Abstract
Cyanobacteria are ancient photosynthetic organisms inhabitant of various habitats including arid and semi- arid water bodies. These are effective producers of various biologically active compounds that have been widely used in food, medicine, cosmetics and pharmaceutical industry. However, non-heterocystous cyanobacteria of arid and semi- arid water bodies are less explored for biotechnological applications. The aim of the present study was to investigate non-heterocystous filamentous cyanobacteria of freshwater bodies of semi-arid region for their pigment profile, phenolic contents and antioxidant properties. Our results revealed that among 8 cyanobacterial strains, D. tharense AT2015/7 and S. subsalsa AT2016/1 possessed higher amount of valuable pigments, phenolic contents and also showed good antioxidant properties. S. subsalsa AT2016/1 was the most promising cyanobacterial taxa for the production of phycocyanin when compared to so far studied non-heterocystous cyanobacteria inhabit aquatic habitats. In the LC-HRMS/MS analysis, 11 phenolic compounds were tentatively identified in D. tharense AT2015/7 and nine phenolic compounds detected in S. subsalsa AT2016/1. The present study revealed that cyanobacteria thriving in the semi-arid region could be promising candidates for the production of valuable pigments, phenolic compounds and could be a potential source of antioxidants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cyanobacteria thriving in arid and semi-arid reservoirs experience the changes in limnological parameters (low to high nutrients, minerals, pH, temperature, salinity, etc.) caused by an annual cycle of drought and rainy season (de Castro Medeiros et al. 2015). One-third land of the planet encompasses arid and semi-arid regions, where most of the tropical developing countries including Latin America, India and Southeast Asia are covered by semi-arid environment (Barbosa et al. 2012). In the past few years, water bodies of arid regions have drawn attention of some of the countries, including Australia, Africa and North America for its biological diversity (Stanley and Fisher 1992; King et al. 1996; Witham et al. 1998; Brendonck and Williams 2000). However, the water reservoirs of arid and semi-arid regions of Asia are less explored for its biological diversity as well as their potential to produce antioxidant molecules (Gopal 2003).
Oxidative stress has long been known in the biological system, arising as a result of an imbalance between oxidants (reactive oxygen and nitrogen species) and antioxidants, which ultimately causes improper metabolism and damage to the cellular system (Sies 1997). An increase in the levels of reactive oxygen and nitrogen species (ROS and RNS) during metabolism leads to incidence of diseases such as cancer, cardiovascular and neurological diseases (Araújo et al. 2016). In order to overcome the menacing effects of free radicals, antioxidant defence system possessing both enzymatic as well as non-enzymatic antioxidants could be introduced (Sharma et al. 2012). In this context, antioxidants derived from natural sources and the foods rich in antioxidants have markedly paved its way as a superfood over synthetic products for the prevention of oxidative-stress related diseases (Vaz et al. 2010; Agyei et al. 2015). Primarily, terrestrial plants are main source of commercially available natural antioxidants. However, in the current world’s scenario, algae have taken over the lead as a functional food that is commonly used in Asian and Western countries. Further, addition of algal constituents in conventional food can present as an attractive approach for producing healthy food products (Blagojević et al. 2018).
In recent decades, cyanobacteria has drawn tremendous attention owing to its ability to synthesise a variety of biologically active secondary metabolites with wide range of medicinal activities such as anti-proliferative, anti-inflammatory and anti-infective activities (Nunnery et al. 2010). The non-heterocystous cyanobacterium Arthrospira commercially known as Spirulina is the well-established genus for its beneficial compounds as well as proteins for alimentary or industrial use (Belay 2013). It is also known as a potential source of antioxidants that includes pigments (C-phycocyanin and carotenoids) as well as phenolic compounds (Kepekçi and Saygideger 2012). Phycobiliproteins, one of the unique photosynthetic pigment endowed by cyanobacteria have also presented as a major candidate for its application in biotechnology, food industry and medicine (Amchova et al. 2015; Dasgupta 2016). These are composed of three basic components, phycocyanin, allophycocyanin and phycoerythrin (Bennett and Bogobad 1973). Several non-heterocystous cyanobacteria were also studied for total phenolic compounds, antioxidant activity, and antibacterial activity (Prakash et al. 2011; Hossain et al. 2016). Kumar et al. (2016) reported that the nutritional value of non-heterocystous cyanobacteria Phormidium foveolarum, and Arthrospira platensis increased under UV-exposure in comparison to heterocystous cyanobacteria Nostoc muscorum. Thus, these attributes have made non-heterocystous cyanobacteria an emerging source of pigments, phenolic compounds and antioxidants for its application in medicine and food industry. Moreover, non-heterocystous cyanobacteria showed dominance over heterocystous cyanobacteria in nitrogenous aquatic system (Havens et al. 2003; Loza et al. 2014). Thus, owing to paucity of research towards the biotechnological potential of non-heterocystous cyanobacteria thriving in aquatic habitats of the semi-arid regions, the present study was designed to explore antioxidant properties of non-heterocystous cyanobacteria inhabit water bodies of the semi-arid regions of Rajasthan.
