Abstract
The effectiveness of the “attract and kill” approach for the management of Bactrocera dorsalis in citrus orchards using autoinoculation strategy was evaluated in three locations in Senegal (Sindia, Sébikhotane and Ndoyene), between 2016 and 2018. Attractant Contaminant Traps (ACT) were treated with 0.3 g of Metarhizium acridum, and methyl eugenol was then deployed at densities of 25, 50 and 100 ACT to infect the flies. Recovery Traps (RT) containing methyl eugenol and a toxicant, Timaye were used to monitor the B. dorsalis population and the contamination rate. Results showed that the rate of contaminated flies increases with the number of ACT, at an average daily rate of contaminated flies of 68.1%, 85.44% and 99.67% at 25, 50, 100 traps, respectively. No contaminated flies were found in the control. The number of flies caught decreased from 21.7, 4.2 and 6.2 flies per day, respectively, for 25, 50, 100 ACTs and control in the first week, to 0.64, 0.71, 0.71 and 99.9 flies per day, respectively, for 25, 50, 100 ACTs and the control. All the flies caught at M. acridum treated sites were contaminated. No significant difference between the incidence of fruit damage in the three ACT densities and the control was found in the first week; however, there was a significant difference over time, from 90.0, 96.7 and 83.3% in the first week, to 30, 50 and 46.7% at the 14th week, respectively, for 25, 50 and 100 ACTs. No significant differences were found in the control. This present study demonstrated the efficacy of autoinoculative systems based-M. acridum for the management of B. dorsalis in citrus orchards in Senegal. This strategy is economical as it uses very little amounts of inoculum with locally made materials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The horticultural sector is a growing industry in Senegal (FAO 2018), contributing significantly to the Agricultural Gross Domestic Product (GDP) (Konta et al. 2016). It also generates revenues in terms of foreign exchange earnings and employment creation through exports, not only for Senegal but also for the entire region (Binta and Barbier 2015). In Senegal, citrus fruits are mainly grown in the Niayes Zone and Casamance areas and comprise a wide range of fruit crops. Although very dynamic, the sector is permanently threatened by various emerging pests, among which are tephritid fruit flies (Ndiaye et al. 2012). The Tephritidae family comprises of 4000 species belonging to 500 genera, and constitutes one of the most economically significant insect groups (Ekesi et al. 2011). Ceratitis cosyra (Walker), C. fasciventris, C. rosa, C. anonae, C. capitata were the most prominent species before the arrival of the oriental or Asian invasive fruit fly, Bactrocera dorsalis (Hendel), on the continent (Ndiaye et al. 2008, 2012; Vayssières et al. 2012; Ekesi et al. 2016). Initially reported in 2003 in Kenya, B. dorsalis spread very rapidly in several countries (Lux et al. 2003; Schutze et al. 2017). In West Africa, losses due to B. dorsalis exceed 50% for cultivars of commercial interest, resulting in considerable income losses for growers (Vayssières et al. 2014). In addition to direct losses in orchards, fruit flies are responsible for the destruction of many mango export consignments, annually, from West Africa to Europe (De Meyer et al. 2009). In Senegal, crop losses attributable to B dorsalis are estimated at 40–60% in the Niayes region, and up to 70–80% in the South in Casamance (Vayssières et al. 2011; Ndiaye et al. 2012). Losses due to fruit flies seriously affect the livelihoods of producers and actors in the sector, including vendors, retailers, negotiators, sellers, exporters, and food processors (Grechi et al. 2013; Maertens 2009). Female fruit flies lay their eggs, in small groups, under the fruit epidermis, and emerging larvae develop in the pulp of the fruit.
Chemical control remains the main control strategy (Badji and Coly 2014). However, the recent introduction of strict quarantine measures and maximum residue limits of pesticides has motivated the search for alternatives to chemical control (Toledo et al. 2006; Deguine et al. 2016).
Fruit fly management includes several steps, ranging from monitoring, mass-trapping, control, to eradication. The Integrated Management Package includes the use of lures and baits, traps, orchard sanitation, an augmentorium, and mass release of parasitoids and biopesticides (Agunloye 1987; Warthen et al. 1998; McQuate et al. 2005; Ekesi 2016; Fombong et al. 2016). There is a growing interest in the use of the entomopathogenic fungi Metarhizium sp. in the management of tephritid fruit flies. However, huge quantities are required for blanket spraying, and the spore have limited viability due to UV. One innovative approach to minimize high volumes of inoculum and persistence is the “attract and kill concept”, which combines a male annihilation technique using the parapheromone Methyl eugenol (ME) and biopesticides (Ekesi and Billah 2007) in an autoinoculation approach. Methyl eugenol is a powerful male attractant of plant origin extensively used for trapping several species of fruit flies. The autoinoculation system allows male B. dorsalis to enter Metarhizium sp. treated traps where they can get infected with conidia of M. anisopliae before returning to the environment to disseminate it among mates or during copulation.
