Abstract
Endophytes are potential source of various novel compounds that help to promote plant growth, eliminate plant pathogens, and enable the plant to resist stress-like conditions. The present study aimed at selecting stress-tolerant bacterial endophytes with plant growth-promoting ability from Adhatoda vasica. Salt-tolerant bacterial endophytes were isolated on nutrient agar with 2.5% NaCl from the leaves of Adhatoda vasica collected from Manipur, India. The isolates were screened for stress tolerance, plant growth-promoting traits, antagonism against fungal phytopathogens and plant growth promotion. Sixteen morphologically distinct salt-tolerant bacterial endophytes were isolated from Adhatoda vasica. All bacterial endophytes showed auxin production while phosphate solubilization was shown by 81% isolates, siderophore production by 75%, ACC deaminase by 43.8% isolates, HCN by 50% isolates, and ammonia by 62.5% isolates. Four bacterial isolates showed antagonistic activity against all the test fungal phytopathogens Fusarium verticillioides (MTCC 3322), Curvularia lunata (MTCC 283), and Alternaria alternata (MTCC 1362). Dendrogram generated based on stress tolerance of the bacterial isolates against salinity, temperature, pH, and calcium salts showed 3 clusters and two independent branches. Two bacterial isolates identified based on phenotypic features and 16S rRNA gene sequencing as Bacillus thuringiensis A1B3 and Bacillus sp. A1B6 significantly increased the growth parameters of pea and maize in comparison to uninoculated control in pots under natural conditions. The attributes of stress tolerance, antagonism against fungal pathogens, and plant growth promotion indicated the potential of Bacillus thuringiensis A1B3 and Bacillus sp. A1B6 to be used as microbial inoculant in agriculture under stressed environment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
An environmentally sound and sustainable crop production is one of the main challenges for agriculture in the twenty-first century. Excessive use of fertilizers and pesticides for enhanced agricultural yield has placed an extensive burden on the agriculture. Ecologically safe, efficient, and cheap biological alternatives are required to reduce the consumption of fertilizers and for improving agriculture productivity. Microorganisms have huge potential offering an alternative to the use of inorganic fertilizers and chemical pesticides. Most of the research related to plant growth-promoting potential of microorganisms is focused on rhizobacteria; however, there is an increasing interest in the huge potential offered by endophytic bacteria for plant growth promotion (Ribeiro et al. 2018). Endophytic microorganisms produce plant growth-promoting metabolites including phytohormones, enzymes like ACC deaminase, organic acids aiding in phosphate solubilization, siderophores, cellulases, and chitinases (Vyas and Kaur 2017). Endophytic microorganisms are not only the promising source of growth metabolites but also enable the plant to resist stress-like conditions. The application of endophytic microorganisms with multiple plant growth-promoting activities, biocontrol mechanisms, and stress tolerance could be beneficial in reducing the use of chemical fertilizers and pesticides for sustainable agriculture in the fragile ecosystems.
Plant disease control has also remained a major challenge for improving crop productivity. Fusarium species are well-known human, animal, and plant pathogens. Fusarium oxysporum and Curvularia lunata cause diseases in wide range of plant species belonging to different families (Lal et al. 2013). Similarly, Alternaria alternata with a wide host range cause leaf spots and blights in many plants while Fusarium verticillioides is an economic fungal pathogen reported to cause stalk rot, ear rot, and kernel rot of maize (Ghosh et al. 2016). In addition to phytopathogens, different biotic and abiotic factors also affect the performance of microorganisms and plant growth (Vyas et al. 2010; Dodd and Pérez-Alfocea 2012; Das et al. 2015). Abiotic stress includes temperature, pH, moisture status, salinity, and salts present in soils. Many plant growth-promoting bacteria showing good results in vitro fail to give the same results in fields, when applied as microbial inoculants due to the stress imposed by the sudden change in the environment (Vyas et al. 2009). Therefore, screening for stress tolerance is an important parameter in the selection of bacterial strains for the development of microbial inoculants. Many bacteria have adapted themselves to the fluctuations in the temperature, pH, and osmolarity while living in the stressed environments. Limited reports are available on the selection of bacterial strains based on stress tolerance for the development of microbial inoculants (Vyas et al. 2009, 2010). One approach is to select stress-tolerant bacteria with multiple plant growth-promoting (PGP) and biocontrol activities.