Materials and methods
Cyanobacterial strains and culture conditions
Eight filamentous non-heterocystous cyanobacteria were isolated from the water samples collected from different aquatic habitats of Ajmer district of Rajasthan, India and identification of cyanobacterial isolates was done based on conventional method using recent taxonomic literatures (Komárek and Anagnostidis 2005; Dadheech et al. 2012a, b; Heidari et al. 2018). The cyanobacterial strains AT2015/3, AT2015/5, AT2015/7, AT2016/2, AT2017/2, AT2016/9 and AT2016/21 were inoculated in 100 mL of BG-11 medium (Rippka et al. 1979) in 250 mL Erlenmeyer flasks, whereas cyanobacterial strain AT2016/1 was inoculated in 100 mL of SP media (Aiba and Ogawa 1977) in 250 mL Erlenmeyer flasks with initial OD of 0.1 for the biomass production. The inoculated cultures were incubated at 25 ± 2 °C under cool white fluorescence lamp (10 Wm− 2) with 14 h:10 h light–dark photoperiod. The cultures were hand-shaken three times in a day. After 15 days of incubation, the cyanobacterial biomass was harvested by centrifugation at 5000 g for 10 min at 4 °C and washed three times with autoclaved double distilled water. The wet biomass of cyanobacteria was freeze-dried at − 80 °C under vacuum in a lyophilizer (AF-5, India) and stored at − 20 °C for further use.
Pigments analysis
Extraction of chlorophyll-a and carotenoids were carried out by weighing 2 mg freeze-dried biomass of cyanobacteria into a microcentrifuge tube (1.5 mL) with the addition of 1 mL of cold methanol (99.9%) followed by homogenization using micropestle. The mixture was vortexed and incubated overnight at 4 °C in dark. After incubation, the pigment loaded extract was centrifuged at 9000 g for 10 min at 4 °C and supernatant collected into a clean Falcon tube (15 mL). The pellet was re-suspended in 1 mL of cold methanol (99.9%) and the suspended cells were completely disrupted using a probe sonicator (CPX 130 Cole-Parmer, USA) with 72 µm amplitude and 20 kHz frequency for 120 s (10 s “on” and 10 s “off” cycle) followed by centrifugation at 9000 g for 10 min at 4 °C. This step was repeated until transparent (without pigment) supernatant was obtained. The supernatants of all the steps were pooled together in a round bottom flask and concentrated using a rotary evaporator (Hei-VAP, Heidolph, Germany). The concentrated extract was re-dissolved in 1 mL of methanol (99.9%) and absorbance of extract was measured at 666 nm, 653 nm and 470 nm using a UV–Visible spectrophotometer (SECTROstarNano, BMG LABTECH, Germany) against methanol (99.9%) as a blank. Chlorophyll-a (Chl-a) and carotenoids (Car) contents were quantified according to the equation described by Wellburn (1994).
The total phycobiliproteins were extracted by weighing 2 mg freeze-dried biomass of cyanobacteria in 1.5 mL microcentrifuge tube and suspended in 1 mL of 0.1 M phosphate buffer (pH 7.2) followed by homogenization using micropestle. The cell suspension was frozen (at − 20 °C) and thawed at room temperature repeatedly three times followed by centrifugation at 9000 g for 10 min at 4 °C. After that, supernatant was collected into a clean centrifuge tube (15 mL). The pellet was again suspended in 1 mL of 0.1 M phosphate buffer (pH 7.2) and the suspended cells were completely disrupted using a probe sonicator (CPX 130 Cole-Parmer, USA) with 72 µm amplitude and 20 kHz frequency for 120 s (10 s “on” and 10 s “off” cycle) followed by centrifugation at 9000 g for 10 min at 4 °C. This step was repeated until transparent (without pigment) supernatant was obtained. The supernatant was pooled together into a Falcon tube (15 mL) and freeze-dried at − 80 °C under vacuum in a lyophilizer (AF-5, India). The freeze-dried extract was re-dissolved in 1 mL of 0.1 M phosphate buffer (pH 7.2). The absorbance of extract was measured at 620 nm, 652 nm and 562 nm using a UV–Visible spectrophotometer (SECTROstarNano, BMG LABTECH, Germany) against phosphate buffer as a blank. The phycobiliprotein concentration was determined using the equations as suggested by Bennett and Bogobad (1973).
