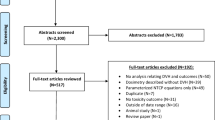
Abstract
Purpose
This study aims to estimate the risk of secondary lung cancer, expressed as the excess absolute risk (EAR), in pediatric patients treated with radiotherapy for Hodgkin’s lymphoma. The project was approved by the INCA Ethics Committee under CAAE 55933222.4.0000.5274.
Methods
The mechanistic and the OED (Organ Equivalent Dose) models were applied for this estimate. Two cases were included in this study, the bell-shaped and the plateau dose–response relationships obtained from the full mechanistic model. OED results were slightly lower using the full mechanistic and plateau models compared with the bell-shaped model.
Results
For an attained age 45 years after exposure, taking into account the three models, the EAR (cases per 104 person-years) values ranged from 19.71 to 25.07 for patients with age at exposure of 15 and 16 years; from 6.98 to 7.22 for patient with age at exposure of 12 years; from 9.73 to 11.29 for patients with age at exposure of 5 and 6 years; and from 16.05 to 16.84 for patient with age at exposure of 13 years. Inadequate coverage and lack of conformity of the treatment target reflect high-dose-volume parameters and finally the estimated risk.
Conclusion
The mechanistic model makes it possible to estimate the secondary cancer risk and compare it with regard to dose distributions, without having to wait for several years to observe the actual risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A serious potential side effect of radiation therapy is the induction of a secondary cancer, that is, a new cancer resulting from the treatment. This is becoming increasingly important in the case of pediatric patients, because of the growing number of young individuals undergoing radiation therapy (RT) and improving survival times after treatment (Shuryak et al. 2011). These patients are probably more sensitive to radiation-induced carcinogenesis than adults, and in addition, have a longer life expectancy (Shuryak et al. 2011). Therefore, it is interesting to predict the probability of second cancer induction in pediatric patients treated with radiation therapy.
Currently there is large uncertainty about the shape of the dose–response relationship for carcinogenesis induced by radiation for most cancer types at radiotherapy doses. Consequently, it is necessary to model cancer induction for radiotherapy patients and thus the underlying dose–response relationship. Such modeling can be based on epidemiological studies, where the usual method for obtaining the dose–response relationship for radiation-associated cancer is to perform a case–control study (Schneider et al. 2011). The advantage of this method is a direct determination of risk as a function of point dose, and the major disadvantage are the large errors involved when determining the location and dose to the origin of the tumor (Schneider et al. 2011). This problem can potentially be solved by developing radiobiological models, i.e., biologically based mathematical models that focus on radiation-induced carcinogenesis processes such as cell killing and mutagenesis, which occur during and shortly after irradiation (Shuryak et al. 2009). This models can predict the second cancer risk of any given radiotherapy protocol using organ dose distributions.
However, models that express the risk of secondary cancer can also be implemented from a combination of radiobiological and epidemiological considerations. Thus, an mechanistic model (Schneider 2009) was developed to determine a possible dose–response relationship for radiation-induced cancer after fractionated radiotherapy by combining the linear-quadratic model of cell kill, the linear-no-threshold (LNT) model for radiation-induced cancer at low dose, the tumor induction through a mutational process, and the repopulation/repair effects.
The dose–response relationship for cancer induction obtained from a combined formalism can be implemented with other models such as the organ-equivalent dose (OED) model (Schneider et al. 2005). The OED concept assumes that any dose distribution in an organ is equivalent and corresponds to the same OED if it causes the same radiation-induced cancer incidence. In order to calculate OED, information about the three-dimensional dose distribution is necessary. This is usually not provided in epidemiological studies on second cancers after radiotherapy. Thus, the dose distributions used are the differential dose-volume histogram (DVH) obtained from the treatment planning systems (TPS).
For the mechanistic model, parameters for site-specific dose–response relationships were obtained by fit of a combination of the Japanese A-bomb survivor data with secondary cancer data from Hodgkin’s patients (Schneider et al. 2011).
There is evidence towards the induction of site-specific second primary cancers after radiotherapy treatment for Hodgkin’s lymphoma (HL). The five most common secondary cancers were breast cancer, lung cancer, acute leukemia, non-Hodgkin lymphoma, and gastrointestinal cancer (Ng et al. 2002).
The aim of this study was to estimate the individualized risk of secondary lung cancer, expressed as the excess absolute risk (EAR), associated with 3D conformal radiotherapy, a technique that uses computed tomography images to design the treatment, for pediatric Hodgkin’s lymphoma patients, based on the mechanistic and OED models.
