (1982), substrate-induced respiration (SIR) using the method described by Alef and Nannipieri (1995), soil microbial biomass carbon (MBC) by the fumigation-extraction procedures (Jenkinson and Ladd 1981), and dehydrogenase activity (Alef and Nannipieri 1995), as important and common soil microbial indicator of soil dehydrogenase activity (DHA) is considered an indicator of overall soil microbial activity which occur intracellular in all living microbial cells (Karimi et al. 2020), were measured (Table 1).
2.4 Data Analysis
Two-factor analysis of variance (ANOVA) was carried out to evaluate statistical analysis of experimental data through the SAS 9.4 software. Mean comparison of different treatment was determined by the Duncan’s at P < 0.05.
3 Results
3.1 Characterization of the Biochars
The results showed that the physicochemical traits of biochar changed after being modified with FeCl2 (Table 2). The Fe-BC had a higher pH, EC, total Fe concentration, and O and H content than BC (Table 2). FTIR spectroscopy was carried out to evaluate the extent to which the Fe-modification of biochar impacted the presence of surface functional groups in the Fe-BC (Fig. 1). The FTIR spectroscopy results showed that the intensities of bands of oxygen-containing functional groups in Fe-BC treatment were significantly higher than in the BC treatment (Fig. 1).
3.2 Soil pH and SOC
The soil pH increased after the application of BC (0.30–0.33 unit) and Fe-BC (0.16–0.23 unit) for all Cd concentration levels (Fig. 2). Significant differences in soil pH were observed when the BC and Fe-BC treatments were compared. However, no significant differences in soil pH were found when the Cd0 and other Cd concentrations (Cd15 and Cd30) were compared.
Relative to the control condition, the soil SOC content increased proportionally with the application of BC (3.29–3.3 fold) and Fe-BC (2.93–2.99 fold) for all soil Cd concentrations (Fig. 2). Again, no significant differences in soil SOC content were observed when comparing the different soil Cd concentration levels (Cd0, Cd15, and Cd30).
3.3 Availability and Fractionation of Cd in Soil
The results indicated that for different Cd levels, adding BC and Fe-BC to the soil significantly decreased the availability of Cd (DTPA-extractable Cd) in the soil by 22.1–28.6% and 33.1–39.5%, respectively, when compared to the control treatment (Fig. 3). Moreover, the soil exchangeable Cd fraction significantly decreased at the Cd15 and Cd30 levels after the addition of BC (by 11.3 and 21.5%), and Fe-BC (by 25.1 and 24.6%) when compared with the control treatment (Fig. 3).
DTPA-extractable Cd and Cd distribution in exchangeable (Exch), bound to carbonates (Car), bound to iron–manganese oxides (Fe-MnOx), bound to organic matter (OM), and residual fractions (Res) in different treatments. Different letters show the significant difference according to Duncan’s test at 5% probability level (n = 3). CK, control; BC, raw biochar; Fe-BC, Fe-modified biochar
The addition of BC and Fe-BC biochars decreased the carbonate Cd fraction in the soil when compared to the control at all Cd levels (Fig. 3). At all Cd concentration levels, the application of Fe-BC reduced the carbonate Cd fraction more substantially than the application of BC in the soil. The concentration of Cd bound to Fe-MnOx in the soil increased after the addition of BC (7.3–14.3%) and Fe-BC (11.3–21.5%) at all Cd levels (Fig. 3). Furthermore, when compared to the control treatment, the application of both biochar types significantly enhanced the binding of Cd to OM (2.3- to 5.5-fold) and residual (1.3–2.15-fold) fractions at all Cd levels (Fig. 3).
The relative percentage of Cd in control soil at all Cd levels in the soil was as follows: Car > Fe-MnOx > Exch > Res > OM (36.5–46.1% and 2.1–4.8% for Car and OM fraction, respectively); whereas in the Fe-BC-amended soil at Cd15 and Cd30, the trend was as follows, respectively: Car > Fe-MnOx > Res > OM > Exch, and Car > Fe-MnOx > Res > Exch > OM (Fig. 4). Also, in BC treatment, Cd fractions at Cd15 and Cd30 were in the following order, respectively: Car > Fe-MnOx > Res > Exch > OM, and Car > Fe-MnOx > Exch > Res > OM. In all treatments, up to 35% of the total Cd in the soil was in Car fraction, and 20.7% in Fe-MnOx Cd fraction (Fig. 4).
