Abstract
Due to the traditional rearing conditions in Algerian farms, a broad panel of ectoparasites can infest ruminants and domestic birds. Among them, lice (Phthiraptera) could be a severe source of discomfort, disturbance, and diseases for the infested animals and cause decreased productivity and economic losses. The current study was conducted for two years and aimed to investigate ruminant and domestic bird lice diversity in northeastern Algeria and argue their veterinary importance. The entomological field investigations were conducted on various animal species, including cattle, sheep, goats, backyard chickens, turkeys, and pigeons, in six regions of northeastern Algeria. Lice were collected manually on their hosts and kept in Eppendorf containing 70% ethanol. They were then morphologically identified according to several morphological keys. Among all 4488 collected lice, five species in ruminants and ten in domestic birds were identified. The most common lice species were Bovicola caprae in goats, Haematopinus eurysternus in cattle, Menacanthus stramineus in backyard chickens, Chelopistes meleagridis in turkeys, and Columbicola columbae in pigeons. We also identified other species with various abundance such as Linognathus africanus, Bovicola ovis, Bovicola bovis, Menopon gallinae, Goniocotes gallinae, Goniodes dissimilis, Goniodes gigas, Lipeurus caponis, Cuclotogaster heterographus, and Campanulotes bidentatus s.l.
The survey results suggest that lice infestations are widespread in the studied areas. Further investigation is needed to evaluate such pests’ impact on overall animal health and production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lice (Phthiraptera) are hemimetabolous insects taxonomically divided into two main morphologically distinct groups: blood-sucking lice (Anoplura) and skin-chewing lice (Mallophaga) (Marcondes 2017; Durden 2019). The bodies of these insects are bilaterally symmetric and dorsoventrally flattened, facilitating their movement through the host’s fur or feathers (Meyer and Madon 2002; Marcondes 2017). Their host and nutrition specificity make them challenging to keep alive once separated from the host (Jassem et al. 2023; Springer et al. 2023). The impact of a lice infestation depends on the species’ aggressivity and the host’s overall health (Ahmed et al. 2009; Gharbi et al. 2013). Light infestations may cause mild discomfort, itching, and irritation. In contrast, heavy infestations can significantly induce anemia, dermatitis, secondary bacterial infections, weight loss, and decreased animal productivity (Al-Saffar and Al-Mawla 2008; Da Silva et al. 2013; Mirzaei et al. 2016; Gharbi et al. 2020; Eren et al. 2022). All these consequences could lead to economic losses for breeders who depend on animal products for sustenance and income (Benelli et al. 2018; Meguini et al. 2018; Muhammad et al. 2021).
In Algeria, animal lice infestations were considered benign entomological epizootic for a long time (Meguini et al. 2018; Ouarti et al. 2020b). This is why there were few surveys on these ectoparasites. The first entomological investigations, dated 2013 and 2014, focused on free-range chicken lice (Medjouel et al. 2013; Medjouel et al. 2014). Since then, lice have aroused very little interest in the Algerian scientific community, and only two studies on nesting birds’ lice were published (Baziz-Neffah et al. 2015; Touati et al. 2015). In early 2018, animal lice started increasing gradually in scope, moving from a sporadic topic to a highly discussed research axe. Studies that followed assessed the diversity of lice species in domestic mammals and farmyard chickens (Meguini et al. 2018), wild boars (Sus scrofa) (Zeroual et al. 2018), and cattle egrets (Bubulcus ibis) (Amina et al. 2018). Later various works investigated lice infestation in wildlife birds such as Miropidae (Torki et al. 2020), the Eurasian coot (Fulica atra) (Ziani et al. 2020), the Common Moorhen (Gallinula chloropus) (Ziani et al. 2021), Passeriformes (Ouarab et al. 2021), migratory and sedentary doves (Absi et al. 2021) and the white stork (Ciconia Ciconia) (Touati et al. 2022). Other works on wild mammal lice have also been published, including those on the common gundi (Ctenodactylus gundi) (Meddour et al. 2022) and the black rats (Rattus rattus) (Randa et al. 2022). Overall, the Algerian studies cited above focused on the lice diversity in wild animals. In contrast, few were interested in domestic ruminants and birds (Saidi et al. 2020; Nahal et al. 2021). Consequently, lit is known about the role of these pests as potential vectors of infectious diseases. Only two Algerian molecular studies have highlighted proof of this possibility (Zeroual et al. 2018; Ouarti et al. 2021). Therefore, it can be assumed that since several sucking or chewing lice species feed on blood, they could serve as reservoirs and vectors of infectious diseases (Aleksandravičienė et al. 2021; Ouarti et al. 2021; Kazim et al. 2022). Indeed, many examples of Anatidae or mammals’ lice species (Ansari 1955) and Amblycera were reported as potential vectors of cestodes and some filaria to swans and geese (Clayton et al. 2008; Hugon 2015; Benelli et al. 2018). Animal lice present a complex challenge for managing livestock and wildlife in Algeria and beyond (Ouarti et al. 2020b). To effectively address this issue, it is crucial, from a cognitive science perspective, to understand the intricacies of how animal lice acquire and probably transmit these pathogenic agents (Promrangsee et al. 2019; Ouarti et al. 2020a).
It will also be necessary to delve into these pests’ morphology, mechanisms to locate and infest the hosts, and how the host’s immune system responds to infestation. Overall, drawing up a checklist of all parasitic animal lice and their associated hosts can provide valuable insights into the evolution of lice parasitic behavior and host-parasite interactions and contribute to stopping the spread and persistence of lice infestations. Nevertheless, developing and implementing sustainable control measures will require a multifaceted approach considering the unique challenges of different host species and their environments.
The current study aimed to assess ruminant and domestic bird lice diversity in northern Algeria and debate their veterinary importance.
Materials and methods
Study areas and period of sampling
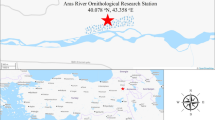
During two years, field collections were carried out on domestic birds and ruminants in six regions of northeastern Algeria: El Tarf (36°51’21.5"N, 8°19’34.5"E), Sétif (36°9′0″N, 5°26′0″E), Oum El Bouaghi (35°52′39″N, 7°06′49″E), Guelma (36°28′0″N, 7°26′0″E), Mila (36°27′0″N, 6°16′0″E) and Bordj Bou Arreridj (36°04′00″N, 4°46′00″E) (Fig. 1).
These cattle-breeding zones belong to similar bioclimatic stages: El Tarf and Guelma have a Mediterranean climate with very hot summers, whereas the rest of the regions have a cold, semi-arid environment with warm summer days (Peel et al. 2007). For our study, we looked for ruminant and bird farms in each study region and adapted the fieldwork according to their disponibility.
Animals
Various animal species, including ruminants and domestic fowl, were involved in this study. One cattle farm (Sétif), four sheep farms (El Tarf n = 3 and Sétif n = 1), and eight goat farms (El Tarf n = 5, Guelma n = 1, Sétif n = 1, Oum El Bouaghi n = 1) were randomly chosen and regularly visited. The study was conducted for backyard chickens in three traditional El Tarf region farms, nine from Bordj Bou Arreridj and one from Mila. In addition, one turkey breeding and two groups of pigeons located in El Tarf (Ain El Kerma) were also checked for the presence of lice. The number of animals on each farm is detailed in Tables 1 and 2.