Extract preparation for phenolic contents and antioxidant activity
Lyophilized biomass of each sample was extracted in methanol (99.9%) and water (10 mg of biomass added to 1 mL of solvent) by sonication at 72 µm amplitude and 20 kHz frequency for 3 min with a 10 s on/off cycle using a probe sonicator (CPX 130 Cole-Parmer, USA). After that, the cell fragments were separated by centrifugation at 10,000 g for 10 min and supernatants collected into clean Falcon tubes. The extraction procedure was repeated thrice and the supernatant pooled together and again centrifuged at 10,000 g for 15 min. The collected supernatant of methanol was dried using a rotatory evaporator (Hei-VAP, Heidolph, Germany) at 35 °C and water extract was lyophilized at − 80 °C under vacuum in a lyophilizer (AF-5, India) to obtain the cell-free dried extract. The dried extracts were used for estimation of total phenolic compounds, and antioxidant activity was measured by two different methods namely 2,2,1-diphenyl-1-picrylhydrazyl (DPPH) and Ferric reducing antioxidant power (FRAP).
Total phenolic content
Folin-Ciocalteu (F–C) assay was performed for determining the total phenolic content (TPC) using 96-well microplates (Zhang et al. 2006). Herein, milli Q water (25 µL) and sample or standard (25 µL) added into well of microplate, followed by the addition of F–C reagent (25 µL). Thereafter, the mixture was incubated for 6 min at room temperature and 100 µL of Na2CO3 (7.5% w/v) was added to it. The reaction mixture was further incubated in dark condition for 90 min. The absorbance was measured spectrophotometrically at 765 nm (SECTROstarNano, BMG LABTECH, Germany). A calibration curve was plotted using galiic acid at a concentration of 20–100 µg mL− 1 and phenolic compounds were expressed as mg gallic acid equivalents (GAE) g− 1.
Antioxidant activity
DPPH assay
DPPH assay was performed in order to evaluate the free radical scavenging efficacy of cyanobacterial extracts (Espín et al. 2000). The experiment was executed by adding 20 μL of extract (12.5–800 μg mL− 1) and 180 μL of 100 μM DPPH reagent to the 96-well plate. For reagent blank, the solvent was added to the DPPH reagent instead of extract. Similarly, methanol and extract were mixed in case of matrix blank. The samples with DPPH reagent including reagent blank and matrix blank were measured at 517 nm using a spectrophotometric microplate reader (SECTROstarNano, BMG LABTECH, Germany) after incubating for 30 min in the dark. The antioxidant activity was then expressed in terms of IC50 of the extract using non-linear regression analysis. Further, ascorbic acid was taken as a positive control in support of the aforementioned experiment.
FRAP assay
The FRAP reagent was prepared by mixing a definite proportion of TPTZ (2,4,6-tripyridyl-s-triazine), aqueous FeCl3 and acetate buffer (pH 3.6) in 1:1:10 ratio (Benzie and Strain 1996). Freshly prepared 200 µL of FRAP reagent (warmed to 37 °C) was added to the 20 µL of extract and incubated for 6 min at room temperature. The sample mixture was then measured at 593 nm using a spectrophotometric microplate reader (SECTROstarNano, BMG LABTECH, Germany). A standard curve was plotted using ascorbic acid and the reducing power displayed in terms of ascorbic acid equivalents (AAE) per gram of dry weight. All the results were shown as mean ± standard deviations (SDs) of triplicate experiments.
LC-HRMS/MS analysis
Liquid chromatography high resolution mass spectrometry (LC-HRMS/MS) analysis of the extracted phenolic compounds was performed on Xevo G2-S Qtof (Waters, USA). Chromatographic separation was attained in an ACQUITY UPLC BEH C18 column (1.7 µm, 2.1 × 50 mm, Waters, USA). The flow rate was 250 μL min− 1, and a volume of 10 μL of the sample injected into the column. The mobile phase was composed of solvent A (water with 0.1% formic acid) and solvent B (methanol with 0.1% formic acid). The mobile phase gradient was programmed as follows: 98–90% A (v/v) from 0 to 5.0 min, 90–70% A (v/v) from 5.0–10.0 min, 70–98% A (v/v) from 10.0–15.0 min. The ionization parameters were as follows: capillary voltage 4000 V, endplate voltage − 500 V; nebulizing gas of nitrogen at 35.0 p.s.i.; drying gas of 10 L min− 1 nitrogen at 350 °C and mass analyzer scanned from 50 to 1500 m/z (mass to charge ratio). The fragmentation amplitude was set to 1.0 V. Mass spectra was simultaneously acquired using electrospray ionization in the positive mode. The phenolic compounds were tentatively identified by comparing MS data of detected peaks with freely available mass spectra of pure reference compounds at MassBank of North America-MoNA (https://mona.fiehnlab.ucdavis.edu) and the MS data reported in the literature.