Methods
Hodgkin’s lymphoma treatment data
Eight 3D conformal radiotherapy treatments were selected. The patient inclusion criteria were pediatric patients who were treated for Hodgkin’s lymphoma at the Brazilian National Cancer Institute (INCA). The linear accelerators (Linacs) used in these treatments were Varian: Trilogy, Clinac 600C, and CL2300C, with a dose rate of 400 MU/min. The common characteristics to all treatments were the use of the Varian Millennium 120 MLC, the field-in-field (FIF) technique, and an isotropic planning target volume (PTV) margin of 0.5 cm around the clinical target volume (CTV). Age at exposure, gender, linac model and energy, prescription doses, and the fractionation scheme for these patients are shown in Table 1. DVHs for the PTV and lungs were extracted from the Eclipse TPS.
Secondary cancer risk
The risk of secondary lung cancer, expressed as the excess absolute risk (EAR), was estimated using the mechanistic model as described by Schneider (2009), for predicting cancer induction after fractionated radiotherapy, and the organ equivalent dose (OED) concept (Schneider et al. 2005), a generalized dose average weighted with the dose–response relationship (measured in Gy) proportional to the probability for the induction of a malignancy.
The excess absolute risk (EAR) was estimated as a linear function of the OED, adjusted for population-specific variables such us the modifying function (µ) and the initial slope (β) (Schneider et al. 2011). Thus, the EAR was calculated according to:
The OED was calculated as:
where V(D) is the differential dose-volume histogram (DVH), the sum is taken over all dose intervals (i) of the DVH, V is the total organ volume, and RED (the risk equivalent dose) is the dose–response relationship for carcinoma induction obtained from the mechanistic model, and it was defined as:
Where it was assumed that the tissue was irradiated with a fractionated treatment schedule of equal dose fractions d up to a dose D. The repopulation/repair parameter (R) characterizes the repopulation/repair-ability of the tissue between two dose fractions. Repopulation effect cancels out the effects of cellular inactivation (cell killing), primarily because some of the proliferating cells carry and pass on pre-malignant damage produced earlier in the treatment (Schneider 2009). The number of cells is reduced by cell killing, which is proportional to the cell kill parameter (α′) and is defined using the linear quadratic model, according to:
In this notation, D and d are the total dose and dose per fraction in lung tissue and DT and dT are the prescribed dose to the target volume with the corresponding fractionation dose, respectively. Moreover, α and β are the usual parameters from the linear-quadratic model for the tissues of interest and are used to calculate the value of α/β value, a way of expressing the sensitivity of tumors and normal tissues to radiation.
Two cases derived from Eq. 3 (full mechanistic model) were included in this study. The first one, commonly named bell-shaped dose–response relationship (Eq. 5), is derived by taking Eq. 3 in the limit of R → 0 (Schneider et al. 2011), and this is completely neglecting any repopulation/repair effect and thus fractionation.
The second case is called plateau dose–response relationship (Eq. 6), which is derived by taking Eq. 3 in the limit of R → 0 (Schneider et al. 2011), and this is considering a full repopulation/repair.
The modifying function (μ) contains population-dependent variables such as age at exposure (e), age attained (a), and the age-modifying parameters γe and γa, as described by:
In this form, the fit parameters are gender-averaged and centered at an age at exposure of 30 years and an attained age of 70 years. The initial slope (β) is the slope of the dose–response curve at low dose, and was defined also for persons exposed at age 30 years and attaining age 70 years (Schneider et al. 2011).
The following organ-specific model parameters were used. For the full model: α = 0.042 Gy−1 and R = 0.83; bell-shaped: α = 0.022 Gy−1 (R = 0); and plateau: α = 0.056 Gy−1 (R = 1). In all cases was considered an α/β value of 3 Gy. The initial slope was β = 8.0 excess cases per 104 person-years Gy, based on A-bomb survivors exposed at 30 years and surviving to 70 years, and modified for a western population. The age-modifying parameters were as follows: γe = 0.002 and γa = 4.23.3 All parameters for second cancer risk calculation were taken from Schneider et al. (2011), based on the risk of selected second malignancies of Hodgkin’s patients after radiotherapy, quantified by Dores et al. (2002).
Results
This section presents the dosimetric evaluation of the radiotherapy treatment plans in terms of target dose coverage (PTV) and dose to organ at risk (lung), and the secondary lung cancer risk estimate, in terms of the excess absolute risk (EAR), according to the mechanistic and OED and models.
