Abstract
The leaching processes and mechanisms of weathered crust elution-deposited rare earth (RE) ores with ammonium sulfate were investigated for their high efficiency and low consumption exploitation. The leaching process is a typical reversible heterogeneous ion exchange reaction in liquid–solid systems. The raw ore and leached tailings were analyzed by SEM and FTIR. Results indicated that no dissolution or structural changes of the ores occurred during the leaching process. The migrations of RE3+, Al3+, and \( {\mathrm{NH}}_4^{+} \) in the leaching process were studied under various liquid–solid ratios. With increasing liquid–solid ratio, the content of \( {\mathrm{NH}}_4^{+} \) adsorbed on clay mineral by physical effect decreased, \( {\mathrm{NH}}_4^{+} \) adsorbed by ion-exchange increased, and the content of exchanged RE and Al increased until reaching equilibrium. The adsorption capacity of the RE ores for \( {\mathrm{NH}}_4^{+} \) remained about 0.61–0.63 mg/g. It was difficult to desorb \( {\mathrm{NH}}_4^{+} \) from RE ores with washing water alone. The leaching mechanism of Al was the same as RE, but the leaching of Al slightly lagged behind RE. At the optimal liquid–solid ratio of 0.4:1, the leaching efficiencies of RE and Al were 95.51% and 90.99%, respectively, and 56.65% of \( {\mathrm{NH}}_4^{+} \) remained in the leachate. After impurity removal and RE precipitation, the leachate can be reused by replenishing fresh leaching agent. The aim of this study is to enhance the leaching process of RE, restrain the leaching of impurities, and decrease the consumption of leaching agents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
With the rapid development of advanced technology, the demand for rare earth (RE) for the international market is quickly growing, especially the demand for middle and heavy RE [1]. Almost 80% of the middle and heavy RE exists in weathered crust elution-deposited RE ore, also called ion-adsorbed RE ore [2]. Owing to its great commercial value, exploitation of weathered crust elution-deposited RE ore has attracted great attention. The RE in the weathered crust elution-deposited RE ore mainly exists as hydrated or hydroxyl hydrated ions and are adsorbed on the clay minerals [3, 4]. Because of the specific occurrence states, RE can be exchanged easily from the weathered crust elution-deposited RE ore into liquid phase with an electrolyte solution through ion exchange. Then RE can be recovered from the leachate using a precipitator—ammonium bicarbonate [5].
During the past 60 years, instead of sodium chloride, ammonium sulfate became the most popular leaching agent due to its high leaching efficiency of RE and low environmental pollution [6]. The leaching technology of the weathered crust elution-deposited RE ore has also been innovated from barrel/bath leaching to heap leaching, and then to in situ leaching [7]. Currently, in situ leaching has serval advantages, including good protection of the surrounding environmental of the mine, low labor intensity, etc., which makes it the main leaching technology for RE recovery [8]. As shown in Fig. 1, the leaching agent is injected into the RE ore body through the injection holes and slowly permeates from top to bottom. During this process, the ammonium ion (\( {\mathrm{NH}}_4^{+} \)) in the leaching agent will exchange with the RE ions (RE3+) on the surface of the ore particle, and RE3+ will transfer into liquid phase from the RE ore [9]. Besides RE, the impurity ions, such as Al3+, Mg2+, K+, Ca2+, etc., exist as ion-exchangeable phase can also be exchanged into liquid phase by \( {\mathrm{NH}}_4^{+} \) [10]. The leachate containing RE and impurities is collected from the deflector pipe at the bottom of the RE ores.
However, in situ leaching technology still has some drawbacks, such as low utilization rate of RE resource, high content of impurities in the leachate, large consumption of leaching agent, etc. [11]. In order to solve the above problems, many researchers studied the theoretical basis and production practice of the weathered crust elution-deposited RE ore. Novel leaching agents such as magnesium sulfate [12], organic acids [13], aluminum sulfate [14], carboxymethyl sesbania gum [15], etc. were selected to replace the traditional leaching agent, ammonium sulfate. The optimization of the leaching technology was also tested [11, 16]. However, it is still difficult to apply these novel leaching agents widely in actual production due to their high cost, introduction of new impurities and pollutants, or the tough treatment of leachate. Recently, there has been a stagnation of leaching technology development. Therefore, research on the leaching process and mechanism of weathered crust elution-deposited RE ore is proposed in this study. It will strengthen the theoretical basis of weathered crust elution-deposited RE ore and provide theoretical guidance for its high efficiency and low consumption exploitation.
The column leaching process was used to simulate the in situ leaching process. Al accounts for 35–75% of the total amount of impurities in the leachate and is the main impurity [17]; therefore, it was chosen as the investigative impurity ion. The leaching chemical basis of RE and Al from the weathered crust elution-deposited RE ore was discussed in detail. The raw ore and leached tailings were analyzed using SEM and FTIR to reveal the leaching mechanism of RE and impurity. The migrations of RE3+, Al3+, and \( {\mathrm{NH}}_4^{+} \) in the leaching process were studied in order to enhance the leaching process of RE, reduce the leaching of impurities, and decrease the consumption of leaching agents. The effects of liquid–solid ratio on the migrations of RE3+, Al3+, and \( {\mathrm{NH}}_4^{+} \) were also investigated to further explore the leaching mechanism. Moreover, the distribution of \( {\mathrm{NH}}_4^{+} \) in the leaching process was calculated. This study intends to provide guidance for the efficient utilization of the leaching agent and decrease ammonia nitrogen pollution.
