Abstract
This study was purposely designed to evaluate the potential of faba bean (Vicia faba) residues as green manure in a maize-faba bean rotation system. Seed germination test and pot experiments were conducted to measure the effects of aqueous extract on maize seed germination and the effects of residues on crop growth and soil microbial activities. Litterbag experiment was conducted to analyze the decomposition patterns of faba bean residues during maize growing season in a regosol soil. The faba bean was separated into roots, stems, leaves, aboveground parts, and whole plants. Seed germination and the lengths of radicle and plumule were lower than those of the control, particularly with 1% aqueous extract concentrations. Aboveground biomass of maize at heading stages was negatively reduced by whole plant, aboveground part, and stem, whereas positively increased by leaf. Faba bean residues varied in their influences on the contents of soil microbial nitrogen and carbon as well as enzyme activities, mainly attributing to their differences in nutrient quality and allelophathy. Faba bean residues showed a quick decomposition rate during the first 5 weeks and slightly decomposed thereafter. The amount of nitrogen (N) released into soil ranged from 3.74 kg N ha−1 in root to 34.67 kg N ha−1 from leaf in the first 5 weeks. Compared with other plant parts, there were more remaining residues in root and stem residues. These results suggest that different management strategies should be taken to balance their negative and positive effects when using faba bean residues as green manure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Faba bean (Vicia faba L) is an annual legume crop widely distributed in temperate and subtropical areas (Rubiales 2010). The green pod of faba bean can be used as vegetables while the dry cotyledons are the principal protein sources for human and animal in some countries (Karkanis et al. 2018). Faba bean is also used as green manure in many agricultural systems. For example, intercropping faba bean (green manure) with carrot (Daucus carota) and cabbage (Brassica oleracea) could ensure sustainable farming and environmentally friendly horticultural production (Lepse et al. 2017). Incorporation of faba bean residues favored the highest fructose accumulation in melon fruits (Stagnari and Pisante 2010). The ear yields of corn following green manuring faba bean were 33–39% higher when compared with fallow under unfertilized conditions on a clay soil (Turgut et al. 2005). However, there are several limitations for faba bean to be used as green manure, such as the high seed cost due to higher thousand-grain weight (500 to 900 g) (Etemadi et al. 2017), allelopathy effect on subsequent crop (Alvarez-Iglesias et al. 2014), and its long-growing period (particularly fall sowing cultivar) (Lopez-Bellido et al. 2005).
When faba bean residues are applied into soils, they will produce toxic compounds such as lactic, benzoic, p-hydroxybenzoic, vanillic, adipic, succinic, malic, glycolic, and p-hydroxyphenylacetic acids (Asaduzzaman and Asao 2012), affecting seed germination and root growth of subsequent crop (Kadioglu et al. 2005), and thus crop yield. In a temperate forage-based system, faba bean aqueous extracts significantly inhibited the germination and early growth of weeds and limited crop growth (lettuce, maize and soybean) under high extract concentrations (Alvarez-Iglesias et al. 2014). In a pot experiment, faba bean concentrations of 1% and 2% of soil dry weight reduced the germination, root and shoot elongation, and aerial biomass of Echinochloa crusgalli and Amaranthus retroflexus by ca. 70% when the seeds were sown at the time of faba bean incorporation (Alvarez-Iglesias et al. 2018). Studies have also shown that aqueous leachates of root, stems, and leaf differed in their chemical constitutes and influences on seed germination and seedling growth (Li et al. 2016; Tefera 2002). Therefore, it is necessary to understand the allelopathy effect of different parts of faba bean on subsequent crops.
Faba bean is not only an important protein source for animal and human, but also a component in different cropping systems to improve soil quality. Rotation with faba bean would improve the physical and chemical properties of soil, enhance soil enzyme activities, and increase the crop yields (Cao et al. 2017; Stagnari and Pisante 2010). In a green manuring-maize-wheat crop rotation system in Carsamba Plain located in the north region of Turkey, application of whole aboveground or underground stubbles of faba bean provided an increase in urease and dehydrogenase activities of soils compared with the control (Surucu et al. 2014). Changing rotation crop by introducing faba bean 2 years before wheat modified the surrounding habitat of microbial communities by providing available C and N as well as suitable soil pH (Aschi et al. 2017). However, due to the difference in nutrient quality as well as allelochemicals of different faba bean parts, their influences on soil properties might also be different.