Dosimetric study
The cumulative DVHs for the PTV extracted from the Eclipse TPS are shown in Fig. 1.
In radiation therapy, the goal is to deliver the maximum dose to the target volume in a homogeneous manner, avoiding the dose to surrounding normal structures. Thus, tools have to be used for this purpose. The homogeneity index (HI) and the conformity index (CI) are two of these tools for the analysis of the treatment plan. The analysis of dose coverage of the planning target volume (PTV) was made taking into account the HI and CI index, defined as, HI = (D2% − D98%)/D50% and CI = V95%/VPTV, respectively (Ma et al. 2015). Here, the dose-volume constraints Vx% and Dx% represent the volume receiving ≥ x% of the prescribed dose and the dose received by x% of the volume, respectively. A higher HI value, ranging from 0 to 1, represents worse homogeneity, and a higher CI value, ranging from 0 to 1, represents better conformity (Ma et al. 2015). Other dosimetric parameters for the PTV, such as the mean dose (Dmean), the V95%, V107% (to analyze the volume confined in 95 to 107% of the prescribed dose, guide for dose homogeneity and dose conformity on the PTV), D2%, D98%, D50% constraints, besides the HI and CI indexes, are shown in Table 2.
Table 2 shows a better target dose coverage for patients 4 and 5, each one with a CI of 0.99 and HI of 0.14, 0.08, and 0.15, respectively. However, the worse was for patient 9, with CI of 0.63 and HI of 1.01. In general, the lack of conformity in these plans was shown by the high value of V107%, except for patient 5, who has the lowest PTV volume.
The cumulative and differential DVHs for the lung are shown in Fig. 2.
In the case of the lung, the analysis was made taking into account dosimetric parameters such as maximum (Dmax), minimum (Dmin), and mean doses (Dmean), and the V5Gy (%), V10Gy (%), V15Gy (%), and V20Gy (%) constraints (VxGy represents the percentage of volume receiving ≥ xGy), which are shown in Table 3.
It is known that V20Gy in lung is a dosimetric parameter for identifying risk of radiation-induced pneumonitis. A generally accepted V20Gy ≤ 30–35% constraint is use in adult patients (Marks et al. 2010). In the case of pediatric patients, this is less clear. Data available on the relationship between radiation dosimetric parameters in the treatment of HL and pulmonary toxicity is sparse for children. In this study, the V20Gy parameter ranged from 12.68 to 33.98%, while the mean dose varied from 4.35 to 11.34 Gy. The dose-volume constraints were higher for patient 7, except for parameter V20Gy, which the higher value corresponds to patient 2. Patient 5 shows lower dose-volume constraints compared to other patients.
Figure 2, for the lung differential DVHs, shows a clearly established distribution with two peaks, one at high and another at low dose. Being that most of the lung volume receives low and intermediate doses. This distribution will influence the risk estimate of secondary cancer, especially in the case of high doses.
Secondary cancer risk evaluation
The dose–response relationships (RED) for the full, bell-shaped, and plateau models and the full model considering the number of fractions (14, 13, 11, and 10) are shown in Fig. 3.
Figure 4 shows the modifying function as a function of age at exposure and attained age and the EAR of secondary lung cancer as a function of punctual dose for age at exposure of 16 years and attained age of 30 years.
The excess absolute risk for secondary lung cancer was estimated for an age at exposure (e) of 5, 6, 12, 13, 15, and 16 years and for an attained age (a) of 7, 15, 30, and 45 years after radiation therapy, as shown in Tables 4, 5, and 6, respectively.
As shown in Tables 4, 5, and 6, the OED ranged from 2.16 for the patient 5 using the full and plateau models to 6.16 for the patient 6 using the bell-shaped model. The OED results were slightly lower using the full and plateau models compared with the bell-shaped model. For the three models considered individually, the lowest and highest values of the OED were observed for patients 5 and 6, respectively. This higher OED calculated for patient 6 is justified by the fact that a higher percentage of volume receives high doses, compared to the other patients, as observed in the lung differential DVHs shown in Fig. 2b.