Statistical analysis
All the results were expressed as mean ± standard deviations for triplicate experiments. Tukey’s test of One-Way ANOVA was used to analyze the significant differences between the means. Non-linear regression analysis was implicated for the determination of IC50 values. Pearson correlation coefficient was calculated to find the extent of correlation between antioxidant activity and total phenolic compounds. Here, p-value < 0.05 was considered as statistically significant. All the statistical analysis was done using GraphPad Prism 6 (GraphPad Software, San Diego, California).
Results and discussion
Identification of cyanobacterial taxa
Based on the phenotypic features, four cyanobacterial isolates (AT2015/3, AT2015/7, AT2016/2 and AT2017/2) were assigned to the species Desertifilum tharense. Two isolates AT2016/1 and AT2016/9 belonged to the species Spirulina subsalsa and Haloleptolyngbya alcalis, respectively, while two other isolates AT2015/5 and AT2015/10 were identified as Leptolyngbya sp. and Laspinema sp., respectively (Table 1). Microphotographs of identified cyanobacterial isolates are given in the supplementary file.
Biomass production and pigment analysis
The cyanobacteria which can produce higher biomass and bioresource for the production of valuable products are in great interest for the industrial purposes. In this study, the biomass yield of 15 days old culture of the tested cyanobacterial strains ranged from 283 to 724 mg L− 1. Among the cyanobacterial strains, Desertifilum tharense AT2015/7 and AT2016/2, Spirulina subsalsa AT2016/1 and Haloleptolyngbya alcalis AT2016/9 were found to produce higher biomass as compared to others (Table 2). In the earlier study, non-heterocystous filamentous cyanobacteria belonging to the genus Lyngbya and Limnothrix were studied for the production of biomass, where strain 15–2 of Lyngbya genus was found to yield 600 mg L− 1 of biomass and four strains of the genus Limnothrix were found to produce biomass in the range from 500–1200 mg L− 1 (Gantar et al. 2012). In the present study four cyanobacteria belonging to the genus Dersertifilum were observed to produce a different range of biomass yield (335–548 mg L− 1). Recently, seven species of cyanobacteria were evaluated for high biomass production (Patel et al. 2018). Among the seven species, three were non-heterocystous cyanobacteria i.e., Oscillatoria sp., Phormidium sp. and Lyngbya sp. where Phormidium sp. gave maximum yield (1070 ± 10.1 mg L− 1) of biomass in 19-days-old culture (Patel et al. 2018). In our study, H. alcalis AT2016/9 yielded maximum biomass equal to 724 ± 17 mg L− 1 from 15-days-old culture (Table 2). The biomass yield of tested cyanobacterial strains could be enhanced by changing culture conditions (Silva et al. 1994; Clark et al. 2018).
Researchers have constantly been screening cyanobacterial strains for their valuable pigments such as Chl-a, carotenoids and phycobiliprotein. In this study, dry biomass of tested cyanobacteria was used to determine their pigment profile. Amongst all the strains, a significant difference was observed in the amount of Chl-a, carotenoids and total phycobiliproteins content (Table 2). The maximum amount of Chl-a (15 ± 0.36 µg mg−1) was observed in D. tharense AT2015/7. S. subsalsa AT2016/1 possessed a higher amount of total carotenoids (4.7 ± 0.13 µg mg−1) as well as total phycobiliproteins (251 ± 12.5 mg g−1) as depicted in Table 2. In our previous study, among six cyanobacteria, the strain PD2001/4 of cyanobacteria D. tharense was found to possess maximum total phycobiliproteins (113.1 mg g−1) (Tomer et al. 2018). The genus Arthrospira (non-heterocystous) is known as an excellent source of phycocyanin (PC) (Pagels et al. 2019). Recently, Arthrospira plentesis IFRPD 1182 was studied for the production of PC that showed an yield of 103 ± 9.23 mg g−1 PC content (Pan-utai and Iamtham 2019). However, in our study, maximum PC (192 ± 10.01 mg g−1) was observed in S. subsalsa AT2016/1 (Fig. 1) that is 1.8 times more than the earlier reported PC in Arthrospira plentesis IFRPD 1182. Hence, S. subsalsa AT2016/1 could be considered as a promising cyanobacterial strain for the production of natural PC.