Overall, the results of the proportion of Cd fractions at different levels of Cd in the soil clearly demonstrated that the application of BC and Fe-BC treatments considerably changed the Cd distribution in the soil and caused the Cd to convert from the Exch fraction to the OM and residual fractions (Fig. 4), thus reducing Cd bioavailability. The results also indicted that Fe-BC had a greater effect on decreasing the proportion of Cd Exch and Car fractions than that of BC.
3.4 Cd Mobility in Soil
The mean comparison of the mobility factor (MF) values at different Cd concentrations in the soil (Fig. 5) demonstrated that this factor significantly decreased with the addition of BC (10.8–11.4%) and Fe-BC (11.4–14.5%) compared to the control treatment (Fig. 5). In addition, the Cd mobility increased with increasing the Cd levels from Cd0 (44.2–56.8) to Cd30 (56.1–66.1) in the soil (Fig. 5). There were no significant differences in the biochar impact on the changes in Cd mobility factor at various levels of Cd in the soil (Fig. 5), which revealed that both BC and Fe-BC treatments had high efficiency in the Cd immobilization at different concentrations of Cd in the soil.
3.5 Soil Microbial Attributes
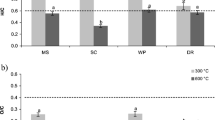
The results indicated that with increasing the Cd concentration in the soil, the soil MBC, BR, and SIR values significantly decreased (Fig. 6). The increases in Cd levels in the soil also led to a decrease in soil dehydrogenase activity (Fig. 6). At all Cd levels in the soil, the BC and Fe-BC addition significantly increased the soil MBC (40.5–75.1%), BR (16.6–48.0%), SIR (12.5–41.9%), and dehydrogenase activity (25.5–102.2%) values compared to those of the control treatment.
Soil basal respiration (BR), substrate-induced respiration (SIR), microbial biomass carbon (MBC), and dehydrogenase (DEH) activity in different treatments. Different letters show the significant difference according to Duncan’s test at 5% probability level (n = 3). CK, control; BC, raw biochar; Fe-BC, Fe-modified biochar
4 Discussion
The results of the biochar traits (Table 2) indicated that Fe was successfully loaded onto the biochar. This result is supported by the SEM image of the biochars, which showed that the Fe particles were more prominent in the Fe-BC than in the BC (Fig. S1). Overall, the results regarding the physicochemical traits and FTIR spectra of the biochars suggest that Fe-BC has a better adsorption capability than BC and perhaps more sorption sites for Cd stabilization.
Soil pH was most strongly affected by biochar alkalinity (Karimi et al. 2019; Tang et al. 2020a). The results showed that the biochar applied to the soil in this study has a high content of alkaline ions, such as potassium (K) (Table 2), which can neutralize the hydrogen ions in soil. Therefore, the increase in soil pH that accompanied the biochar treatments (BC and Fe-BC) could be attributed to the alkaline nature of these biochars. Moreover, the liming effect of the biochars could increase the soil CEC and decrease the amount of H+ (Tang et al. 2020a), which could further explain the increase in soil pH after the addition of biochars.
In other work, Karimi et al. (2020) also reported that a significant increase in the pH of calcareous soil that was amended with corn-residue biochar. The biochars used in the current study had a high carbon content (Table 2). Therefore, the application of the biochars used in this study caused a significant increase in soil SOC content. Our results agreed with the findings of Karimi et al. (2020), who reported that the addition of corn biochar increased the SOC content of calcareous soil under incubation conditions. Saffari et al. (2020) also reported that the SOC content in a sandy loam soil significantly increased due to the incorporation of corn-residue biochar.
The decrease in the DTPA-extractable Cd observed in the present study after the addition of biochars could be attributed to changes in Cd fractions in the soil. Soil pH strongly influences the reactions and availability of PTEs in calcareous soil (Karimi et al. 2019). In comparison to the control treatment, treatments that involved the addition of biochars to soil increased the soil pH in the current study (Fig. 2). The increased soil pH values accelerated the formation of sorption sites on the negatively charged soil colloids and clay particles (Xu et al. 2020). In addition, the increased soil pH due to the application of biochars can result in the formation of Cd hydroxide (Cd(OH)2) or phosphate precipitation, which can reduce the availability of Cd and promote its transition to low labile fractions (Zhao et al. 2016).