Metarhizium spp. has been widely studied because of its relatively narrow host range and environmental safety on non-target pests (Bischoff et al. 2009). It belongs to the class of Hyphomycetes. Some species of Metarhizium are effective biological control agents and are prescribed for various insect pests (Fernandes and Biteencourt 2008; Gao et al. 2011; Pattemore et al. 2014). Metarhizium anisopliae var. acridium IMI 330189 available as Green Muscle® has been shown to induce significantly high mortalities in B. dorsalis (between 93.7% to 94.8%) with a lethal time to 50% mortality (LT50) between 2.8 to 3.6 days (Ouna 2010).
The use of the autoinoculation approach was successfully proven by Zakari-Moussa et al. (2012), for the management of fruit flies B. dorsalis and Ceratitis cosyra (Walker) in the northern part of Guinea-Bissau. However, the effectiveness of such approach in Senegal is unknown.
Therefore, the aims of this study were (i) to demonstrate the field efficacy of various densities of autoinoculation devices-based M. acridum for the management of B. dorsalis populations in citrus orchards, and (ii) to determine the effect of such management approach on fruit damage.
Material and methods
Study site
The study was conducted in various parts of the country, such as Sebikhotane (14°44′29.3”N, 17°07′34.0”W and 14°44′27.3”N 17°08′24.5”W), Ndoyene (14°45′24.1”N, 17°07′27.0”W) and Sindia (14°33′54.5”N, 17°02′31.5”W) (Fig. 1). Sebikhotane has a savanna climate, with limited annual rainfall (300 mm–600 mm). It is classified as semi-arid, according to the Köppen-Geiger scale (BSh). The temperature average is 26.1 °C throughout the year and the precipitation average is 533 mm (Table 1). The vegetation is composed of citrus and mango trees (CLIMATE-DATA.ORG 2019). Sindia is also characterized by a savanna climate with limited rainfall. The classification of Köppen-Geiger is of semi-arid type (BSh). The temperature average is 25.9 °C throughout the year, and the precipitation average is 531 mm (Table 1). The vegetation is dominant composed of citrus (Citrus sinensis, C. reticulata et C. paradisi) and a few feet of mango trees (CLIMATE-DATA.ORG 2020).
Biopesticides
The entomopathogenic fungi M. acridum (IMI 330189) (Bischoff et al. 2009) was sourced from SenBiotech SA and stored at −30 °C. The viability of conidia was determined by spread-plating 0.1 ml of the conidial suspension (5 × 106 conidia mL−1) on Sabouraud Dextrose Agar (SDA) plates. Sterile microscope cover slips were placed on each plate. The plates were incubated at a temperature of 25 °C and examined after 20 h for germination, using an optical microscope. Percentage germination was determined by counting approximately 100 spores for each Petri dish at 200 x magnification. The percentage germination was at 93.6% prior to the experiment.
Trap design and field experiments
Trap design
Two types of traps were used in the trials. Both traps were locally made, one using 0.5-l plastic bottles (24 cm in length), and the other, 1.5-l plastic bottles (32 cm in length).
The Attractant Contaminant Trap (ACT) was painted in yellow and the bottoms of the bottles were cut. The trap contained a dish made of cardboard rolled with velvet material, onto which 0.3 g of M. acridum (1.5 × 1010 conidia mL−1) was applied. Methyl eugenol (4 ml) was applied on a cotton wick and placed at the middle of the tray to attract males of B. dorsalis (Fig. 2a). The inoculum was renewed every week, and the Methyl eugenol was renewed every 15 days.
The Recovery Traps (RT) comprised of a bottle with two opposite holes in the upper part. Ten (10) grams of Timaye (Methyl-eugenol + Deltamethrin), rolled in gauze fabric and tied with galvanized steel wire, are introduced into the RT (Fig. 2b). Methyl-eugenol attracts contaminated males B. dorsalis from ACTs to the RT, where they are killed by Deltamethrin. The Timaye is renewed every 30 days. Recovery traps are used to monitor fruit fly population and assess the contamination rate. Traps were hung on trees with galvanized steel wire, at a height of 1.5 m from the ground under the canopy.
Field experiments
Four trials were conducted in the field during the rainy seasons of 2016, 2017 and 2018. The first three trials assessed the number of fruit flies contaminated at various densities (25, 50 and 100) of the ACT over 12 days. The fourth trial evaluated the effect of ACT densities in the control of B. dorsalis population and their impact on fruit damage during three months between July and November 2018.