Plants which possess ethnobotanical history offer enormous opportunities for the recovery of novel endophytic microorganisms. Adhatoda vasica (Justicia adhatoda), commonly known as Malabar nut or vasaka, belonging to the family Acanthaceae grows in the Indian plains or lower Himalayas, up to a range of 1000 m above sea level (Kumar et al. 2013). In Ayurvedic medicine, Adhatoda vasica is used for a multitude of disorders including leprosy, blood disorders, heart troubles, fever, vomiting, loss of memory, leucoderma, jaundice, tumors, mouth troubles, sore eye, and gonorrhea. No reports are available on the stress-tolerant plant growth-promoting endophytic bacteria isolated from Adhatoda vasica. Therefore, the present study was aimed at isolating stress-tolerant endophytic bacteria from Adhatoda vasica with multiple plant growth-promoting and antagonistic activities against plant fungal pathogens.
2 Materials and Methods
2.1 Isolation of Culturable Bacterial Endophytes
Culturable bacterial endophytes were isolated from leaf samples of Adhatoda vasica growing at Manipur, India, located between the latitude 24° 48′ 50.2812″ N and longitude 93° 57′ 1.0044″ E. Leaf samples were collected from healthy plants growing at three different locations and brought to the Microbiology Laboratory, Lovely Professional University, Jalandhar, India, in polythene bags after keeping the leaf samples between the folds of brown sheets. The samples were processed immediately by washing with distilled water. The leaves were surface sterilized by dipping in ethanol (75%) for 30 s followed by 0.2% HgCl2 for 3 min and washed with sterilized distilled water five times (Tan et al. 2015). The surface-sterilized leaves were cut into thin sections of about 1–2 mm and placed on agar-solidified nutrient agar amended with 2.5% NaCl. The plates were incubated in the dark at 28 °C for 24–72 h. The bacterial isolates growing in the immediate vicinity of leaf tissue were purified and preserved under 30% glycerol for further studies.
2.2 Screening of Salt-Tolerant Bacterial Endophytes for Plant Growth-Promoting Attributes
2.2.1 Phosphate Solubilization
Phosphate solubilization by salt-tolerant bacterial isolates was detected qualitatively on modified Pikovskaya (PVK) agar. Zone of phosphate solubilization around bacterial colonies was measured on fifth day of incubation at 28 °C.
For quantitative estimation, 50 ml NBRIP (National Botanical Research Institute Phosphate) broth was inoculated with 500 μl of the bacterial cultures (~ 3 × 109 CFU/ml). The flasks were incubated in a refrigerated incubator shaker at 180 rpm at 28 °C. The uninoculated sterilized NBRIP medium served as control. The liberated P was estimated by yellow color method on fifth day of incubation as described earlier (Nautiyal 1999; Gulati et al. 2008). The total soluble phosphorus was calculated from the regression equation generated from the standard curve prepared from KH2PO4 in the range of 0 to 900 μg P per milliliter. The values of phosphorus liberated were expressed as microgram per milliliter over uninoculated control.
2.2.2 IAA-Like Auxin Production
Indole-3-acetic acid production was quantified in nutrient broth with 0.1% DL-tryptophan using Salkowski reagent (Loper and Schroth 1986). In brief, 100 μl of 24 h old bacterial cultures were inoculated in the medium and incubated for 48 h in the dark at 28 °C. Thereafter, the cultures were centrifuged at 10,000 rpm and 4 ml Salkowski reagent was added to 1 ml culture supernatant. After 10 min, the absorbance of the resultant pink color was measured at 535 nm and the values of IAA-like auxins were expressed as microgram per milliliter over uninoculated control. The total IAA was calculated from the regression equation generated from the standard curve prepared from IAA in the range of 0 to 50 microgram IAA per milliliter.
2.2.3 ACC (1-Aminocyclopropane-1-Carboxylate) Deaminase Activity
The salt-tolerant bacterial isolates were screened for ACC deaminase activity on DF salts minimal medium with ACC as sole nitrogen source (Glick 2014). The DF agar plates were streaked with the bacterial cultures and incubated at 28 °C. The plates were observed for the appearance of growth after 48 h.
2.2.4 Siderophore Production
The glassware used for siderophore detection were rinsed with 20% HCl to remove iron and washed with de-ionized water. Screening of the bacterial isolates for siderophore production was carried out on chrome azurol sulfonate (CAS) agar plates (Schwyn and Neilands 1987). The CAS agar plates were spot inoculated with the bacterial cultures and incubated at 28 °C. The diameter of orange color halo was measured on the fifth day of incubation.
2.2.5 Ammonia Production
Ammonia production was detected by adding Nessler’s reagent to bacterial isolates grown in peptone broth for 24 h. The change in color from faint yellow to dark brown indicated the production of ammonia (Cappuccino and Sherman 1992).