Therefore, the increase in soil pH resulting from biochar addition can be responsible for the decrease in soil Cd availability. This conclusion is confirmed by the negative linear regressions observed between the soil pH and DTPA-extractable Cd (R2 > 0.83, P < 0.01, Fig. S2). Congruent with our results, Tu et al. (2020) found that the application of maize biochar led to a significant decrease in the DTPA-extractable Cd in alkaline soil. The biochar application can enhance the exchangeable content of alkaline cations such as K, Ca, and Mg, which might promote the adsorption of Cd through the cation exchange and, in turn, reduce the concentration of exchangeable Cd in the soil (Xu et al. 2020). The results revealed that the Fe-BC reduced the Cd availability in the soil to a greater extent than BC. This result was probably due to the higher Cd stabilization of the Fe-BC treatment, which promotes adsorption (either by ionic exchange or by complexation), electrostatic attraction, and precipitation due to its unique physicochemical properties (Table 2).
The results showed that the application of both biochars decreased the carbonate Cd fraction in the soil (Fig. 3). This result may be due to the adsorption of Cd bound to carbonate by biochars through different mechanisms like complexation, precipitation, and physicochemical sorption (Chen et al. 2020). Furthermore, Cd bound to carbonate fraction was probably transformed to Cd bound to OM fraction. Therefore, the Cd carbonate fractions declined when BC and Fe-BC were added to the soil. The applied biochars, particularly Fe-BC, had abundant oxygen-containing functional groups on their surfaces (Fig. 1), which can react with the organic-bounded Cd, thus increasing Fe-MnOx fractions. Moreover, the increase in soil pH transforms Cd from other fractions into the Fe-MnOx fraction following the formation of precipitate complexes and increased negative charges, which, in turn, decreases Cd mobility (Chen et al. 2020). This finding is in line with previous observations of other PTE-contaminated calcareous soils amended with different biochars (Hamzenejad Taghlidabad and Sepehr 2018; Boostani et al. 2019).
The increased OM-Cd fractions associated with biochar treatments was probably due to the complexation between the elevated SOM with BC and Fe-BC addition and mobile metal ions in the soil. The results also indicate that the OM-Cd fraction was higher in the Fe-BC treatment than in the BC treatment in Cd-contaminated soil, which may be due to the greater oxygen-containing functional groups of the former (Fig. 1). Perhaps Cd showed a high preference for the carboxyl and hydroxyl functional groups of biochar surface (Tu et al. 2020). Therefore, the increased binding of Cd to OM fraction in this study might generate more oxygen-containing functional groups, mineral oxides, and OM of biochars. Ahmad et al. (2014) reported that the high immobilization of PTEs in biochar-amended soils was mainly due to the presence of mineral oxides and OM in biochar, which coprecipitated and made inner-sphere complexes with PTEs. Similar to the current study, other studies indicated that biochar concentration of PTEs bounded to OM in calcareous soil increased with the addition of different biochars (Boostani et al. 2019; Kabiri et al. 2019; Karimi et al. 2019).
In terms of the MF, which is a relative dynamic index of Cd, among the chemical fractions of Cd, the Exch and Car fractions are identified as mobile and having a high environmental risk (Kabala and Singh 2001). A high MF value is indicative of high PTE bioavailability (Kabala and Singh 2001; Boostani et al. 2019). The most significant relative reduction in MF value (14.4%) was observed with the application of Fe-BC by for the Cd30 treatment (Fig. 5). The results demonstrated that the applied biochars (especially Fe-BC) significantly immobilized the Cd in the soil. General mechanisms for soil PTE immobilization with the addition of biochars were as follows: (1) the enhanced soil pH (Fig. 2) and the introduction of ligands, which can reduce the Cd mobility via the formation of insoluble precipitate, which is supported by the strong negative relationship between the MF of Cd and soil pH (R2 > 0.88, P < 0.01) (Fig. S3), and (2) the increased soil OM (Fig. 2) that can bind to Cd and increase the adsorption and complexity of Cd through the activated surfaces of functional groups and, thus, the formation of stable complexes (Lahori et al. 2017).
In addition, mobile Cd cations transformed into low labile fractions (Fig. 4), perhaps via the formation of Cd (hydro-)oxide phosphate or carbonate. The formation of these compounds could be due to the existence of these anions, as shown in a previous study by Zhang et al. (2016). The lower values of MF associated with the Fe-BC treatment than BC demonstrated that Fe-BC had a substantial impact on Cd immobilization, which could be explained by some of its characteristics (e.g., higher pH, CEC, and specific surface area (Table 2), and its surface functional groups (Fig S2). These factors could have reduced Cd mobility via adsorption and the formation of surface complexes.
