Abstract
As the impacts of climate change continue to be felt around the world, understanding the effects on groundwater quality and quantity has become an important area of investigation. As a global source of water that contributes to preserving the environment, a better understanding of the effect of precipitation seasonal pattern on these systems is crucial; though studies connected to groundwater quality in this era of environmental crisis are at infancy. This study aims to evaluate the effect of precipitation seasonal pattern on groundwater quality in a coal enriched environment of developing city of west African sub-region with particular reference to Enugu, a coal city in Nigeria. Three residential areas (Abakpa, Achara, and Independence Layout) were randomly selected from high, medium, and low neighbourhood densities in the metropolis. Within the period spanning from April 2018 to March 2019, a physiochemical analysis was conducted on twelve deep wells utilizing weighted arithmetic index method. This technique was implemented in order to facilitate the assessment of the degree of water quality by translating a number of variables to just one metric value. The results of the investigation showed that the groundwater resources in the study region are mildly acidic, presumably as a result of the presence of pyrite, which is a byproduct of coal weathering, and chloride-ion-charged rains. Additionally, noticeable distinctions in the properties of water samples were observed between the dry and rainy seasons. Specifically, just 1% of the sampled water had excellent ratings, while 58.3% were considered good, 29.1% were deemed poor, and 8.3% of samples were categorized as very poor. The study concluded that coupled with climate crisis, seasonal precipitation patterns affect groundwater resources by reducing recharge, discharge, and the overall quality of water. These results have important implications for the management of groundwater resources in the region and highlight the need for continued monitoring and assessment of water quality in the face of ongoing environmental changes.
Article Highlights
-
Groundwater quality in Enugu varies between dry and rainy seasons, with higher pollution levels during the wet months.
-
Local hydrogeology and land use affect groundwater quality, with some areas more susceptible to contamination.
-
Regular monitoring and sustainable water management are crucial to ensure safe drinking water in the face of climate crisis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Water occupies about 70% of the earth’s surface, however according to Behailu et al. [1] only around 3% of the total volume of water is available in potable form. Implying its inadequacy to the demands of the global rising human population and for industrial needs. Globally, groundwater comprises nearly 30% of freshwater reserves [2], making it indispensable for fulfilling 50% of drinking needs in urban zones and 85% in rural areas [3]. However, as climate change, land use changes, pollution and overexploitation threaten surface water bodies [4]; reliance on subterranean aquifers is rising, particularly across water scarce regions of sub-Saharan Africa like Enugu that extract over 60% of their drinking supply from groundwater [5]. According to Nnaemeka-Okeke [6], rapid urbanization and its impact on climate change are two of the most pressing issues facing cities in the twenty-first century. The rapid population growth propelling humanity into Anthropocene [7, 8] combined with impacts of climate change is exacerbating water scarcity globally. In Enugu city, increased industrialization, vehicular emissions, waste generation and latrines amidst growing density have degraded environmental quality [9, 10]. Continued flux of biological, chemical, and particulate species through unsanitary municipal conduits and localized landfills is deteriorating shallow aquifer quality across Nigerian cities [3]. Heavy seasonal precipitation flushes substantial suspended solids while altering flow velocities and residence times, regulating dissolution, leaching and subsurface mobility of ions and metals. Prolonged interaction between infiltrating water and geological media determines groundwater chemistry [11]. Hence, climate variability and urban pollutive patterns can cooperatively impact potability. In Africa’s sub region, the majority of its inhabitants reside in an environment where available water resources do not satisfy global standards [12]. In fact, the World bank statistics [13] reveal that sub–Saharan Africa requires about $93 billion to fill the deficits of infrastructure. Subsequently, third world country like Nigeria would need $1.2–1.5 billion as yearly investments for basic services like potable water, power, and good roads [14]; whereas Estache [15], proposed that infrastructure spending will be not less than 8% of annual GDP.
