Abstract
Arboviral infections are re-emerging diseases of public health concern circulating among wild animals and human populations due to shortage of strong entomological data in addition to increasing urbanization, transportation and mosquito adaptation to complex environment in Ethiopia. This study assessed the response of Aedes mosquito to interventions (environmental management and Chemical (Temephos and Propoxure) spray) made to control the Chikungunya outbreak in Dire Dawa, Eastern Ethiopia. A before and after intervention entomological study design was used to address all villages, with simple random sampling technique. To determine the vector density, entomological indices (House index, Container index, Bretaeu index and Pupae index) were calculated. A total of 1297 containers were inspected in 800 households for the presence of Aedes immature and adult. Out of 1297 containers inspected, 1128 (87%) were found be Aedes larvae/pupae positive. A total 1289 mosquitoes were collected, 1264 (98%) were Aedes and the rest 25 (2%) were Culex mosquitoes. The house index, container index, bretaue index and pupae index before and after control intervention was (90.125%, 7.4%), (92.87%, 14.75%), (141.75%, 20.5%) and (1431.5, 4%) respectively. The infestation indices of Aedes mosquitoes drastically reduced after control interventions which imply that deploying integrated vector control intervention, like environmental management, insecticidal applications are effective in Aedes mosquito control during outbreaks of arboviral diseases. Permanent and comprehensive sentinel sites should be established fort continuous surveillance of vector status and diseases to halt cyclic transmission.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Arboviral diseases circulating among wild animals and humans populations are the leading morbidity to human (Kuna et al. 2019). The majority of arboviral diseases can be grouped under three virus genus: Flavivirus, Alphavirus, and Bunyavirus (Washington State Departement of Health 2019). Currently, high percentage of arboviral diseases that have serious public health impact at global scale are originated from Africa (Braack et al. 2018). A wide variety of the RNA virus, such as Dengue (DENV), Yellow fever (YFV), Zika (ZIKV), Chikungunya (CHIKV), and West Nile fever (WNV) are important causes of human morbidity worldwide (ECDC 2014). Animals and other reservoir hosts are impacted and aided to maintain arboviral diseases circulating in populations. Blood sucking insects, mostly mosquitoes are the best-known disease vectors that transmit important zoonotic infectious diseases (Menchaca-Armenta et al. 2018). They ingest disease-causing microorganisms by feeding on infected host and then later inject to a new host during their subsequent blood meal (Bartlow et al. 2019). The continental emergence and re-emergence and burden of arboviral diseases are raising global concerns because of threats to health and the absence of feasible prevention and control strategies (Paixão et al. 2017).Vector-borne diseases account more than 17% of all infectious diseases, causing more than 700,000 deaths annually . For instance, in 128 countries, about 4 billion peoples are at risk of dengue with 96 million estimated cases per year (Bartlow et al. 2019).Environmental and climate factors, the interaction between host and pathogen, and the development of immunity in the population can influence the dynamics of transmission of those diseases (Palaniyandi 2014). This also highly related with increased human population migration that lead to maximize the human-vector contact in expanding urban environments (Braack et al. 2018). According to WHO statement, the burden of arboviral diseases, particularly CHIKV, DENV, YF and ZIKV viral diseases are alarming (World Health Organization (WHO) 2019a).
The vector species ability to transmit a pathogen is favored by location and time (WHO 2015). Aedes aegypti frequently bites human and is responsible for urban viral diseases transmission. Ae. Albopictus is resilient and aggressive mosquito, is the second vector of arboviral diseases that have large geographical distribution (Kraemer et al. 2015). The biting activity of both vectors is primarily limited to outdoor day time impairing the effectiveness of insecticide treated mosquito nets (Paixão et al. 2017). Controlling adult Aedes mosquito population and their bites are not seem to be easy activities, especially in tropical climates (Epicenters 2016). Along with an increase of emerging zoonotic diseases, there have been range expansions of reservoir hosts, vectors, and the pathogens they harbor. CHIKV is the current enzootic mosquito borne viral disease being spread to new area especially in Africa due to increased human migration (Rezza and Weaver 2019). Though considerable arboviral diseases without full documentation are known to be endemic in Ethiopia, infection remains unreported due to lack of laboratory facilities and inaccessibility of some endemic area. Since 2016, CHIKV has occurred in different parts of Ethiopia and spread rapidly causing severe impacts on public health (Getachew et al. 2015).