2.2.6 Hydrogen Cyanide Production
Hydrogen cyanide (HCN) production was detected using Castric method (1975). Briefly, the bacterial isolates were streaked on nutrient agar amended with glycine. Filter paper discs soaked in 2% Na2CO3 prepared in 0.5% picric acid solution were kept inside the lid of the Petri plates and incubated for 3 days at 28 °C. Color change of filter paper discs from orange to brown indicated HCN production.
2.3 Screening for Antagonism on Solid Medium by Well Diffusion Assay
The bacterial isolates were evaluated for antagonism against fungal phytopathogens Fusarium verticillioides strain 1 (MTCC 3322), Curvularia lunata strain 716 (MTCC 283), and Alternaria alternata strain 6663 (MTCC 1362) on yeast extract medium by well diffusion assay (Harris et al. 1989). The bacterial cultures were grown in nutrient broth for 48 h at 28 °C and centrifuged at 10,000 rpm. The fungal cultures were grown in potato dextrose agar for 7 days and a spore suspension was prepared by homogenizing the fungal cultures and suspending in sterile normal saline. One milliliter of fungal suspension was spread plated onto yeast extract agar plates in triplicates. A well is bored in the center of plates with the help of a sterile cork borer and 50 μl bacterial supernatant is added into each well in comparison to control plates where 50 μl of sterile distilled water was added. The zone of inhibition was measured after incubating the plates at 28 °C for 7 days.
2.4 Stress Tolerance Screening
Stress tolerance screening was carried out by growing the bacterial isolates at different stress levels of temperature, pH, salinity, and calcium salts. Temperature effect was studied by streaking the bacterial isolates on nutrient agar plates and incubating at 10, 30, and 40 °C for 48 h and observed for growth. Similarly, for pH stress, the nutrient agar with pH 5, 7, and 9 was prepared using citrate-phosphate buffer and the bacterial isolates were grown at 28 °C. Likewise, for salinity stress, the nutrient agar medium with 5 and 7.5% NaCl was prepared and the bacterial isolates were grown on the medium at 28 °C. The bacterial isolates were also screened for tolerance against calcium salts by growing on nutrient agar plates with 2.5 and 5% CaSO4, 2.5 and 5% CaCl2, and 2.5 and 5% CaCO3.
The results on growth of bacterial strains were scored as binary numbers, 1 representing growth and 0 indicating no growth. The data was subjected to cluster analysis using PAST software.
2.5 Effect on Plant Growth Promotion
Two selected bacterial isolates A1B3 and A1B6 were evaluated for growth promotion of pea (Pisum sativum var. Palam Priya) and maize (Zea mays var. Parbhat) as described earlier (Gulati et al. 2009; Kaur et al. 2017). Briefly, maize and pea seeds were sterilized by dipping for 3 min in 20% sodium hypochlorite and washed thrice with sterile distilled water. Thereafter, the surface sterilized seeds were dipped for 30 min in 48 h old bacterial cultures (OD adjusted to 1.0~109 CFU/ml). The initial count of bacteria per seed was determined by serially diluting a single bacterized seed in normal saline up to 10−6. From each dilution, 100 μl was spread plated on to nutrient agar plates and the plates were incubated for 24–48 h at 28 °C. Two seeds were sown in each 10-cm-diameter pots containing unsterilized garden soil. Seeds dipped in sterilized nutrient broth and sown in pots served as uninoculated controls. The experiment consisted of three treatments with 4 replicates each. The pots were kept under natural conditions in randomized block design and data was collected after 45 days on shoot length, root length, and total dry weight. For dry weight calculation, the plants were dried at 70 °C for 3 days in an oven till constant weight is obtained.
2.6 Characterization and Identification of the Bacterial Isolate
The bacterial isolate A1B3 and A1B6 were partially characterized and identified on the basis of phenotypic characters and 16S rRNA gene sequencing. For phenotypic characterization, Gram’s staining, motility, endospore staining, methyl red, Voges-Proskauer, catalase, citrate, oxidase, and urease tests were performed following the standard methods (Krieg and Holt 1984).
16S rRNA gene sequence analysis was carried out as described earlier (Gulati et al. 2008). RNA was isolated using the Qiagen DNeasy Plant Mini Kit. Gene amplification, thermocycling conditions, and sequence analysis have been described earlier in detail (Gulati et al. 2008). The sequences were aligned with ClustalW and MEGA software package version 7 using Kimura’s two-parameter model was used to calculate the evolutionary distance of the stress-tolerant strains A1B3 and A1B6 and their related taxa. The gene sequences of A1B3 and A1B6 have been submitted to NCBI GenBank with the accession numbers MG779636 and MG779637, respectively.