Globally, groundwater is the commonest source of potable water and till date remains a vital source of freshwater for meeting the everyday demand for water, particularly in third world countries [16, 17]. It compensates for the water supply constraint occasioned by failed infrastructure. However, despite growing dependency upon groundwater for urban water supplies concerns remain over the sustainability of ground water, in terms of quantity and quality. Thus, to ensure safe consumption of groundwater as a source of potable water, it must meet compliance of established quality standards that are based on the physicochemical composition of the water. This composition in turn is influenced by various hydro-chemical processes that are themselves subject to the effects of climatic conditions. The chemical composition of groundwater is a good determinant of its suitability for human and livestock consumption. The quality of groundwater refers to the state of water that is located beneath Earth’s surface (gathered in cracks in subsurface rocks and in between soil particles). The deteriorating trend in water quality is assumed to be exacerbated by climate change and related extreme events [18]. Studies revealed that the phenomena of climate change are currently having a measurably negative impact on both human and ecological systems [19], and its repercussions are already a reality that are felt worldwide. There is evidence in the literature that human activity is warming the atmosphere and aquatic bodies over time; and rise in sea level, ocean surge and coastal plain floods are all projected consequences of climate change [20]. When this is combined with pollution from waste disposal and urbanization; garbage collected in open waste sites is transported via drainage channels [21]. As a result of breakdown, advection, and absorption, mineralization and nutrient loading in surface water occurs, eventually percolating into shallow aquifers [22,23,24]. Subsequently, the complex process of recharging groundwater from stormwater typically spans a period of several days to weeks, during which time various bio-geochemical processes take place. The outcome of these processes can result in elevated concentrations of ions and metals, which have the potential to negatively impact the quality of groundwater. This phenomenon is especially pronounced in rural settlements located throughout Nigeria, where access to clean and potable groundwater is often hindered, thus posing significant health risks associated with water-borne diseases.
According to IPCC [20], the average surface temperature of the earth has risen by approximately 0.6 °C ± 0.2 °C since the year 1861. Furthermore, the IPCC has been predicted that the average temperature is anticipated to increase within the range of 2 °C to 4 °C over the next century. This phenomenon significantly disrupts the water cycle with an observed increase in the rate of evaporation from water bodies and impact on the magnitude, length, and force of rainfall. The combination of high evaporation rates and low precipitation levels can also have the effect of reducing the volume of surface water available, resulting in greater reliance on groundwater sources [25]. Anecdotal evidence among residents in the urban area of Enugu reveals a longstanding reliance on groundwater sources, particularly hand-dug wells, which serve as a viable alternative to traditional mains water supplies. Edeonovo [9] highlights contamination risks, with most of the sampled wells not meeting national potable standards. Rural areas, mirroring wider Nigerian issues, face contamination from septic tank, landfill seepage, and unsanitary practices [3]. Furthermore, the region's coal-bearing geology naturally degrades water quality, as pyrite oxidation in abandoned mines produces acidic, metal-rich leachates. Also, the geological composition of the soil strata has made it impossible to get potable water from mechanically drilled boreholes. As such the middle and high-income group frequently employ private water supply trucks as an alternative means of accessing water resources, albeit with questionable levels of purity. Given that so much of daily life and future planning are predicated on the availability of reliable hydrological systems, it is of paramount importance to gain a thorough understanding of the impact of climate change on groundwater supply.
The Fourth Assessment Report of the Intergovernmental Panel on Climate Change did not consider the detail implications of climate change on water quality [26]. Evidence in literature have established the influence of climate change-driven flooding on surface water quality, particularly in coastal areas where excessive precipitation is prevalent [27,28,29,30], though research on climate change’s impact on groundwater quality in sub-Saharan region is at infancy and still budding [31, 32]. This can be likened to the fact of groundwater invisibility and built on the premise that groundwater quality monitoring statistics are rarely collected in the research area. Previous authors like [17, 29, 33, 34] discovered higher levels of ions, heavy metals, and trace metals in shallow aquifers in Nigeria's coastal metropolis, with many of them crediting groundwater contamination on sewage effluents, indiscriminate dumping of domestic, agricultural, and industrial waste. However, among various studies, none has hitherto examined the impact of seasonal precipitation patterns on groundwater quality in regions characterized by an abundance of coal resources in this climate change era. The study therefore, was designed to evaluate the impact of seasonal precipitation patterns on the quality of groundwater in a coal enriched environment of developing city of west African sub-region with particular reference to Enugu, a coal city in Nigeria. A Knowledge of this will help drive policies of the Sustainable Development Goals (SDG1 that emphasizes leaving no one out and ending poverty, as access to clean water is not only a necessity but speaks to SDG6—clean water and sanitation, hence SDG3—good health and wellbeing), resource management, and resilient city advancement in the face of global climatic consequences. The present study aims to achieve several specific objectives, namely: (i) to assess the quality of water in selected sample areas through the use of physicochemical analysis and evaluation of specific parameters; (ii) to use the water quality index approach for assessing the overall quality of the water by converting the various parameter to a single value indicator; (iii) to make results comparison against established standards for drinkable water; and (iv) to formulate suggestions based on the research results, especially in light of global climate change impact.