2 Experimental
2.1 Materials
The experimental RE ore sample is original RE ore collected from Dingnan (DN) County in Jiangxi Province in southern China. The RE ore sample was mixed thoroughly and dried in a drying oven at 70 °C for 8 h.
All chemicals in this study were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China) of analytical grade. The deionized water used was produced by a specific system of water quality.
2.2 Column Leaching Experiments
In each column leaching experiment, 250 g of the RE ore sample was packed uniformly in a glass leaching column with an inner diameter of 45 mm and a column length of 500 mm. A sand core filter plate was fixed to the bottom of the leaching column to retain the soil particles and double layers of filter papers were placed on the top of the RE ore sample to resist preferential flow. The leaching agent (0.2 mol/L of ammonium sulfate at an initial pH of 5.5-6.0) solution with different volume was added from the top of the column at a flow rate of 0.5 mL/min by a peristaltic pump at room temperature. Once a certain volume of leaching agent was pumped into the RE ore sample, the deionized water instead of the leaching agent solution was added as washing water to leach the RE ore sample until the concentration of RE3+, Al3+, and \( {\mathrm{NH}}_4^{+} \) in the leachate fell below the detection limit. Leachate was collected from the bottom of the column.
2.3 Analytical Methods
The total content of RE in the leachate was determined by ethylene diamine tetraacetic acid (EDTA) titration using xylenol orange as indicator and hexamethylene tetramine as buffer solution. Afterward, excess EDTA solution was added into the studied sample solution and placed in a water bath at 90 °C for 10 min. The concentration of Al3+ was analyzed using titration with a known concentration of zinc solution. The concentration of \( {\mathrm{NH}}_4^{+} \) was measured by an ammonia gas sensing electrode (PNH3-1-01, Shanghai INESA Scientific Instrument Co., Ltd.). All analysis was repeated at least three times, and the average value was obtained. The error range of the analytical method was within ±3%.
3 Results and Discussion
3.1 Characterization of RE Ore
The main chemical composition of the RE ore was determined by X-ray fluorescence (Axios advanced, Panalytical B.V.), and the result is listed in Table 1. As shown in Table 1, the main chemical composition of the RE ore is SiO2 and Al2O3, which account for 58.09% and 19.53% of the total mass, respectively, and the content of RE is 0.10%. The occurrence states of RE and Al in the RE ore were analyzed by the sequential fractionating extraction methods [18, 19]. RE in the minerals often exists as aqueous soluble state, ion-exchangeable state, colloid sediment state, and mineral state [20], and the percentage of each phase is 0.0083%, 90%, 3.2%, and 6.8%, respectively. There are obvious differences in the content of RE among various states. Results indicated that RE in the weathered crust elution-deposited RE ore mainly exists in the ion-exchangeable state. The exchangeable RE grade is 0.090%.
The occurrence states of Al include soluble state, exchangeable state, adsorption inorganic hydroxyl state, iron oxide combined state, interlaminar state, non-crystalline metasilicate state, and mineral state [5], and the percentage of each phase is 3.44 × 10−5%, 0.077%, 0.19%, 0.61%, 0.16%, 15.26%, and 83.70%, respectively. It is found that Al mainly exists as mineral state and non-crystalline metasilicate state in the weathered crust elution-deposited RE ore. The grade of exchangeable Al is 0.015%. The grade of the exchangeable impurity ions can be obtained by a column leaching experiment; 250 g of RE ore sample were leached with 0.4 mol/L (NH4)2SO4 solution until the concentration of every kind of impurity ion in the leachate was below the detection limit. Chemical composition of the leachate was analyzed using inductively coupled plasma mass spectrometry (ICP-MS, Agilent 7700x, Agilent Technologies Inc.). The grade of the exchangeable K, Mg, Ca, Mn, and Zn is 0.012%, 0.011%, 0.0028%, 0.0022%, and 0.00024%, respectively. It can be easily seen that the grade of the exchangeable Al is larger than other impurity ions. Among all the different states, only the exchangeable state can be exchanged from the RE ore into liquid phase by cation. Aluminum, being the uppermost impurity ion in the leaching process of RE, is often studied as the typical impurity ion.
The partitioning of the exchangeable RE in the leachate was analyzed by ICP-MS and the results are listed in Table 2; 52.06% of RE is the middle and heavy RE with tremendous commercial value.
The particle size distribution of RE ores and distribution of exchangeable RE and Al are shown in Table 3. It can be seen that the particle size of 60.27% RE ore is less than 0.25 mm. Most RE ore samples are fine particles, implying a high content of clay minerals. The original rock is weathered completely, and the unweathered mineral with large particle size, such as feldspar, quartz, and other accessory minerals, is negligible. Of the exchangeable RE, 78.33% and, of the exchangeable Al, 78.21% existed in the RE ore with a particle size below 0.25 mm, and the average grade is 0.10% and 0.019%, respectively. Usually, the smaller the particle size of RE ore is, the higher the content of the clay minerals. Clay minerals with large specific surface area and multiple adsorption activity centers can adsorb more cations [5]. Therefore, the exchangeable RE and Al are mainly in the fine particles.