The first trial was conducted in 2016 (September 19 to October 01, 2016) on a 6-ha citrus orchard field in Sebikhotane. Four blocks of 1 ha each were identified and in each block, 25 ACTs and 10 RT were applied. In another hectare of citrus orchard located at Ndoyene, a control comprising 10 RTs was installed. Males flies of B. dorsalis were collected daily in each block. From the flies recovered in each treated block, three batches of 100 flies were randomly selected to determine the rate of contamination. Flies contaminated with M. acridum were then counted in each batch, and the average number was used as the contamination rate in the block. The contamination rate was estimated as the proportion of trapped flies that had at least 3 of the 4 following characters that indicated their contamination: a pink-colored cuticle, modified eyes, flat and curved abdomen, and translucent femur (Ekesi et al. 2007; Faye et al. 2017; Toledo et al. 2017). Two other trials were conducted at Sindia in 2017. The first one (August 18 to August 30, 2017) was conducted in a 12.5 ha-field containing citrus orchards with 220 trees per hectare. Four blocks, receiving 50 ACT and 10 RTs per hectare, were identified in the first field. In a 2.5-ha of citrus orchard at Sebikhotane, one hectare was delimited and considered as a control, containing 10 RTs. Flies were collected daily. The second one was conducted in the same field (September 18 to September 30, 2017) with 100 ACT and 10 RTs per hectare for the field test, and 10 RTs in the control. For all three tests, RTs were installed one day after ACTs and monitored daily for 12 days to find out the average daily rate of contaminated flies for each density.
Field trials on the efficacy of three ACT densities
The last experiment was conducted in 2018 (July 27 to November 3) in a citrus orchard in Sindia, which had an area of 12.5 ha, with 220 trees per hectare. A citrus orchard with an area of 2.5 ha, composed mainly of citrus and some mango trees served as a control in Sebikhotane. Seven hundred (700) ACT and 52 RTs were deployed and monitored weekly for three months. The treatments were as follows:
-
25 ACT and 4 RT per hectare;
-
50 ACT and 4 RT per hectare;
-
100 ACT and 4 RT per hectare.
The RTs were situated away from the experimental site and considered as a control treatment. Each trap density was repeated 4 times, and the twelve treated blocks were placed in a completely randomized design (Fig. 3a and b). The 4 RTs in each block carrying ACT were placed centrally, as shown in Fig. 2, to monitor the B. dorsalis population. RT handles were oiled to prevent ants from eating caught flies. The flies caught were counted weekly and the contamination rate assessed.
Dynamics of damage on fruits
The infestation rate was assessed at the start, and at the 5th, 9th and 14th weeks of the trial in the plots, with the three separate trap densities. At each site, ten trees with ripening fruits were randomly selected and on each tree, 10 fruits were randomly chosen from the accessible fruits of the tree and inspected for signs of female oviposition. The symptoms observed on fruits included signs of decay, sap flow and sting scars. Pressure was applied with the thumb to release the sap from the already stung fruit. Inspected trees were tagged in red to avoid double counting.
Data analysis
The figure for Daily trapped flies (DTF) was evaluated by using the following formula:
The infestation rate was evaluated at the beginning of the trial, and at 5, 9 and 14 weeks later, from 100 fruits (10 trees sampled and 10 fruits per tree per hectare), for the 25, 50 and 100 ACT densities, as follows:
A One-Way ANOVA was used to compare the daily contamination rate for each density against the control plot. For the field trials on efficacy of 3 ACT densities, the weekly data on captured oriental fruit flies were transformed to number of flies per trap per day. Count data were transformed with \( \sqrt{\mathrm{X}+1} \) to normalize data and for the homogeneity of variances. The Student-Newman-Keuls (SNK) test, at α = 5%, was used for mean separation. GenStat 9éme Edition software was used for carrying out after the ANOVA (GenStat 2006). A Pearson’s correlation test was performed with InfoStat software (2018 version) to determine the influence of ACT density, contamination rate, and time on the dynamics of fruit fly populations (Di Rienzo et al. 2018).
Results
Effect of ACT density of fruit fly contamination
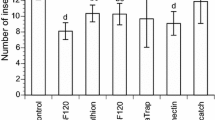
The results of preliminary trials conducted in 2016 and 2017 are given in Table 2. There was a significant variation in the average rate of contaminated flies for each of the three ACT densities and the control (DF = 3; F = 1065.56; p < 0.001). The rate of contaminated flies increases with the number of ACT (Fig. 4a and b). The Pearson correlation test, in Table 3, showed a positive correlation (r = 0.92; p < 0,0001) between trap densities and the contamination rate. With 25 ACT, the average daily rate of contaminated flies was 68.1 ± 1.87%, while at 50 ACT, the rate was at 85.44. At 100 ACT, the rate was 99.67 ± 0.180%. No contaminated fly was found in the control during the two years (Table 2).