The presence of Fe in the Fe-BC treatment (Table 2) might have decreased Cd mobility via ion exchange mobility. Decreased PTEs mobility was also reported by Xu et al. (2016) in Cd- and Pb-contaminated soil that was treated with rice and wheat straw. Nonetheless, further research is needed to understand the interaction between Cd and biochars in the pedosphere in the long term. Ahmad et al. (2017) reported that a surface complexation including Fe-/Al-minerals or carboxyl groups and PTEs-phosphate precipitation significantly reduce PTE mobility in alkaline soil amended with biochars. Boostani et al. (2018) also reported that the MF of soil Cd in contaminated calcareous soil considerably decreased due to the addition of various types of biochars.
The decreases in MBC, BR, and SIR values due to Cd contamination (Fig. 6) could be attributed to increased Cd availability (Fig. 3) and mobility (Fig. 6) and possible Cd toxicity. This is confirmed by the significant negative correlation between soil Cd availability and soil microbial attributes (Table 3). Similarly, Xu et al. (2018) noticed that the microbial biomass values in Pb- and Cd-spiked soils were significantly lower than in non-spiked soil. In addition, the decrease in soil dehydrogenase activity (Fig. 6) associated with increased soil Cd levels could be due to Cd ions reactions with enzyme-substrates or their interactions with protein active groups (Tang et al. 2020b).
High concentrations of Cd in soil have a toxic effect on almost all microbes by affecting metabolic functions such as protein synthesis (Tang et al. 2019; Pan et al. 2020), thus causing variations in microbial biomass and activity (Kaurin et al. 2018). Although the total contents of Cd can exert either positive or negative impacts on microbial activity (Fajardo et al. 2019; Pan et al. 2020), in the present research, Cd fractions were dominant drivers of changes in the microbial activity of soil.
In the current study, the Cd level affected the microbial attributes of soil. However, the effects of its fractions, especially its mobile fractions (Exch and Car), were of particular interest since they might be more toxic to soil microorganisms than other fractions. The present findings also demonstrated that the Fe-MnOx fraction of Cd significantly impacts the microbial activity of soil. Pearson’s correlation coefficients (r) between chemical fractions of Cd and soil microbial attributes demonstrated that, among the five fractions of Cd in the soil, the Exch, Car, and Fe-MnOx fractions had the strongest effects on soil microbial attributes. These fractions were also toxic for soil microorganisms and inhibited microbial activity (Table 3). These effects can be attributed to the high mobility and bioavailability of these fractions in soil. In contrast, no significant correlations were noted between OM and the residual fractions of Cd and soil microbial attributes (Table 3).
The biochar amendments applied in the present study had significant positive impacts on all investigated soil microbial attributes (Fig. 6). Increases in the soil microbial biomass and respiration owing to the addition of biochar can be explained by the following factors: (1) increased soil SOC (Fig. 2) and supplemented labile carbon and soluble nutrients for microbial utilization (Xu et al. 2018; Herrmann et al. 2019); (2) reduced soil Cd availability (Fig. 3) and mobility (Fig. 5) and the subsequent decrease in Cd toxicity; (3) increased substrate availability and the release of accessible organic compounds (Karimi et al. 2020); and (4) the provision of suitable microbial habitat (Xu et al. 2018; Karimi et al. 2020). Biochar also acts as a source of energy for soil microbes and facilitates their survival in contaminated soils (Ali et al. 2020).
Similar results were reported for PTE-contaminated soils amended with various biochars (Xu et al. 2018; Tang et al. 2020b). Dehydrogenase is an important intracellular enzyme that is associated with soil microbial activity (Karimi et al. 2020; Tang et al. 2020b). The enhanced dehydrogenase activity associated with biochar addition can be explained by the resultant increases in soil SOC (Fig. 2) and microbial biomass (Fig. 6). In addition, the higher dehydrogenase values of the Fe-BC treatment (in comparison to the BC treatment) might be due to the greater microbial biomass of the Fe-BC treatment (Fig. 6). This difference was confirmed by the significant correlation between dehydrogenase activity and MBC values (R = 0.95, P < 0.05; Table S2).