After the plants are buried in soils, the plant residues start to decompose with the help of soil microbes. On the Canadian prairies, faba bean residues released 70% and 63% of their C and N, respectively, in the first year (Lupwayi and Soon 2015). A study in the cool temperate agro-ecosystem indicated that the retardation of C mineralization was the least strong in Melilotus alba which had a relatively low cellulose content and a higher content of low molecular compounds (Magid et al. 2001). Using a range of vegetable root residues and plant parts of two green manures (ryegrass and white mustard), Chaves et al. (2004) reported that the leaves of the green manures released more N than both stems (only for white mustard) and roots. These results suggest that plant N and C show different release patterns mainly due to their difference in plant quality and nutrient mineralization rates under various environmental conditions (Havstad et al. 2010).
The plant quality factors influencing the N and C mineralization in soils include the C/N ratio, nutrient concentrations, and the contents of cellulose and lignin (Erinle and marschner 2019; Jensen et al. 2005). The decomposed C is either mineralized as CO2 or assimilated by the soil microflora (Truong and Marschner 2018), while the N dynamics are governed by the C rates and the C/N ratio (Nicolardot et al. 2001). Based on a simple dynamic model, Nicolardot et al. (2001) found that C/N ratio of the decomposers increased with the residue C/N ratio, and higher humification was predicted for substrates with lower C/N ratios. In a calcareous soil, net N mineralization was highly correlated with plant N concentration and lignin content (Vandat et al. 2011). However, based on a study with large number of plant materials (76 samples, covering 37 species and several plant parts), Jensen et al. (2005) reported that the plant residue C/N or lignin/N ratio was not closely correlated to C decomposition and N mineralization. These results imply that factors influencing residue nutrient mineralization are complicated and the nutrient release pattern might be depended upon combined effects from soil properties, plant species, and the local environments.
As a multi-purpose green manure, there are several ways to use faba bean: (1) returning roots to soil after forage harvest, (2) returning aboveground parts after pod harvest, or (3) returning whole plant to soil. However, due to the difference in quality properties of these faba bean parts being returned to the soil, their nutrient release pattern might also be different, which is still unclear. Therefore, in the current study, plants of fall sowing faba bean were collected after the fresh pod were harvested in April 2017, which were further separated into roots, stem, leaf, aboveground parts (stem and leaf), and whole plants. We first analyzed the allelopathy effects of aqueous extract on seed germination and seedling growth of maize, then measured the soil microbial activities as well as maize yield in a pot experiment. Next, we analyzed the nutrient release and the residue remaining of faba bean parts using a litterbag experiment, following 4-month decomposition in subsequent crop. We hypothesized that different parts of faba bean varied in their allelopathy effects and nutrient release patterns due to residue quality difference. The main objectives of the study were to determine (1) the effects of faba bean parts on seed germination and soil microbial activities; (2) the quantity of dry matter, C, and N returned to the soil; (3) C and N release patterns of the different faba bean parts; and (4) to evaluate the potential of faba bean residues as green manure in a maize-faba bean rotation system.
2 Materials and Methods
2.1 Experimental Site Description
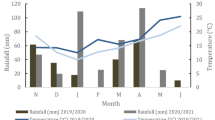
The experiment was conducted at Beibei Research Station of Southwest University (29° 49′ N, 106° 25′ E), located in Chongqing, China. The area has a mean temperature of 17.8 °C and a mean annual rainfall of 1100 mm during the last 30 years. The average maximum month temperature ranged between 22.9 °C in May and 31.3 °C in July during the experiment period from May to September in 2017 (Fig. 1). The soil is a purple regosol, one of the most important types of agricultural soil in Southern China. The chemical properties of the soils at 0–20-cm depth were as follows: pH (soil/water = 1:5) was 7.8, the concentrations of total N, total phosphorus (P), total potassium (K), and total organic carbon were 0.51 ± 0.04, 0.38 ± 0.06, 43.67 ± 0.29, and 21.13 ± 0.21 g kg−1, respectively; the concentrations of alkali dispelled N (AN), available phosphorus (AP, Bray), and available potassium (AK) were 41.94 ± 3.48, 6.81 ± 1.01, and 56.67 ± 3.51 mg kg−1, respectively. The measurements of the soil chemical properties followed the methods from Bao (2005).
2.2 Plant Materials
Seeds of faba bean (Vicia faba L. cv Chenghu 13) were sowed in early October 2016. Before sowing, the soil was applied with 80 kg N, 80 kg P2O5, and 40 kg K2O per hectare. No irrigation or further fertilization was applied during the following growing period. The faba beans were mainly used for harvesting fresh vegetable beans during March and April. In April 20, 2017, the faba bean plants were collected after the pod harvest, and the roots were dig out using shovel and washed free of soils on tap water. The plants were further separated into roots, stem, leaf, aboveground parts (no pod), and whole plants (no pod). Then, the plant materials were dried at 65 °C for 48 h and cut into 1-cm pieces. About 5 g of each sample was grounded through 2-mm sieve for chemical analyses. In total, 200 plants were harvested, and this enabled sufficient amounts of samples for pot experiment, litterbag experiment, and aqueous extract for seed germination test.