Daily rates of contaminated flies at 25 ACT varied from 83.5% on the second day to 55.25% on the sixth day. The 0.3 g dose of the fungus was renewed on the sixth day, with the result of an increase in the rate of infected flies to 73% on the ninth day, which decreased to 62% on the twelfth day. The rate of infected flies at 50 ACT varied from 88.6% on the second day to 79% on the sixth day. The rate increased after the renewal of the fungus on the sixth day to 90.4% on the eighth day, and then decreased to 83% on the twelfth day. The rate of infected flies at 100 ACT varied from 99% on the second day to 100% on the sixth day. It did not change after the fungus was renewed on the sixth day, and it was 100% by the twelfth day (Fig. 4b).
Field efficacy for three ACT densities
Evaluation of daily trapped flies
The population dynamics of the B. dorsalis in the fungus-treated plot (Sindia) and in the control (Sebikhotane), during August to November 2018, are shown in Fig. 5. The flies trapped per day decreased with the various treatments of ACT, whereas in the control, the DTF increased over a period of 5 weeks, with an DTF of 126.8 ± 4.8 flies. There was a significant positive correlation between density of trap and DTF (r = 0.65; p < 0.0001). An ANOVA test showed significant differences in number of flies caught between the control block and the three densities of ACT treated blocks (DF = 3; F = 63.45; p < 0.001) (Fig. 5). The first week surveys indicated an average fly daily trapped of 21.71 for the density of 25 ACT, 4.21 ± 4.82 for the density of 50 ACT, 6.21 ± 4.82 for the density of 100 ACTs, and 69.11 ± 4.82 flies for the control. At the sixth week, the DTF for the density of 25 ACTs, was 0.64 ± 4.82 flies, against 0.71 ± 4.82 for the density of 50 ACTs, and 0.71 ± 4.82 flies for the density than 100 ACTs. At the 14th week, the DTF was 1.14 ± 4.82 flies for the density of 25 ACTs, 0.24 ± 4.82 flies for the density of 50 ACTs, and 0.56 ± 4.82 flies for the density than 100 ACTs. All the flies caught on the treated sites were contaminated.
There is no significant difference between treated orchards from week 4 until the end of the trial. In contrast, throughout the experiment, the DTF was significantly higher in the control orchard than in the treated ones. All the flies caught at the treated sites were contaminated. The Pearson correlation test showed no significant correlation (r = −0.12; p = 0.3602) between trap catches and the rate of contaminated flies. The same test showed no significant correlation (r = 0.59; p < 0,0001) between the sites and the number of contaminated flies.
Dynamics of fruits damage
Analysis of variance showed that there was no significant difference between the incidence of fruit damage in the three ACT densities and the control (DF = 3; F = 1.75; p < 0.174), with 64.17% for 25 ACTs, 74.17% for 50 ACTs, 69.17% for 100 ACTs, and 92.5% for the control.
However, significant difference of the incidence of fruit fly damage were found over time (DF = 3; F = 24.24; p < 0.001). Damage decreased significantly from 90.0%, 96.7%, and 83.3% in the first week, to 30%, 50%, and 46.7% in the 14th week, respectively, for 25, 50 and 100 ACT in the treated orchard (Fig. 6). For the control, there was no significant reduction in the incidence of damage, over time.
Discussion
The use of autoinoculation systems is seen as an innovative approach for pest management control especially when combined with semiochemicals and biopesticides. Methyl eugenol is a natural chemical compoundfound in many plants. When ingested by Bactrocera males, it acts as a booster to the male sex pheromonal components, thereby increasing their mating success (Ekesi et al. 2016). One of the advantages of entomopathogenic fungi is that they infect their host through direct contact and do not need to be ingested. Results of the current study showed variations in contamination rates with M. acridum as a function of the density of ACTs. This implies that male B. dorsalis picked infection from ACTs which have caused mortality with minimal dose of 0.3 g of M. acridum spores.
The maximum amount of fungus inoculum used in this study was 30 g of spores per hectare for the highest density of ACT. Ekesi and Billah (2007) suggested an application of 80 to 100 g of spores per week of M. anisopliae for 100 self-inoculating devices for a mango orchard with 100 trees per hectare. It can be concluded that if appropriate number of traps are used, less inoculum will be required thereby confirming the cost-effectiveness of the lure and kill approach.