In general, the soil SOC in the BC treatment was higher than that in the Fe-BC treatment. Nevertheless, the soil microbial biomass values of the Fe-BC-treated soil were higher than those of the BC-treated soil for all soil Cd levels, most notably at the Cd30 level. These results could be attributed to the higher SSA of the Fe-BC soil, the presence of Fe (Table 2), and the lower Cd availability, mobility, and toxicity associated with the Fe-BC soil. These results are supported by the strong negative correlations found among Cd availability, Cd mobility, and soil microbial attributes (Table 3). Moreover, the results of the current study revealed that the improvements in the microbial attributes of Cd-contaminated soil were due not only to the OC supplied by the biochar but also to its Cd immobilization capability.
5 Conclusions
In the current study, raw and FeCl2-modified common reed biochars were applied to Cd-contaminated calcareous soil. These biochar efficacies to immobilize Cd in soils with different Cd concentrations were evaluated for both types of biochar. Associated improvements in the soil microbial attributes were also assessed. The results showed that the Cd concentration did not affect biochars efficiencies to immobilize Cd. Both the applied biochars, particularly the FeCl2-modified biochar, increased the alteration of potential mobile fractions (exchangeable and carbonate) of Cd to organic and residual fractions. This effect was found to reduce Cd’s mobility and enhance the soil microbial activity. Soil pH and OM significantly changed the Cd distribution of the tested soils. Among the different chemical fractions of Cd in the soil, exchangeable Cd had the most significant correlation with variations in soil microbial attributes. The FeCl2-modified biochar outperformed the raw biochar in terms of Cd immobilization and soil microbial attribute improvements. Cadmium availability was found to be the main factor that regulated microbial activity. Based on the results of the present work, FeCl2-modified common reed biochar can be used as a soil remediation material to improve soil microbial activity and minimize Cd mobility in soil. However, further long-term studies are needed to explore the interactions among Fe-modified biochars, soil microorganisms, and Cd in contaminated calcareous soils.
References
Ahmad M, Rajapaksha AU, Lim JE, Zhang M, Bolan N, Mohan D, Vithanage M, Lee SS, Ok YS (2014) Biochar as a sorbent for contaminant management in soil and water: a review. Chemosphere 99:19–33. https://doi.org/10.1016/j.chemosphere.2013.10.071
Ahmad M, Lee SS, Lee SE, Al-Wabel MI, Tsang DC, Ok YS (2017) Biochar-induced changes in soil properties affected immobilization/mobilization of metals/metalloids in contaminated soils. J Soils Sediments 17(3):717–730. https://doi.org/10.1007/s11368-015-1339-4
Alef K, Nannipieri P (1995) Methods in applied soil microbiology and biochemistry. Academic Press
Ali A, Shaheen SM, Guo D, Li Y, Xiao R, Wahid F, Azeem M, Sohail K, Zhang T, Rinklebe J, Li R (2020) Apricot shell-and apple tree-derived biochar affect the fractionation and bioavailability of Zn and Cd as well as the microbial activity in smelter contaminated soil. Environ Pollut 10:114773. https://doi.org/10.1016/j.envpol.2020.114773
Boostani HR, Hardie A, Najafi-Ghiri M, Khalili D (2018) Investigation of cadmium immobilization in a contaminated calcareous soil as influenced by biochars and natural zeolite application. Int J Environ Sci Technol 15:2433–2446. https://doi.org/10.1007/s13762-017-1544-3
Boostani HR, Najafi-Ghiri M, Hardie AG, Khalili D (2019) Comparison of Pb stabilization in a contaminated calcareous soil by application of vermicompost and sheep manure and their biochars produced at two temperatures. J Appl Geochem 102:121–128. https://doi.org/10.1016/j.apgeochem.2019.01.013