3.2 Leaching Mechanism of Weathered Crust Elution-Deposited RE Ore
3.2.1 Leaching Chemical Basis of Weathered Crust Elution-Deposited RE Ore
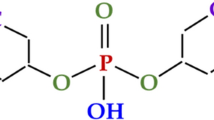
The weathered crust elution-deposited RE ores are mainly composed of clay minerals, like kaolinite, halloysite, white mica, etc. [21]. These clay minerals are all aluminosilicates and are considered as natural inorganic ion-exchangers. The exchangeable RE and impurity ions are adsorbed on the clay minerals, which can be desorbed into the solution by cations (\( {\mathrm{NH}}_4^{+} \), Na+, Mg2+, etc.) through ion exchange reactions [22]. Therefore, the leaching chemical reaction of RE and impurity ions (e.g., Al) with ammonium salts may be written as [23]:
where s and aq represent solid phase and aqueous phase, respectively.
As shown above, the leaching reaction is a typical reversible heterogeneous reaction in liquid–solid systems. That is the leaching theoretical basis of the weathered crust elution-deposited RE ores [24].
Current leaching techniques used for weathered crust elution-deposited RE ores are in situ leaching and heap leaching. Despite the leaching method chosen, the leaching agent all slowly permeates in the ore from top to bottom. The leaching process of RE can be described concretely as shown in Fig. 2. Once the leaching agent makes contact with the ore, an immobile liquid film will first form on the surface of the ore particle. After the immobile liquid film becomes stable and is saturated, the solution begins to flow among the ore particles and form a mobile liquid layer. \( {\mathrm{NH}}_4^{+} \) in the mobile liquid layer needs to diffuse to the surface of the clay minerals passing through the immobile liquid film and exchange with RE3+, which is adsorbed on the surface of the clay minerals. Owing to the consumption of \( {\mathrm{NH}}_4^{+} \) by ion-exchange, the concentration of \( {\mathrm{NH}}_4^{+} \) in the immobile liquid film gradually decreases, and the concentration of \( {\mathrm{NH}}_4^{+} \) decreases with the decreasing distance from the reaction zone (C1 > C2). On the contrary, RE3+ exchanged by \( {\mathrm{NH}}_4^{+} \) also needs to diffuse to the mobile liquid layer across the immobile liquid film. The concentration of RE3+ increases with the decreasing distance from the reaction zone (C3 > C4). The concentration gap causes the diffusion of the reactants (\( {\mathrm{NH}}_4^{+} \)) from the mobile liquid center to the phase interface and the diffusion of the reaction product (RE3+) from the phase interface center to the mobile liquid. Therefore, the leaching process of weathered crust elution-deposited RE ores can be considered as a mass transfer between the mobile liquid layer and the immobile liquid film [25].
3.2.2 SEM Analysis of Raw Ore and Leached Tailings
The raw ore and leached tailings were analyzed by scanning electron microscope (SEM, JSM-6700F, JEOL Ltd.) and the results are shown in Fig. 3. There are almost no differences between the morphology of raw ore and leached tailings. It can be inferred that the leaching process of weathered crust elution-deposited RE ore is only an ion exchange process, and no dissolution or structural change of the ores occurred.
3.2.3 FTIR Analysis of Raw Ore and Leached Tailings
The raw ore and leached tailings were determined by fourier transform infrared spectrum (FTIR, Nicolet Nexus 470 FT-IR, Thermo Electron Corp.) and the results are shown in Fig. 4. It indicates that the two absorption peaks at 3695 cm−1 and 3620 cm−1 are caused by the internal hydroxyl vibration of the clay minerals. The three absorption peaks at 1114 cm−1, 1031 cm−1, and 1007 cm−1 are generated by the Si–O stretching vibration of the clay minerals and microcline. The absorption peak at 912 cm−1 is produced by the O–H vibration of the clay minerals. The three absorption peaks at 795 cm−1, 756 cm−1, and 698 cm−1 are caused by the Si–O–Si and Si–O–Al vibrations of the layered silicates of clay minerals. The two absorption peaks at 536 cm−1 and 469 cm−1 are produced by the Si–O flexural vibrations and Al–O stretching vibration of the clay minerals. The FTIR spectra of the raw ore is consistent with that of the leached tailings, suggesting that there is nearly no obvious change on the surface of the RE ores after leaching.
3.3 Leaching Process of Weathered Crust Elution-Deposited RE Ore
3.3.1 Migration of RE in the Leaching Process
The migration of RE in the leaching process under various liquid–solid ratios is shown in Fig. 5. Initially, the concentration of RE increased to a maximum value and then decreased to zero with the increasing volume of leachate. In the initial stage of the leaching process, there were not enough \( {\mathrm{NH}}_4^{+} \) ions in the leaching agents to exchange all the RE ions that were adsorbed on the surface of the clay minerals. Moreover, RE3+ ions that were exchanged into the solution migrated downward with the migration of the solution and might be reabsorbed by the clay minerals. This explains why the concentration of RE in the leachate was lower in the initial leaching stage. However, with the inflow of leaching agent, the content of RE3+ exchanged into the solution increased, and the increasing concentration of surplus \( {\mathrm{NH}}_4^{+} \) in the solution would restrain the reabsorption of RE3+. The concentration of RE in the leachate increased rapidly with the increasing volume of leachate. In the later stage of the leaching process, almost all RE3+ had been exchanged into the solution and the residual RE3+ on the clay minerals continued to decrease. The concentration of RE in the leachate decreased until zero.