There was a significant reduction in B. dorsalis population in Sindia, as compared with the control orchard in Sebikhotane. According to the International Atomic Energy Agency (IAEA 2003), an orchard in which DTF <1 is considered to have been the object of a fruit fly suppression/removal. All the three trap densities were effective in the management of B. dorsalis in the orchard treated after six weeks at an DTF less than 1 for all densities. This low DTF, could be explained by possible horizontal transmission of M. acridum from infected flies to healthy flies. Fungal disease is transmitted by simple contact, or during mating, or by ingestion in ticks that devour their dead infected congeners (Sanjaya et al. 2013). Studies on the horizontal transmission of a fungal infection from infected insects to healthy insects have already been reported in termites, butterflies and houseflies (Daniel and Wyss 2008; Carcamo et al. 2015; Opisa et al. 2019). In the laboratory, Dimbi et al. (2013) and Sookar et al. (2014) showed that contaminated flies, before dying, were capable of transmitting fungal disease to healthy flies, causing mortality between 71 and 100%, at 10 to 15 days after inoculation. Hedström and Monge-Nájera (1998) reported that infected females or males that copulate more than once increase the chances of fungal disease being transferred to healthy populations of fruit flies. In addition to the horizontal transmission of the fungus from an infected male to the healthy female, an infected male can spread the fungal disease through contact with another healthy male during leks (Banelli et al. 2014; Toledo et al. 2017). Additionally, Shelly et al. (2016) demonstrated that males of Bactrocera produce and diffuse sex pheromones that invite the receptive females over long distances. Furthermore, in Hawaii, Shelly and Kaneshiro (1991) observed groupings of males, or leks, in a citrus orchard, which attracted females for mating in the canopy.
Males of B. dorsalis that visited the ACTs spent much time there around the cotton wick soaked in methyl eugenol. Haq et al. (2016) demonstrated that a methyl eugenol diet of males of the B. dorsalis complex improves their mating competitiveness, thereby increasing their ability to transmit fungal infection to healthy females. San Andrés et al. (2014) demonstrated in the laboratory the effectiveness of the use of ACTs based on M. anisopliae against C. capitata. Navarro-Llopis et al. (2015) showed that a density of 24 ACTs with M. anisopliae was effective against C. capitata in a citrus orchard in Valencia, Spain.
There was a density relatedness between ACT traps and population reduction and infection with M. acridum resulted in reduced fruit damage. Blocks with the density of 25 ACT have the lowest fruit damage rate, which dropped from 90% to 30%. Fungal infection has been shown to have an effect in infected females by reducing fertility (Dimbi et al. 2013). Orchard sanitation was not applied during our study and the fallen fruits served as breeding grounds for the surviving population of B. dorsalis. Re-infestation of the orchard by flies was noted from these fallen citrus fruits. Vargas et al. (2010), Deguine et al. (2013) and Ekesi et al. (2016) showed that sanitation is the foundation of integrated fruit fly control, without which any other control method is effective (Vargas et al. 2010; Deguine et al. 2013; Ekesi et al. 2016).
This study demonstrated the effectiveness of the combination of M. acridum treated traps and parapheromone for the dissemination of fungal spores by male of B. dorsalis. This technique significantly reduced the fruit fly population, uses minimal amount of fungus inoculum and is made of locally available and affordable materials. The study suggests a more in-depth study on the cost-benefit analysis and the development of strategies for the scaling of this technique as a first-line management strategy during early seasons, in conjunction with other IPM techniques such as orchard sanitation.
References
Agunloye OJ (1987) Trapping and chemical control of Ceratitis capitata (Wied.) (Diptera: Tephritidae) on sweet orange (Citrus sinensis) in Nigeria. J Horticult Sci Ashford 62(2):269–271
Badji H, Coly EV (2014) Gestion des Attaques de la Mouche des Fruits sur les Cultures de Cucurbitacées au Moyen de Pesticides Naturels au Sénégal. Acta Hortic 1021:421–426. https://doi.org/10.17660/actahortic.2014.1021.39