2.3 Seed Germination Test
Seed germination test was conducted in petri dishes under the conditions of 25 °C, 80% relative humidity, and 12-h light and 12-h dark in an illumination incubator. Maize seeds were surface sterilized with 10% H2O2 for 10 min and rinsed three times under tap water. Twenty seeds were placed in each petri dish, and then were supplemented everyday with 0.5% root extract, 1% root extract, 0.5% aboveground part extract, and 1% aboveground part extract, respectively. The total amount of extract supplemented every day was about 5 mL in each petri dish. The control was supplemented with the same amount of distilled water. Each treatment replicated three times. The seed germination was recorded when the radicle broke through the seed coat and reached half of the length of the seed.
Germination potential (%): the ratio of the number of normal germination seeds to the number of tested seeds at the initial germination stage (third day).
Germination rate (%): the ratio of the number of normal germinating seeds to the number of tested seeds at the end (seventh day) of germination.
After the germination test, five representative seedlings were selected for each treatment to determine the radicle length and the germ length.
2.4 Pot Experiment
The pot experiment was a one-way random design with six treatments, mixing 20-g faba bean root, stem, leaf, aboveground part, or whole plants with 5-kg soil in each pot (15 cm × 20 cm), with only soil for control. The soils (0–20-cm depth) were sampled from experimental site, air dried, and sieved through 2-mm sieve. Maize seeds (Zea mays cv Jingkenuo 2000) were surface sterilized with 10% H2O2 for 10 min, rinsed three times under tap water, and then were seeded. Each pot had two plants. The pots were placed under a glass rain shelter and watered every 3 days to keep water content around 60–80% field capacity. The pot positions were changed every other day. No fertilizer was applied. Each treatment replicated three times.
When plants reached four-leaf stage (20 days), about 100-g fresh soil were collected from each pot, quickly screened through a 2-mm sieve, and then used to measure the contents of soil microbial carbon (MBC) and microbial nitrogen (MBN) and the activities of soil urease and dehydrogenase.
Two months after sprout, the plants were harvested at their heading stages, dried at 65 °C for 48 h, and weighed for aboveground biomass.
2.5 Litterbag Experiment
The litterbag experiment was a one-way random design with five treatments, including roots, stems, leaves, aboveground parts, and whole plants, with four replicates. For each treatment, about 20,000 g of dried plant materials was placed in a nylon litterbag (20 × 20 cm, mesh size 1.5 mm) and buried in soils growing maize (Zea mays cv Jingkenuo 2000) at a depth of 10 cm in May 20, 2017. In total, 36 bags per treatment were prepared, among which 32 were buried and the remaining 4 bags were used as initial one (control). The maize was planted with 60 cm between rows and 20 cm between plants. The maize land was randomly divided into four plots (12 × 12 m) with 1-m distance between each other. Eight bags in each treatment were randomly buried in each plot. Each bag occupied about 0.25-m2 area, equaling to 800 kg DM ha−1. Such application rate accounted for about 70% of the total plant biomass harvested excluding the beans. During the experimental period, the maize received no additional fertilization except for initial fertilization with 100 kg N ha−1, 80 kg P2O5 ha−1, and 40 kg K2O ha−1. After 1, 2, 3, 4, 5, 7, 11, and 17 weeks, one litterbag for each treatment was collected from each plot, washed under tap water first, and cleaned free of soil particles with distilled water; then, the remaining residues were taken out, dried at 65 °C for 48 h, and weighed. The initial samples in bags were washed under tap water, cleaned, and dried as those buried in soils, to calculate the DM and nutrient release from the initials. All dried samples were grounded through 1-mm mesh before chemical analysis.
About 0.5-g dried plant sample was digested in 5 mL H2SO4 until the solution color turned to transparent; then, the nutrients were analyzed by the methods of Bao (2005). Then, total N was analyzed by Kjeldahl method, total P was measured using Mo-Sb colorimetric procedure, and total K was measured using flame photometry (Bao 2005). About 0.3-g dried plant sample was oxidized by potassium dichromate (K2Cr2O7)-sulfuric acid mixture; then, the solution was titrated by FeSO4 solution to measure total organic carbon (Nelson and Sommers 1982).
2.6 Data Analysis for Decomposition Pattern
All data were the average from four replicates. One-way ANOVA analysis was applied to analyze the difference between the nutrient contents in initial samples of different faba bean parts using SPSS 13.0 (SPSS Inc., Chicago, USA).
The remaining of DM, N, C, and alkanes in residues after decomposition and their release percentage were calculated with the following equation:
where DMs was the dry matter of each sample in bag after decomposition and DMi was the DM in initial sample.
where nutrient was either N or C.
where DMps was DM in the pre-decomposed sample and DMfs was DM in the following decomposed sample.