Carter MR, Gregorich EG (2008) Soil sampling and methods of analysis. Canadian Society of Soil Science, CRC Press, Taylor and Francis Group, Boca Raton
Chen D, Wang X, Wang X, Feng K, Su J, Dong J (2020) The mechanism of cadmium sorption by sulphur-modified wheat straw biochar and its application cadmium-contaminated soil. Sci Total Environ 714:136550. https://doi.org/10.1016/j.scitotenv.2020.136550
Fajardo C, Costa G, Nande M, Botías P, García-Cantalejo J, Martín M (2019) Pb, Cd, and Zn soil contamination: monitoring functional and structural impacts on the microbiome. Appl Soil Ecol 135:56–64. https://doi.org/10.1016/j.apsoil.2018.10.022
Feng Z, Chen N, Feng C, Gao Y (2018) Mechanisms of Cr (VI) removal by FeCl3-modified lotus stem-based biochar (FeCl3@LS-BC) using mass-balance and functional group expressions. Colloids Surf A Physicochem Eng Asp 551:17–24. https://doi.org/10.1016/j.colsurfa.2018.04.054
Feng Y, Liu P, Wang Y, Liu W, Liu Y, Finfrock YZ (2020) Mechanistic investigation of mercury removal by unmodified and Fe-modified biochars based on synchrotron-based methods. Sci Total Environ 719:137435. https://doi.org/10.1016/j.scitotenv.2020.137435
Hamzenejad Taghlidabad R, Sepehr E (2018) Heavy metals immobilization in contaminated soil by grape-pruning-residue biochar. Arch Agron Soil Sci 64(8):1041–1052. https://doi.org/10.1080/03650340.2017.1407872
Herrmann L, Lesueur D, Robin A, Robain H, Wiriyakitnateekul W, Bräu L (2019) Impact of biochar application dose on soil microbial communities associated with rubber trees in North East Thailand. Sci Total Environ 689:970–979. https://doi.org/10.1016/j.scitotenv.2019.06.441
IBI (2015) Standardized product definition and product testing guidelines for biochar that is used in soil. International Biochar Initiative
Jenkinson DS, Ladd JN (1981) Microbial biomass in soil. Measurement and turnover. In: Paul EA (ed) Soil biochemistry. Marcel Dekker, New York, pp 415–471
Kabala C, Singh BR (2001) Fractionation and mobility of copper, leadand zinc in soil profiles in vicinity of a copper smelter. J Environ Qual 30:485–492. https://doi.org/10.2134/jeq2001.302485x
Kabiri P, Motaghian H, Hosseinpur A (2019) Effects of walnut leaves biochars on lead and zinc fractionation and phytotoxicity in a naturally calcareous highly contaminated soil. Water Air Soil Pollut 230(11):263. https://doi.org/10.1007/s11270-019-4316-5
Kamran M, Malik Z, Parveen A, Zong Y, Abbasi GH, Rafiq MT, Shaaban M, Mustafa A, Bashir S, Rafay M, Mehmood S (2019) Biochar alleviates Cd phytotoxicity by minimizing bioavailability and oxidative stress in pak choi (Brassica chinensis L.) cultivated in Cd-polluted soil. J Environ Manag 250:109500. https://doi.org/10.1016/j.jenvman.2019.109500
Karimi A, Khodaverdiloo H, Rasouli Sadaghiani MH (2017a) Characterisation of grawth and biochemical response of Onopordum acanthium L. under lead stress as affected by microbial inoculation. Chem Ecol 33(10):963–976. https://doi.org/10.1080/02757540.2017.1391798
Karimi A, Khodaverdiloo H, Rasouli Sadaghiani MH (2017b) Fungi and bacteria as helping agents for remediation of a Pb - contaminated soil by Onopordum acanthium. Casp J Environ Sci 15(3):249–262. https://doi.org/10.22124/CJES.2017.2466
Karimi A, Khodaverdiloo H, Rasouli-Sadaghiani MH (2018) Microbial-enhanced phytoremediation of Lead contaminated calcareous soil by Centaurea cyanus L. Clean (Weinh) 46(2):1700665. https://doi.org/10.1002/clen.201700665
Karimi A, Moezzi A, Chorom M, Enayatizamir N (2019) Chemical fractions and availability of Zn in a calcareous soil in response to biochar amendments. J Soil Sci Plant Nutr 19(4):851–864. https://doi.org/10.1007/s42729-019-00084-1
Karimi A, Moezzi A, Chorom M, Enayatizamir N (2020) Application of biochar changed the status of nutrients and biological activity in a calcareous soil. J Soil Sci Plant Nutr 20(2):450–459. https://doi.org/10.1007/s42729-019-00129-5