Total phenolic content (TPC)
Phenolic compounds are known to play crucial role in antioxidant activity as they can donate hydrogen atom or electron in order to form a stable complex. In this study, two solvent systems (methanol and water) were used for the extraction of phenolic compounds. The methanolic extract of tested cyanobacterial strains was observed to have TPC in the range from 10.8 ± 0.7 to 21.2 ± 1.8 µg GAE mg− 1, whereas water extracts possessed TPC in the range from 14.3 ± 1.4 to 28.6 ± 1.3 µg GAE mg− 1 (Table 2). Earlier, 12 cyanobacterial strains were tested for the TPC in three different solvents, water fraction was found to possess TPC in range from 0.3 ± 0.02 to 19.15 ± 0.04 µg GAE mg− 1, while hexane and ethyl acetate fraction were observed to have 0.02 ± 0.0002 to 2.11 ± 0.11 µg GAE mg− 1 and 0.02 ± 0.006 to 4.47 ± 0.02 µg GAE mg− 1 respectively (Hajimahmoodi et al. 2010). Recently, four cyanobacterial strains were isolated from a stressed aquatic system and their TPC was in the range from 6.25 ± 0.12 to 13.58 ± 0.17 µg GAE mg− 1 (Badr et al. 2019). In our study, amongst 8 cyanobacterial strains, the TPC was found to be higher in water extract of D. tharense AT2015/7 and S. subsalsa AT2016/1 in comparison to its methanolic extracts (Table 2). However, these results were also in agreement with the earlier studies where water fraction was found to possess high TPC (Hajimahmoodi et al. 2010; Machu et al. 2015). The lowest phenolic content was observed in the methanolic as well as aqueous extract of D. tharense AT2016/2 (10.8 ± 0.7 and 14.3 ± 1.4 µg GAE mg− 1 respectively). The variability in the TPC can be well attributed to several governing factors such as the type of strain, geographical origin, physiological and environmental variations (Marinho-Soriano et al. 2006). Further, another most important factor is the extraction procedure used, that plays a decisive role in obtaining TPC of any cyanobacteria. Hence, extraction with water showed higher content of phenolic compounds from non-heterocystous filamentous cyanobacteria isolated from aquatic bodies of the semi-arid region.
In vitro antioxidant activity
Antioxidant potential of cyanobacteria is commonly determined by DPPH and FRAP assay (Babić et al. 2016; Blagojević et al. 2018). In this study, DPPH radical scavenging activities of both extracts (methanol and water) of cyanobacteria were tested against the DPPH radicals. For this purpose, an antioxidant assay was performed with different concentrations of extracts in the reaction. The IC50 values of DPPH were observed to be different among all the tested cyanobacteria (Table 3). The S. subsalsa AT2016/1 and D. tharense AT2015/7 showed most effective scavenging activity (64.3 ± 2.7 and 67.2 ± 3.6 µg mL− 1, respectively), whereas the strains Laspinema sp. AT2015/10 exhibited least effective scavenging activity (347 ± 19.4 µg mL− 1) in the water extract. However, the methanolic extract of S. subsalsa AT2016/1 also showed remarkable scavenging activity (118.4 ± 5.6 µg mL− 1) and Leptolyngbya sp. AT2015/5 displayed poor scavenging activity (333.8 ± 7.2 µg mL−1). Two non-heterocystous cyanobacteria Oscillatoria M2 and Phormidium M1 were found to have IC50 values equal to 93.25 ± 8.63 and 47.62 ± 7.59 µg mL− 1, respectively (Babić et al. 2016). Similarly, non-heterocystous cyanobacteria belonging to the genus Arthrospira and Leptolyngbya were studied for the radical scavenging activity where IC50 for the strain S1 and S2 of Arthropsira was 120 µg mL− 1 and 110 µg mL− 1 respectively, whereas Leptolyngbya sp. showed scavenging activity (22.94 ± 0.47%) at 50 µg mL− 1 (Blagojević et al. 2018; Badr et al. 2019). Hence, S. subsalsa AT2016/1 and D. tharense AT2015/7 could be more potent as antioxidant. Interestingly, it was also noted that the scavenging activity was strain-specific as well as dependent on the solvent used for extraction as some of the strains showed good scavenging activity in water extract, whereas others showed in methanolic extract (Table 3). These results were in concordance to earlier study where antioxidant activity by DPPH was found highest in water extract of some microalgae while other strains showed good scavenging in ethyl acetate and hexane extract (Hajimahmoodi et al. 2010). However, phenolic compounds were found maximum in water extract of all the strains in comparison to methanolic extract except Laspinema sp. AT2015/10 (Table 2). Thus, the results indicate that the scavenging activity was also governed by other compounds present in the extracts with phenolic compounds. Promisingly, cyanobacteria thriving in aquatic habitats of the semi-arid regions could be good candidates for scavenging the free radicals.