Lice collection and identification
For birds, the collections were made manually or using entomological grippers by inspecting the entire body of the birds, paying specific attention to the head and feathers of the neck, wings, belly, and tail. When the birds were overly infested, a bioinsecticide powder (based on lavender and geranium essential oils) with no side effects was sprinkled on their bodies. After that, they were placed in a carton box for 30 min (Clayton and Walther 1997). In the case of ruminants, the parasitized sheep’s fleeces were separated or, in some cases, cut with scissors, and the lice were then retrieved from the deepest parts of the wool. The skin was brushed using a stainless-steel lice comb for cattle and goats. All the lice found on each examined animal were collected and directly stored in individual prelabelled Eppendorfs containing 70% ethanol.
The morphological identification of sampled lice was realized at the species level under a Leica® binocular lens with LED light. The dichotomous keys and criteria provided by Tuff (Tuff 1977), Wall (Wall and Shearer 2001), Pajot (Pajot 2000), and Hugon (Hugon 2015) were used for the morphological identification of adult specimens, while nymphal stages were excluded. The nomenclature of the lice species used in this study was chosen based on names provided by earlier investigations and for which there is a scientific consortium (Hopkins and Clay 1953; Barnard 2011; Dik and Halajian 2013; Meguini et al. 2018; Ouarti et al. 2020a). A microscope (Zeiss Axio Zoom.V16, Zeiss, Marly le Roi, France) was used to acquire dorsal and ventral photos of each species at a magnification of x56 (Fig. 2a and Fig. 2b).
Dorsal and ventral photographs of some of the collected lice species x56. a: Bovicola caprae[♀:A, B; ♂ C, D; Nymphe: E]; Bovicola bovis[♀:F, G]; Haematopinus eurysternus[♀:H, I; ♂ J, K; Nymphe: L, M]; Linognathus africanus[♀:N, O; ♂ P, Q]; Lipeurus caponis[♀:R, S]; Columbicola columbae[♀:T, U; ♂ V, W]; Chelopistes meleagridis[♀:X, Y; ♂ Z, Z’]. b: Goniodes dissimilis[♀:1, 2; ♂ 3, 4]; Goniocotes gallinae[♀:5, 6; ♂ 7, 8]; Menacanthus stramineus[♀:9, 10; ♂ 11, 12]; Menopon gallinae[♀:13, 14; ♂ 15, 16]; Campanulotes bidentatus[♀:17, 18; ♂ 19, 20]
Results
Ruminants and domestic bird’s lice diversity
A total of 4488 lice (1355 from ruminants and 3133 from birds) were collected from more than 184 infested hosts belonging to various animal species. The outcome of the morphological identification summarized in Tables 1 and 2 confirmed the presence of five lice species belonging to three different genera in ruminants and ten species affiliated with nine genera in birds.
Among all infested ruminants, the most common lice were from goats, more particularly, Bovicola caprae (Gurlt, 1843) with 474/1355 (34.98%) collected specimens, followed by Linognathus africanus (Kellogg & Paine, 1911) with 393/1355 (29%) samples. Haematopinus eurysternus (Nitzsch, 1818) was more common in cattle with 314/1355 (23.17%) lice. However, the infestation rates of Bovicola ovis (Schrank, 1781) and Bovicola bovis (Linnaeus, 1758) were only 10.84% and 1.99%, respectively.
In backyard chickens, seven species were sampled. The amblyceran chewing lice Menacanthus stramineus (Nitzsch, 1818) lice were the most frequently collected with 1704/2667 (63.89%) specimens. They were followed by another chewing louses Menopon gallinae (Linnaeus, 1758), with 751/2667 (28.15%) samples. Three other ischnoceran species, including Goniocotes gallinae (de Geer, 1778), Goniodes dissimilis (Denny, 1842), and Goniodes gigas (Taschenberg, 1879), were collected with low abundances of 3.78%, 2.58%, and 1.01%, respectively. We also identified 14 specimens of Lipeurus caponis (Linnaeus, 1758) and only one Cuclotogaster heterographus female (Nitzsch, 1866).
All the examined turkeys were infested with one or two genera. Chelopistes meleagridis (Linnaeus, 1758) was the most common species (66.27%), while Me. stramineus (Nitzsch, 1818) was the second most common (33.72%). Lice infested all the pigeons in both groups. Two species were identified on them: 105/128 (82.03%) Columbicola columbae (Linnaeus, 1758) and 23/128 (17.96%) Campanulotes bidentatus s.l. (Scopoli, 1763).
3.2. Geographical distribution of lice species
During our investigation in El Tarf localities, farms from this region were the most infested with 2365/4488 (52.70%) collected lice belonging to 13 species, followed by those from Mila (three species), Sétif (five species) and Bordj Bou Arreridj (four species). On the other hand, only two goat species (B. caprae and L. Africanus) were identified in Oum EL Bouaghi and Guelma (Fig. 1). The relative abundance of each species according to the sampled region is detailed in Figs. 3 and 4.
Discussion
Lice species have probably always been associated with humans, animals, and their ancestors (Light et al. 2010; Durden 2019; Patel et al. 2021). They have been scattered worldwide through the migrations humans have made during their existence. (Reed et al. 2004; Duvallet et al. 2017). Because of this intimate association between lice and their hosts, these insects showed strong co-speciation and coevolution (Boyd et al. 2017; Sweet et al. 2022). Their origin is still unknown. However, phylogenetic studies suggested that Anoplura and Mallophaga are monophyletic and that all lice derive from a non-hematophagous ancestor that developed its mouthparts to adapt to a hematophagous feeding behavior (Reed et al. 2007; Johnson et al. 2022).
Due to this nutrition mode, the vectorial competence of these arthropods has been highlighted throughout human evolution (Houhamdi et al. 2005; Amanzougaghene et al. 2020). However, this ability to transmit pathogens has often been restricted to some Anoplurans blood-sucking lice species of public health importance, such as Pediculus humanus humanus (Linnaeus, 1758) and P. humanus capitis (Linnaeus, 1758) (Amanzougaghene et al. 2017; Mana et al. 2017; Louni et al. 2018; Boumbanda Koyo et al. 2019).
Although domestic and wild animals have specific lice species, they are seldom considered a significant problem in Algeria. As a result, breeders often ignore them and consider them harmless to their animals. Furthermore, according to previous studies, relatively few of these animal lice species are potential vectors of pathogens (Kumsa et al. 2012; Zeroual et al. 2018; Promrangsee et al. 2019; Ouarti et al. 2021). Instead, the frequently highlighted pathogens in literature were the swinepox virus and the murine Mycoplasma associated with Haematopinus suis (Linnaeus, 1758) and Polyplax spinulosa (Burmeister, 1839) (Hornok et al. 2015; Ramakrishnan and Ashokkumar 2019; Delhon 2022).