Banelli G, Daane KM, Canale A, Niu CY, Messing RH, Vargas RI (2014) Sexual communication and related behaviours in Tephritidae: current knowledge and potential applications for integrated Pest management. J Pest Sci 87:385–405. https://doi.org/10.1007/s10340-014-0577-3
Binta BAA, Barbier B (2015) Economic and environmental performances of organic farming system compared to conventional farming system: a case study of the horticulture sector in the Niayes region of Senegal. Procedia Environ Sci 29:17–19. https://doi.org/10.1016/j.proenv.2015.07.132
Bischoff JF, Rehner SA, Humber RA (2009) A multilocus phylogeny of the Metarhizium anisopliae lineage. Mycologia 101(4):512–530
Carcamo MC, Felchicher F, Duarte JP, Bernardi E, Ribeiro PB (2015) Horizontal transmission of Beauveria bassiana (Hypocreales: Cordycipitaceae) and Metarhizium anisopliae (Hypoceales: Clavicipitaceae) in Musca domestica (Diptera: Muscidae). J Econ Entomol 108:1579–1586. https://doi.org/10.1093/jee/tov163
CLIMATE-DATA.ORG (2020) Senegal climate: Average Temperature, weather by month, Senegal weather averages.URL:https://fr.climate-data.org/afrique/senegal/sindia/sindia-29237/ (Accessed 8 Jan 2020)
CLIMATE-DATA.ORG (2019) Senegal climate: Average Temperature, weather by month, Senegal weather averages. URL:https://fr.climate-data.org/afrique/senegal/ndoyene/ndoyene-1044525:/ (Accessed 29 Dec 2019)
Daniel C, Wyss E (2008) Field applications of entomopathogenic fungi against Rhagoletis cerasi. In: book, Markus (Ed.) Ecofruit - 13th International Conference on Cultivation Technique and Phytopathological Problems in Organic Fruit-Growing: Proceedings to the Conference from 18thFebruary to 20th February 2008 at Weinsberg/Germany pp 87-92
De Meyer M, Robertson M, Mansell M, Ekesi S, Tsuruta K, Mwaiko W, Vayssières JF, Peterson T (2009) Ecological niche and potential geographic distribution of the invasive fruit fly Bactrocera invadens (Diptera, Tephritidae). Bull Entomol Res 100(1):35–48
Deguine JP, Augusseaux X, Insa G, Jolet M, Le Roux K, Marquier M, , Rousse P, Roux E, Soupapoullé Y, Suzanne W (2013) Gestion Agroécologique des Mouches des Légumes à la Réunion. Innov Agronom 28: 59–74
Deguine JP, Gloanec C, Aubertot JN et al (2016) Protection agroécologique des cultures. Editions Quae, p 288
Di Rienzo JA, Casanoves F, Balzarini MG, Gonzalez L, Tablada M, Robledo CW. (2018) InfoStat versión 2018. InfoStat Group, Facultad de Ciencias Agropecuarias, Universidad Nacional de Córdoba, Argentina. URL http://www.infostat.com.ar
Dimbi S, Maniania NK, Ekesi S (2013) Horizontal transmission of Metarhizium anisopliae in fruit flies and effects of fungal infection on egg laying and fertility. Insects 4(2):206–216
Ekesi S (2016) Baiting and male annihilation techniques for fruit fly suppression in Africa pp 275–292. In: Fruit Fly Research and Development in Africa - Towards a Sustainable Management Strategy to Improve Horticulture
Ekesi S, Billah MK (2007) A field guide to the management of economically important Tephritid fruit fly in Africa. Nairobi: ICIPE Science Press. (English version-ISBN: 92-9064-209-2)
Ekesi S, Chabi-Olaye A, Subramanian S, Borgemeister C (2011) Horticultural pest management and the African economy: successes, challenges and opportunities in a changing global environment. pp 165–183 In: Acta Horticulturae
Ekesi S, Dimbi S, Maniania NK (2007) The role of entomopathogenic fungi in the integrated management of fruit flies (Diptera: Tephritidae) with emphasis on species occurring in Africa. In: Ekesi S, Maniania NK, eds. Use of Entomopathogenic Fungi in Biological Pest Management. Research, SignPost, Kerala, pp. 239–274
Ekesi S, Mohamed SA, de Meyer M (2016) Fruit fly research and development in Africa-Towards a sustainable management strategy to improve horticulture. First Edition Springer Editions pp. 778 https://doi.org/10.1007/978-3-319-43226-7
FAO (2018) Food Outlook - Biannual Report on Global Food Markets – November 2018. Rome. 104 pp. Licence: CC BY-NC-SA 3.0 IGO
Faye PD, Sangare YK, Coly EV, Badji K, Bal AB, Dieng EO (2017). Evaluation of the biological effectiveness of Metarhizium acridum against wild populations of fruit fly Bactrocera dorsalis (Hendel) in Niayes region. (Conference poster) AAIS 22nd Meeting and Scientific Conference, October 23-27, ARC Wad Medani, Sudan. https://doi.org/10.13140/RG.2.2.18829.36324