The EAR estimate is also strongly dependent on the initial slope of the different organ-specific dose–response curves. The EAR was estimated taking into account the age at exposure and attained age. The EAR values, taking into account the three models, were less than or equal to 0.4 cases per 104 person-years, for an attained age 7 years after exposure. For an attained age 15 years after exposure, taking into account the three models, the EAR was slightly higher than 1.05 cases per 104 person-years for patients with age at exposure of 15 and 16 years while it was lower than 0.74 cases per 104 person-years for patients with age at exposure less than 13 years. For an attained age 30 years after exposure, taking into account the three models, the EAR values ranged from 5.85 to 7.59 cases per 104 person-years for patients with age at exposure of 15 and 16 years (patients 1, 2, 3, and 4). For patient 5, the EAR was 1.92 and 1.98 cases per 104 person-years, for patients 6 and 7 ranged from 2.23 to 2.49 cases per 104 person-years, and for patient 8 ranged from 4.53 to 4.75 cases per 104 person-years. For an attained age 45 years after exposure, taking into account the three models, the EAR values ranged from 19.71 to 25.07 cases per 104 person-years for patients with age at exposure of 15 and 16 years (patients 1, 2, 3, and 4). For patient 5, the EAR ranged from 6.98 to 7.22 cases per 104 person-years, for patients 6 and 7 ranged from 9.73 to 11.29 cases per 104 person-years, and for patient 8 ranged from 16.05 to 16.84 cases per 104 person-years. In general, for all three models, an increase in risk was observed with the increase in the attained age, this is due to how was defined the modifying function. At this point, no specific differences were considered regarding the sex of the patient.
When the EAR is compared for different ages at exposure, for example, for patients with 5 and 6 years with those of 15 or 16 years, a lower risk was observed for the patients with younger ages at exposure, for all attained ages after exposure, considering the three models used here. Thus, considering the full model, 45 years after exposure were estimated an EAR of 20.70 and 24.02 cases per 104 person-years for patients with an age at exposure of 16 years, while an EAR of 10.60 and 9.73 per 104 person-years for patients with 5 and 6 years at exposure, respectively.
Figure 5 summarizes the results obtained in Tables 4, 5, and 6. It shows the excess absolute risk of secondary lung cancer (per 104 person-years), for different age at exposure, 45 years after treatment, and for an age at exposure of 16 years and different attained.
In general, different parts of each organ at risk are exposed to a broad range of doses, from low to higher doses. In Fig. 6, it is shown the estimated contribution of individual doses, as percentage of the prescribed dose, to the OED, using the full mechanistic model. The largest predicted contributions to the OED, per unit dose, came from high doses, where the contribution peaks above the prescribed dose, while a lower contribution was observed in the intermediate dose region, and with a small maximum, in most cases, at quite low doses. As the EAR is proportional to the OED, it shows that, when the proportion of irradiated lung volume at high doses increases, the secondary cancer risk also increases.
Discussion
The mechanistic model includes several assumptions to simplify the biological processes leading to cancer induction, and the model parameters were obtained through a combined fit from atomic bomb survivors and Hodgkin’s lymphoma patient data (Schneider et al. 2011).
Considerable uncertainties are involved in modeling the underlying biology of radiation-induced cancer and in the determination of model parameters (Nguyen et al. 2015), and in the same way, epidemiological data from the atomic-bomb survivors and the Hodgkin’s patients are subject to many sources of uncertainty, for example, in radiation dose estimate (Gilbert 2009; UNSCEAR 2012). Therefore, using this model implies considerable uncertainties in the risk estimations, as shown by Nguyen et al. (2015), where the uncertainty in the mechanistic model parameters was estimated and finally the uncertainty in the estimated absolute risk. These uncertainties can be reduced by estimating relative risks because absolute risks are less reliable than these (Nguyen et al. 2015). This can be done, for example, by comparing the risk produced by different radiotherapy techniques.
The mechanistic model allows estimating the individual risks of developing a secondary malignant neoplasm. This fact is common in radiotherapy where dose–response relationships that are considered population-averaged, as in this case, are used in individual estimates.
From the modifying function used here, there are important issues to be taken into account. First, EAR is not influenced significantly by age at exposure. This fact can be observed by varying the age at exposure (for all attained ages). However, it is known that the risk is higher for younger children than older children because young children have longer post-treatment lives and their bodies are growing and changing more rapidly than older children (Shin 2011). Second, EAR increases with attained age for any age at exposure (Tables 4, 5, and 6). However, the risk of developing a radiation-induced lung secondary cancer does not start to increase until an attained age of approximately 30 years (Figura 4a).
Figure 4b shows significant differences in the dose–response relationships of EAR at high doses, especially in the case of the bell-shaped dose–response relationship. This difference is marked by the process of cell repopulation and begins to become significant from 30 Gy. The prescribed doses used in the treatment of Hodgkin lymphoma in pediatric patients in this paper were less than 25.2 Gy (Table 1). Thus, the portions of the dose–response relationships used were the upward sloping part of the curves.