Entomological study of arboviral disease vector mosquitoes is limited and the vector host interaction is not clearly understood, which in turn makes the disease control programs difficult to design (Lilay et al. 2017). As a result, more entomological information regarding the viral transmission rate of CHIKV and the status of the vector in its transmission activity is required to design and implement affordable and safe vector control intervention that aid in prevention of the transmission of virus to new area of Ethiopia. In the scenario of vector control, source reduction is the primary methods focused with environmental sanitation; health promotion and community participation (Abeyewickreme et al. 2012; Arunachalam et al. 2012). The effectiveness or impacts of environment is not clearly understood that supported by research in Ethiopia. The current challenges in adult Aedes control are due to lack of appropriate vector biology including behavioral information as the virus spread becomes a serious threat in the country. An improved understanding of the relation of entomological factors to risk must be a priority. The complex natural history of vector and arboviral transmission contributes to the difficulty in setting goals and executing effective control. The most cost-effective means of preventing mosquito-borne disease is targeting the adult vector, which transmits the pathogen. Evidence based Aedes mosquito specific chemical-based control strategies have yet to receive the attention they merit, despite their obvious promise.
In Ethiopia, chemical spray, developed initially for malaria control, have not been sufficiently valuated against Aedes mosquitoes. Information generated from studying mosquitoes and their response to intervention needs to be synthesized into meaningful control models of virus transmission risk. In the outbreak of the CHIKV, adult and larvae control spraying were performed on the outside of the mosquito population to significantly reduce the number of adult mosquitoes in terms of giving response in addition to removing breeding sites with vector control campaign. Chemical control is not used for routine Chikungunya prevention and is also not reserved for the control of epidemics in Ethiopia. Although, chemical control is recommended only as a last resort in an IPM principle, it was decided to spray Propoxur, for aerial space spray for both indoor and outdoor as per WHO recommended dose for other vectors like Anopheles mosquitoes. In addition, abate chemical was used as larvicidal control for immature stages in stored water sources where difficult to remove. It was performed in a judicious way i.e. after clear and detail training and orientation for all concerned bodies by professional entomologists. This study was conducted to assess the impact on interventions of adult Aedes mosquitoes in Dire Dawa from the beginning of August 2019 for two months consecutive.
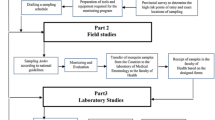
Materials and methods
Study area and setting
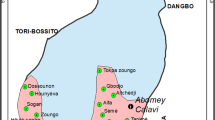
The study was conducted in Dire Dawa city which is located at a distance of 515 km east of Addis Ababa, the capital city of Ethiopia. It is located at 9∘ 35 N and 41∘51E with an altitude of 1193 masl with the average maximum annual temperature of 32.8°C and 637mm average annual rainfall (Fig. 1). The municipality has a non-contiguous water supply (every 2 days) and irregular garbage collection (Getachew et al. 2015). The informal settlements with slums or sub-standard housing because of a number of people living in precarious sites such as mountains and stream sides are commonly known (UN-HABITAT 2008). Due to the high-water shortage in the city, the people used to store rain water for use. There is also a lack of sufficient flood drainage system and sanitation problem, hampering the health of the community of Dire Dawa city (Getachew et al. 2015). A before and after control intervention entomological study design with simple random sampling of the household (HHs) was done from August 2019 to October 2019, during the major outbreak of Chikungunya in Dire Dawa, Eastern Ethiopia. All city kebeles with high and low Health facility-based Chikungunya case report were investigated in the basis of the house to house visiting. Both domestic and peri-domestic immature stage mosquito habitats were identified with their level of infestation. Based on reported cases, the Chikungunya risk transmission or target kebeles included were Goro, Sabian, Diredawa (01), Gendekore, Addis ketema, Legehare and Gendegerada (Fig. 1) within urban settlements.