Kaurin A, Cernilogar Z, Lestan D (2018) Revitalisation of metal-contaminated, EDTA washed soil by addition of unpolluted soil, compost and biochar: effects on soil enzyme activity, microbial community composition and abundance. Chemosphere 193:726–736. https://doi.org/10.1016/j.chemosphere.2017.11.082
Khajavi-Shojaei S, Moezzi A, Norouzi Masir M, Taghavi M (2020) Characteristics of conocarpus wastes and common reed biochars as a predictor of potential environmental and agronomic applications. Energy Source A:1–18. https://doi.org/10.1080/15567036.2020.1783396
Khodaverdiloo H, Han FX, Hamzenejad Taghlidabad R, Karimi A, Moradi N, Joseph Kazery JA (2020) Potentially toxic element contamination of arid and semi-arid soils and its phytoremediation. Arid Land Res Manag 34:361–391. https://doi.org/10.1080/15324982.2020.1746707
Lahori AH, Zhanyu GUO, Zhang Z, Ronghua LI, Mahar A, Awasthi MK, Feng SHEN, Sial TA, Kumbhar F, Ping WANG, Jiang S (2017) Use of biochar as an amendment for remediation of heavy metal-contaminated soils: prospects and challenges. Pedosphere 27(6):991–1014. https://doi.org/10.1016/S1002-0160(17)60490-9
Li B, Yang L, Wang CQ, Zhang QP, Liu QC, Li YD, Xiao R (2017) Adsorption of cd (II) from aqueous solutions by rape straw biochar derived from different modification processes. Chemosphere 175:332–340. https://doi.org/10.1016/j.chemosphere.2017.02.061
Lyu H, Tang J, Cui M, Gao B, Shen B (2020) Biochar/iron (BC/Fe) composites for soil and groundwater remediation: synthesis, applications, and mechanisms. Chemosphere 246:125609. https://doi.org/10.1016/j.chemosphere.2019.125609
Palansooriya KN, Shaheen SM, Chen SS, Tsang DC, Hashimoto Y, Hou D, Bolan NS, Rinklebe J (2020) Soil amendments for immobilization of potentially toxic elements in contaminated soils: a critical review. Environ Int 134:105046. https://doi.org/10.1016/j.envint.2019.105046
Pan D, Liu C, Yu H, Li F (2019) A paddy field study of arsenic and cadmium pollution control by using iron-modified biochar and silica sol together. Environ Sci Pollut Res 26(24):24979–24987. https://doi.org/10.1007/s11356-019-05381-x
Pan X, Zhang S, Zhong Q, Gong G, Wang G, Guo X, Xu X (2020) Effects of soil chemical properties and fractions of Pb, Cd, and Zn on bacterial and fungal communities. Sci Total Environ 715:136904. https://doi.org/10.1016/j.scitotenv.2020.136904
Qiao JT, Liu TX, Wang XQ, Li FB, Lv YH, Cui JH, Zeng XD, Yuan YZ, Liu CP (2018) Simultaneous alleviation of cadmium and arsenic accumulation in rice by applying zero-valent iron and biochar to contaminated paddy soils. Chemosphere 195:260–271. https://doi.org/10.1016/j.chemosphere.2017.12.081
Qiao J, Yu H, Wang X, Li F, Wang Q, Yuan Y, Liu C (2019) The applicability of biochar and zero-valent iron for the mitigation of arsenic and cadmium contamination in an alkaline paddy soil. Biochar 1(2):203–212. https://doi.org/10.1007/s42773-019-00015-4
Rajendran M, Shi L, Wu C, Li W, An W, Liu Z, Xue S (2019) Effect of sulfur and sulfur-iron modified biochar on cadmium availability and transfer in the soil–rice system. Chemosphere 222:314–322. https://doi.org/10.1016/j.chemosphere.2019.01.149
Saffari N, Hajabbasi MA, Shirani H, Mosaddeghi MR, Mamedov AI (2020) Biochar type and pyrolysis temperature effects on soil quality indicators and structural stability. J Environ Manag 261:110190. https://doi.org/10.1016/j.jenvman.2020.110190
Singh B, Camps-Arbestain M, Lehmann J (2017) Biochar: a guide toanalytical methods. Caliton: CSIRO Publishing, Boca Raton
Tan Z, Yuan S, Hong M, Zhang L, Huang Q (2020) Mechanism of negative surface charge formation on biochar and its effect on the fixation of soil Cd. J Hazard Mater 384:121370. https://doi.org/10.1016/j.jhazmat.2019.121370
Tang J, Zhang J, Ren L, Zhou Y, Gao J, Luo L, Yang Y, Peng Q, Huang H, Chen A (2019) Diagnosis of soil contamination using microbiological indices: a review on heavy metal pollution. J Environ Manag 242:121–130. https://doi.org/10.1016/j.jenvman.2019.04.061