2.7 Experimental Design and Statistical Analysis
Randomized block design was implemented for carrying out the experiments. Unless stated otherwise, all values are the means of three replicates. Data on plant growth promotion was analyzed by analysis of variance (ANOVA) using XLSTAT 2016. The mean of the treatments were compared by Fisher’s significant difference (LSD) test at p values of 0.05.
3 Results
3.1 Isolation and Screening of Salt-Tolerant Bacterial Endophytes for Plant Growth-Promoting Attributes
In the present study, a total number of 16 morphologically distinct salt-tolerant endophytic bacteria were isolated from the leaves of Adhatoda vasica on nutrient agar plates with 2.5% NaCl. The salt-tolerant endophytes were further assessed for PGP activities including phosphate solubilization, auxin production, ACC deaminase activity, siderophore production, ammonia production, and HCN production.
Out of the 16 salt-tolerant bacterial endophytes, 13 isolates showed phosphate solubilization ranging from 12.8 to 424.4 μg/ml over uninoculated control at 28 °C on the fifth day of incubation in liquid medium (Table 1). Three isolates A1B5, A2B4, and A3B4 did not show any phosphate solubilization (Table 1). The bacterial isolates differed in their ability to solubilize phosphate with the highest solubilization shown by A1B6 and lowest by A3B1.
All 16 salt-tolerant endophytic bacteria showed the production of IAA-like auxins in tryptophan-amended media after 48 h incubation as detected using Salkowski reagent. However, the isolates varied in their ability to produce auxins ranging from 9.3 to 27.5 μg/ml. The highest production was shown by A1B3 while the lowest by A3B5 (Table 1). In addition, 12 isolates showed siderophore production, 10 showed ammonia production, 7 isolates showed ACC deaminase activity, and six isolates showed hydrogen cyanide production (Table 1).
3.2 Screening for Antagonism
The salt-tolerant bacterial endophytes were screened for antagonism against fungal pathogens. Eight bacterial isolates showed antagonistic activity against Fusarium verticillioides, seven against Alternaria alternata, and four against Curvularia lunata by well diffusion assay (Table 1). The zone of inhibition ranged from 8 to 22 mm against Fusarium verticillioides, 4–18 mm against Curvularia lunata, and 5–19 mm against Alternaria alternata. Four isolates showed antagonism against all tested phytopathogens with the highest antagonistic activity shown by A1B6 followed by A1B3 (Table 1, Fig. 1).
3.3 Screening for Stress Tolerance
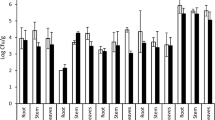
The 16 bacterial endophytes were tested for tolerance against different levels of pH, temperature, salinity, and calcium salts. The bacterial isolates exhibited difference in tolerating stress conditions (Fig. 2). All bacterial isolates showed growth on agar medium with 2.5 and 5% CaSO4, and 2.5% CaCO3. None of the isolates showed growth on 5% CaCl2. Seven isolates could grow at 5% NaCl, five at 7.5% NaCl, nine at 2.5% CaCl2, and 11 at 5% CaCO3. Among the different levels of pH tested, all isolates showed growth on pH 7 and four at pH 5 and 9. Likewise, among the different tested temperatures, all isolates exhibited growth at 30 °C, five at 10 °C, and four at 40 °C.
Similarity coefficient dendrogram of plant growth-promoting bacterial endophytes from Adhatoda vasica derived from their growth pattern under different stress levels of temperature (10 °C, 30 °C, and 40 °C), pH (5, 7, and 9), salinity (NaCl 5% and 7.5% NaCl), and calcium salts (CaSO4 2.5 and 5%, CaCO3 2.5 and 5%, and CaCl2 2.5 and 5%)
The dendrogram generated based on the stress tolerance of these bacterial isolates at different levels of tested temperatures, pH, salinity, and calcium salts showed three clusters and two independent branches with cluster 1 including 8 isolates, cluster II including 3 isolates, and cluster III including 2 isolates (Fig. 2). The bacterial isolates A1B3 and A1B6 showing the highest stress tolerance stood separately as an independent branch (Fig. 2).
3.4 Plant Growth Promotion
The bacterial isolates A1B3 and A1B6 selected based on initial studies on PGP activities, antagonism, and stress tolerance were tested in pots for growth promotion of pea and maize. The initial count of A1B3 and A1B6 per seed was 4.3 × 105 and 3.9 × 105 colony forming units, respectively. Both the bacterial isolates significantly enhanced the growth of both pea and maize (Table 2). However, among the two isolates, A1B3 showed significantly higher growth promotion than A1B6, except for the dry weight of both maize and pea where both the treatments are statistically at par with one another.