Mosquito based surveillance: sample collection
To carry out this survey, a total of 800 households were selected with simple random sampling techniques. At the kebele level, 100 houses were purposively planned and visited in line with WHO, 2019 updated formula for screening and predicting future disease risk (World Health Organization (WHO) 2019b). A comparative study i.e. before and after vector control measures implementation was used. So that the results of this study were presented as obtained before any mosquito control method undertaken, and after one week campaign of community education, environmental management and chemical spray (propoxure for adult and temophos (Abate) for water stage control) and see the difference of mosquito density on different containers type. The survey was carried throughout the kebele after all campaign intervention.
Adult mosquito collection
Resting collection
During periods of inactivity, adult mosquitoes were collected from typically rested indoors, especially in bedrooms, resided houses and mostly in dark places, such as cloth closets and other sheltered sites and outdoor from open empty barrels, fallen leaf and walls of houses with systematic searching of these sites. Adult mosquito collection was initiated in and around HHs early in the morning (5:00 -11:00am) to late evening (14:00 -19:00pm). Standard manual mouth aspirators were used to collect mosquito from all premises by entomological expertise. Under this operational collection technique, all resting places of Aedes mosquitoes were systematical searched with the aid of flush light based on the location and time of the mosquitoes resting once before and one after community mobilization and awareness creation. Aedes Mosquitoes were sequentially collected from each resting site using constant effort at predefined intervals. In aspiration methods, collector entered the house and collected mosquitoes resting in all rooms and other dark places for a period of 10 minutes continually for six (6) hours in the morning and five (5) hours in the afternoon. As collection proceeded, each aspirator collection cup was labeled with all mosquitoes related behavioral information on well prepared observational checklist during collection.
Immature mosquito sample collection
A house-to-house immature stage survey was carried out at the peri-domestic environs to detect larval breeding. Sampling of immature (aquatic) stage of mosquitoes i.e. larvae of different instars, and pupa) was made by standard dipper and pipette from all water containing containers, and HH GPS information was taken. Three dips were taken from each mosquito breeding habitat accordingly volume of water for a total of two months. This survey, carried out to collect the following information: larval habitat type especially in house and around house containers, presence of immature stages of Aedes, number of Aedes pupae and the state of sewage and trash collection services, potential breeding sites in public areas such as catch basins (roadside storm drains), storm water runoff channels, and waste disposal to identify potential peri-domestic larval habitats. The inspection for the presence of larvae/pupae were through visual and hand lenses considering both potentially artificial and natural breeding habitats with water type and status. In the houses where mosquitoes were found and a list of all kind of containers and the number of mosquitoes were recorded. The collected immature stages were moved to be reared in temporary established insectary to adult for species identification based on their morphological characters using identification keys under compound light microscope (Huang and Rueda 2017). The density of Aedes mosquito was determined through counting the number of the immature stages in different types of containers considering any activities of vector control. The morphologically identified mosquito were preserved in 96% ethanol and then, stored in liquid nitrogen for further viral processing with RT-PCR in the future.
Data management and analysis
All information collected during entomological investigation were entered into Excel sheet and exported to SPSS software version 20 (IBM Corporation, Java)) for statistical analysis. Descriptive statistics were used to summarize observed positive differences in the total number of containers on a property by different container type. A Kruskal–Wallis test was used to determine the most prevalent container type in the area with container type as the independent variable. A paired sample t-test was used to analyze and compare the vector abundance in different study kebeles/villages. In this analysis (P– value <0.05) was considered as significant value. The larval survey data were calculated and analyzed in terms of different larval survey techniques like House index (HI), Container index (CI), and Breteau index (BI).The calculation of larval indices were based on the following mathematical formula as of WHO (World Health Organization (WHO) 2019c) Eq. (1):
Results
Adult Aedes mosquito survey
Relatively high number of Aedes mosquito were collected from open empty barrels which followed by Bedroom corridor and Bed respectively. In the before interventions campaign study, the number of mosquitoes were relatively high where, found to be reduced at the end of the control intervention campaign study. Open empty barrels, and bed rooms were found to be preferred resting sites of adult Aedes mosquito during day time in comparison with other sites (P < 0.04). Fallen leaf, wall/corridor and room corridor were less infested as compared to other resting sites during the day time post intervention adult mosquito count was significantly lower as compared to pre-intervention in all collection sites (P = 0.031). Still there was mosquito number that collected from the open empty barrels even after chemical spray (Fig. 2).