The release amount per hectare was calculated based upon the DM application rate at 800 kg ha−1 and the percentage of DM (N or C) released during certain period.
Regression analysis between the dry matter and nutrient remaining in residue and the decomposition days indicated that linear model did not give the best fit. Therefore, the data was fitted into the following non-linear model:
where y 0 was the adjusted remaining percentage at day 0 and t was the day after residue placement.
Linear model was applied between C remaining (%), the N concentrations, and the C/N ratio and the days after residue placement according to Pearson correlation analysis using SPSS13.0.
3 Results
3.1 Seed Germination and Aboveground Biomass
The responses of maize seed germination to water extract of faba bean residues varied at different stages. Seed germination at day 3 was negatively influenced under the conditions of 1% root and 1% aboveground extracts (Fig. 2). No significant difference in seed germination was observed between treatments under the conditions of 0.5% root and 0.5% aboveground extracts at day 3 and at both extract concentrations at day 7 (no seed germinated after day 7). However, the lengths of radicle and plumule at day 3 and day 7 significantly decreased when water extracts of faba bean residues were applied, except non-significant changes at 0.5% root extract at both days. The lengths of radicle and plumule with 1% water extract were significantly lower than those with 0.5% water extract at both days.
The faba bean residues also significantly influenced the aboveground biomass of maize in pot experiment (Fig. 2e). Compared with the control, the aboveground biomass significantly reduced when residues of whole plant, aboveground part, or stem were applied, significantly increased when leaf residue was applied, and not significantly changed when root residue was applied. Among the residues, the aboveground biomass with leaf residue was the highest, followed by root residue, with other parts the lowest.
3.2 Soil Microbial Activities
Application of faba bean residues significantly influenced the soil enzyme activities and soil microbial nitrogen and carbon contents. The dehydrogenase activities in soils applied with faba bean residues were higher than those in soils of the control, except a non-significant increase with whole plant and leaf (Fig. 3a). The urease activities showed non-significant responses to residue applications when compared with the control (Fig. 3b). The responses of the contents of soil microbial nitrogen (MBN) and microbial carbon (MBC) varied between residues (Fig. 3c, d). The contents of MBC in soils applied with whole plant and stem were significantly lower than those in control, whereas not significantly changed in soils with aboveground parts, root, and leaf. The contents of MBN in soils with aboveground part, stem, and leaf were significantly higher than those of the control, whereas not significantly changed in soils with whole plant and root. The ratios of MBC/MBN in residue incorporated soils were significantly lower than that in control excepting with root residue (Fig. 3e).
Effects of faba bean residues on soil dehydrogenase activity (a), urease activity (b), the contents of soil microbial carbon (c) and microbial nitrogen (d), and the ratio of MBC/MBN (e). MBC/MBN the content ratio of soil microbial carbon and microbial nitrogen. Different lowercase letters above the data bar represented significance at P = 0.05 level according to least significant difference test
3.3 Nutrient Contents in Faba Bean
Nutrient contents in faba bean varied greatly between plant parts (Table 1). The C contents in whole plant, aboveground part, and stem were significantly higher than those in leaf and root, whereas the N contents in leaf were significantly higher than those in other parts, with the lowest N content in stem and root. Consequently, stem and root had the highest C/N ratio, followed by whole plant and aboveground part, with the lowest in leaf. Stem had the lowest P and K contents among different faba bean parts, whereas leaf had the highest K content. During the 4-month placement in soil, the concentrations of residue C (Fig. 4a) and N (Fig. 4b) varied slightly. However, the C/N ratio increased during the first 3 weeks and then reduced, except that in leaf which almost unchanged (Fig. 4c).
3.4 Dry Matter, Carbon, and Nitrogen Remaining in Residues
The faba bean residues showed a quick decomposition during the first 5 weeks after they were buried in soils of subsequent maize land, with the remaining of dry matter (DM), N, and C averaged 45.30%, 30.32%, and 41.30%, respectively, across all plant parts at fifth week (Fig. 5). From the fifth week to 17th week, the faba bean residues decomposed slightly, with the remaining of DM, N, and C averaged 37.19%, 36.89%, and 36.26%, respectively, at 17th week. The N showed an increased remaining percentage in residue from the fifth week to 17th week. Among the plant parts, root and stem had the highest remaining of DM, N, and C, when compared with the other plant parts.
Regression analysis indicated that the relationship between the DM, N, and C remaining percentages and the days after residue placement negatively fitted quadratic functions, except N remaining in whole plant, aboveground part, and root (Table 2, Fig. 5).