In Algeria, less is known about the diversity of sucking and chewing lice and their part in vector-borne disease epidemiology. Several studies were conducted on human lice’s phylogeny and vector competence (Mana et al. 2017; Louni et al. 2018; Ouarti et al. 2022), while few were interested in animal lice (Medjouel et al. 2013; Medjouel et al. 2014; Meguini et al. 2018; Nahal et al. 2021; Ouarab et al. 2021). Therefore, a survey of ruminants’ most prevalent lice species and domestic fowl is imperative to understand host-lice interaction. Furthermore, the detailed findings could update any outdated information regarding the diversity of lice and host records in northeastern Algeria.
During our survey and among 4488 collected specimens, five ruminant and ten fowl lice species were identified. Our results regarding the identified lice species on ruminants and poultry are consistent with those described in previous Algerian studies (Meguini et al. 2018; Nahal et al. 2021; Ouarab et al. 2021). The goat lice B. caprae and the poultry body louse Me. stramineus were the most common lice species. For a long time, the goat-biting louse, B. caprae, was morphologically confused with its close parent, B. limbatus (syn. B. limbata). Ancient studies have even suggested convergent evolution between both species (Der Breite 1985; Benitez Rodriguez et al. 1986). However, they can be differentiated by examining the male genitalia and the female’s size, where B. limbatus specimens are more robust (Der Breite 1985; Price and Graham 1997).
Interestingly, B. caprae can only be hosted by goats (Benelli et al. 2018). In our survey, the goats were often co-infested by B. caprae and L. africanus. Previous studies confirmed these findings, where chewing and sucking lice were present on the same host (Rashmi and Saxena 2017; Corke and Matthews 2018; Mansur et al. 2019; González-Álvarez 2020).
The African blue-sucking louse L. africanus can be hosted by sheep and goats (Aimee and Patrick 2019). It can be distinguished from L. stenopsis by observing the head’s posterolateral margins bulging in L. africanus(O’callaghan et al. 1989; Nizamov and Prelezov 2021). Unlike Bovicols, which are limited to a direct pathogenic role by damaging the host skin and reducing its resistance to other diseases (White et al. 2022), L. africanus can carry various pathogenic agents such as Anaplasma ovis, Coxiella burnetti, and Rickettsia spp. (Ehlers et al. 2020; Ouarti et al. 2021). The hematophagous short-nosed cattle louse H. eurysternus identified in our study alongside B. bovis can be responsible for severe anemia and mortalities (Lasisi et al. 2010). It may also be involved in transmitting pathogens such as Theileria orientalis, Rickettsia spp., and C. burnetti(Reeves et al. 2006; Hornok et al. 2010; Lakew et al. 2021). However, the vectorial competence of these lice cannot be confirmed as the pathogenic agents could mechanically be acquired during a blood meal on an infected host.
The chicken body louse Me. stramineus was reported in several studies in Europe and other countries as the most common bird lice species (Paliy et al. 2018; Adly et al. 2022; Chambless et al. 2022; Kouam et al. 2022; Shaikh et al. 2023). Domestic poultry can be infested by this species via lice transfer after direct contact with wild turkeys and birds (Hugon 2015). In addition, this amblyceran louse can parasitize a wide range of hosts and is often localized in areas with a low density of feathers (Smith 2004; Martinů et al. 2015; Paliy et al. 2018; Adly et al. 2022; Chambless et al. 2022; Kouam et al. 2022). Heavily-infested chickens were reported to have skin with scabs, dried blood, dander, serous oozing, and areas carrying lice aggregates. Minor punctiform wounds in the bleeding area of young feathers were also noticed. This is in agreement with all symptoms reported in previous studies (Devaney 1976; Pavlovic et al. 1989; Shanta et al. 2006).
Over fieldwork, the shaft lice M. gallinae were more frequently collected on young birds than adults. They can be morphologically differentiated from Menacanthus sp. by the absence of a spine-like sclerotinized ventral process on the head (Yevstafieva 2015; Saikia et al. 2017). Organized in a single file along the feathers shaft, they usually feed on feather particles (Price and Graham 1997; Saxena et al. 1997; Sudiana et al. 2020). Although it is generally admitted that chicken body louse damages are more severe than those induced by shaft louse, several studies have emphasized that M. gallinae-infested chickens are less egg productive than uninfested (Ikpeze et al. 2008; Abdullah and Mohammed 2013; Mohammed and Mohammad 2021).
Despite their classification among chewing lice, Me. stramineus and M. gallinae can adopt a hematophagous behavior by grinding fledgling feathers under the skin and absorbing thus tiny blood droplets visible through their digestive tract inducing thus, in some cases, severe anemia (Sychra et al. 2008; Kumar et al. 2017; Abdullah et al. 2018). Due to this behavior, these lice species have been suspected as potential vectors of bacteria, virus, and protozoa. Pathogens such as Borrelia sp, Wolbachia, the equine encephalomyelitis virus, and Toxoplasma gondii were isolated from Me. stramineus(Covacin and Barker 2007; Al-Lebawi and Hadi 2015; Ahmed et al. 2020; Corrin et al. 2021; Ouarab et al. 2021), while Chlamydia psittaci the psittacosis agent in birds was detected in M. gallinae(Mirzaei et al. 2016; Kaboudi et al. 2019).
Three ischnoceran species were also identified in backyard chickens: Go. gallinae (chicken fluff louse), G. dissimilis (brown chicken louse), and G. gigas (large chicken louse); these low pathogenic species are often confused because of their close general morphology (Sychra et al. 2008; Hugon 2015). Nevertheless, the study of their phylogenetic relationship shows that they belong to the same cluster and form a monophyletic group (Nasser et al. 2020; Mohammed Adnan and Shamal Abdullah 2021).
Our survey identified C. meleagridis alongside M. stramineus as the most frequent turkeys lice. This species is usually localized on wild and domesticated turkeys’ neck and breast feathers (Reeves et al. 2007; Sanchez-Montes et al. 2018; Adly et al. 2022). C. meleagridis lice feed on their host’s feathers, causing significant skin damage, irritation, and discomfort in severe infestations (Camacho-Escobar et al. 2014; Maturano and Daemon 2014; Rabana et al. 2019; Shaikh et al. 2021). To the best of our knowledge, there is no molecular evidence of the vector potential of C. meleagridis(Ouarti et al. 2021). However, as noticed for other chewing lice species, it is theoretically possible for the large turkey louse to act as a vector of specific diseases or pathogens.
All the examined pigeons of the study were infested with Co. columbae (slender pigeon louse) and Ca. bidentatus s.l. (small pigeon louse). These two species are recognized worldwide as the most common pigeon lice (Alajmi et al. 2021; Olejkov et al. 2021; Al-Badrani and Al-Muffti 2023; Jassem et al. 2023). Co. columbae lice live between the barbs of the primary and secondary tail feathers, deposit their eggs under the wings and feed on the chest feathers (Price and Graham 1997; Hugon 2015; Soliman et al. 2022). Ca. bidentatus s.l. adults are more versatile; they can be found in all body regions or mainly on fluffy parts of body feathers (Hugon 2015; Marcondes 2017). Studies have shown that the combined infestations of these two lice species interfere negatively with male sexual parades and induce female pigeons to choose uninfested males for mating (Clayton 1990,1991; Price and Graham 1997).