Tang X, Shen H, Chen M, Yang X, Yang D, Wang F, Chen Z, Liu X, Wang H, Xu J (2020a) Achieving the safe use of Cd- and As-contaminated agricultural land with an Fe-based biochar: a field study. Sci Total Environ 706:135898. https://doi.org/10.1016/j.scitotenv.2019.135898
Tang J, Zhang L, Zhang J, Ren L, Zhou Y, Zheng Y, Luo L, Yang Y, Huang H, Chen A (2020b) Physicochemical features, metal availability and enzyme activity in heavy metal-polluted soil remediated by biochar and compost. Sci Total Environ 701:134751. https://doi.org/10.1016/j.scitotenv.2019.134751
Tessier A, Campbell PG, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51(7):844–851. https://doi.org/10.1021/ac50043a017
Tu C, Wei J, Guan F, Liu Y, Sun Y, Luo Y (2020) Biochar and bacteria inoculated biochar enhanced Cd and Cu immobilization and enzymatic activity in a polluted soil. Environ Int 137:105576. https://doi.org/10.1016/j.envint.2020.105576
Wan X, Li C, Parikh SJ (2020) Simultaneous removal of arsenic, cadmium, and lead from soil by iron-modified magnetic biochar. Environ Pollut 261:114157. https://doi.org/10.1016/j.envpol.2020.114157
Wang J, Wang S (2019) Preparation, modification and environmental application of biochar: a review. J Clean Prod 227:1002–1022. https://doi.org/10.1016/j.envpol.2020.114133
Wang L, Bolan NS, Tsang DC, Hou D (2020) Green immobilization of toxic metals using alkaline enhanced rice husk biochar: effects of pyrolysis temperature and KOH concentration. Sci Total Environ:137584. https://doi.org/10.1016/j.scitotenv.2020.137584
Xu P, Sun CX, Ye XZ, Xiao WD, Zhang Q, Wang Q (2016) The effect of biochar and crop straws on heavy metal bioavailability and plant accumulation in a cd and Pb polluted soil. Ecotoxicol Environ Saf 132:94–100. https://doi.org/10.1016/j.ecoenv.2016.05.031
Xu Y, Seshadri B, Sarkar B, Wang H, Rumpel C, Sparks D, Farrell M, Hall T, Yang X, Bolan N (2018) Biochar modulates heavy metal toxicity and improves microbial carbon use efficiency in soil. Sci Total Environ 621:148–159. https://doi.org/10.1016/j.scitotenv.2017.11.214
Xu C, Zhao J, Yang W, He L, Wei W, Tan X, Wang J, Lin A (2020) Evaluation of biochar pyrolyzed from kitchen waste, corn straw, and peanut hulls on immobilization of Pb and Cd in contaminated soil. Environ Pollut 261:114133. https://doi.org/10.1016/j.envpol.2020.114133
Yu Y, An Q, Jin L, Luo N, Li Z, Jiang J (2020) Unraveling sorption of Cr (VI) from aqueous solution by FeCl3 and ZnCl2-modified corn stalks biochar: implicit mechanism and application. Bioresour Technol 297:122466. https://doi.org/10.1016/j.biortech.2019.122466
Zhang G, Guo X, Zhao Z, He Q, Wang S, Zhu Y, Yan Y, Liu X, Sun K, Zhao Y, Qian T (2016) Effects of biochars on the availability of heavy metals to ryegrass in an alkaline contaminated soil. Environ Pollut 218:513–522
Zhang H, Shao J, Zhang S, Zhang X, Chen H (2020) Effect of phosphorus-modified biochars on immobilization of Cu (II), Cd (II), and As (V) in paddy soil. J Hazard Mater 390:121349. https://doi.org/10.1016/j.jhazmat.2019.121349
Zhao B, Xu R, Ma F, Li Y, Wang L (2016) Effects of biochars derived from chicken manure and rape straw on speciation and phytoavailability of Cd to maize in artificially contaminated loess soil. J Environ Manag 184:569–574. https://doi.org/10.1016/j.jenvman.2016.10.020
Funding
This study was supported by the Research Vice Chancellor of Shahid Chamran University of Ahvaz, Ahvaz, Iran (grant number: AG1397-3-02-26247-Grant- Faculty of Agriculture).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1216 kb)
Rights and permissions
About this article
Cite this article
Moradi, N., Karimi, A. Fe-Modified Common Reed Biochar Reduced Cadmium (Cd) Mobility and Enhanced Microbial Activity in a Contaminated Calcareous Soil. J Soil Sci Plant Nutr 21, 329–340 (2021). https://doi.org/10.1007/s42729-020-00363-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-020-00363-2