The C remaining percentage was negatively correlated with the N concentration in plants (y = 67.83 − 0.56x, P < 0.0001) and positively correlated with the C/N ratio in plants (y = 33.91 + 8.95x, P < 0.0001) (Fig. 6). Due to the extreme high N concentration in leaf, no model fit N concentration and C remaining percentage when data from leaf were included. Therefore, the data in leaf was not included in the model (see supplemental data from leaf in Fig. S1).
3.5 Release Pattern of Dry Matter, Carbon, and Nitrogen
The amount of DM, N, and C released to the soil varied greatly during different periods and between plant parts (Fig. 7). During 0–5 weeks, the released DM reached 58%, 62%, 52%, 62%, and 40% in whole plant, aboveground parts, stem, leaf, and root, respectively, equaling to 465, 496, 415, 493, and 319 kg DM/ha. During 6–11 weeks, the amounts of released DM were about 8% in whole plant and root, less than 4% in stem and aboveground part, and almost no release in leaf. During 12–17 weeks, the released amount of DM was the highest in stem (11%), followed by root (5%) and aboveground part (3%), with almost no release in whole plant and leaf.
The amount of released N reached above 65% in all plant parts during 0–5 weeks, then showed slight accumulation during 6–11 weeks in all plant parts except for about 1% release in leaf. During 12–17 weeks, the whole plant released about 7% of N, whereas the other plant parts accumulated N. The stem accumulated the highest amount of N during 6–11 weeks (20%) and 12–17 weeks (11%), followed by root (about 7% during 6–11 weeks and 12–17 weeks). The amount of N released in soil ranged from 3.74 kg N ha−1 in root to 34.67 kg N ha−1 from aboveground parts during 0–5 weeks.
The C release pattern was much different from DM and N. The leaf had the highest C release reaching 70% during 0–5 weeks, followed by whole plant (63%) and aboveground part (65%), with stem (53%) and root (42%) the lowest. During 6–11 weeks, whole plant, aboveground part, and root showed further C release, whereas leaf showed slight accumulation and no change in stem. During 12–17 weeks, leaf and stem showed about 17% and 10% C accumulation, respectively; aboveground part released about 3% C, whereas no much changes could be observed in whole plant and root. The amount of C released in soil ranged from 6.86 kg C ha−1 in root to 16.31 kg C ha−1 from aboveground part during 0–5 weeks.
4 Discussion
Aqueous extracts of faba bean negatively influenced early germination (day 3) of maize seed, particularly under higher extract concentrations, suggesting that faba bean might exert allelopathy effects on subsequent crop when it is incorporated into the agricultural system as green manure. This is common in many agricultural systems, where green manures are applied to mainly control early weed growth, including green manure Eucalyptus globulus (Puig et al. 2019), faba bean (Alvarez-Iglesias et al. 2014), and red clover (McKenna et al. 2018). In this study, though no significant difference in seed germination could be observed at later stage (day 7), the lengths of radicle and plumule at day 3 and day 7 significantly decreased when aqueous extracts of faba bean residues were applied, particularly with 1% extract concentrations at both days. Results from the pot experiment further indicated that aboveground biomass of maize at heading stages was also negatively influenced by faba bean residues, such as whole plant, aboveground part, or stem. Such negative influence on maize growth might reduce biomass accumulation during early growth stages (Alvarez-Iglesias et al. 2018), and thus the final maize yield (Vyn and Raimbault 1993). All these indicated that aqueous extract of faba bean influenced not only early seedling growth but also later development of maize. Long-term accumulation of allelochemicals might be existed during the decomposition of faba bean residues (Kraus et al. 2002), which thus resulted in reduced aboveground biomass. This also suggested that allelopathy effect should be considered when using faba bean residues as green manure in subsequent maize planting. However, interestingly, the aboveground biomass was significantly increased when leaf residue was applied and unchanged when root residue was applied. This implied that the allelochemicals released from faba bean residues might differ between plant parts and more toxic chemical might be extracted from stems (Javaid et al. 2015).
The most important role of green manure is to improve soil quality. This is proved in many studies where green manure increases soil organic carbon content and improves soil physical properties and soil fertility (Yang et al. 2012). However, risk also exists when faba bean is applied as green manure. Besides negative influences on early crop growth and plant biomass, the contents of soil MBN and MBC as well as enzyme activities were also influenced by faba bean residues. For example, the dehydrogenase activities in soils applied with faba bean residues were higher than those in soils of the control, the contents of MBC in soils applied with whole plant and stem were significantly lower than those in control, whereas the contents of MBN in soils with aboveground part, stem, and leaf were significantly higher than those of the control. On one hand, such inconsistent changes between plant residues might be attributed to their difference in releasing allelochmicals (Javaid et al. 2015). Though we had not analyzed and quantified the allelochemicals between faba bean residues, the contents and the constituents of allelochemicals have been shown to be different in many plant species (Chon and Kim 2002; Deng et al. 2014). On the other hand, the difference in nutrient concentrations and nutrient release patterns between faba bean residues might also attribute to their difference in influencing soil microbial activities.