Larvae and pupae survey
A total of 800 houses were investigated for the presence of mosquito breeding containers. The main mosquito breeding containers were: upper surface of tombs designed by Muslim community, discarded tires, flower pots, barrel, jerrycan, bucket, roto, birka or long-term water storage and ditches. In the first phase, before vector control application, nearly all inspected houses were found to be positive for larvae/pupae (Table 2).
Before and after vector control intervention
In total, 721 houses were infested by mosquitoes larvae and pupae. Sabian, Addis ketema, Goro, Legehare, Dechatu, and Gendegerada kebeles were highly infested with high number of mosquitoes in the kebele before public awareness and chemical sprays campaigns (Table 2). Of 800 houses inspected for the presence or absence of mosquito larvae after intervention, only 59 houses were positive (Fig. 4).
From each kebeles, the number of mosquitoes collected was identified to indicate the infestation status. Goro, Sabian, Legehare and Diredawa (01) kebeles were highly infested (Fig. 3).
In both pre and post intervention a total of 1297 containers were inspected for the presence or absence of larvae/pupae. In pre-intervention, a total of 1128 containers were larvae/pupae positive (Table 3) where, 161 containers were larvae/pupae positive in post intervention survey (Fig. 4). The locations of containers were also observed during the entomological assessment with water status and types. The most containers that found larvae/pupae positive were placed outdoor with clean rain water. It has been identified that the mosquito has been able to breed vigorously in barrels, tires, flower pots, buckets, and tomb pools. Tire (25.2), Flower pots (17.64%), Barrel (14.9%), Jerrycan (15.9%) and Roto (11.6%) were the most potential breeding sites of Aedes larvae/pupae. The least infested breeding sites and containers found in the community were Ditches (5.6), Bucket/bowl (56) Tomb (26) and Birka/pool (2.3%) like water storage (Table 3).
After application of all control intervention like: Community education how to manage breeding sites of Aedes mosquito, chemical spray to adult and water stage control and removing habitats; the density of mosquito was highly decreased. From all larvae positive containers, Barrels (46), Jerrycan (28), Tires (26), Flower pots (22), Bucket (7), Ditches (10), Birka (9), Tomb pool (7) and Roto (6) were larvae positive in respective number. Accordingly, it has been proven that mosquito populations have decreased significantly after intervention (Fig. 4).
The number of positive containers with Aedes mosquito’s immature stages was significantly varied by container types after and before intervention (Kruskal–Wallis test, P < 0.01).
To determine the status of mosquito density, the entomological Indices were calculated by kebeles before and after control intervention. The before indices indicated that all kebeles were at risk of any arboviral diseases since the density of the vectors were beyond the WHO established risk estimation criteria. The house index (HI), Container index, Breteau index (BI) and Pupae index (PI) in Dire Dawa city were 91%, 92.875%, 141.75 % and1431.5 respectively. The larval density of Aedes mosquito was high in each kebeles prior to vector control intervention. The immature stage of Aedes mosquito density decreased with less sensitivity of future arboviral diseases risk in post vector control intervention survey. Though the sensitivity of the indices (HI, CI and PI) were low, BI (20.5%) after control intervention shows still there is high probability of future viral disease occurrences in the city. Sabian, Goro, Legehare and Dire Dawa kebeles in the city were the most risky area because of high sensitivity with high predictive criteria for other arboviral diseases (Table 4).
The density of Aedes mosquito was significantly varied when compared by study kebeles (villages) in both the before and after intervention analysis (96% CI: 40 – 74; t: 7; P < 0.001).
Discussion
In the present study, the density of Aedes mosquito and species were detected before and after control interventions methods that could help to measure the effectiveness of control methods and predict arboviral diseases status and risk. The density of Aedes mosquitoes was significantly different in relation to applied vector control methods.