Fombong AT, Kachigamba DL, Torto B (2016) Chemical ecology of African tephritid fruit flies. In: Ekesi S, Mohamed S, De Meyer M (Editors) Fruit fly research and development in africa - towards a sustainable management strategy to improve horticulture. pp. 133-165
Fernandes E, Bittencourt V (2008) Entomopathogenic fungi against south American tick species. Exp Appl Acarol 46:71–93
Gao Q, Jin K, Ying SH, Zhang Y, Xiao G, Shang Y, Duan Z, Hu X, Xie XQ, Zhou G, Peng G, Luo Z, Huang W, Wang B, Fang W, Wang S, Zhong Y, Ma LJ, St Leger RJ, Zhao GP, Pei Y, Feng MG, Xia Y, Wang C (2011) Genome sequencing and comparative transcriptomics of the model entomopathogenic fungi Metarhizium anisopliae and M acridum. PLoS Genet 7(1). https://doi.org/10.1371/journal.pgen.1001264
GenStat (2006) Ninth Edition, (Version −9.1.0.147. Supplied by VSN International Ltd, 5 The Waterhouse Street, Hemel Hempstead HP1 1ES. United Kingdom. http://www.vsni.co.uk
Grechi I, Sane CAB, Diame L, De Bon H, Benneveau A, Michels T, Huguenin V, Malézieux E, Diarra K, Rey JY (2013) Mango-based orchards in Senegal: diversity of design and management patterns. Fruits 68(06):447–466
Haq I, Veysen MJB, Schutze M, Hendeicks J, Shelly T (2016) Effects of methyl Eugenol feeding on mating compatibility of Asian population of Bactrocera dorsalis (Diptera: Tephritidae) with African population and with B. carambolae. J Econ Entomol 1(109):148–153
Hedström I, Monge-Nájera J (1998) Is sexually transmitted fungal infection evidence for size-related mating success in Neotropical guava fruit flies? Rev Biol Trop 46(4):1129–1132
IAEA (2003) Insect Pest control section. Trapping guidelines for AreaWide fruit Fly Programmes. Int Atom Energy Agency Vienna Austria 26:7–46
Konta IS, Djiba S, Sane S, Diassi L, Ndiaye AB, Noba K (2016) Etude de la dynamique de Bactrocera dorsalis (Hendel) (Diptera : Tephritidae) dans les vergers de mangues en Basse Casamance : influence des facteurs climatiques. Int J Biol Chem Sci 9:2698. https://doi.org/10.4314/ijbcs.v9i6.15
Lux SA, Copeland RS, White IM, Manrakhan A (2003) A new invasive fruit Fly species from the Bactrocera dorsalis (Hendel) group detected in East Africa. Int J Tropic Insect Sci 23:355–361. https://doi.org/10.1017/s174275840001242x
Maertens M (2009) Horticulture exports, agro-industrialization, and farm-nonfarm linkages with the smallholder farm sector: evidence from Senegal. Agric Econ 40:219–229. https://doi.org/10.1111/j.1574-0862.2009.00371.x
McQuate G, Silva C, Jang E (2005) Mediterranean fruit fly (Diptera: Tephritidae) suppression in persimmon trough bait sprays in adjacent coffee plantings. J Appl Entomol 129:110–117
Navarro-Llopis V, Ayala I, Sanchis J, Primo J, Moya P (2015) Field efficacy of a Metarhizium anisopliae-based attractant–contaminant device to control Ceratitis capitata (Diptera: Tephritidae). J Econ Entomol 108(4):1570–1578
Ndiaye M, Dieng E, Delhove G (2008) Population dynamics and on-farm fruit fly integrated pest management in mango orchards in the natural area of Niayes in Senegal. Pest Manag Horticult Ecosyst 14(1):1–8
Ndiaye O, Vayssières JF, Rey JY, Ndiaye S, Diedhiou PM, Ba CT, Diatta P (2012) Seasonality and the importance of fruit fly (Diptera: Tephritidae) host plants in orchards at Niayes and the Thiès plateau. Fruits 67:311–331. https://doi.org/10.1051/fruits/2012024
Opisa S, Plessis D, Akutse KS, Fiaboe KKM, Ekesi S (2019) Horizontal transmission of Metarhizium anisopliae between Spoladea recurvalis (Lepidoptera: Crambidae). Microb Pathogen 131:197–204
Ouna E (2010) Entomopathogenicity of Hyphomycetes Fungi to fruit fly Bactrocera invadens (Diptera, Tephritidae) and their potential for biological control on mango, Jomo Kenyatta University, Thesis research in entomology-miceobiology. Nairobi, 116p. https://ir-library.ku.ac.ke/handle/123456789/682
Pattemore JA, Hane JK, Williams AH, Wilson BA, Stodart BJ, Ash GJ (2014) The genome sequence of the biocontrol fungus Metarhizium anisopliae and comparative genomics of Metarhizium species. BMC Genomics 15(1):660. https://doi.org/10.1186/1471-2164-15-660
San Andrés V, Ayala I, Abad MC, Primo J, Castanera P, Moya P (2014) Laboratory evaluation of the compatibility of a new attractive contaminant device containing Metarhizium anisopliae with Ceratitis capitata sterile males. Biol Control 72:54–61