Our results indicated that faba bean residues showed a quick decomposition rate during the first 5 weeks, with almost 55% of DM, 70% of N, and 59% of C released into soils, suggesting that the fall sowing faba bean is also a good legume green manure for summer crops. With more nutrient released into the soil from green manure, particularly the N, less N fertilization is needed (Rochester et al. 2001). Based on a meta-analysis, legume winter cover crops increased corn yield by 37% when no N fertilizer was applied, and this benefit decreased with application of N fertilizer (Miguez and Bollero 2005). Over an 8-year study, a rye cover crop has been shown an effective N management tool with no corn yield penalty (Snapp and Surapur 2018). In the current study, the amount of N released into the soil ranged from 3.74 kg N ha−1 from root to 34.67 kg N ha−1 from leaf during 0–5 weeks. This was reasonable when compared with the results from another study with faba bean in a silt loam at Alberta, Canada, where the total amount of N released reached 80 kg N ha−1 during 0–52 weeks (Lupwayi and Soon 2015). Low amount of N released during earlier season may match with the low N requirement of subsequent crop during seedling stage, reducing the risk of leaching surplus N into environment. In a 2-year lysimeter study, Bergstrom and Kirchmann (2004) reported that the total cumulative N leached reached 73 kg ha−1 (red clover manure) and claimed that the water quality benefits of legume-based green manures should be evaluated with regard to the timing of N release and demand for N by the plant. However, Yao et al. (2018) reported that growing legume green manures could maintain high crop yield and mitigate the environmental impact of residual nitrate by substantially replacing the synthetic N to avoid nitrate leaching to deeper soils. Further study is needed to clarify the relationship between the amounts of faba bean nutrients released into the soil and the amounts of nutrients leached to deeper soils.
C release to the soil is an important way for green manures to improve the SOC. Correlation analysis indicated that the C remaining (%) was negatively correlated with the N concentrations and positively correlated with their C/N ratios in faba bean plants (data from leaf were not included), suggesting that higher N concentration in plant residue would result in high amount of C release in plant residue. This was in consistent with the results from a 3-years study with faba bean in Canada (Lupwayi and Soon 2015). The plant residue in soils will be mainly degraded by microbial decomposers, including bacteria and fungi, and their grazers, which have lower C/N values when compared with most litter species (Berg and McClaugherty 2003). In the current study, the C/N ratio ranged from 0.33 in leaf to 1.77 in stem. Increased N concentration in plant residue might benefit the high N demand by decomposers, and thus resulting in higher C release.
Both N and C showed a slight release during the 6–17 weeks when compared with 0–5 weeks. On one hand, this was related to the low nutrient concentrations in residues during late season. On the other hand, the low decomposition rate during the late season might also be attributed to changes of residue quality such as the relatively increased lignin content when most easily decomposable C was released (Klotzbucher et al. 2011). Field incubations of plant litter also demonstrated that increasing lignin concentration reduced biotic decomposition (Austin and Ballare 2010). Accumulation of N and C was also observed during the late season. This might be attributed to the soil bacterial and fungi, which propagated on plant residue and deposited exterior N or C from soils into plant residue, resulting in increased N and C amount (Santa-Regina et al. 1997). However, a final nutrient release would be observed when all DM was degraded after a long period of decomposition (Lupwayi and Soon 2015).
5 Conclusions
As a multi-purpose pulse crop, the fall sowing faba bean could release about 3.74 kg N ha−1 from root to 34.67 kg N ha−1 from leaf into soils during 0–5 weeks after the residues were returned into the soils of the following summer cash crop, suggesting that farmers could use either whole plant, or aboveground parts, or stem (leaf) or root of the faba bean, to improve the soil quality and reduce the amounts of chemical fertilizations. However, due to their significant difference in residue qualities, the root, stem, leaf, aboveground parts, and whole plant exhibited various N and C release patterns in soils. Meanwhile, faba bean residues also exerted allelopathy effects on maize seed germination, seedling growth, and soil microbial activities, suggesting that different management strategies should be taken to balance their negative and positive effects when using faba bean as green manure to improve soil quality and subsequent crop productivity.