Arboviral diseases like CHIKV have been spreading in many parts of the country lately. The nature of Aedes mosquitoes varies greatly from other mosquitoes like Malaria mosquito making it difficult to control. This also because of environmental changes and interactions among hosts, reservoirs, vectors, and pathogens on transmission of infectious diseases (Bartlow et al. 2019). There is no control system designed at national level for this dangerous mosquito so called Aedes and has nothing to do with technology that simply focused to remove its breeding site. As the study done in Bangladesh showed that focusing on the consistent breeding containers and houses that consistently accommodate Aedes larvae can be important to adopt new control measures for the specific needs (Ferdousi et al. 2015). According to the findings, chemical spray for adult and immature stage mosquito control was very effective in addition to the regular removal of possible Aedes mosquito breeding sites. In Ethiopia, targeting chemical based killing of adult Aedes mosquitoes, there is no research report conducted to prove the effectiveness of chemical that being used for for malaria vector control. Efforts were made to study all possible entomological aspects that could clarify and quantify human risk because of vector density. In the study area, the mosquito abundance was very high that highly linked with the community practices regarding water management. This study findings showed that Aedes aegypti was present in all the kebeles visited. This agrees with the findings of reported that Aedes aegypti occurs high in urban area (Chukwuekezie et al. 2018).
In terms of breeding habitats, different types of containers were surveyed for the presence of the vector responsible for mosquito borne disease transmission. This study revealed that water storage containers mentioned below were consistently more likely to contain Aedes larvae/pupae. These all observed containers, preferred for water storage and preservation are provide favorable breeding conditions for Ae. aegypti mosquitoes throughout the year regardless of the season (Limbaso et al. 2018). Containers that observed as the common breeding habitats in Dire Dawa city were tires, flower pots, barrels, buckets, jerrycan and roto. According to previous study conducted by (Getachew et al. 2015) and now confirmed by this study in Dire Dawa, the majorities of the residents in the study area store tap and rain water in containers for domestic use. In contrast to this findings, most containers these larvae positive were placed indoor as of conducted research in Bangladesh by Ferdousi et al. (2015). Surprisingly, there were a number of tombs like pools which were found as breeding habitats of Aedes mosquito found as other at outdoor. All containers with larvae/pupae positives were filled with clean rain water most preferred by this vector in the Dire Dawa city.
The current entomological data shows that almost all homestead containers used for water storage were importantly preferred by Aedes mosquito to breed in as observed in the study of (Chukwuekezie et al. 2018) in Nigeria. It is also observed that the habit of littering the environment with discarded containers among dwellers provides good breeding sites for Aedes mosquito (Lutomiah et al. 2013). Of all inspected houses, about 90% of houses were infested with immature stages of Aedes mosquito. This study found to be near similar with the study conducted in Northeastern Kenya by (Limbaso et al. 2018). Planting flower in a suitable containers for Aedes mosquitoes breeding were more common activities that practiced by the communities of Dire Dawa city.
In this study, efforts have been made to understand the significance of entomological surveillance linked with Aedes mosquito density using HI, CI, BI and PI. These entomological indices help to design the implementation of the outbreak prevention and control measure and further predict future outbreak. The larval indices shown in this study are relatively in comparable with the study conducted in the same place, Dire Dawa (Getachew et al. 2015). The larval indices were also associated with the risk of arboviral disease transmission, which is in parallel with the study conducted in Indonesia (Siregar and Makmur 2018). The presence of suitable containers for larvae breeding can increase the density of Aedes mosquito population, associated with the risk of arboviral disease transmission (Lutomiah et al. 2013). In line with the study conducted by Menon et al. (2019) in KonikkaraPanchaya, all the investigated sites had high entomological indices which indicate the fact of high risk of arboviral disease transmission before intervention (Sharma et al. 2015) in contrast with the study conducted by Huang et al. (2014).
Conclusion
According to the current study carried out in house to house for mosquito density analysis in Dire Dawa town, the number of mosquitoes has been found to be the highest at delayed and lack of integrated vector control interventions. This was because of scarcity or a problem with drinking water supply in the city as to make the community to use stored rainwater for long periods of time for laundry, toilet and flower. Almost all the domestics containers in and around the home used for storage of water are mosquito larvae positive and found to be as suitable for mosquito breeding. High infestation of disease vectors breeding in households of affected kebeles visited, barrels and abandoned or used tyre were common in households that are used for washing purposes, garages, and heavy vehicle owners. Rain water filled containers such as barrels/drum, jerry cans, flower pots and plastic container or bowls were also important breeding sites. Based on larval density indicators, all the kebeles studied had high risk before any intervention and moderate-high risk with low sensitivity after intervention for dengue/chikungunya transmission. In this study, the mosquito density after spray indirectly shows that the use of outside chemical spray for adult mosquito control is effective. Source reduction or environmental management, done by households themselves, is the most cost-effective approach for chikungunya and other arboviral diseases control including dengue.