As shown in Fig. 5, all elution curves are nearly the same in the studied range of leachate volume except the liquid–solid ratios of 0.2:1 and 0.3:1 after the leachate volume reached 50 mL and 75 mL, respectively. This is caused by the differences between the volume of the leaching agent solution under various liquid–solid ratios. Take the liquid–solid ratio of 0.2:1 as an example, the added 50 mL of leaching agent solution could not completely soak 250 g of the RE ores (the saturated water absorption amount of 250 g RE ores is about 110 mL). The leaching agent solution would migrate downward under the driven force of the washing water until discharging from the bottom of the RE ores. Once the leachate volume reached 50 mL, the liquid filled in the RE ores was almost all washing water. The washing water added subsequently could only make a small amount of RE3+ desorb from the clay minerals and transfer into the solution. Therefore, the concentration of RE in the leachate decreased sharply to zero after the leachate volume reached 50 mL. For the same reason, the concentration of RE began to decrease after leachate volume reached about 75 mL. It can be inferred that \( {\mathrm{NH}}_4^{+} \) is the key to the desorption of RE3+ from the clay minerals. Once all the leaching agent solution discharges from the bottom of the RE ores, it is difficult to desorb RE3+ from the clay minerals by the washing water.
From liquid–solid ratio of 0.4:1 to 0.6:1, the elution curves are almost the same in the studied range of leachate volume. This indicates that almost all the RE had been leached under the liquid–solid ratio of 0.4:1, and there was little effect on the elution curves under high liquid–solid ratios. As shown in Fig. 6, when the liquid–solid ratio increased from 0.2:1 to 0.6:1, the leaching efficiency of RE was 57.73%, 85.23%, 95.51%, 96.58%, and 97.54%, respectively. When the liquid–solid ratio reached above 0.4:1, there was little effect of liquid–solid ratio on the leaching efficiency of RE.
From Fig. 6, it also can be found that the curves of the leachate volume (V) versus the RE leaching efficiency (\( {\upalpha}_{{\mathrm{RE}}_2{\mathrm{O}}_3} \)) nearly overlaps except for the liquid–solid ratio of 0.2:1 and 0.3:1 after the leachate volume of 50 mL and 75 mL, respectively. The results are consistent with that shown in Fig. 5. It can be concluded that the optimal liquid–solid ratio is 0.4:1 and 95.51% of the RE leaching efficiency can be obtained. In the actual exploitation, the liquid–solid ratio should be chosen according to the characteristic and RE grade of the RE ores to realize a high efficiency and low consumption exploitation of weathered crust elution-deposited RE ores.
3.3.2 Migration of Al in the Leaching Process
The migration of Al in the leaching process under various liquid–solid ratios are shown in Fig. 7. Results indicates that with increasing leachate volume, the concentration of Al in the leachate increases to a maximum value and then decrease to zero. The elution curves of Al are the same as that of RE. This implies that Al is also exchanged by \( {\mathrm{NH}}_4^{+} \), which is similar to RE; the leaching process and mechanism of RE and Al are the same. Unlike the migration of RE, the improvement of the liquid–solid ratio had little effect on the elution curves of Al after the liquid–solid of 0.5:1 instead of 0.4:1. The leaching of Al slightly lagged behind RE. This may be because the adsorption capacity of the clay minerals on Al is stronger than that on RE, or the exchangeability of \( {\mathrm{NH}}_4^{+} \) with Al is weaker than that of \( {\mathrm{NH}}_4^{+} \) with RE. In the leaching process of weathered crust elution-deposited RE ores, there is a competitive desorption between RE and the impurity ions.
Leaching efficiency of Al under various liquid–solid ratios is shown in Fig. 8. It can be seen that the leaching efficiency of Al increases with increasing volume of the leachate. For liquid–solid ratios from 0.2:1 to 0.6:1, the leaching efficiency of Al reached a maximum value at the leachate volume of 50 mL, 75 mL, 100 mL, 125 mL, and 125 mL, respectively, and then reached equilibrium. The results are consistent with that shown in Fig. 8. The leaching efficiency of Al increased slightly when the liquid–solid ratio was greater than 0.5:1. The optimal liquid–solid ratio is 0.5:1 with maximum leaching efficiency of Al. However, the exploitation of weathered crust elution-deposited RE ores aims to obtain RE, instead of Al, and the impurity ions in the leachate will increase the cost of impurity removal and decrease the quality of the RE products. Compared with the liquid–solid ratio of 0.4:1, the leaching efficiency of Al increased by 7.34%, but the leaching efficiency of RE only rose by 1.07% at the liquid–solid ratio of 0.4:1. Therefore, the optimal liquid–solid ratio is 0.4:1 for a maximum leaching efficiency of RE (95.51%) and a relative lower leaching efficiency of Al (90.99%). In conclusion, it is very important to choose an optimal liquid–solid ratio to leaching the weathered crust elution-deposited RE ores.