In case of FRAP assay, the antioxidant activity of the prepared extracts was tested by assessing the reduction of ferric to coloured ferrous-tripyridyltriazine complex (Benzie and Strain 1996). It is an inexpensive and simple approach with high reproducibility to determine the reducing power of biological extract along with various fruits and vegetables (Wong et al. 2006). The maximum reducing power of methanolic extract was observed for the D. tharense AT2015/7 (56.5 ± 2.6 µg AAE mg− 1). Similarly, FRAP activity of water extract was observed to be higher in D. tharense AT2015/7 (21.7 ± 0.5 µg AAE mg− 1) and Leptolyngbya sp. AT2015/5 (22.8 ± 1.6 µg AAE mg− 1) (Table 3). However, in one of the studies, highest FRAP activity was observed in the ethanolic extract of a cyanobacterium Phormidium M1 (22.48 ± 2.18 µg AAE mg− 1) (Babić et al. 2016) and Arthrospira S1 (21.01 ± 1.66 µg AAE mg− 1) (Blagojević et al. 2018). In our study, D. tharense AT2015/7 was found to have maximum reducing power with the methanolic extract. It could be due to the presence of majority of total carotenoids and phenolic compounds in methanolic extract of D. tharense AT2015/7 (Table 2).
The degree of correlation was evaluated with the phenolic compounds following the DPPH and FRAP assays. Herein, Pearson correlation analysis showed significant relation of water extract with DPPH assay (r = 0.7447, p = 0.034) (Table 4). However, TPC in methanolic extract of tested cyanobacterial strains did not show a significant relationship with DPPH activity. Similarly, in case of FRAP assay, no significant correlation was observed between total phenolics and antioxidant activity in both the extracts, which showed the probability of other compounds playing a major role in FRAP activity. Machu et al. (2015) reported that the water-soluble compounds present in edible algal products showed a correlation with the antioxidant activity. However, Blagojević et al. (2018) reported that TPC extracted in aqueous ethanol did not show a significant correlation with DPPH acitivity. Thus, our results depicted that phenolic compounds extracted in water showed good correlation with the radical scavenging activity. The DPPH activity in methanolic and FRAP activity in the both extracts may be majorly governed by some other compounds (Babić et al. 2016; Blagojević et al. 2018). Based on the maximum phenolic contents and good antioxidant activity in water extract, S. subsalsa AT2016/1 and D. tharense AT2015/7 were selected for the characterization of phenolic compounds.
LC-HRMS/MS analysis of phenolic compounds
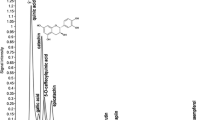
A total of 11 phenolic compounds were tentatively identified in D. tharense AT2015/7, while 9 phenolic compounds were identified in S. subsalsa AT2016/1 based on the protonated molecular ion m/z and their MS/MS fragmentation (Table 5). The phenolic compounds namely p-coumaroylquinic acid, resveratrol 3-O-glucoside, vanillin, 4-hydroxyphenylacetic acid, caffeic acid ethyl ester, 3-sinapoylquinic acid, ellagic acid glucoside, rosmarinic acid, 4-sinapoylquinic acid, ferulic acid 4-O-glucoside, and resveratrol were detected in D. tharense AT2015/7. Whereas, S. subsalsa AT2016/1 showed the presence of compounds including chlorogenic acid, 4-hydroxybenzoic acid 4-O-glucoside, caffeic acid ethyl ester, rosmadial, gallic acid ethyl ester, gallic acid, ellagic acid, vanillic acid and caffeoyl tartaric acid. Amongst all the phenolic compounds, caffeic acid ethyl ester was found to be common in both strains (D. tharense AT2015/7 and S. subsalsa AT2016/1). It can be noted that most of the phenolic compounds reported in the current study have also been found previously in different taxa of cyanobacteria (Machu et al. 2015; Babić et al. 2016; Singh et al. 2017; Blagojević et al. 2018) except some of the compounds such as ellagic acid, rosmadial, resveratrol 3-O-glucoside, rosmarinic acid, 4-sinapoylquinic acid, and resveratrol. Thus, D. tharense AT2015/7 and S. subsalsa could prove to be a promising source of diverse phenolic compounds.
Conclusions
Our study reported bioprospecting potential of non-heterocystous cyanobacteria isolated from freshwater bodies of semi-arid region. Cyanobacterial strains D. tharense AT2015/7 and S. subsalsa AT2016/1 were found to be promising candidates in terms of valuable pigments, phenolic contents and their antioxidant properties. The phycocyanin pigment-producing capacity of S. subsalsa AT2016/1 indicates its putative use as food colorant, which can be further scaled up for large scale production. Furthermore, statistical analysis showed significant correlation between phenolic compounds extracted in water and free radical scavenging activity. In addition, LC-HRMS/MS analysis revealed that D tharense AT2015/7 and S. subsalsa AT2016/1 possessed diverse phenolic compounds.