3.5 Characterization and Identification of the Bacterial Endophytes
The bacterial isolates A1B3 and A1B6 were characterized based on phenotypic features, biochemical tests, and 16S rRNA gene sequencing. Both the bacterial isolates were Gram positive, motile, rod shaped, arranged in chains, positive for endospore, catalase, and citrate utilization. The isolates were tested negative for oxidase, methyl red, Voges-Proskauer, indole, and urease. Based on the results, the bacterial isolates A1B3 and A1B6 were tentatively identified belonging to the genus Bacillus.
To confirm the identity of the bacterial isolates A1B3 and A1B6, 16S rRNA gene sequencing was carried out. 16S rRNA gene analysis of 1504-bp sequence of A1B3 showed 99% similarity with Bacillus thuringiensis strain IHB B 7117 while 1502-bp sequence of A1B6 showed 99% similarity with Bacillus sp. strain M1B2 (Fig. 3). The phylogenetic tree constructed on the basis of 16S rRNA gene sequences of A1B3 and A1B6 and their nearest neighbors showed two distinct groups. The first group consisted of A1B3 along with 4 Bacillus thuringiensis strains, 4 Bacillus sp. strains, and B. pumilus strain, whereas the second group consisted of A1B6 and Bacillus thuringiensis strain IHB B 7117.
4 Discussion
4.1 Isolation of Bacterial Endophytes and Screening for Plant Growth-Promoting Attributes
In view of the increasing cost and pollution related with chemical pesticides and fertilizers, interest has increased to find alternative methods of fertilization and control of pests. Bacterial endophytes are important components of sustainable agriculture playing a significant role in the nutrition of plants in view of their capability to produce large array of agriculturally important metabolites. However, different biotic and abiotic factors affect the performance of these microorganisms (Vyas et al. 2010; Das et al. 2015; Vyas and Kaur 2018). Abiotic stresses include temperature, pH, moisture status, salinity, and salts present in soils. Importantly, even though many plant growth-promoting bacteria show good results in vitro, they fail to give the same results in fields, when applied as microbial inoculants. One main reason for their failure is the stress imposed on them by the sudden changes in the environment (Vyas et al. 2010). Screening for stress tolerance is an important parameter in the selection of bacterial strains for the development of microbial inoculants. Therefore, with the objective of selecting stress-tolerant PGP and antagonistic bacteria, 16 morphologically distinct salt-tolerant endophytic bacteria were isolated from the leaves of Adhatoda vasica on nutrient agar plates with 2.5% NaCl. Endophytic bacteria have been isolated earlier also from different medicinal plants; however, no reports are available on the stress-tolerant endophytic bacteria isolated from Adhatoda vasica.
Phosphorus is an important nutrient limiting the growth of plants due to its fixation in soil. Microorganisms have the ability to solubilize insoluble phosphates in the soil and enhance growth of plants under conditions of poor phosphorus availability (Gulati et al. 2008). Herein, the 16 salt-tolerant bacterial endophytes showed phosphate solubilization but differed in their ability to solubilize tricalcium phosphate (Table 1). The results are in accordance with the earlier report where Pseudomonas strains showed difference in the solubilization of various phosphate substrates (Gulati et al. 2008). Recently, Aneurinibacillus sp., Bacillus sp., and Lysinibacillus sp. isolated from banana roots have been reported to exhibit phosphate solubilization (Matos et al. 2017). The application of these strains could prove to be highly beneficial in calcareous soils where phosphorus deficiency is attributed to the binding of phosphate with calcium.
Phytohormone production is another mechanism for plant growth promotion used by bacterial endophytes. All bacterial endophytes showed IAA production as detected spectrophotometrically (Table 1). IAA production has been reported for many PGP bacterial endophytes in the presence of tryptophan (Tsavkelova et al. 2007; Vyas and Kaur 2017). In addition, siderophore production and hydrogen cyanide production are important attributes of the microorganisms that influence plant growth by suppressing fungal pathogens. In the present studies, six isolates showed hydrogen cyanide production ranging from weak to strong as indicated by the color change of filter paper discs from yellow to orange/brown (Table 1). Siderophore-producing bacteria suppress fungal pathogens by making iron unavailable for fungal growth (Sayyed and Chincholkar 2009). Siderophore production zones by 12 bacterial isolates ranged from 3.5 to 21.2 mm after 5 days incubation on CAS agar plates.