3.3.3 Migration of Ammonium Ion in the Leaching Process
The migration of \( {\mathrm{NH}}_4^{+} \) in the leachate under various liquid–solid ratios is shown in Fig. 9. It is found that with increasing volume of the leachate, the elution curves can be divided into three stages (I, II, and IV) under the liquid–solid ratios of 0.2:1 to 0.4:1 and four stages (I, II, III, and IV) under the liquid–solid ratios of 0.5:1 to 0.6:1.
In the first stage (I), the concentration of \( {\mathrm{NH}}_4^{+} \) in the leachate was approximately zero. This is because almost all the \( {\mathrm{NH}}_4^{+} \) in the leaching agent solution was used to exchange RE3+ and Al3+ that were adsorbed on the surface of the clay minerals in the initial stage of the leaching process.
In the second stage (II), the concentration of \( {\mathrm{NH}}_4^{+} \) in the leachate increased rapidly to a maximum value. With the inflow of leaching agent and the outflow of RE3+ and Al3+, the residual RE3+ and Al3+ adsorbed on the clay minerals reduced, and the surplus \( {\mathrm{NH}}_4^{+} \) in the solution increased. This is why the concentration of \( {\mathrm{NH}}_4^{+} \) in the leachate increased rapidly.
In the third stage (III), the concentration of \( {\mathrm{NH}}_4^{+} \) in the leachate was close to the initial concentration of 0.2 mol/L. This stage can only be observed under the liquid–solid ratios larger than 0.5:1. This is because 95.51% of RE3+ and 90.99% of Al3+ had been exchanged and transferred into the solution using 100 mL of the leaching agent solution (the liquid–solid ratio of 0.4:1, shown in Figs. 6 and 8). Under higher liquid–solid ratios of 0.5:1 and 0.6:1, there were no significant changes in \( {\mathrm{NH}}_4^{+} \) concentration in the leachate, and it was approximately the same with the initial concentration once the leachate volume exceeded 100 mL. However, under lower liquid–solid ratios of 0.4:1 to 0.2:1, all \( {\mathrm{NH}}_4^{+} \) in the leaching agent solution was used to exchange RE3+ and Al3+ that were adsorbed on the clay minerals, and there was no surplus \( {\mathrm{NH}}_4^{+} \) discharged from the RE ores. Hence, the third stage did not appear under the liquid–solid ratio of 0.4:1 to 0.2:1.
In the fourth stage (IV), the concentration of \( {\mathrm{NH}}_4^{+} \) in the leachate decreased sharply to zero. Under the liquid–solid ratios of 0.2:1 to 0.6:1, the RE ores were nearly filled with washing water when the leachate volume exceeded 50 mL, 75 mL, 100 mL, 125 mL, and 150 mL, respectively. \( {\mathrm{NH}}_4^{+} \) adsorbed on the surface of the clay minerals was difficult to be desorbed into the solution by the washing water. Therefore, the concentration of \( {\mathrm{NH}}_4^{+} \) in the leachate began to decrease sharply, once all the added leaching agent solution was discharged from the RE ores.
Therefore, it can be concluded that the appearance of the third stage means some of the leaching agent will be wasted. In the actual production, the usage amount of the leaching agent should be controlled reasonably.
As shown in Fig. 10, the migration of \( {\mathrm{NH}}_4^{+} \) in the RE ore under various liquid–solid ratios can be divided into three situations. All the column leaching experiments were proceeded using 250 g RE ores, of which the saturated water absorption amount was about 110 mL. In the first situation, the volume of the leaching agent solution was smaller than 110 mL, such as the liquid–solid ratios of 0.2:1 (50 mL), 0.3:1 (75 mL), and 0.4:1 (100 mL). The content of \( {\mathrm{NH}}_4^{+} \) in the RE ore remained stable at the beginning, then decreased to a constant value with increasing volume of the leachate. When the leachate outflowed from the bottom of the leaching column, the influent liquid from the top of the leaching column was replaced by washing water. No more \( {\mathrm{NH}}_4^{+} \) would be added into the RE ores. Moreover, there was almost no \( {\mathrm{NH}}_4^{+} \) outflowing from the RE ores in the initial stage of the leaching process (the first stage shown in Fig. 9). Thus, the content of \( {\mathrm{NH}}_4^{+} \) in the RE ore was similar to that in the leaching agent solution, and the higher the liquid–solid ratio, the higher the content of \( {\mathrm{NH}}_4^{+} \) in the RE ores. When \( {\mathrm{NH}}_4^{+} \) outflowed rapidly from the RE ores (the second stage shown in Fig. 9), the content of \( {\mathrm{NH}}_4^{+} \) in the RE ore also decreased sharply. At the last stage of the leaching process, the concentration of effluent \( {\mathrm{NH}}_4^{+} \) decreased sharply to zero (the fourth stage shown in Fig. 9), and the content of \( {\mathrm{NH}}_4^{+} \) in the RE ore approached to a constant.