Secondary cancer risk is known to be greater for larger target volumes because this implies larger volumes of the healthy tissue irradiated (Paganetti 2012); this is the case of Hodgkin’s lymphoma patients treated with 3D conformal radiotherapy. At this point, the volume of the lung within the irradiation field, i.e., in the high dose region, will be important even for large PTVs.
From studies, it is also known that most secondary cancers develop within the treatment field or at the margins, reflecting a major contribution from the primary radiation, i.e., high doses (Gilbert et al. 2003). Hence, a smaller PTV potentially results in a decrease of second cancer risk (Zwahlen et al. 2016). Outside the treatment field, the risk of secondary cancer is mainly due to scattering. To provide a complete description of the cancer-induction risks for a specific RT technique, it is necessary to study the dose deposition produced by the different kinds of secondary radiation present during the treatment. Thus, in order to assess the doses deposited by scattered out-of-field radiation, Monte Carlo simulations can be performed. It because is expected that the second cancer risks estimation from Monte Carlo calculated dose distributions would be more representative.
Few population-based studies quantify organ-specific excesses, including lung, among large numbers of long-term survivors; address second cancer risk by age at HL diagnosis; provide estimates of absolute excess risk; or consider attained age, especially in the case of patients treated for childhood cancer.
In the literature, studies about secondary lung cancers are found that comprise patients treated for Hodgkin’s lymphoma over a broad range of ages, including pediatric patients. For pediatric HL, there has been little detailed documentation of secondary solid cancer at sites other than thyroid and breast. Adults, unlike children, often develop secondary lung cancer after treatment for HL (Hodgson et al. 2007; Ng et al. 2010). However, the EAR in children also increased, although the elevation is less compared to adults (Lin and Teitell 2005). The model parameters were obtained from the data fit in the study by Dores et al. (2002). Study of 32,591 patients, whose mean age at diagnosis HL was 37 years and mean follow-up time was 8 years (mean age 45 years). For secondary lung cancer, the EAR was 9.7 per 104 person-years. For patients aged ≤ 21 years at diagnosis of LH, the EAR of this secondary cancer was 0.7 per 104 person-years. For patients with a diagnosis of HL ≤ 20 years (18.2% of total patients) and attained age < 40 years, the EAR of this secondary cancer was 0.6 per 104 person-years. It was also observed that the EAR of secondary lung cancer increases as age at diagnosis of HL increases. Thus, the EAR (104 person-years) was 0.7 for children < 21 and 3.3, 8.2, 21.8, 44.6, and 12.3 for the age ranges of 21–30, 31–40, 41–50, 51–60, and ≥ 61 years. Bhatia et al. (2003) presented a report of the Late Effects Study Group cohort, for children with Hodgkin’s lymphoma aged 16 years or younger. For patients that developed a secondary lung cancer, a median age at diagnosis of HL was 11.6 years, a median age at diagnosis of subsequent neoplasm was 37.2 years, and a median time to subsequent neoplasm was 25.7 years. The EAR was 2 cases per 104 person-years. Metayer et al. (2000) quantified the long-term risk of second cancers among 5925 HL patients diagnosed before the age of 21 years, and who survived 1 or more years. The mean (median) age at HL diagnosis was 16 (17) years. Average duration of follow-up was 10.5 years. Solid tumors occurred an average of 16 years (median, 15 years; range, 1 to 45 years) after HL diagnosis at a mean age of 32 years. When considered the lung and respiratory system, second cancers, according the age at diagnosis of HL, the EAR were 3, 0.3, and 1 cases per 104 person-years for patients between 0–9 years, 10–16 years, and 17–20 years, respectively. Maule et al. (2007) quantified the risks of second malignant neoplasms in survivors of childhood leukemia and lymphoma using a large dataset from 13 population-based cancer registries that survived for at least one day. A total of 133 s malignant neoplasms (leukemias, Hodgkin’s lymphomas, and non-Hodgkin’s lymphomas) were observed in 16,540 patients, after a mean follow-up of 6.5 years. Mean age and follow-up time after lymphoma were 26.0 and 16.3 years, respectively. The EAR (for 105 person-years) of secondary lung neoplasm was 13.6, when the primary neoplasm was Hodgkin’s lymphoma. Our estimates of EAR for secondary lung cancer are in reasonable accordance with results of epidemiological studies. Since the estimated risks varied slightly for the variants of the mechanistic mode studied, any of them could be used without significant differences. However, knowing that the full model takes into account the effects of repopulation and tissue repair, we consider this model more realistic.