Sanjaya Y, Ocampo V, Caoili BL (2013) Transmission effect of entomopathogenic fungi on population of Tetranychus kanzawai (Kishida) (Tetranychidae:Acarina). Arthropods 2(1):36–41
Schutze M, Bourtzis K, Cameron SL, Clarke AR, De Meyer M, Hee AKW, Hendricks J, Krosch MN, Mwatawala M (2017) Integrative taxonomy versus taxonomic authority without peer review: the case of the oriental fruit fly, Bactrocera dorsalis (Tephritidae). System Entomol 42:609–620
Shelly TE, Epsky N, Jang EB, Reyes-Flores J, Vargas R (2016) Trapping and the detection, control, and regulation of Tephritid fruit flies: lures, area-wide programs, and trade implications. Springer, New York
Shelly TE, Kaneshiro KY (1991) Lek behavior of the oriental fruit fly, Dacus dorsalis, in Hawaii (Diptera: Tephritidae). J Insect Behav 4:235–241
Sookar P, Bhagwant S, Allymanod M (2014) Effect of Metarhizium anisopliae on the fertility and fecundity of two species of fruit flies and horizontal transmission of Mycotic infection. J Insect Sci 14(100):1–12. https://doi.org/10.1673/031.014.100
Toledo J, Flores S, Campos S, Villasenoe A, Enkerlin W, Liedo P, Valle A, Montoya P (2017) Pathogenicity of three formulations of Beauveria bassiana and efficacy of autoinoculation devices and sterile fruit fly males for dissemination of conidia for control of Ceratitis capitata. https://doi.org/10.1111/eea.12608
Toledo J, Liedo P, Flores S, Campos SE, Villasenor A, Montoya P (2006) Use of Beauveria bassiana and Metarhizium anisopliae for Fruit Fly control: A novel Approach. In book: Fruit Flies of Economic Importance: From Basic to Applied knowledge, Edition: 1, Publisher: Press Color, Bahia, Brazil., pp.127-132
Vargas RI, Pinero JC, Mau RFL, Jang EB, Klungness LM, McInnis DO, Harris EB, McQuate GT, Bautista RC, Wong L (2010) Area-wide suppression of the Mediterranean fruit Fly, Ceratitis capitata, and the oriental fruit Fly, Bactrocera dorsalis, in Kamuela, Hawaii. J Insect Sci 10:1–17. https://doi.org/10.1673/031.010.13501
Vayssières J-F, Adandonon A, N’Diaye O, Sinzogan A, Kooyman C, Badji K, Rey J-Y, Wharton RA (2012) Native parasitoids associated with fruit flies (Diptera: Tephritidae) in cultivated and wild fruit crops in Casamance, Senegal. African Entomol 20:308–315. https://doi.org/10.4001/003.020.0221
Vayssières JF, Sinzogan A, Adandonon A, Rey JY, Dieng EO, Camara K, Sangaré M, Ouedraogo SN, Hala N, Sidibe A, Kéita YF, Gogovor G, Korie S, Coulibaly O, Kikissagbé C, Tossou A, Billah M, Biney K, Nobime O, Diatta P, N'Dépo OR, Noussourou M, Traore L, Saizonou S, Tamo M (2014) Annual population dynamics of mango fruit flies (Diptera:Tephritidae) in West Africa: socio-economic aspects, host phenology and implication for management. Fruits 69(011):207–209
Vayssières JF, Vannière H, Gueye PS, Barry O, Hanne AM, Korie S, Niassy A, Delhove G (2011) Preliminary inventory of fruit fly species (Diptera, Tephritidae) in mango orchards in the Niayes region, Senegal, in 2004. Fruits 66(002):91–107
Warthen JD, Cunningham RT, Leonhardt BA, Cook JM, Avery JW, Harte E (1998) Comparison of Ceralure and Trimedlure controlled-release formulations for male Mediterranean fruit flies in C&C Traps. J Chem Ecol 24(8):1305–1314
Zakari-Moussa O, Ratnadass A, Vayssières JF, Nikiema A, Fatondji D, Salha H, Aboubacar K, Rickewaert P, Pasternak D (2012) GF-120 effects on fruits fly species (Diptera, tephritidae) in Sahelian agroforestery-based horticultural cropping systems. Fruits 67(025):333–339
Acknowledgements
We would like to thank the Senegalese Plant Protection Directorate for making the work possible, the team of technicians and trainees from the agricultural zoology laboratory for the follow-up and Dr. Charles Haddad, producer for facilitating access to his orchard.
Funding
- Plant Protection Directorate of Senegal make the M. acridum strain available free of charge
- Dr. Charles Haddad paid the Methyl eugenol and fund some material to make traps.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Faye, P.D., Bal, A.B., Ndiaye, N.M. et al. Field efficacy of Metarhizium acridum (Hypocreales: Clavicipitaceae) in the control of Bactrocera dorsalis (Diptera: Tephritidae) in citrus orchards in Senegal. Int J Trop Insect Sci 41, 1185–1195 (2021). https://doi.org/10.1007/s42690-020-00306-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42690-020-00306-5