In view of the fact that there is no available vaccine for treatment of chikungunya and dengue fever, the only best method to control disease epidemics is source reduction of vector breeding habitats, create awareness among the people, and prevention measures to protect from aggressive day biting Aedes mosquitoes. Community based educational programs aiming to train householders to use water containers appropriately would be a favorable intervention program to reduce the larval breeding sites. Sustained entomological surveillance and vector control programs targeting the most productive containers are needed to prevent possible outbreaks. Detection and early warning systems need to be strengthened in the city to determine the true range of chikungunya and its, overlap with dengue. A permanent and comprehensive sentinel sites should be set up so that continuous study can be conducted on regular basis. Chemicals and spray materials should be ready for prevention of mosquito density and mosquito borne diseases that will help in the immediate response to outbreak and Intersectoral collaboration among partners is a key component of the chikungunya and dengue control strategy.
Data availability
Datasets generated or analyzed during this study are available from the corresponding Author up on reasonable request.
References
Abeyewickreme W, Wickremasinghe AR, Karunatilake K, Sommerfeld J, Kroeger A (2012) Community mobilization and household level waste management for dengue vector control in Gampaha district of Sri Lanka; an intervention study. Pathog Glob Health 106:479–487
Arunachalam N, Tyagi BK, Samuel M, Krishnamoorthi R, Manavalan R, Tewari SC et al (2012) Community-based control of Aedes aegypti by adoption of eco-health methods in Chennai. Pathog Glob Health 106
Bartlow AW, Manore C, Xu C, Kaufeld KA, Valle S Del, Ziemann A, Fair JM (2019) Forecasting zoonotic infectious disease response to climate change : Mosquito Vectors and a Changing Environment. Vet Sciences 6
Braack L, Almeida APG De, Cornel AJ, Swanepoel R, Jager C De (2018) Mosquito-borne arboviruses of African origin : Review of key viruses and vectors. Parasit Vectors 1–26
Chukwuekezie OC, Nwankwo AC, Nwosu EO, Chukwuekezie OC (2018) Diversity and distribution of Aedes mosquitoes in Nigeria. New York Sci J 11
ECD (2014) Mosquito Borne Arboviruses In the Mediterranian and Black sea regions. The MedLabSecure Network, Network 2014
Epicenters N (2016) Aedes aegypti vector control and prevention measures in the context of Zika , Yellow Fever , Dengue or Chikungunya Technical Guidance WASH Regional Group (West and Central Africa) The vector “Know your Enemy” Understand the Aedes mosquito while in different and moving contexts Vector control strategy From the Interim Technical Note for UNICEF in the context of Zika.