The model uses the information of how much volume of an organ receives a certain dose of radiation, represented in a DVH, and that basically depends on the prescribed dose and the number of fractions of treatment. Other parameters that intervene in the configuration of the treatment affect the final dose deposited, such as the energy of the beam, the number of fields, and the size of each field used.
One of the limitations of the mechanistic model is that when using data from epidemiological studies, it leads to errors in the correlation of risk and radiation dose, and therefore in the establishment of dose–response relationships. The strength of the model is that estimates can be established by considering risk-modifying factors, such as those related to age in radiation exposure and age at which risk is estimated.
Studies that estimate the risk of secondary neoplasms for radiation doses from exposure to imaging techniques, such as computed tomography, use a linear model, in which there is a linear relationship between risk and radiation dose. Yuasa et al. (2019) estimated that the risk of secondary lung neoplasm after four-dimensional cone-beam computed tomography for men and women was 7.3 and 10.7 cases per 106 person-years (Yuasa et al. 2019). Thus, the risk due to imaging techniques influence to a lesser extent compared to the treatment of primary cancer.
Finally, the mechanistic model, even considering that the dose–response relationships were obtained for patients with a broader age range (including pediatric patients) and high doses, allowed the estimation of the risk of secondary lung cancer using the dose distribution in the organ and considering modifying risk parameters, such as age at exposure and attained age, in this estimate.
Conclusion
The mechanistic model, based on radiobiological considerations and adjusted to epidemiological data to obtain the model parameters, allows estimating the individualized secondary risk due to radiotherapy treatments, using the dose distribution in the organ of interest. Here we use this model to estimate the risk of secondary lung neoplasm in pediatric patients treated for Hodgkin lymphoma.
From the dosimetric parameters, it is observed that some treatment plans present a worse coverage of the dose and lack of conformity; in this way, we recommend establishing quality guidelines of the treatment plans to improve these parameters since they will influence the radiation dose received by the lung. Thus, it is essential to implement modern pediatric radiotherapy techniques that can provide good target coverage while minimizing radiation doses to normal structures that may result in a reduction in the risk for radiation-induced second cancers.
To estimate the risk of secondary lung neoplasms in this study, three variants of the mechanistic model were used. The complete model takes into account the cell repopulation/repair effect, considering the ability to repopulate and repair from tissues, and the bell-shaped and plateau models, which are obtained from the complete model, considering whether the repopulation/repair effect is completely neglected or full considered. From these relationships, it can be observed that they differ mainly at high doses, where the complete and plateau models show the same behavior, but presenting a slightly higher risk in the case of the complete model. The same is the case with increasing the number of dose fractions. However, the risk estimate is altered by the use of risk modifiers, and these are the factors, in addition to the radiation dose, that affect the final estimate. The risk modifiers considered here were age at exposure and attained age, age at which we estimate the risk of developing secondary malignant neoplasm. These factors similarly influence the estimate. Thus, for early age at exposure, we have observed that the risk is lower compared to more advanced ages. In the case of the attained age, the risk increases as it increases, and the most significant increase being for the higher attained ages. These factors similarly influence the estimate. Thus, for early age at exposure, we have observed that the risk is lower compared to more advanced ages, and in the case of the attained age, the risk increases as it increases, the most significant increase being for higher attained ages. Therefore, any of these models can be used in estimating the risk of a secondary malignant neoplasm for pediatric patients, but since the complete model considers the ability of the tissues to repopulate and repair, we consider the best alternative for this purpose.
While the model used in this study produces results that are consistent with expectations, they were based solely on radiation dose, normal tissue volume, and patient age, and second cancer risk is not just a function of these factors. Second cancer risk also involves complex interactions of host, environmental, and non-radiation treatment factors. Epidemiological studies with long years of follow-up are necessary to establish the relationship between these factors and the risk of developing a second malignancy. This represents the main limitations of the model. The strengths are that it allows us to estimate risk using age-modifying factors, without having to wait years to observe actual risk. Thus, future work involves the incorporation of this methodology into a radiotherapy treatment planning system to serve as an additional tool for planning evaluation.