Overall, the high prevalence of some lice species in our study could be attributed to poor management practices in ectoparasite control, poor hygiene, and inadequate use of insecticides that favor the lice species’ reproduction (Mulugeta et al. 2010; Meguini et al. 2018; Durden 2019). A conducive environment in terms of weather and climate could also be incriminated (Mansur et al. 2019). Indeed, climatic conditions, including temperature and humidity, are essential in the lice’ life cycle (Chen and Mullens 2014; Durden 2019). As it was noticed for the El Tarf region, where we collected 2365 lice, favorable and optimum climatic conditions, host availability, and abiotic factors would potentiate the increase in abundance, thus increasing the lice infestation rate.
In order to preserve animal productivity, lice infestation control should typically involve using insecticides (pyrethroid). However, reasonable insecticide use should be mandatory to avoid overuse that can lead to environmental pollution and resistance among lice species. Alternatively to these chemical control methods, good sanitation practices and breeding conditions can also help to prevent and control lice infestations. This includes regularly cleaning and disinfecting breeding locals, coops, and equipment and removing and destroying dead-infested animals’ bodies. Overall, combining chemical and non-chemical control methods can effectively manage lice infestations in birds and ruminants. Further research will be needed to expand the Algerian list of chewing and sucking lice -by including wild mammalian species- and to access their ability to transmit pathogenic agents.
References
Abdullah SH, Mohammed AA (2013) Ecto and Endo parasites prevalence in domestic chickens in Sulaimani region. Iraqi J Veterinary Med 37(2):149–155
Abdullah SH, Mohammed AA, Saeid NM (2018) Study of ecto and haemo parasites in domestic pigeons (Columba livia Domestica) in Sulaimani province. Kurdistan region/Iraq Journal of Zankoy Sulaimani 20(1):37–44
Absi K, Dik B, Farhi K, Belhamra M (2021) New data concerning ectoparasites infesting two species of doves, the migratory turtle dove (Streptopelia turtur) and the exotic sedentary eurasian collared dove (Streptopelia decaocto) in south-eastern oases of Algeria (Biskra). Bull Soc Zool Fr 146(2):57–67
Adly E, Alkhalaf AA, Nasser M, Al Ashaal S (2022) Contribution to the knowledge of chewing lice of Turkey Meleagris gallopavo Domesticus Linnaeus, 1758 (Galliformes: Phasianidae) encountered in Egypt and Saudi Arabia with new records and identification key. Int J Trop Insect Sci 42(3):2693–2700. https://doi.org/10.1007/s42690-022-00798-3
Ahmed W, Habeeb SM, El Moghazy FM, Hanafi EM (2009) Observation on pediculosis in buffalo-cows with emphasis on its impact on ovarian activity and control by herbal remedies. World Appl Sci J 6(8):1128–1138
Ahmed SE, Thamar NK, Othman RM (2020) Molecular detection of Toxoplasma Gondii in chicken lice (Menacanthus Stramineus). Basrah J Veterinary Res 19(3)
Aimee D-KC, Patrick YK (2019) Infestation of ruminant species by ticks and lice in port-bouët cattle market (Abidjan-Cote D’ivoire). Archives of Veterinary and Animal Sciences 1(1)
Al-Badrani MA, Al-Muffti SA (2023) Survey and Prevalence of Lice Infestation the pigeons (Columba livia Domestica) in Kurdistan Region-Iraq. Rafidain J Sci 32(1):1–8. https://doi.org/10.33899/RJS.2023.177282
Al-Lebawi FI, Hadi M (2015) Molecular detection of Toxoplasmosis in biting lice (Menacanthus Stramineus) on naturally infected chickens using polymerase chain reaction. AL-Qadisiyah J Veterinary Med Sci 14(2):37–40
Al-Saffar T, Al-Mawla E (2008) Some hematological changes in chickens infected with ectoparasites in Mosul. Iraqi J Veterinary Sci 22(2):95–100. https://doi.org/10.33899/ijvs.2008.5726
Alajmi RA, Metwally DM, El-Khadragy MF, Yehia HM, El-Ashram S, Almusawi Z, Bashir MA, Alotaibi NJ, Abdel-Gaber R (2021) Molecular identification of Campanulotes Bidentatus Scopoli, 1763 (Phthiraptera, Philopteridae) infecting the domestic pigeon Columba livia from Saudi Arabia. Saudi J Biol Sci 28(4):2613–2617. https://doi.org/10.1016/j.sjbs.2021.02.006
Aleksandravičienė A, Paulauskas A, Stanko M, Fričová J, Radzijevskaja J (2021) New records of Bartonella spp. and Rickettsia spp. in lice collected from small rodents. Vector-Borne and Zoonotic Diseases 21(5):342–350. https://doi.org/10.1089/vbz.2020.2722
Amanzougaghene N, Fenollar F, Sangare AK, Sissoko MS, Doumbo OK, Raoult D, Mediannikov O (2017) Detection of bacterial pathogens including potential new species in human head lice from Mali. PLoS ONE 12(9):e0184621. https://doi.org/10.1371/journal.pone.0184621
Amanzougaghene N, Fenollar F, Raoult D, Mediannikov O (2020) Where are we with human lice? A review of the current state of knowledge. Front Cell Infect Microbiol 9:474. https://doi.org/10.3389/fcimb.2019.00474
Amina A, Yassine N, Menouar S, Bilal D, Ramzi H (2018) First data on identification of avian lice Ciconiphilus Decimfasciatus (Boiduvale and Lacordaire, 1835). Species parasitizing cattle egrets (Bubulcus ibis) in Eastern of Algeria. World 7(2):45–49
Ansari M (1955) Synoptic table for the determination of Mallophaga infesting the domestic fowl (Gallus gallus Domesticus). Indian J Entom 17:245–270
Barnard PC (2011) Order Phthiraptera: The Sucking and Biting Lice. The Royal Entomological Society Book of British Insects 106–111
Baziz-Neffah F, Bitam I, Kernif T, Beneldjouzi A, Boutellis A, Berenger J-M, Zenia S, Doumandji S (2015) Contribution to the knowledge of bird ectoparasites in Algeria. Bull Soc Zool Fr 140(2):81–98
Benelli G, Caselli A, Di Giuseppe G, Canale A (2018) Control of biting lice, Mallophaga – a review. Acta Trop 177:211–219. https://doi.org/10.1016/j.actatropica.2017.05.031