In the second situation, the volume of the leaching agent solution was only slightly larger than 110 mL, such as the liquid–solid ratio of 0.5:1 (125 mL). There was a rising trend of \( {\mathrm{NH}}_4^{+} \) content in the RE ore before reaching steady state, compared to the first situation, and this was due to the increase of the leaching agent solution. When the leachate outflowed from the bottom of the leaching column, about 15 mL of the leaching agent solution was still added into the RE ores. Moreover, there was almost no \( {\mathrm{NH}}_4^{+} \) outflowing from the RE ores at the start of the leaching process (the first stage shown in Fig. 9). Thus, the content of \( {\mathrm{NH}}_4^{+} \) in the RE ore increased. After all the leaching agent solution was added into the RE ores, no \( {\mathrm{NH}}_4^{+} \) was detected from the outflows of the RE ores. The content of \( {\mathrm{NH}}_4^{+} \) in the RE ore remained stable. The subsequent migration trend of \( {\mathrm{NH}}_4^{+} \) in the RE ore was the same as the first situation.
In the third situation, the volume of the leaching agent solution was far larger than 110 mL, such as the liquid–solid ratio of 0.6:1 (150 mL). Different from the previous situations, there was no balance stage like liquid–solid ratios of 0.2:1 to 0.5:1. At a higher liquid–solid ratio (≥0.6:1), a large amount of \( {\mathrm{NH}}_4^{+} \) started to outflow from the RE ores (the second stage shown in Fig. 9) after all the leaching agent solution was added into the RE ores. Thus, the content of \( {\mathrm{NH}}_4^{+} \) in the RE ores first increased to a maximum value, and then decreased to a constant value with increasing leachate volume. The above phenomenon shown in Fig. 9 was consistent with that in Fig. 8.
As shown in Fig. 10, there was little change in the content of the residual \( {\mathrm{NH}}_4^{+} \) in the RE ores at the studied liquid–solid ratios and it remains around 0.61–0.63 mg/g. The adsorption of \( {\mathrm{NH}}_4^{+} \) by clay minerals was already saturated, and it is difficult to desorb \( {\mathrm{NH}}_4^{+} \) from the clay minerals by washing water alone. Some harmless leaching agents can be used to leach the RE ores in order to reduce the residual \( {\mathrm{NH}}_4^{+} \) in the RE ores and avoid the pollution of the RE ores and the underground water.
In the leaching process, the distribution of \( {\mathrm{NH}}_4^{+} \) can be divided into the following four parts: the remaining \( {\mathrm{NH}}_4^{+} \) in the leachate (\( {\mathrm{NH}}_4^{+} \)-1), the \( {\mathrm{NH}}_4^{+} \) adsorbed on the clay minerals by exchanging with RE3+ (\( {\mathrm{NH}}_4^{+} \)-2), the \( {\mathrm{NH}}_4^{+} \) adsorbed on the clay minerals by exchanging with Al3+ (\( {\mathrm{NH}}_4^{+} \)-3), and the \( {\mathrm{NH}}_4^{+} \) adsorbed on the clay minerals by exchanging with other cations and physical effects (\( {\mathrm{NH}}_4^{+} \)-4). Except for \( {\mathrm{NH}}_4^{+} \)-1, the other three portions of \( {\mathrm{NH}}_4^{+} \) are all left in the RE ores. In order to further understand the consumption of \( {\mathrm{NH}}_4^{+} \) in the leaching process, the distribution of \( {\mathrm{NH}}_4^{+} \) was calculated under different liquid–solid ratios and the results are shown in Fig. 11.
As shown in Fig. 11, the residual \( {\mathrm{NH}}_4^{+} \) in the RE ores including \( {\mathrm{NH}}_4^{+} \)-2, \( {\mathrm{NH}}_4^{+} \)-3 and \( {\mathrm{NH}}_4^{+} \)-4 increased slowly from 8.47 mmol to 8.81 mmol with increasing liquid–solid ratio. The liquid–solid ratio played a small role in the residual \( {\mathrm{NH}}_4^{+} \) in the RE ores. The saturation adsorption of \( {\mathrm{NH}}_4^{+} \) by the clay minerals was obtained at the liquid–solid ratio of 0.2:1.
With increasing liquid–solid ratio, the contents of \( {\mathrm{NH}}_4^{+} \)-2 and \( {\mathrm{NH}}_4^{+} \)-3 both increased, then remained stable after the liquid–solid ratios of 0.4:1 and 0.5:1, respectively. The results are consistent with that in Fig. 6 and Fig. 8. It can be inferred that the content of the \( {\mathrm{NH}}_4^{+} \) adsorbed on the clay minerals by exchanging with other cations also increased with the increase of the liquid–solid ratio. However, the content of \( {\mathrm{NH}}_4^{+} \)-4 decreased and then remained stable after liquid–solid ratio of 0.5:1. This indicated the content of the \( {\mathrm{NH}}_4^{+} \) adsorbed on the clay minerals by physical effects must decrease until the liquid–solid ratio reaches 0.5:1 and then remains constant. It is because the adsorption of \( {\mathrm{NH}}_4^{+} \) on the clay minerals by physical effects is easier and faster than that by chemical effects. In the initial stage of the leaching process, a large amount of \( {\mathrm{NH}}_4^{+} \) in the leaching agent was adsorbed on the clay minerals by physical effects, the van der Waals force. This binding force between \( {\mathrm{NH}}_4^{+} \) and the clay minerals was so weak that the \( {\mathrm{NH}}_4^{+} \) was easily desorbed from the clay minerals. With the leaching proceeding, the \( {\mathrm{NH}}_4^{+} \) adsorbed by the physical effects would desorb from the clay minerals and transfer into the solution, and then reach equilibrium. Thus, with increasing liquid–solid ratio, the content of \( {\mathrm{NH}}_4^{+} \) adsorbed by the physical effects decreased to a constant value, while that by the chemical effects (exchange reactions) increased to a constant value. Whether it was the chemical adsorption or the physical adsorption, the adsorption capacity of the clay minerals to \( {\mathrm{NH}}_4^{+} \) was limited. Once the clay minerals reached adsorption saturation, the residual \( {\mathrm{NH}}_4^{+} \) in the RE ores would not increase any more. That is why the residual \( {\mathrm{NH}}_4^{+} \) in the RE ores observes nearly no increase with the increase of the liquid–solid ratio from 0.2:1 to 0.6:1.