References
Agyei D, Danquah MK, Sarethy IP, Pan S (2015) Antioxidative peptides derived from food proteins. In: Rani V, Yadav U (eds) Free Radicals in human health and disease. Springer, New Delhi, pp 417–430
Aiba S, Ogawa T (1977) Assessment of growth yield of a blue–green alga, Spirulina platensis, in axenic and continuous culture. J Gen Microbiol 102(1):179–182
Amchova P, Kotolova H, Ruda-Kucerova J (2015) Health safety issues of synthetic food colorants. Regul Toxicol Pharmacol 73(3):914–922
Babić O, Kovač D, Rašeta M et al (2016) Evaluation of antioxidant activity and phenolic profile of filamentous terrestrial cyanobacterial strains isolated from forest ecosystem. J Appl Phycol 28(4):2333–2342
Badr OA, El-Shawaf II, El-Garhy HA et al (2019) Antioxidant activity and phycoremediation ability of four cyanobacterial isolates obtained from a stressed aquatic system. Mol Phylogenet Evol 134:300–310
Barbosa JEDL, Medeiros ESF, Brasil J et al (2012) Aquatic systems in semi-arid Brazil: limnology and management. Acta Limnol Bras 24(1):103–118
Belay A (2013) Biology and industrial production of Arthrospira (Spirulina). In: Richmond A, HU Q (eds) Handbook of Microalgal Culture: Applied Phycology and Biotechnology: Second Edition. Blackwell, Oxford, pp 339–358
Bennett A, Bogobad L (1973) Complementary chromatic adaptation in a filamentous blue-green alga. J Cell Biol 58(2):419–435
Benzie IFF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239(1):70–76
Blagojević D, Babić O, Rašeta M et al (2018) Antioxidant activity and phenolic profile in filamentous cyanobacteria: the impact of nitrogen. J Appl Phycol 30(4):2337–2346
Brendonck L, Williams WD (2000) Biodiversity in wetlanands of dry regions (Drylands). In: Gopal B, Junk WJ, Davis JA (eds) Biodiveristy in wetlands: Assessment, function and conservation. Backhuys, Leiden, pp 181–194
Clark RL, McGinley LL, Purdy HM et al (2018) Light-optimized growth of cyanobacterial cultures: growth phases and productivity of biomass and secreted molecules in light-limited batch growth. Metab Eng 47:230–242
Dadheech PK, Abed RMM, Mahmoud H et al (2012a) Polyphasic characterization of cyanobacteria isolated from desert crusts, and the description of Desertifilum tharense gen. et sp. nov. (Oscillatoriales). Phycologia 51(3):260–270
Dadheech PK, Mahmoud H, Kotut K, Krienitz L (2012b) Haloleptolyngbya alcalis gen. et sp. nov., a new filamentous cyanobacterium from the soda lake Nakuru, Kenya. Hydrobiologia 691(1):269–283
Dasgupta CN (2016) Algae as a source of phycocyanin and other industrially important pigments. In: Das D (ed) Algal biorefinery: an integrated approach. Springer, Cham, pp 253–276
de Araújo RFF, Martins DBG, Borba MACSM (2016) Oxidative stress and disease. In: Morales-Gonzalez JA, Madrigal-Santillan EO (eds) A master regulator of oxidative stress—the transcription factor Nrf2. IntechOpen, London
de Castro ML, Mattos A, Lürling M, Becker V (2015) Is the future blue-green or brown? The effects of extreme events on phytoplankton dynamics in a semi-arid man-made lake. Aquat Ecol 49(3):293–307
Espín JC, Soler-Rivas C, Wichers HJ (2000) Characterization of the total free radical scavenger capacity of vegetable oils and oil fractions using 2,2-diphenyl-1-picrylhydrazyl radical. J Agric Food Chem 48(3):648–656
Gantar M, Simović D, Djilas S et al (2012) Isolation, characterization and antioxidative activity of C-phycocyanin from Limnothrix sp. strain 37-2-1. J Biotechnol 159(1–2):21–26
Gopal B (2003) Aquatic biodiversity in arid and semi-arid zones of Asia and water management. In: Lemons J, Victor R, Schaffer D (eds) Conserving Biodiversity in Arid Regions. Springer, Boston, pp 199–215
Hajimahmoodi M, Faramarzi MA, Mohammadi N et al (2010) Evaluation of antioxidant properties and total phenolic contents of some strains of microalgae. J Appl Phycol 22(1):43–50
Havens KE, James RT, East TL, Smith VH (2003) N: P ratios, light limitation, and cyanobacterial dominance in a subtropical lake impacted by non-point source nutrient pollution. Environ Pollut 122(3):379–390
Heidari F, Zima J, Riahi H, Hauer T (2018) New simple trichal cyanobacterial taxa isolated from radioactive thermal springs. Fottea 18(2):137–149
Hossain MF, Ratnayake RR, Meerajini K, Wasantha Kumara KL (2016) Antioxidant properties in some selected cyanobacteria isolated from fresh water bodies of Sri Lanka. Food Sci Nutr 4(5):753–758
Kepekçi RA, Saygideger SD (2012) Enhancement of phenolic compound production in Spirulina platensis by two-step batch mode cultivation. J Appl Phycol 24(4):897–905