References
Bhatia S, Yasui Y, Robison LL, Birch JM, Bogue MK, Diller L, DeLaat C, Fossati-Bellani F, Morgan E, Oberlin O, Reaman G, Ruymann FB, Tersak J, Meadows AT. High risk of subsequent neoplasms continues with extended follow-up of childhood Hodgkin’s disease: report from the Late Effects Study Group. J Clin Oncol. 2003;21(23):4386–94.
Dores GM, Metayer C, Curtis RE, Lynch CF, Clarke EA, Glimelius B, Storm H, Pukkala E, van Leeuwen FE, Holowaty EJ, Andersson M, Wiklund T, Joensuu T, van’t Veer MB, Stovall M, Gospodarowicz M, Travis LB. Second malignant neoplasms among long-term survivors of Hodgkin’s disease: a population-based evaluation over 25 years. J Clin Oncol. 2002;20(16):3484–94.
Gilbert ES, Stovall M, Gospodarowicz M, Van Leeuwen FE, Andersson M, Glimelius B, Joensuu T, Lynch CF, Curtis RE, Holowaty E, Storm H, Pukkala E, van’t Veer MB, Fraumeni JF, Boice JD Jr, Clarke EA, Travis LB. Lung cancer after treatment for Hodgkin’s disease: focus on radiation effects. Radiat Res. 2003;159(2):161–73.
Gilbert ES. The impact of dosimetry uncertainties on dose-response analyses. Health Phys. 2009;97(5):487–92.
Hodgson DC, Gilbert ES, Dores GM, Schonfeld SJ, Lynch CF, Storm H, Hall P, Langmark F, Pukkala E, Andersson M, Kaijser M, Joensuu H, Fosså SD, Travis LB. Long-term solid cancer risk among 5-year survivors of Hodgkin’s lymphoma. J Clin Oncol. 2007;25(12):1489–97.
Lin HM, Teitell MA. Second malignancy after treatment of pediatric Hodgkin disease. J Pediatr Hematol Oncol. 2005;27(1):28–36.
Lorigan P, Radford J, Howell A, Thatcher N. Lung cancer after treatment for Hodgkin lymphoma: a systematic review. Lancet Oncol. 2005;6(10):773–9.
Ma C, Zhang W, Lu J, Wu L, Wu F, Huang B, Lin Y, Li D. Dosimetric comparison and evaluation of three radiotherapy techniques for use after modified radical mastectomy for locally advanced left-sided breast cancer. Sci Rep. 2015;5:12274.
Maule M, Scélo G, Pastore G, Brennan P, Hemminki K, Tracey E, Sankila R, Weiderpass E, Olsen JH, McBride ML, Brewster DH, Pompe-kirn V, Kliewer EV, Chia KS, Tonita JM, Martos C, Jonasson JG, Merletti F, Boffetta P. Risk of second malignant neoplasms after childhood leukemia and lymphoma: an international study. J Natl Cancer Inst. 2007;99(10):790–800.
Marks LB, Bentzen SM, Deasy JO, Kong FM, Bradley JD, Vogelius IS, El Naqa I, Hubbs JL, Lebesque JV, Timmerman RD, Martel MK, Jackson A. Radiation dose volume effects in the lung. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S70–6.
Metayer C, Lynch CF, Clarke EA, Glimelius B, Storm H, Pukkala E, Joensuu T, van Leeuwen FE, van’t Veer MB, Curtis RE, Holowaty EJ, Andersson M, Wiklund T, Gospodarowicz M, Travis LB. Second cancers among long-term survivors of Hodgkin’s disease diagnosed in childhood and adolescence. J Clin Oncol. 2000;18(12):2435–43.
Ng AK, Bernardo MV, Weller E, Backstrand K, Silver B, Marcus KC, Tarbell NJ, Stevenson MA, Friedberg JW, Mauch PM. Second malignancy after Hodgkin disease treated with radiation therapy with or without chemotherapy: long-term risks and risk factors. Blood. 2002;100(6):1989–96.
Ng AK, Kenney LB, Gilbert ES, Travis LB. Secondary malignancies across the age spectrum. Semin Radiat Oncol. 2010;20(1):67–78.
Nguyen J, Moteabbed M, Paganetti H. Assessment of uncertainties in radiation-induced cancer risk predictions at clinically relevant doses. Med Phys. 2015;42(1):81–9.
Paganetti H. Assessment of the risk for developing a second malignancy from scattered and secondary radiation in radiation therapy. Health Phys. 2012;103(5):652–61.
Schneider U, Zwahlen D, Ross D, Kaser-Hotz B. Estimation of radiation-induced cancer from three-dimensional dose distributions: concept of organ equivalent dose. Int J Radiat Biol Phys. 2005;61:1510–5.