Several plant growth-promoting bacteria produce the enzyme 1-aminocyclopropane-1-carboxylate (ACC) deaminase which breaks the plant ethylene precursor ACC to ammonia and α-ketobutyrate (Glick 2014). ACC deaminase-producing bacteria help to promote root elongation and plant growth by hydrolyzing ACC from germinating seeds and increasing the active rhizosphere zone. In the present studies, seven isolates exhibited growth on DF salts minimal medium with ACC as sole nitrogen source (Table 1). ACC deaminase-producing bacteria are known to protect plants against drought, flooding, salts, heavy metals as well as from bacterial and fungal pathogens (Glick 2014; Saikia et al. 2018). Therefore, bacteria producing ACC deaminase are important components of agriculture in stressed environments. Ammonia production is also an important trait as it is used by plants for their growth as a source of nitrogen (Glick 2014). Herein, ammonia production was shown by 10 isolates (Table 1). No reports are available on the plant growth-promoting endophytic bacteria from Adhatoda vasica.
4.2 Screening for Antagonism
Plant pathogens cause huge damage to the crop plants worldwide. Large-scale use of chemicals for controlling phytopathogens has led to the buildup of pesticide resistance among pathogens (Zalila-Kolsi et al. 2016). Biological control of plant diseases is an effective alternative to the use of chemical pesticides and fungicides. In the present studies, bacterial endophytes showed antagonistic activity against Fusarium verticillioides, Alternaria alternata, and Curvularia lunata (Table 1, Fig. 1). The antagonistic activity can be correlated with the production of HCN as all isolates showing antagonism also exhibited HCN production. HCN is one of the most important compounds inhibiting the growth of fungal pathogens, among the volatile compounds produced by bacteria (Reetha et al. 2014). The bacterial isolates showing antagonism also produced siderophores, which have also been implicated in inhibiting the growth of fungal pathogens. Strains of Bacillus amyloliquefaciens, Bacillus subtilis, and Paenibacillus polymyxa have been shown to show antagonistic activity against Fusarium graminearum (Zalila-Kolsi et al. 2016). Recently, endophytic Bacillus atrophaeus isolated from wild ethnomedicinal plant Glycyrrhiza uralensis (licorice) has been reported against multiple phytopathogens including Fusarium oxysporum, Alternaria solani, and Verticillium dahlia (Mohamad et al. 2018).
4.3 Screening for Stress Tolerance
The performance of plant growth-promoting bacteria is constrained by the stress generated by environmental factors including temperature, desiccation, pH, alkalinity/acidity, and salinity in the soil (Vyas et al. 2010; Das et al. 2015; Vyas and Kaur 2018). Salinity is a major stress-limiting plant growth, crop productivity, and performance of microorganisms in soils (Dodd and Pérez-Alfocea 2012). Screening for stress tolerance is an important parameter while selecting bacterial strains for developing biofertilizers. Therefore, the selection of stress-tolerant bacterial strains is essential for consistency in field performance for application as the microbial inoculants. In the present studies, the bacterial endophytes tested for tolerance against different levels of pH, temperature, salinity, and calcium salts exhibited difference in tolerating stress conditions (Fig. 2). The dendrogram generated based on the growth of these bacterial isolates at different stress levels showed three clusters and two independent branches (Fig. 2). The bacterial isolates A1B3 and A1B6 showing the highest stress tolerance stood separately as an independent branch (Fig. 2). Similar results were observed earlier while screening stress-tolerant Pseudomonas strains from Lahaul and Spiti valley in Himachal Pradesh, India (Vyas et al. 2009). Many reports are available on screening rhizobacteria for PGP properties; however, limited reports have shown the selection of bacteria based on stress tolerance along with other activities for the development of microbial inoculants (Vyas et al. 2009; Vyas and Kaur 2018). Selection of bacteria based on stress tolerance is important as bacterial screened in vitro fail to give same response in field due to the stress imposed by the environmental conditions (Vyas et al. 2009; Vyas and Kaur 2017; Kaur et al. 2018).
4.4 Plant Growth Promotion
The bacterial isolates A1B3 and A1B6 selected based on initial studies on PGP activities, antagonism, and stress tolerance were tested in pots for growth promotion of pea and maize. Both the bacterial isolates significantly enhanced the growth of both pea and maize (Table 2). However, among the two isolates, A1B3 showed significantly higher growth promotion than A1B6, except for the dry weight of both maize and pea where both the treatments are statistically at par with one another. Many PGP bacterial species belonging to Bacillus, Pseudomonas, Acinetobacter, and Rahnella have been reported to enhance growth promotion in various plants (Gulati et al. 2009; Vyas et al. 2010; Vyas and Kaur 2017; Kaur et al. 2018). Endophytic Bacillus licheniformis and Bacillus sp. isolated from Eucalyptus leaves and stems have shown growth-promoting effects on Eucalyptus plantlets under controlled environment (Paz et al. 2012). Recently, endophytic Bacillus strains isolated from sap, roots, and leaves of maize plants have been reported to enhance the growth and nutrient uptake of pearl millet (Ribeiro et al. 2018).