King JL, Simovich MA, Brusca RC (1996) Species richness, endemism and ecology of crustacean assemblages in Northern California vernal pools. Hydrobiologia 328(2):85–116
Komárek J, Anagnostidis K (2005) Süßwasserflora von Mitteleuropa, bd. 19/2: Cyanoprokaryota: Oscillatoriales. Spektrum Akademischer Verlag, München
Kumar J, Parihar P, Singh R et al (2016) UV-B induces biomass production and nonenzymatic antioxidant compounds in three cyanobacteria. J Appl Phycol 28(1):131–140
Loza V, Perona E, Mateo P (2014) Specific responses to nitrogen and phosphorus enrichment in cyanobacteria: factors influencing changes in species dominance along eutrophic gradients. Water Res 48(1):622–631
Machu L, Misurcova L, Ambrozova JV et al (2015) Phenolic content and antioxidant capacity in algal food products. Molecules 20(1):1118–1133
Marinho-Soriano E, Fonseca PC, Carneiro MAA, Moreira WSC (2006) Seasonal variation in the chemical composition of two tropical seaweeds. Bioresour Technol 97(18):2402–2406
Nunnery JK, Mevers E, Gerwick WH (2010) Biologically active secondary metabolites from marine cyanobacteria. Curr Opin Biotechnol 21:787–793
Pagels F, Guedes AC, Amaro HM et al (2019) Phycobiliproteins from cyanobacteria: chemistry and biotechnological applications. Biotechnol Adv 37:422–443
Pan-utai W, Iamtham S (2019) Extraction, purification and antioxidant activity of phycobiliprotein from Arthrospira platensis. Process Biochem 82:189–198
Patel VK, Sundaram S, Patel AK, Kalra A (2018) Characterization of seven species of cyanobacteria for high-quality biomass production. Arab J Sci Eng 43(1):109–121
Prakash JW, Johnson M, Jeeva S (2011) Antimicrobial activity of certain fresh water microalgae from Thamirabarani River, Tamil Nadu, South India. Asian Pac J Trop Biomed 1(2):S170–S173
Rippka R, Deruelles J, Waterbury JB et al (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology 111(1):1–61
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot 2012:1–26
Sies H (1997) Physiological society symposium: Impaired endothelial and smooth muscle cell function in oxidative stress. Exp Physiol 82:291–295
Silva HJ, Italiano MC, Ferrari SG (1994) Improved biomass production of cyanobacteria by reutilization of the culture medium. Biotechnol Tech 8(12):889–894
Singh DP, Prabha R, Verma S et al (2017) Antioxidant properties and polyphenolic content in terrestrial cyanobacteria. 3 Biotech 7(2):134
Stanley EH, Fisher SG (1992) Aquatic ecosystems in semi-arid regions: implications for resource management. In: N.H.R.I. Symposium Series 7, Environment Canada. The Institute, pp 271–280
Tomer AK, Neelam DK, Dadheech PK (2018) Pigments profiling of non-heterocystous filamentous cyanobacterial taxa (oscillatoriales) inhabited in biological crusts and soda lake. Vegetos 31(1):43–50
Vaz JA, Heleno SA, Martins A et al (2010) Wild mushrooms Clitocybe alexandri and Lepista inversa: in vitro antioxidant activity and growth inhibition of human tumour cell lines. Food Chem Toxicol 48(10):2881–2884
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144(3):307–313
Witham CW, Bauder ET, Belk D, et al (1998) Ecology, conservation, and management of vernal pool ecosystems: proceedings from the 1996 california native plant society conference. California Native Plant Society
Wong CC, Bin LH, Cheng KW, Chen F (2006) A systematic survey of antioxidant activity of 30 Chinese medicinal plants using the ferric reducing antioxidant power assay. Food Chem 97(4):705–711
Zhang Q, Zhang J, Shen J et al (2006) A simple 96-well microplate method for estimation of total polyphenol content in seaweeds. J Appl Phycol 18(3–5):445–450
Acknowledgements
We are thankful to the Department of Microbiology, Central University of Rajasthan for providing necessary facilities and Material Research Centre, MNIT, Jaipur for providing LC-HRMS/MS facility. This work was financially supported by the Department of Science and Technology, Rajasthan (P.7(3) DST/BTR&D/EAC/2018/3158). Authors are thankful to Dr. Kiran Kumar Tejavath for providing Elisa Reader facility and also Dr. Tarun Kumar Bhatt for providing Sonicator facility.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tomer, A.K., Dadheech, P.K. Bioprospecting antioxidants in some non-heterocystous filamentous cyanobacteria inhabit water bodies of semi-arid Rajasthan in India. Vegetos 33, 601–609 (2020). https://doi.org/10.1007/s42535-020-00147-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42535-020-00147-0