References
Alvarez-Iglesias L, Puig CG, Garabatos A, Reigosa MJ, Pedrol N (2014) Vicia faba aqueous extracts and plant material can suppress weeds and enhance crops. Allelopath J 34:299–313
Alvarez-Iglesias L, Puig CG, Revilla P, Reigosa MJ, Pedrol N (2018) Faba bean as green manure for field weed control in maize. Weed Res 58:437–449
Asaduzzaman M, Asao T (2012) Autotoxicity in beans and their allelochemicals. Sci Hortic-Amsterdam 134:26–31
Aschi A, Aubert M, Riah-Anglet W, Nelieu S, Dubois C, Akpa-Vinceslas M, Trinsoutrot-Gattin I (2017) Introduction of faba bean in crop rotation: impacts on soil chemical and biological characteristics. Appl Soil Ecol 120:219–228
Austin AT, Ballare CL (2010) Dual role of lignin in plant litter decomposition in terrestrial ecosystems. PNAS 107:4618–4622
Bao SD (2005) Agricultural chemical analysis of soil. China Agriculture Press, Beijing
Berg B, McClaugherty C (2003) Plant litter: decomposition, humus formation, Carbon sequestration. Springer-Verlag, Berlin
Bergstrom L, Kirchmann H (2004) Leaching and crop uptake of nitrogen from nitrogen-15-labeled green manures and ammonium nitrate. J Environ Qual 33:1786–1792
Cao Y, Wu C, Wang L, Chen M, Zhao H, Bian X, Chen Y, Xia L (2017) Rotation of broad bean improves the soil quality of facility green house. Legum Res 40:710–715
Chaves B, De Neve S, Hofman G, Boeckx P, Van Cleemput O (2004) Nitrogen mineralization of vegetable root residues and green manures as related to their (bio) chemical composition. Eur J Agron 21:161–170
Chon SU, Kim JD (2002) Biological activity and quantification of suspected allelochemicals from alfalfa plant parts. J Agron Crop Sci 188:281–285
Deng WH, Chen BY, Zhang YQ, Zhang JQ, Qin SG, Wang R, Wang NN, Wu B, Shen YB (2014) Effects of extraction conditions on allelochemicals release from the Artemisia ordosica. Allelopath J 34:215–226
Erinle K, Marschner P (2019) Soil water availability influences P pools in the detritusphere of crop residues with different C/P ratios. J Soil Sci Plant Nutr:1–9. https://doi.org/10.1007/s42729-019-00076-1
Etemadi F, Hashemi M, Shureshjani RA, Autio WR (2017) Application of data envelopment analysis to assess performance efficiency of eight faba bean varieties. Agron J 109:1225–1231
Havstad L, Aamlid T, Henriksen T (2010) Decomposition of straw from herbage seed production: Effects of species, nutrient amendment and straw placement on C and N net mineralization. Acta Agric Scand Sect B Soil Plant Sci 60:12
Javaid MM, Bhan M, Johnson JV, Rathinasabapathi B, Chase CA (2015) Biological and chemical characterizations of allelopathic potential of diverse accessions of the cover crop Sunn hemp. J Am Soc Hortic Sci 140:532–541
Jensen LS, Salo T, Palmason F, Breland TA, Henriksen TM, Stenberg B, Pedersen A, Lundstrom C, Esala M (2005) Influence of biochemical quality on C and N mineralisation from a broad variety of plant materials in soil. Plant Soil 273:307–326
Kadioglu I, Yanar Y, Asav U (2005) Allelopathic effects of weeds extracts against seed germination of some plants. J Environ Biol 26:169–173
Karkanis A, Ntatsi G, Lepse L, Fernandez JA, Vagen IM, Rewald B, Alsina I, Kronberga A, Balliu A, Olle M, Bodner G, Dubova L, Rosa E, Savvas D (2018) Faba bean cultivation—revealing novel managing practices for more sustainable and competitive. European cropping systems. Front Plant Sci 9:14
Klotzbucher T, Kaiser K, Guggenberger G, Gatzek C, Kalbitz K (2011) A new conceptual model for the fate of lignin in decomposing plant litter. Ecology 92:1052–1062
Kraus E, Voeten M, Lambers H (2002) Allelopathic and autotoxic interactions in selected populations of Lolium perenne grown in monoculture and mixed culture. Functi Plant Bio 29:1465–1473
Lepse L, Dane S, Zeipina S, Dominguez-Perles R, Rosa EAS (2017) Evaluation of vegetable-faba bean (Vicia faba L) intercropping under Latvian agro-ecological conditions. J Sci Food Agric 97:4334–4342
Li J, He SY, Qin XD (2016) Allelopathic potential and volatile compounds of Manihot esculenta Crantz against weeds. Allelopath J 37:195–206
Lopez-Bellido FJ, Lopez-Bellido L, Lopez-Bellido RJ (2005) Competition, growth and yield of faba bean (Vicia faba L.). Eur J Agron 23:359–378
Lupwayi NZ, Soon YK (2015) Carbon and nitrogen release from legume crop residues for three subsequent crops. Soil Sci Soc Amer J 79:1650–1659