Schneider U. Mechanistic model of radiation-induced cancer after fractionated radiotherapy using the linear-quadratic formula. Med Phys. 2009;36(4):1138–43.
Schneider U, Stipper A, Besserer J. Dose-response relationship for lung cancer induction at radiotherapy dose. Z Med Phys. 2010;20(3):206–14.
Schneider U, Sumila M, Robotka J. Site-specific dose-response relationships for cancer induction from the combined Japanese A-bomb and Hodgkin cohorts for doses relevant to radiotherapy. Theor Biol Med Model. 2011;8:27.
Shin N (2011) Modeling secondary cancer risk following paediatric radiotherapy: a comparison of intensity modulated proton therapy and photon therapy. Master’s Thesis. Department of Medical Physics, McGill University, Montreal. https://escholarship.mcgill.ca/concern/theses/0k225g20w.
Shuryak I, Hahnfeldt P, Hlatky L, Sachs RK, Brenner DJ. A new view of radiation-induced cancer:integrating short- and long-term processes. Part I: Approach. Radiat Environ Biophys. 2009;48:263–74.
Shuryak I, Sachs RK, Brenner DJ. A new view of radiation-induced cancer. Radiat Prot Dosim. 2011;143(2–4):358–64.
Sud A, Thomsen H, Sundquist K, Houlston RS, Hemminki K. Risk of second cancer in Hodgkin lymphoma survivors and influence of family history. J Clin Oncol. 2017;35(14):1584–90.
Swerdlow AJ, Barber JA, Hudson GV, Cunningham D, Gupta RK, Hancock BW, Horwich A, Lister TA, Linch DC. Risk of second malignancy after Hodgkin’s disease in a collaborative British cohort: the relation to age at treatment. J Clin Oncol. 2000;18(3):498–509.
Swerdlow AJ, Schoemaker MJ, Allerton R, Horwich A, Barber JA, Cunningham D, Lister TA, Rohatiner AZ, Vaughan Hudson G, Williams MV, Linch DC. Lung cancer after Hodgkin’s disease: a nested case-controlled study of the relation to treatment. J Clin Oncol. 2001;19(6):1610–8.
Travis LB, Gospodarowicz M, Curtis RE, Clarke EA, Andersson M, Glimelius B, Joensuu T, Lynch CF, van Leeuwen FE, Holowaty E, Storm H, Glimelius I, Pukkala E, Stovall M, Fraumeni JF Jr, Boice JD Jr, Gilbert E. Lung cancer following chemotherapy and radiotherapy for Hodgkin’s disease. J Natl Cancer Inst. 2002;94(3):182–92.
UNSCEAR (2012) Report of the United Nations Scientific Committee on the Effects of Atomic Radiation. Fifty-ninth session (21–25 May 2012). https://www.unscear.org/unscear/uploads/documents/unscear-reports/UNSCEAR_2012_Report.
van Leeuwen FE, Klokman WJ, Veer MB, Hagenbeek A, Krol AD, Vetter UA, Schaapveld M, van Heerde P, Burgers JM, Somers R, Aleman BM. Long term risk of second malignancy in survivors of Hodgkin disease treated during adolescence or young adulthood. J Clin Oncol. 2000;18(3):487–97.
Yuasa Y, Shiinoki T, Onizuka R, Fujimoto K. Estimation of effective imaging dose and excess absolute risk of secondary cancer incidence for four-dimensional cone-beam computed tomography acquisition. J Appl Clin Med Phys. 2019;20(11):57–68.
Zwahlen DR, Bischoff LI, Gruber G, Sumila M, Schneider U. Estimation of second cancer risk after radiotherapy for rectal cancer: comparison of 3D conformal radiotherapy and volumetric modulated arc therapy using different high dose fractionation schemes. Radiat Oncol. 2016;11(1):149.
Acknowledgements
The authors would like to acknowledge to Pedro Paulo Pereira Junior for her fundamental contribution to the development of this work.
Funding
This study recived the financial support of the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil (Grant number 141540/2014–1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Melgarejo, H.L., de Menezes, A.F., da Silva, L.P. et al. Estimating the risk of secondary lung cancer from radiotherapy for pediatric Hodgkin’s lymphoma patients using a mechanistic model. Res. Biomed. Eng. 38, 857–869 (2022). https://doi.org/10.1007/s42600-022-00231-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42600-022-00231-1