It also can be seen in Fig. 11 that with liquid–solid ratio increasing from 0.2:1 to 0.6:1, the content of \( {\mathrm{NH}}_4^{+} \)-1 increased from 1.53 mmol to 21.19 mmol, and the proportion of \( {\mathrm{NH}}_4^{+} \)-1 in the leaching agent solution rose from 15.30% to 70.63%. Even though the leaching work used the optimal liquid–solid ratio of 0.4:1, 56.65% of \( {\mathrm{NH}}_4^{+} \) in the leaching agent solution still remained in the leachate. In the actual exploitation, the leachate, after the removal of impurity and RE precipitation, can be reused by replenishing a certain amount of fresh leaching agent. In this way, the effective utilization of the leaching agent can be improved greatly, and the consumption of the leaching agent and the cost of production can also be obviously decreased.
4 Conclusions
For a high efficiency and low consumption exploitation, the leaching process and mechanism of weathered crust elution-deposited RE ores with ammonium sulfate were investigated. The exchangeable RE and impurity ions are adsorbed on the clay minerals that can be desorbed into the solution by \( {\mathrm{NH}}_4^{+} \) through ion exchange reactions. There was no dissolution or structural change of the ores in the leaching process, which was proved by the SEM and FTIR analysis of raw ore and leached tailings.
The migrations of RE3+, Al3+, and \( {\mathrm{NH}}_4^{+} \) in the leaching process were studied under various liquid–solid ratios. The concentration of RE and Al in the leachate increased, then decreased to zero with the increasing volume of the leachate. Compared to RE, the leaching mechanism of Al was the same, but the leaching of Al was relatively difficult. The maximum leaching efficiency of RE and Al were obtained at the liquid–solid ratios of 0.4:1 and 0.5:1, respectively. \( {\mathrm{NH}}_4^{+} \) was the key for the desorption of RE3+ and impurities from the clay minerals. At the optimal liquid–solid ratio of 0.4:1, 56.65% of \( {\mathrm{NH}}_4^{+} \) was left in the leachate. After impurity removal and RE precipitation, the leachate can be reused to leach RE ores by replenishing fresh leaching agents, which will improve the efficient utilization of the leaching agent.
With the increase of the liquid–solid ratio, the content of \( {\mathrm{NH}}_4^{+} \) adsorbed physically on clay mineral decreased, \( {\mathrm{NH}}_4^{+} \) adsorbed by ion-exchange increased, and the content of exchanged RE and Al also increased until equilibrium. The adsorption capacity of clay mineral with \( {\mathrm{NH}}_4^{+} \) always stayed at 0.61–0.63 mg/g. It was difficult for washing water to desorb \( {\mathrm{NH}}_4^{+} \) from RE ores. Harmless eluent is suggested to restore the RE ore by reducing the residual ammonium. The above results would benefit the high efficiency and low consumption exploitation of weathered crust elution-deposited RE ores.