Magid J, Henriksen O, Thorup-Kristensen K, Mueller T (2001) Disproportionately high N-mineralisation rates from green manures at low temperatures—implications for modeling and management in cool temperate agro-ecosystems. Plant Soil 228:73–82
McKenna P, Cannon N, Conway J, Dooley J (2018) The use of red clover (Trifolium pratense) in soil fertility-building: a review. Field Crop Res 221:38–49
Miguez FE, Bollero GA (2005) Review of corn yield response under winter cover cropping systems using meta-analytic methods. Crop Sci 45:2318–2329
Nelson D, Sommers L (1982) Total carbon, organic carbon and organic matter. In: Page AL, Miller RH, Keenay DR (eds) Methods of Soil Analysis. American Society of Agronomy and Soil Science Society of American, Madison, pp 101–129
Nicolardot B, Recous S, Mary B (2001) Simulation of C and N mineralisation during crop residue decomposition: a simple dynamic model based on the C : N ratio of the residues. Plant Soil 228:83–103
Puig CG, Revilla P, Esther Barreal M, Reigosa MJ, Pedrol N (2019) On the suitability of Eucalyptus globulus green manure for field weed control. Crop Prot 121:57–65
Rochester IJ, Peoples MB, Hulugalle NR, Gault RR, Constable GA (2001) Using legumes to enhance nitrogen fertility and improve soil condition in cotton cropping systems. Field Crop Res 70:27–41
Rubiales D (2010) Faba beans in sustainable agriculture Introduction. Field Crop Res 115:201–202
Santa-Regina I, Rapp M, Martin A, Gallardo JF (1997) Nutrient release dynamics in decomposing leaf litter in two Mediterranean deciduous oak species. Ann For Sci 54:747–760
Snapp S, Surapur S (2018) Rye cover crop retains nitrogen and doesn’t reduce corn yields. Soil Tillage Res 180:107–115
Stagnari F, Pisante M (2010) Managing faba bean residues to enhance the fruit quality of the melon (Cucumis melo L) crop. Sci Hortic 126:317–323
Surucu A, Ozyazici MA, Bayrakli B, Kizilkaya R (2014) Effects of green manuring on soil enzyme activity. Fresenius Environ Bull 23:2126–2132
Tefera T (2002) Allelopathic effects of Parthenium hysterophorus extracts on seed germination and seedling growth of Eragrostis tef. J Agron Crop Sci 188:306–310
Truong THA, Marschner P (2018) Addition of residues with different C/N ratio in soil over time individually or as mixes—effect on nutrient availability and microbial biomass depends on amendment rate and frequency. J Soil Sci Plant Nutr 18:1157–1172
Turgut I, Bilgili U, Duman A, Acikgoz E (2005) Effect of green manuring on the yield of sweet corn. Agron Sustain Dev 25:433–438
Vandat E, Nourbakhsh F, Basiri M (2011) Lignin content of range plant residues controls N mineralization in soil. Eur J Soil Biol 47:243–246
Vyn TJ, Raimbault BA (1993) long term effect of five tillage systems on corn response and soil structure. Agron J 85:1074–1079
Yang ZP, Xu MG, Zheng SX, Nie J, Gao JS, Liao YL, Xie J (2012) Effects of long-term winter planted green manure on physical properties of reddish paddy soil under a double-rice cropping system. J Integr Agric 11:655–664
Yao ZY, Zhang DB, Yao PW, Zhao N, Li YY, Zhang SQ, Zhai BN, Huang DL, Ma AS, Zuo YJ, Cao WD, Gao YJ (2018) Optimizing the synthetic nitrogen rate to balance residual nitrate and crop yield in a leguminous green-manured wheat cropping system. Sci Total Environ 631-632:1234–1242
Acknowledgments
The authors would like to express thanks to Miss Qing Yang from the College of Agronomy and Biotechnology of Southwest University (China) for her help during field sampling.
Funding
This study was funded by National Natural Science Foundation of China (31670407), Science and Technology Innovation Project for Social Livelihood of Chongqing (cstc2016shmszx80107), and the Fundamental Research Funds for the Central Universities (XDJK2018B021).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(JPG 245 kb)
Rights and permissions
About this article
Cite this article
He, Z., Yao, L., Zhang, X. et al. Faba Bean Organs Differed in Their Effects on Maize Seed Germination Rate and Soil Microbial Activities as well as Their Decomposition Patterns in a Regosol Soil. J Soil Sci Plant Nutr 20, 367–379 (2020). https://doi.org/10.1007/s42729-019-00117-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-019-00117-9