Benitez Rodriguez R, Cruz S, Florido Navio A, Perez Jimenez J (1986) Bovicola caprae and Bovicola Limbata (Mallophaga) parasites of Capra hircus. Is there a possible phenomenon of convergent evolution between both species? Cahiers ORSTOM Serie Entomologie Medicale et Parasitologie (France)
Boumbanda Koyo CS, Amanzougaghene N, Davoust B, Tshilolo L, Lekana-Douki JB, Raoult D, Mediannikov O, Fenollar F (2019) Genetic diversity of human head lice and molecular detection of associated bacterial pathogens in Democratic Republic of Congo. Parasit. Vectors 12(1):1–9. https://doi.org/10.1186/s13071-019-3540-6
Boyd BM, Allen JM, Nguyen NP, Vachaspati P, Quicksall ZS, Warnow T, Mugisha L, Johnson KP, Reed DL (2017) Primates, lice and Bacteria: speciation and genome evolution in the symbionts of Hominid Lice. Mol Biol Evol 34(7):1743–1757. https://doi.org/10.1093/molbev/msx117
Camacho-Escobar MA, Arroyo-Ledezma J, Avila-Serrano N, Jerez-Salas M, Sanchez-Bernal E, García-López J (2014) Ectoparasites and their damage in backyard turkeys in Oaxaca’s coast. Mexico Eur J Veterinary Med 2014(7):1–18
Chambless KN, Cornell KA, Crespo R, Snyder WE, Owen JP (2022) Diversity and prevalence of ectoparasites on Poultry from Open Environment farms in the Western-United States of Washington, Idaho, Oregon, and California. J Med Entomol 59(5):1837–1841. https://doi.org/10.1093/jme/tjac093
Chen BL, Mullens BA (2014) Temperature and humidity effects on off-host survival of the northern fowl mite (Acari: Macronyssidae) and the chicken body louse (Phthiraptera: Menoponidae). J Econ Entomol 101(2):637–646. https://doi.org/10.1093/jee/101.2.637
Clayton DH (1990) Mate choice in experimentally parasitized rock doves: lousy males lose. Am Zool 30(2):251–262. https://doi.org/10.1093/icb/30.2.251
Clayton DH (1991) Coevolution of avian grooming and ectoparasite avoidance. Bird–parasite interactions: ecology. Evol Behav 14:258–289
Clayton DH, Walther BA (1997) Collection and quantification of arthropod parasites of birds. Host-parasite evolution: general principles and avian models. Oxford University Press, Oxford, pp 419–440
Clayton DH, Adams RJ, Bush SE (2008) Phthiraptera, the chewing lice. Parasitic diseases of wild birds 515–526
Corke M, Matthews J (2018) Diagnosing and treating Skin Diseases in goats: an update. Pract 40(4):149–156
Corrin T, Ackford R, Mascarenhas M, Greig J, Waddell LA (2021) Eastern equine encephalitis virus: a scoping review of the global evidence. Vector-Borne and Zoonotic Diseases 21(5):305–320. https://doi.org/10.1089/vbz.2020.2671
Covacin C, Barker SC (2007) Supergroup F Wolbachia bacteria parasitise lice (Insecta: Phthiraptera). Parasitol Res 100:479–485. https://doi.org/10.1007/s00436-006-0309-6
Da Silva AS, Lopes LS, Diaz JDS, Tonin AA, Stefani LM, Araújo DN (2013) Lice outbreak in buffaloes: evidence of Anaplasma marginale transmission by sucking lice Haematopinus tuberculatus. J Parasitol 99(3):546–547. https://doi.org/10.1645/ge-3260.1
Delhon G (2022) Poxviridae. (eds.), Vet Microbiol.pp. 522–532. https://doi.org/10.1002/9781119650836.ch53
Der Breite G (1985) Morphologische Unterschiede Der Weibchen Von Bovicola caprae und B. Limbata (Mallophaga). Angew Parasitol 26:241–243
Devaney JA (1976) Effects of the chicken body louse, Menacanthus Stramineus, on caged layers. Poult Sci 55(1):430–435
Dik B, Halajian A (2013) Chewing lice (Phthiraptera) of several species of wild birds in Iran, with new records. J Arthropod-Borne Dis 7(1):83
Durden LA (2019) In: Lice, Phthiraptera (eds) (eds.), Med Vet Entomol. Elsevier., pp 79–106
Duvallet G, Fontenille D, Robert V (2017) Entomologie médicale et vétérinaire. 1ère édition ed, IRD Éditions, Quae, MRS. 688. https://doi.org/10.4000/books.irdeditions.21923
Ehlers J, Krüger A, Rakotondranary SJ, Ratovonamana RY, Poppert S, Ganzhorn JU, Tappe D (2020) Molecular detection of Rickettsia spp., Borrelia spp., Bartonella spp. and Yersinia pestis in ectoparasites of endemic and domestic animals in southwest Madagascar. Acta Trop 205(105339). https://doi.org/10.1016/j.actatropica.2020.105339
Eren G, Özkoç ÖÜ, Acici M (2022) Contributions to the knowledge of the diversity of the chewing lice fauna in Turkey. Turkish J Zool 46(6):444–455. https://doi.org/10.55730/1300-0179.3099
Gharbi M, Ben Abdallah H, Mbarek Y, Jedidi M, Darghouth M (2013) Cross-sectional study of cattle lice infestation in the region of Nabeul in north-east Tunisia. Rev. sci. Tech. Off int Epiz 32(3):1–8. https://doi.org/10.20506/rst.32.2.2208
Gharbi M, Labibi W, Jedidi M, Zouari M (2020) Cattle infestation by lice in Northern Tunisia. Revue d’élevage et de médecine vétérinaire des pays tropicaux 73(2):141–144. https://doi.org/10.19182/remvt.31860
González-Álvarez VH (2020) Presence of two lice species (Insecta: Phthiraptera) in a goat (Capra hircus) from La Comarca Lagunera, Mexico: a case report. Int J Res Appl Sci Biotechnol 7(5):152–155. https://doi.org/10.31033/ijrasb.7.5.22
Hopkins GHE, Clay T (1953) XLII.—Additions and corrections to the check list of Mallophaga. J Nat Hist 6(66):434–448. https://doi.org/10.1080/00222935308654443
Hornok S, Hofmann-Lehmann R, De Mera IGF, Meli ML, Elek V, Hajtós I, Répási A, Gönczi E, Tánczos B, Farkas R, Lutz H, De La Fuente J (2010) Survey on blood-sucking lice (Phthiraptera: Anoplura) of ruminants and pigs with molecular detection of Anaplasma and Rickettsia spp. Vet Parasitol 174(3–4):355–358. https://doi.org/10.1016/j.vetpar.2010.09.003
Hornok S, Földvári G, Rigó K, Meli ML, Gönczi E, Répási A, Farkas R, Papp I, Kontschán J, Hofmann-Lehmann R (2015) Synanthropic rodents and their ectoparasites as carriers of a novel haemoplasma and vector-borne, zoonotic pathogens indoors. Parasit. Vectors 8(1):1–6. https://doi.org/10.1186%2Fs13071-014-0630-3
Houhamdi L, Parola P, Raoult D (2005) Lice and lice-borne diseases in humans. Medecine tropicale: revue du Corps de sante colonial 65(1):13–23