4.5 Characterization and Identification of the Bacterial Endophytes
The bacterial isolates A1B3 and A1B6 were characterized based on phenotypic features, biochemical tests, and 16S rRNA gene sequencing. 16S rRNA gene analysis of A1B3 showed highest similarity with Bacillus thuringiensis while A1B3 was most closely related to Bacillus sp. strain M1B2 (Fig. 3). Plant growth-promoting and antagonistic Bacillus spp. have earlier been reported for their stress tolerance against salinity, temperature, and desiccation (Vardharajula et al. 2011). However, no reports are available on the stress-tolerant bacterial endophytes isolated from Adhatoda vasica.
5 Conclusion
Endophytes are essential components of sustainable agriculture due to their ability to produce large number of agriculturally important metabolites. These endophytes not only enhance plant growth but also enable the plants to resist stress-like conditions. The present study characterizes two potential stress-tolerant plant growth-promoting endophytic bacterial strains isolated from the leaves of Adhatoda vasica as Bacillus thuringiensis A1B3 and Bacillus sp. A1B6. In addition to plant growth-promoting traits and antagonism against fungal pathogens, these strains also showed stress tolerance against temperature, pH, salinity, and calcium salts, which is essential for promoting plant growth under stressful conditions. The endophytic strains A1B3 and A1B6 also enhanced the growth of pea and maize plants in comparison to uninoculated control under natural conditions. Therefore, the strains Bacillus thuringiensis A1B3 and Bacillus sp. A1B6 selected as potential candidates based on multiple plant growth-promoting traits, antagonism against fungal pathogens, stress tolerance, and plant growth promotion can be used as microbial inoculants for agriculture in the stressed environment.
References
Cappuccino JC, Sherman N (1992) Microbiology: a laboratory manual. Benjamin/Cummings Publishing Co., New York, pp 125–179
Castric PA (1975) Hydrogen cyanide, a secondary metabolite of Pseudomonas aeruginosa. Can J Microbiol 21(5):613–618
Das P, Behera BK, Meena DK, Azmi SA, Chatterjee S, Meena K, Sharma AP (2015) Salt stress tolerant genes in halophilic and halotolerant bacteria: paradigm for salt stress adaptation and osmoprotection. Int J Curr Microbiol App Sci 4(1):642–658
Dodd IC, Pérez-Alfocea F (2012) Microbial amelioration of crop salinity stress. J Exp Bot 63(9):3415–3428
Ghosh R, Barman S, Khatun J, Mandal NC (2016) Biological control of Alternaria alternata causing leaf spot disease of Aloe vera using two strains of rhizobacteria. Biol Control 97:102–108
Glick BR (2014) Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol Res 169(1):30–39
Gulati A, Rahi P, Vyas P (2008) Characterization of phosphate-solubilizing fluorescent pseudomonads from the rhizosphere of seabuckthorn growing in the cold deserts of Himalayas. Curr Microbiol 56(1):73–79
Gulati A, Vyas P, Rahi P, Kasana RC (2009) Plant growth-promoting and rhizosphere-competent Acinetobacter rhizosphaerae strain BIHB 723 from the cold deserts of the Himalayas. Curr Microbiol 58(4):371–377
Harris LJ, Daeschel MA, Stiles ME, Klaenhammer TR (1989) Antimicrobial activity of lactic acid bacteria against Listeria monocytogenes. J Food Prot 52:384–387
Kaur R, Devi MA, Vyas P (2017) Endophytic Pseudomonas sp. TCA1 from Tinospora cordifolia stem with antagonistic and plant growth-promoting potential. Res J Pharmacy Technol 10(2):456–460
Kaur A, Devi SR, Vyas P (2018) Stress-tolerant antagonistic plant growth-promoting rhizobacteria from Zea mays. J Plant Prot Res 58:115–123
Krieg NR, Holt JG (1984) Bergey’s manual of systematic bacteriology Vol 1. Williams and Willkins, Baltimore, p 964
Kumar M, Dandapat S, Kumar A, Sinha MP (2013) Determination of nutritive value and mineral elements of five-leaf chaste tree (Vitex negundo L.) and Malabar nut (Adhatoda vasica Nees). Atmosphere 7:8
Lal M, Kumar S, Ali M, Khan A, Singh V, Murti S (2013) Host range, susceptibility period of Curvularia lunata causing leaf spot of black gram and germplasm screening. Agriways 1:142–146