The current research topic is considered beneficial in the ongoing discourse on sustainability imperatives and water quality at broader scales, and the associated groundwater interactions influenced by climate and land use; all of which are important for future environmental protection and restoration initiatives. The results of this study are expected to provide valuable insights into the state of groundwater quality in the study area, thereby informing policy making and management strategies aimed at promoting the sustainable use of this important resource.
1.1 Effects of climate variability on groundwater quality
Groundwater is a critical natural resource with multifaceted benefits that support human well-being, ecosystems, and enables ecological development. At the global scale, it serves as a vital source of freshwater, accounting for one-third of total water withdrawals, thereby providing access to safe and reliable water for nearly 85% of rural and 50% of urban water needs. In recent times, the availability and quality of groundwater have faced significant challenges due to a combination of climatic and non-climatic factors. Non-climatic factors such as land-use changes, over-extraction, and pollution have also contributed to the depletion and degradation of groundwater resources. This has emerged as a growing concern in the modern era [35]. Studies indicate that climate change is perceived as a key factor impacting the water cycle, leading to changes in precipitation patterns, temperature regimes, and hydrological processes [36,37,38,39]. The research findings of Dragoni and Sukhija [40] established that water replenished during arid seasons manifests a heightened salinity level and an elevated concentration of total dissolved solids (TDS). Conversely, water recharged during wet periods may exhibit a reverse trend. Furthermore, the presence of specific ions in groundwater can be attributed to prior water–rock interactions taking place during distinct climatic regimes. Temperature fluctuations, heightened concentrations of dissolved CO2 in rainwater, and shifts in precipitation patterns have been observed to collectively facilitate amplified dissolution of carbonate minerals in subterranean water reserves. For instance, in the karstic granitic terrain aquifers of Cotmeana River basin, Romania, Rădulescu et al. [41] noted a strong correlation between wetter climate and increased mineral weathering. Serdeczny et al. [32] assessed climate change effects on coastal aquifers across Sub-Saharan Africa. Rising sea levels are enabling saltwater intrusion and rendering groundwater unusable. As noted in coastal urban centers of Ghana, southward hydraulic gradients have led to enhanced salinization.
Coal is a commonly used term to describe widespread deposits of carbon-rich materials that originate from plant matter and are found in layered formations [42]. It is predominantly composed of organic materials that have undergone significant transformation under immense pressure and elevated temperatures over several years. In Enugu, located in Southeast Nigeria, the coal deposits originate from partially decomposed and macerated plant material, primarily derived from vascular land plants. The metropolis is fondly called “coal city” due to the presence of large deposits of coal and it has affected underground water, as several shallow and deep hand-dug wells have become an alternative source of groundwater in the locality mainly for domestic purposes. However, it is generally understood that the influence of mine on site abandonment has adverse effects on the quality of water. As such, it is imperative to conduct a comprehensive assessment of the physicochemical attributes of water sourced from groundwater resources situated in coal enrich regions. This will enable an accurate determination of the water quality in Enugu Urban. Several researchers have analyzed the impacts of coal mining on underlying aquifers along the Appalachian region in the United States. Martin et al. [43] noted increased mineralization and higher abundances of ions like sulfate in groundwater near mines with extensive subsurface works compared to unmined areas. Elevated concentrations of metallic species were also reported due to oxidation of exposed coal and pyrite weathering. This signifies potential deterioration from coal extraction processes. Martin et al. [44] evidenced in West-Central Indiana that coal mining processes can alter physical aquifer architecture, water budgets and ambient chemistry over prolonged durations due to large-scale landscape disturbance. These case studies demonstrate the pronounced impacts, active and abandoned coal mines can exert on underlying groundwater reserves through physical disturbances and acid rock drainage. Since the study area also has substantial coal deposits, such previous analyses provide helpful context.