Hugon A (2015) Réalisation d’une clef de détermination des espèces de poux présentes sur la poule domestique Gallus gallus domesticus (Thèse de Doctorat) Université Claude Bernard Lyon I
Ikpeze OO, Amagba IC, Eneanya CI (2008) Preliminary survey of ectoparasites of chicken in Awka, South-Eastern Nigeria. Anim Res Int 5(2):848–851
Jassem MI, Alali FA, Al-Ashbal HN, Jawad MH, Alhesnawi AS (2023) Prevalence of chewing lice species on migratory birds in Razzaza lake. Iraqi J Veterinary Sci 37(2):479–485. https://doi.org/10.33899/ijvs.2022.134464.2434
Johnson KP, Matthee C, Doña J (2022) Phylogenomics reveals the origin of mammal lice out of Afrotheria. Nat Ecol Evol 6(8):1205–1210. https://doi.org/10.1038/s41559-022-01803-1
Kaboudi K, Romdhane R, Salem A, Bouzouaia M (2019) Occurrence of ectoparasites in backyard domestic chickens (Gallus gallus Domesticus) in the northeast of Tunisia. J Anim Health Prod 7(3):92–98. https://doi.org/10.17582/journal.jahp/2019/7.3.92.98
Kazim A-R, Houssaini J, Tappe D, Heo C-C (2022) An annotated checklist of sucking lice (Phthiraptera: Anoplura) from domestic and wild mammals in Malaysia, with lists of hosts and pathogens. Zootaxa 5214(3):301–336. https://doi.org/10.11646/zootaxa.5214.3.1
Kouam MK, Fokeng AN, Biekop HF, Touko ABH, Tebug TT (2022) Prevelance and clinical signs of chewing lice in local chickens (Gallus gallus Domesticus) in Menoua Division, Western highlands of Cameroon. Veterinary Parasitology: Regional Studies and Reports 34:100772. https://doi.org/10.1016/j.vprsr.2022.100772
Kumar S, Ahmad A, Ali R, Kumar V (2017) A note on the haematophagous nature of poultry shaft louse, menopon gallinae (Amblycera: Phthiraptera). J Parasitic Dis 41(1):117–119. https://doi.org/10.1007/s12639-016-0760-y
Kumsa B, Socolovschi C, Parola P, Rolain J-M, Raoult D (2012) Molecular detection of Acinetobacter species in lice and keds of domestic animals in Oromia Regional State. Ethiopia PLoS ONE 7(12):e52377–e52377. https://doi.org/10.1371/journal.pone.0052377
Lakew BT, Kheravii SK, Wu S-B, Eastwood S, Andrew NR, Nicholas AH, Walkden-Brown SW (2021) Detection and distribution of haematophagous flies and lice on cattle farms and potential role in the transmission of Theileria Orientalis. Vet Parasitol 298:109516. https://doi.org/10.1016/j.vetpar.2021.109516
Lasisi O, Eyarefe O, Adejinmi J (2010) Anaemia and mortality in calves caused by the short-nosed sucking louse (Haematopinus eurysternus)(Nitzsch) in Ibadan. Nigerian Veterinary Journal 31(4):295–299. https://doi.org/10.4314/nvj.v31i4.68979
Light JE, Smith VS, Allen JM, Durden LA, Reed DL (2010) Evolutionary history of mammalian sucking lice (Phthiraptera: Anoplura). BMC Evol Biol 10(1):1–15. https://doi.org/10.1186/1471-2148-10-292
Louni M, Mana N, Bitam I, Dahmani M, Parola P, Fenollar F, Raoult D, Mediannikov O (2018) Body lice of homeless people reveal the presence of several emerging bacterial pathogens in northern Algeria. PLoS Negl Trop Dis 12(4):e0006397. https://doi.org/10.1371/journal.pntd.0006397
Mana N, Louni M, Parola P, Bitam I (2017) Human head lice and pubic lice reveal the presence of several Acinetobacter species in Algiers. Algeria Comp Immunol Microbiol Infect Dis 53:33–39. https://doi.org/10.1016/j.cimid.2017.06.003
Mansur M, Mahmoud N, Allamoushi S, El Aziz MA (2019) Biodiversity and prevalence of chewing lice on local poultry. J Dairy Veterinary Anim Res 8:26–31. https://doi.org/10.15406/jdvar.2019.08.00238
Marcondes CB (2017) Arthropod borne Diseases. Springer Int Publishing. https://doi.org/10.1007/978-3-319-13884-8
Martinů J, Sychra O, Literák I, Čapek M, Gustafsson DL, Štefka J (2015) Host generalists and specialists emerging side by side: an analysis of evolutionary patterns in the cosmopolitan chewing louse genus. Menacanthus Int J Parasitol 45(1):63–73. https://doi.org/10.1016/j.ijpara.2014.09.001
Maturano R, Daemon E (2014) Reproduction, development and habits of the large Turkey louse Chelopistes Meleagridis (Phthiraptera: Ischnocera) under laboratory conditions. Braz J Biol 74:712–719. https://doi.org/10.1590/bjb.2014.0085
Meddour S, Mlik R, Dik B, Hastriter MW, Sekour M (2022) Ectoparasites of the common gundi (Ctenodactylus gundi Rothmann) from the Aures Region. Algeria Annals of Parasitology 68(3):519–529. https://doi.org/10.17420/ap6803.458
Medjouel I, Benakhla A, Senouci K, Djelil H, Matallah F (2013) Prevalence and distribution of chewing lice (Phthiraptera) in free range chickens from the traditional rearing system in the Algerian North East, Area of El-Tarf. Int J Poult Sci 12(12):721. https://doi.org/10.3923/ijps.2013.721.725
Medjouel I, Benakhla A, Senouci K, Djelil H, Matallah F (2014) Prevalence and seasonal variation of Mallophagan species (Phthiraptera) in free-range chickens from rural localities of Oran. Algeria Sci J Anim Sci 3(3):64–69
Meguini MN, Righi S, Zeroual F, Saidani K, Benakhla A (2018) Inventory of lice of mammals and farmyard chicken in North-eastern Algeria. Vet World 11(3):386–396. https://doi.org/10.14202/vetworld.2018.386-396
Meyer R, Madon M (2002) Arthropods of public health significance in California. California: MVCAC 84–86
Mirzaei M, Ghashghaei O, Yakhchali M (2016) Prevalence of ectoparasites of indigenous chickens from Dalahu region, Kermanshah province. Iran Türkiye Parazitolojii Dergisi 40(1):13. https://doi.org/10.5152/tpd.2016.4185
Mohammed AS, Mohammad MJ (2021) Prevalence of lice parasite, Menopon Gallinae. Local Chickens in Samarra NeuroQuantology 19(9):20–24. https://doi.org/10.14704/nq.2021.19.9.NQ21133
Mohammed Adnan AB, Shamal Abdullah AM (2021) Phylogenetic analysis of lice infested chicken (Gallus gallus Domisticus) with new records in Kurdistan of Iraq. Ann Parasitol 67(2):161–168. https://doi.org/10.17420/ap6702.325
Muhammad A, Bashir R, Mahmood M, Afzal MS, Simsek S, Awan UA, Khan MR, Ahmed H, Cao J (2021) Epidemiology of ectoparasites (ticks, lice, and mites) in the livestock of Pakistan: a review. Frontiers. Veterinary Sci 8:780738. https://doi.org/10.3389/fvets.2021.780738