Ferdousi F, Yoshimatsu S, Ma E, Sohel N, Wagatsuma Y (2015) Identification of essential containers for Aedes larval breeding to control dengue in Dhaka, Bangladesh. Trop Med Health 43:253–264
Getachew D, Tekie H, Gebre-michael T, Balkew M, Mesfin A (2015) Breeding Sites of Aedes aegypti : Potential dengue vectors in Dire Dawa , East Ethiopia. Hindawi 2015: 8 pages
Huang Y, Rueda LM (2017) Pictorial keys to the sections, groups, and species of the Aedes (Finlaya) in the Afrotropical Region (Diptera: Culicidae). Zootaxa 4221:131–141
Huang Yan-Jang S, Higgs Stephen Horne, Kate McElroy Vanlandingham DL (2014) Flavivirus-Mosquito interactions. Viruses 6:4703–4730
Kraemer MUG, Sinka ME, Duda KA, Mylne AQN, Shearer FM, Barker CM et al (2015) The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife 4:1–18
Kuna A, Gajewski M, Biernat B (2019) Selected arboviral diseases imported to Poland – current state of knowledge and perspectives for research. Ann Agric Environ Med 26:385–391
Lilay A, Asamene N, Bekele A, Mengesha M, Wendabeku M, Tareke I et al (2017) Reemergence of yellow fever in Ethiopia after 50 years, 2013 : epidemiological and entomological investigations. BMC Infect Dis 2–7
Limbaso SK, Nyunja A, Ofula V, Owaka S, Koka H, Koskei E et al (2018) Human and entomologic investigations of chikungunya outbreak in Mandera, Northeastern Kenya, 2016. PLoS One 13:1–13
Lutomiah J, Bast J, Clark J, Richardson J, Yalwala S, Oullo D et al (2013) Abundance, diversity, and distribution of mosquito vectors in selected ecological regions of Kenya : Public health implications. J Vector Ecol 38:134–142
Palaniyandi M (2014) The environmental aspects of dengue and chikungunya outbreaks in India : GIS for epidemic control. Int J Mosq Res 1:35–40
Menchaca-Armenta I, Ocampo-Torres M, Hernández- Gómez A, Zamora-Cerritos K (2018) Risk perception and level of knowledge of diseases transmitted by Aedes aegypti. J Sao Paulo Inst Trop Med 60:1–9
Menon VTK, Rachel J, Saju CR, Rafi MM, Joshy VM (2019) A study on mosquito density in rural Kerala before and after floods. Int J Community Med Public Heal 6:659–663
Network TM, ECDC (2014) Mosquito-borne arboviruses in the Mediterranean and Black Sea regions. MediLabSecure Netw
Paixão ES, Teixeira MG, Rodrigues LC, Paixão ES (2017) Zika, chikungunya and dengue : the causes and threats of new and re- emerging arboviral diseases. BMJ Glob Heal 3:1–6
Rezza G, Weaver SC (2019) Chikungunya as a paradigm for emerging viral diseases : Evaluating disease impact and hurdles to vaccine development. Neglected Trop Dis 13:1–12
Sharma AK, Kumar K, Singh S, Sharma AK (2015) Entomological surveillance for the vector of yellow fever/ dengue/chikungunya in and around ports of Goa, India. Int J Pure Appl Zool 3:204–209
Siregar FA, Makmur T (2018) Survey on aedes mosquito density and pattern distribution of aedes aegypti and aedes albopictus in high and low incidence districts in north sumatera province Survey on aedes mosquito density and pattern distribution of aedes aegypti and aedes albopictus. IOP Conf Ser Earth Environ Sci 130:012018
UN-HABITAT (2008) Ethiopia: Dire dawa urban profile Nairobi, Kenya
Washington State Departement of Health (2019) Arboviral Disease (except West Nile virus and Yellow Fever) Washington D.C
WHO (2015) Climate change and vector-borne disease- proceedings of the workshop Fiji
World Health Organization (WHO) (2016) Entomological surveillance for Aedes spp . in the context of Zika virus Interim guidance for entomologists Geneva
World Health Organization (WHO) (2019a) Framework for the implementation of the global vector control responsible in the World health organization (WHO) African region Republic of Congom 19-23
World Health Organization (WHO) (2019b) Framework for the implementation of the global vector control response in the WHO Africa region Brazzaville, Republic of Cong
World Health Organization (WHO) (2019c) Manual on prevention of establishment and control of mosquitoes of public health importance in the WHO European Region (with special reference to invasive mosquitoes) Denmark
Acknowledgements
We would like to thank all colleagues at Ethiopian Public Health Institute, Ministry of Health, all Dire Dawa vector control team, Health centers and facilities staffs, Dire Dawa city administration and Medicine San Frontier (MSF), Spain for their administrative, technical support and help in site guidance during data collection.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
The authors declare there is no competing interest and the funder had no role in the design of the study (collection, analysis and interpretation of data, writing of the manuscript and decision to publish the results).
Rights and permissions
About this article
Cite this article
Waldetensai, A., Gemechu, F., Kinfe, E. et al. Aedes mosquito responses to control interventions against the Chikungunya outbreak of Dire Dawa, Eastern Ethiopia. Int J Trop Insect Sci 41, 2511–2520 (2021). https://doi.org/10.1007/s42690-021-00430-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42690-021-00430-w