The water quality index (WQI) is an established tool employed to provide a consolidated overview of the general quality of a water sample, as described in reference [45]. By condensing multiple water quality parameters into a concise expression, the WQI simplifies the interpretation of water quality data and increases public accessibility to such information. Literature shows that about seven alternative models for evaluating water quality index exist. The Nemerow-Sumitomo Water Quality Index (NWQI) is a reliable and widely used tool for assessing water quality. It has limitations in accurately evaluating surface water quality that falls below class 5 [46]. Despite its robustness, the NWQI may not be sensitive enough to capture subtle changes in water quality parameters, particularly in heavily polluted water bodies. An alternative approach, the single factor pollution index (SFPI), has emerged as a promising tool for assessing surface water quality, even in polluted environments [46]. The SFPI method utilizes a set of water quality parameters, such as pH, dissolved oxygen, and fecal coliform count, to classify water quality based on a scale from I to V. The SFPI approach is advantageous over the NWQI in that it provides a simpler and more straightforward water quality classification system that is less dependent on complex mathematical modeling. Additionally, the SFPI method is more adaptable and can be used to assess water quality across a wide range of environments, including heavily polluted water bodies. However, it assesses water quality based on the lowest categorization among the evaluated water quality indices. This method may provide biased results in cases where the water quality is extremely poor. To address this limitation, a weighted arithmetic index (WAI) method was developed to provide a more accurate and reliable assessment of water quality. The WAI method assigns weights to different water quality parameters based on their relative importance and calculates an aggregate score, which is used to classify water quality into different categories. This method has gained widespread acceptance and has been widely used by various analysts in the field [47, 48] and used also in this current study.
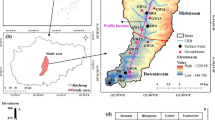
2 Geographical location
Enugu city is situated in Nigeria's south-east and the capital city of both the old eastern region and the present-day Enugu state. The area falls under the Anambra Basin—a large sedimentary depression rich in coal seams [49]. Decades of mining activity has produced substantial waste piles and exposed excavation voids underground. Weathering of the exposed coal measures and pyrite generates acid rock drainage, leading to accelerated leaching of ions under humid tropical conditions [50]. The oxidizing acidic leachates percolating through fractures can alter groundwater chemistry. Additionally, runoff from waste rock piles on the land surface facilitates particulate and dissolved mineral transfer. Hence both natural and anthropogenic processes contribute to water quality variations. On the globe, Enugu is located between 06o21oN and 06o30oN latitude and 07o26oE and 07o37oE longitude (See Fig. 1). The Atlantic coast of West Africa is about 230 km away from this city [51]. It is a rapidly expanding city harbouring a current projected population of 1.2milion people of which over 90% are of the Igbo ethnic group [52]. Nigeria’s city and perhaps Enugu environment has become fragile as a consequence of increased industrialization, urbanization, and increased population growth; raising concern for environmentalist [53]. The city of made up of 19 neighbourhood grouped under 3 clusters according to residential densities which include low, medium, and high densities [54].
Groundwater has remained an indispensable water source in both rural and urban areas of southeastern Nigeria. In Enugu urban, city dwellers rely heavily on hand-dug deep wells for potable water to complement the usual supply from commercial water tankers during the dry season. However, available literature reveals that these resources are threatened by pollution caused by human lifestyle choices, as evidenced by the poor degree of hygiene practiced in most developing cities [55] or hydrology composition of lower soil strata as evident in the metropolis. The climatological data shows that the city is situated within a warm and humid tropical region, with the relative humidity varying between 55 and 85%, and reaching its peak at 75% during the period spanning from March to November [56]. Previously, the daily average temperature was recorded at 26 °C however, with the present global warming impact, this figure ranges between 27 °C and 37 °C [56]. The rainy season within the region typically starts in April through August. It is characterized by high relative humidity and intense rainfall. According to meteorological data, the city experiences its heaviest rainfall during the months of June and July, with July being the wettest month, recording an average of 360 mm of rainfall. The estimated annual rainfall range for the city falls between 1500 and 2000 mm, as reported in [56].
3 Research methodology
3.1 Description of study area
The Study area is in Enugu urban and according to Okeke et al. [54] it is subdivided into 19 residential areas carved out from three local government councils. Three residential areas (Abakpa, Achara and Independence Layout) were selected from high, medium and low neighbourhood densities in the metropolis. The chosen three residential study areas were selected because they are the most populated when rating based on their categorization of different neighborhood densities. Apparently, it is assumed that sample sites are heavily utilized by residents who constantly rely on groundwater supply. To determine places for sample collection, an assessment of the targeted study location of Abakpa, Achara and Independence Layout was conducted (See Figs. 2 and 3 for details).