Mulugeta Y, Yacob HT, Ashenafi H (2010) Ectoparasites of small ruminants in three selected agro-ecological sites of Tigray Region. Ethiopia Trop Anim Health Prod 42(6):1219–1224. https://doi.org/10.1007/s11250-010-9551-0
Nahal A, Righi S, Boucheikhchoukh M, Benakhla A (2021) Prevalence of ectoparasites in free-range backyard chicken flocks in northeast Algeria. Veterinarska Stanica 52(6):693–702. https://hrcak.srce.hr/249013
Nasser M, Adly E, Alahmed A, Shobrak M (2020) Host habitat and position on host affecting the evolution of chewing lice (Phthiraptera): phylogenetic analysis of Ischnocera in Saudi Arabia. J Insect Biodivers Syst 6(1):101–112. https://doi.org/10.52547/jibs.6.1.101
Nizamov N, Prelezov P (2021) First report of Linognathus africanus (Phthiraptera: Anoplura) on goats in Bulgaria. Bulgarian J Veterinary Med 24(2):261–267. https://doi.org/10.15547/bjvm.2297
O’callaghan M, Beveridge I, Barton M, Mcewan D (1989) Recognition of the sucking louse Linognathus africanus on goats. Aust Vet J 66(7):228–229. https://doi.org/10.1111/j.1751-0813.1989.tb09820.x
Olejkov L, Kritofk JN, Trnka A, Sychra O (2021) An annotated checklist of chewing lice (Phthiraptera: Amblycera, Ischnocera) from Slovakia. Zootaxa 5069(1):1–80. https://doi.org/10.11646/zootaxa.5069.1.1
Ouarab S, Baaloudj A, Aouar-Sadli M, Medjdoub-Bensaad F, Abdelkader D (2021) Diversity of ectoparasites and their pathogens in birds (Passeriformes and Columbiformes) in Bouinan Region (Blida-Algeria). Ecology. Environ Conserv 27(1):253–260
Ouarti B, Laroche M, Righi S, Meguini MN, Benakhla A, Raoult D, Parola P (2020a) Development of MALDI-TOF mass spectrometry for the identification of lice isolated from farm animals. https://doi.org/10.1051/parasite/2020026. Parasite 27
Ouarti B, Righi S, Tall ML, Meguini MN, Ouarti K, Parola P, Benakhla A (2020b) Survey of ruminant infestation by lice in north-east Algeria. Revue Algérienne Des Sciences A 5:13–18
Ouarti B, Mediannikov O, Righi S, Benakhla A, Raoult D, Parola P (2021) Molecular detection of microorganisms in lice collected from farm animals in Northeastern Algeria. Comparative immunology. Microbiol Infect Dis 74:101569. https://doi.org/10.1016/j.cimid.2020.101569
Ouarti B, Fonkou DMM, Houhamdi L, Mediannikov O, Parola P (2022) Lice and lice-borne Diseases in humans in Africa: a narrative review. Acta Trop 237:106709. https://doi.org/10.1016/j.actatropica.2022.106709
Pajot F-X (2000) Les Poux (Insecta, Anoplura) de la région afrotropicale. 37 IRD Editions
Paliy A, Mashkey A, Sumakova N (2018) Distribution of poultry ectoparasites in industrial farms, farms, and private plots with different rearing technologies. Biosystems Divers 26(2):153–159
Patel P, Tan A, Levell N (2021) A clinical review and history of pubic lice. Clin Exp Dermatol 46(7):1181–1188. https://doi.org/10.1111/ced.14666
Pavlovic I, Blazin V, Hudina V, Ilic Z, Miljkovic B (1989) Effect of the biting louse Menacanthus stramineus on reducing the egg production of poultry under intensive conditions. Veterinarski Glasnik 43:181–186
Peel MC, Finlayson BL, Mcmahon TA (2007) Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst Sci 11(5):1633–1644. https://doi.org/10.5194/hess-11-1633-2007
Price MA, Graham O (1997) Chewing and sucking lice as parasites of mammals and birds. U.S. Dept. of Agriculture, Agricultural Research Service, USDA publications
Promrangsee C, Khositharattanakool P, Somwang P, Sunantaraporn S, Phumee A, Preativatanyou K, Tawatsin A, Brownell N, Siriyasatien P (2019) The prevalence of Bartonella Bacteria in Cattle Lice Collected from three provinces of. Thail Insects 10(6):152. https://doi.org/10.3390/insects10060152
Rabana J, Adamu L, Dauda J, Abubakar A (2019) Ectoparasitosis in domesticated turkeys (Meleagris gallopavo) in Jere Area, Borno State, Nigeria. Int J 5(1):11–22. https://doi.org/10.18488/journal.110.2019.51.11.22
Ramakrishnan MA, Ashokkumar D (2019) Swinepox Virus. In: Y. S. Malik, R. K. Singh & M. P. Yadav (eds.), Recent Advances in Animal Virology. Springer Singapore, Singapore.pp. 161–169. https://doi.org/10.1007/978-981-13-9073-9_9
Randa M, Meddour S, Bilal D, Souttou K, Sekour M (2022) First report of ectoparasites from black rats (Rattus rattus Linnaeus, 1758) in oasis regions from Algeria. Notulae Scientia Biologicae 14(1):11013–11013. https://doi.org/10.15835/nsb14111013
Rashmi A, Saxena A (2017) Population levels of phthirapteran ectoparasites on the goats in Rampur (UP). J Parasitic Dis 41(3):778–781. https://doi.org/10.1007/s12639-017-0888-4
Reed DL, Smith VS, Hammond SL, Rogers AR, Clayton DH (2004) Genetic analysis of lice supports direct contact between modern and archaic humans. PLoS Biol 2(11):e340. https://doi.org/10.1371/journal.pbio.0020340
Reed DL, Light JE, Allen JM, Kirchman JJ (2007) Pair of lice lost or parasites regained: the evolutionary history of anthropoid primate lice. BMC Biol 5(1):1–11. https://doi.org/10.1186/1741-7007-5-7
Reeves WK, Szumlas DE, Moriarity JR, Loftis AD, Abbassy MM, Helmy IM, Dasch GA (2006) Louse-borne bacterial pathogens in lice (Phthiraptera) of rodents and cattle from Egypt. J Parasitol 92(2):313–318. https://doi.org/10.1645/GE-717R.1
Reeves WK, Durden LA, Ritzi CM, Beckham KR, Super PE, Oconnor BM (2007) Ectoparasites and other ectosymbiotic arthropods of vertebrates in the Great Smoky Mountains National Park. USA Zootaxa 1392(1):31–68. https://doi.org/10.11646/zootaxa.1392.1.2
Saidi R, Mimoune N, Baazizi R, Khelef D, Azzouz MY, Kaidi R (2020) Contribution to studying ecto and mesoparasites in goats in Southern Algeria Vet 69(1)
Saikia M, Bhattacharjee K, Sarmah P, Deka D, Mushahary D (2017) Prevalence of ectoparasitic infestation of pigeon (Columba livia Domestica) in Assam. India J Entomol Zool Stud 5(4):1286–1288