References
Jha MK, Kumari A, Panda R, Kumar JR, Yoo K, Lee JY (2016) Review on hydrometallurgical recovery of rare earth metals. Hydrometallurgy 165:2–26
Yang XJ, Lin AJ, Li XL, Wu YD, Zhou WB, Chen ZH (2013) China’s ion-adsorption rare earth resources, mining consequences and preservation. Environ Dev 8:131–136
Nesbitt HW (1979) Mobility and fractionation of rare earth elements during weathering of a granodiorite. Nature 279:206–210
Gupta CK, Krishnamurthy N (2015) Extractive metallurgy of rare earths. CRC, Boca Raton
Chi RA, Tian J (2008) Weathered crust elution-deposited rare earth ores. Nova Science, New York
He ZY, Zhang ZY, Yu JX, Xu ZG, Chi RA (2016) Process optimization of rare earth and aluminum leaching from weathered crust elution-deposited rare earth ore with compound ammonium salts. J Rare Earths 34:413–419
Yang XL, Zhang JW (2015) Recovery of rare earth from ion-adsorption rare earth ores with a compound lixiviant. Sep Purif Technol 142:203–208
Xiao YF, Feng ZY, Hu GH, Huang L, Huang XW, Chen YY, Long ZQ (2016) Reduction leaching of rare earth from ion-adsorption type rare earths ore with ferrous sulfate. J Rare Earths 34:917–923
Li KZ, Liu HP, Lai FG, Xiao YF, Hu YM, Wang C, Xu HB (2017) Migration of natural radionuclides in the extraction process of the ion-adsorption type rare earths ore. Hydrometallurgy 171:236–244
Qiu TS, Fang XH, Wu HQ, Zeng QH, Zhu DM (2014) Leaching behaviors of iron and aluminum elements of ion-absorbed rare earth ore with a new impurity depressant. Trans Nonferrous Metals Soc China 24:2986–2990
Zhang ZY, He ZY, Yu JX, Chi RA (2016) Novel solution injection technology for in-situ leaching of weathered crust elution-deposited rare earth ores. Hydrometallurgy 164:248–256
Xiao YF, Feng ZY, Huang XW, Huang L, Chen YY, Wang LS, Long ZQ (2015) Recovery of rare earths from weathered crust elution-deposited rare earths ore without ammonia nitrogen pollution: I. leaching with magnesium sulfate. Hydrometallurgy 153:58–65
Wang L, Liao CF, Yang YM, Xu HB, Xiao YF, Yan CH (2017) Effects of organic acids on the leaching process of ion-adsorption type rare earth ore. J Rare Earths 35:1233–1238
Yang LF, Li CC, Wang DS, Li FY, Liu YZ, Zhou XZ, Liu MB, Wang ZF, Li YX (2019) Leaching ion adsorption rare earth by aluminum sulfate for increasing efficiency and lowering the environmental impact. J Rare Earths 37:429–436
Tian J, Yin JQ, Tang XK, Chen J, Luo XP, Rao GH (2013) Enhanced leaching process of a low-grade weathered crust elution-deposited rare earth ore with carboxymethyl sesbania gum. Hydrometallurgy 139:124–131
Zhang ZY, Sun NJ, He ZY, Chi RA (2018) Local concentration of middle and heavy rare earth elements in the col on the weathered crust elution-deposited rare earth ores. J Rare Earths 36:552–558
Zhou F, Feng J, Wang ZQ, Xu YL, Zhang ZY, Chi RA (2017) One step purification of impurities in the leachate of weathered crust elution-deposited rare earth ores. Physicochem Probl Miner Process 53:1188–1199
Lai FG, Huang L, Gao GH, Yang R, Xiao YF (2018) Recovery of rare earths from ion-absorbed rare earths ore with MgSO4-ascorbic acid compound leaching agent. J Rare Earths 36:521–527
Lai FG, Gao GH, Huang L, Xiao YF, Li KZ (2018) Compound leaching of rare earth from the ion-adsorption type rare earth ore with magnesium sulfate and ascorbic acid. Hydrometallurgy 179:25–35
Tian J, Yin JQ, Chi RA, Rao GH, Jiang MT, Ouyang KX (2010) Kinetics of leaching rare earth from the weathered crust elution-deposited rare earth ores with ammonium sulfate solution. Hydrometallurgy 101:166–170
Hu GH, Feng ZY, Dong JS, Meng XL, Xiao YF, Liu XS (2017) Mineral properties and leaching characteristics of volcanic weathered crust elution-deposited rare earth ore. J Rare Earths 35:906–910
Moldoveanu GA, Papangelakis VG (2013) Recovery of rare earth elements adsorbed on clay minerals: II. Leaching with ammonium sulfate. Hydrometallurgy 131:158–166
Moldoveanu GA, Papangelakis VG (2012) Recovery of rare earth elements adsorbed on clay minerals: I. desorption mechanism. Hydrometallurgy 117:71–78
He ZY, Zhang ZY, Yu JX, Zhou F, Xu YL, Xu ZG, Chen Z, Chi RA (2016) Kinetics of column leaching of rare earth and aluminum from weathered crust elution-deposited rare earth ore with compound ammonium salt. Hydrometallurgy 163:33–39
He ZY, Zhang ZY, Yu JX, Xu ZG, Xu YL, Zhou F, Chi RA (2016) Column leaching process of rare earth and aluminum from weathered crust elution-deposited rare earth ore with ammonium salt. Trans Nonferrous Metals Soc China 26:3024–3033
Acknowledgments
The work is financially supported by grants from the National Natural Science Foundation of China (No. 51704339, No. 51734001, and No. 41472071) and “the Fundamental Research Funds for the Central Universities”, South-Central University for Nationalities (No. CZY19033, No. CZP19002, and No. YZZ16002).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• The leaching process of RE and impurity was a typical reversible heterogeneous ion exchange reaction in liquid–solid systems.
• The leaching mechanism of Al was the same as RE, but the leaching of Al slightly lagged behind RE.
• 56.65% of \( {\mathrm{NH}}_4^{+} \) remaining in the leachate can be reused after impurity removal and RE precipitation.
• The residual \( {\mathrm{NH}}_4^{+} \) in RE ores was about 0.61–0.63 mg/g that was difficult to desorb for washing water.
Rights and permissions
About this article
Cite this article
He, Z., Zhang, R., Nie, W. et al. Leaching Process and Mechanism of Weathered Crust Elution-Deposited Rare Earth Ore. Mining, Metallurgy & Exploration 36, 1021–1031 (2019). https://doi.org/10.1007/s42461-019-00116-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42461-019-00116-5