Loper JE, Schroth MN (1986) Influence of bacterial sources of indole-3-acetic acid on root elongation of sugar beet. Phytopathol 76(4):386–389
Matos AD, Gomes IC, Nietsche S, Xavier AA, Gomes WS, Dos Santos Neto JA, Pereira MC (2017) Phosphate solubilization by endophytic bacteria isolated from banana trees. An Acad Bras Cienc 89(4):2945–2954
Mohamad OA, Li L, Ma JB, Hatab S, Xu L, Guo JW, Rasulov BA, Liu YH, Hedlund BP, Li WJ (2018) Evaluation of the antimicrobial activity of endophytic bacterial populations from Chinese traditional medicinal plant Licorice and characterization of the bioactive secondary metabolites produced by Bacillus atrophaeus against Verticillium dahliae. Front Microbiol 9
Nautiyal CS (1999) An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol Lett 170:265–270
Paz ICP, Santin RCM, Guimarães AM, Rosa OPP, Dias ACF, Quecine MC, Azevedo JL, Matsumura ATS (2012) Eucalyptus growth promotion by endophytic Bacillus spp. Genet Mol Res 11(4):3711–3720
Reetha AK, Pavani SL, Mohan S (2014) Hydrogen cyanide production ability by bacterial antagonist and their antibiotics inhibition potential on Macrophomina phaseolina (Tassi.) Goid. Int J Curr Microbiol App Sci 3:172–178
Ribeiro VP, Marriel IE, de Sousa SM, de Paula Lana UG, Mattos BB, de Oliveira CA, Gomes EA (2018) Endophytic Bacillus strains enhance pearl millet growth and nutrient uptake under low-P. Braz J Microbiol 49:40–46
Saikia J, Sarma RK, Dhandia R, Yadav A, Bharali R, Gupta VK, Saikia R (2018) Alleviation of drought stress in pulse crops with ACC deaminase producing rhizobacteria isolated from acidic soil of Northeast India. Sci Rep 8:3560
Sayyed RZ, Chincholkar SB (2009) Siderophore-producing Alcaligenes feacalis exhibited more biocontrol potential Vis-à-Vis chemical fungicide. Curr Microbiol 58:47–51
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56
Tan D, Fu L, Han B, Sun X, Zheng P, Zhang J (2015) Identification of an endophytic antifungal bacterial strain isolated from the rubber tree and its application in the biological control of banana Fusarium wilt. PLoS One 10:e0131974
Tsavkelova EA, Cherdyntseva TA, Botina SG, Netrusov AI (2007) Bacteria associated with orchid roots and microbial production of auxin. Microbiol Res 162:69–76
Vardharajula S, Zulfikar Ali S, Grover M, Reddy G, Bandi V (2011) Drought-tolerant plant growth promoting Bacillus spp.: effect on growth, osmolytes, and antioxidant status of maize under drought stress. J Plant Interact 6:1–14
Vyas P, Kaur R (2017) Plant growth-promoting and antagonistic endophytic bacteria from the medicinal plant Tinospora cordifolia stem. Inter J Res Pharm Sci 8:196–199
Vyas P, Kaur A (2018) Stress-tolerant antagonistic rhizobacteria isolated from the medicinal plant Tinospora cordifolia. Biotechnologia 99:129–136
Vyas P, Rahi P, Gulati A (2009) Stress tolerance and genetic variability of phosphate-solubilizing fluorescent Pseudomonas from the cold deserts of the trans-Himalayas. Microb Ecol 58:425–434
Vyas P, Joshi R, Sharma KC, Rahi P, Gulati A, Gulati A (2010) Cold-adapted and rhizosphere-competent strain of Rahnella sp. with broad-spectrum plant growth-promotion potential. J Microbiol Biotechnol 2:1724–1734
Zalila-Kolsi I, Mahmoud AB, Ali H, Sellami S, Nasfi Z, Tounsi S, Jamoussi K (2016) Antagonist effects of Bacillus spp. strains against Fusarium graminearum for protection of durum wheat (Triticum turgidum L. subsp. durum). Microbiol Res 192:148–158
Acknowledgements
The authors acknowledge the Vice Chancellor, Punjab Agricultural University, Punjab and the Chancellor, Lovely Professional University, Punjab, for providing the necessary facilities.
Funding
This study was financially supported by the Chancellor, Lovely Professional University, Punjab, to carry out the research studies.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vyas, P., Kaur, R. Culturable Stress-Tolerant Plant Growth-Promoting Bacterial Endophytes Associated with Adhatoda vasica. J Soil Sci Plant Nutr 19, 290–298 (2019). https://doi.org/10.1007/s42729-019-00028-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-019-00028-9