Sanchez-Montes S, Colunga-Salas P, Alvarez-Castillo L, Guzman-Cornejo C, Montiel-Parra G (2018) Chewing lice (Insecta: Phthiraptera) associated with vertebrates in Mexico. Zootaxa 4372(1):1–109. https://doi.org/10.11646/zootaxa.4372.1.1
Saxena A, Singh S, Kumar S (1997) Site preference of poultry shaft louse, menopon gallinae (Phthiraptera: Amblycera) on host body. Riv Parassitol 58:383–390
Shaikh F, Naz S, Birmani NA (2021) Infestation of chewing lice (phthiraptera: insecta) on Turkey fowl (aves: galliformes) from district Hyderabad. Sindh Pakistan Pakistan J Parasitol 71:27–35
Shaikh F, Naz S, Birmani NA (2023) 9. Biodiversity of chewing lice and helminthes parasites of domestic fowls Gallus gallus domesticus (Linnaeus, 1758)(Aves: Galliformes) from Hyderabad. Sindh Pakistan Pure and Applied Biology (PAB) 12(1):87–92. https://doi.org/10.19045/bspab.2023.120010
Shanta I, Begum N, Anisuzzaman A, Bari A, Karim M (2006) Prevalence and clinico-pathological effects of ectoparasites in backyard poultry. Bangladesh J Veterinary Med 4(1):19–26. https://doi.org/10.3329/bjvm.v4i1.1520
Smith VS (2004) The Chewing Lice: World Checklist and Biological Overview.—RD Price, RA Hellenthal, RL Palma, KP Johnson, DH Clayton. 2003. Illinois Natural History Survey Special Publication 24. pp. Society of Systematic Zoology. https://doi.org/10.1080/10635150490468521
Soliman D, Adly E, Nasser M, Shehata M, Kamal M (2022) Seasonal population dynamics of the common chewing lice Columbicola columbae infesting the domestic pigeon Columba livia. https://doi.org/10.1080/00305316.2022.2136777. Orient Insects1-11
Springer A, Durden LA, Kiene F, Klein A, Rakotondravony R, Ehlers J, Greiman SE, Blanco MB, Zohdy S, Kessler SE (2023) Molecular phylogenetics of the sucking louse genus Lemurpediculus (Insecta: Phthiraptera), ectoparasites of Lemurs, with descriptions of three new species. Int J Parasitology: Parasites Wildl 20:138–152. https://doi.org/10.1016/j.ijppaw.2023.02.002
Sudiana E, Santoso S, Yani E (2020) Prevalence and diversity of ectoparasites in scavenging chickens (Gallus Domesticus) and their association to body weight. Biodiversitas J Biol Divers 21(7):3163–3169. https://doi.org/10.13057/biodiv/d210738
Sweet AD, Johnson KP, Cameron SL (2022) Independent evolution of highly variable, fragmented mitogenomes of parasitic lice. Commun Biol 5(1):677. https://doi.org/10.1038/s42003-022-03625-0
Sychra O, Harmat P, Literák I (2008) Chewing lice (Phthiraptera) on chickens (Gallus gallus) from small backyard flocks in the eastern part of the Czech Republic. Vet Parasitol 152(3–4):344–348. https://doi.org/10.1016/j.vetpar.2008.01.001
Torki S, Marniche F, Dik B, Guezoul O (2020) First records of the chewing lice (Phthiraptera) associated with Meropidae species in Biskra (northern Sahara, Algeria). Int J Sci Res 76(4/1). https://doi.org/10.21506/j.ponte.2020.4.1
Touati L, Figuerola J, Alfarhan AH, Samraoui B (2015) Distribution patterns of ectoparasites of glossy Ibis (Plegadis falcinellus) chicks. Zool Ecol 25(1):46–53. https://doi.org/10.1080/21658005.2015.1005447
Touati L, Athamnia M, Nedjah R, Boucheker A, Samraoui F, El-Serehy HA, Samraoui B (2022) Composition and distribution on a host of avian lice of White storks in North-Eastern Algeria. Diversity 14(2):77. https://doi.org/10.3390/d14020077
Tuff DW (1977) A key to the lice of man and domestic animals. Tex J Sci 28(1/4):145–159
Wall RL, Shearer D (2001) Veterinary ectoparasites: biology, pathology and control. 2nd ed, Wiley and Blackwell, LDN. 304 pp
White SD, Affolter VK, Molinaro AM, Depenbrock SM, Chigerwe M, Heller MC, Rowe JD (2022) Skin Disease in goats (Capra aegagrus hircus): a retrospective study of 358 cases at a university veterinary teaching hospital (1988–2020). Vet Dermatol 33(3):227–e264. https://doi.org/10.1111/vde.13052
Yevstafieva V (2015) Chewing lice (Order Mallophaga, suborders Amblycera and Ischnocera) fauna of domestic chicken (Gallus gallus Domesticus). Ukraine Вестник зоологии 49(5):393–400. https://doi.org/10.1515/vzoo-2015-0044
Zeroual F, Leulmi H, Benakhla A, Raoult D, Parola P, Bitam I (2018) Molecular evidence of Rickettsia slovaca in Wild Boar Lice. In Northeastern Algeria Vector Borne Zoonotic Dis 18(2):114–116. https://doi.org/10.1089/vbz.2017.2165
Ziani R, Ziani B-EC, Dik B, Marniche F, Lazli A (2020) Louse species (Phthiraptera: Amblycera, Ischnocera) collected on the common coot, Fulica atra (Linnaeus, 1758), and their microhabitat selection. Bull Soc Zool Fr 145:135–153
Ziani R, Lazli A, Marniche F, Ziani B-EC, Dik B (2021) The distribution and diversity of chewing lice (Phthiraptera) on the common Moorhen Gallinula chloropus in Algeria. Bird Study 68(3):359–369. https://doi.org/10.1080/00063657.2022.2092593
Acknowledgements
The authors express their heartfelt gratitude to all individuals who supported this study. Additionally, they extend their appreciation to the entire staff at the Institut Hospitalo-Universitaire (IHU) Méditerranée Infection, Marseille, France.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
was obtained from the Chadli Bendjedid El Tarf University’s Animal Local Ethics Committee. The authors state that the animals were handled and treated following the Algerian legislation (Ordinance No. 06 − 05 of 19 Joumada Ethania 1427, corresponding to 15 July 2006). The breeders and the local agricultural services office verbally approved the field operations.
Competing interests
The authors declared that they have no competing interests. No funding was received for conducting this study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Boucheikhchoukh, M., Leulmi, H., Dib, L. et al. Lice (Phthiraptera) diversity in ruminants and domestic birds in northeastern Algeria. Int J Trop Insect Sci 43, 2233–2244 (2023). https://doi.org/10.1007/s42690-023-01127-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42690-023-01127-y