3.2 Sample determination and collection
Enugu urban comprises three local districts (north, east, and south), each with four sampling points to ensure representative sampling (see Fig. 2). Specifically, Independence Layout in Enugu North, Abakpa in Enugu East, and Achara Layout in Enugu South each had four sampling points, as illustrated in Table 1. Selection criteria for sampling sites was based on distribution across neighbourhood area and usage frequency. Common public wells with heavy usage serving ≥ 15 households were preferred over less frequented ones to assess potential effects of drawdown and recharge cycles. For a period of 12 months, samples were taken at monthly intervals. Every month, samples were taken twice: once in the first week and once in the last week. Samples for the rainy season were taken from April to August 2018, whereas samples for the dry season were taken from September to March 2019. In total, twelve samples were analyzed in this study. Prior to sample collection, the sample bottles and vessels were thoroughly sterilized with a non-phosphate laboratory grade detergent solution (2% non-ionic surfactant) to eliminate any potential contamination. The sample bottles were then stored in a controlled environment with a temperature maintained at around 4 °C to ensure a clean and suitable storage condition. The same well water used to collect the groundwater samples was used to rinse the sample vials before filling them. A total of twelve groundwater samples were collected from wells and placed in clean and sterilized 200 cl plastic bottles with secure lids. To protect the samples from light and external contaminants, the bottles were then wrapped with black polyethylene bags and stored in a makeshift icebox for transportation, maintaining the sample's integrity during transit. 0.5 cl of concentrated HNO3 was added to the samples to preserve them for examination of heavy metals and stop any metals from sticking to the container walls. The samples were then put through a 0.45 mm Millipore membrane paper filtering device, which is made entirely of glass. A pore size of 0.45 μm was selected as it efficiently traps suspended particles including bacterial cells that could interfere with dissolved metal analyses, while allowing complete passage of ionic species [57]. Before filtering, the membrane mesh was cleaned using 1% HNO3 and then rinsed with highly pure water [58]. In order to prevent ground water from coming into contact with the atmosphere, physical parameters like pH, Electrical Conductivity (EC), Total Dissolved Solids (TDS), and temperature were first measured after sampling using a new Hanna Multi-Meter with replaceable electrodes.
3.3 Evaluation procedure
Using a conventional approach as documented in AOAC [59] and Lenore et al. [60] the following parameters were measured: pH, Biochemical Oxygen Demand (BOD), and Chemical Oxygen Demand (COD). Temperature was measured using electrometric method [61,62,63]. Water samples were tested for total acidity and total alkalinity using a standard method (Titrimetric method). The total suspended solid (TSS) was calculated using a gravimetric technique [59, 60]. TDS was measured directly using a HANNA (Model HI 9811-5) multimeter that had been pre-calibrated in accordance with the manufacturer’s instructions. A 10 cl glass beaker was filled with 1 cl of water sample. By lightly depressing the meter's tactile button, the TDS mode was turned on after the probe was immersed into the beaker. Before it was recorded, the metre’s value that was shown on its LCD panel was allowed to stabilize. By calculating the mathematical sum of Total Dissolved Solids and Total Suspended Solids, Total Solid (TS) was derived. Utilizing the Nephelometric Method, turbidity was assessed. Sulphate (SO42−) was determined using Turbidimetric Method [59, 60]. The EDTA (Ethylenediaminetetraacetic acid) Titrimetric Method was used to determine total hardness [59, 60]. According to the test method specified in IS 3025 (Part 14), electrical conductivity was measured. By using the flame photometric method (APHA 3500-K Band and APHA 3500-Na B standard procedures) respectively, potassium (K) and sodium (Na) were calculated. Standard procedures were followed to estimate the amounts of iron (Fe), lead (Pb), and zinc (Zn) as described in reference [59, 60].
3.4 Data analysis
Through the use of specialized software (SPSS), the study's data were statistically analyzed. Descriptive statistical methods, including measures of central tendency and variation, were used to analyze and characterize the data (Tables 2, 3).
3.5 Technique for rating quality of water
To determine if water was suitable for drinking, the weighted arithmetic WQI approach was utilized [64]. The following formulae were used to calculate the water quality rating scale, relative weight, and overall WQI as adopted by Cooray et al. [65].
where qi, = quality rating scale.
Ci, = concentration of i th parameter.
Si = standard value of i th parameter.
Relative weight was calculated by wi = 1/Si,where the I parameter’s standard value is inversely proportional to the relative weight.
The WQI was expressed by the following equation.:
where: WQI = water quality index.
Qi = sub index for the i th water quality parameter.
Wi = weight associated with the i th water quality parameter; n = number of water quality parameter.
During the dry and rainy seasons, there was a substantial change in the total physicochemical water quality index of the research as evident in Table 4.
4 Results and discussion
The Water Quality Index (WQI) provides a quantitative representation of ambient water chemistry from a potability and usage safety perspective across the monitored wells. Lower values within the scaled classification system indicate superior quality. Analysis of the seasonal WQI shifts offers relevant insights. Some wells showcase retained classifications across wet and dry months, despite varying index scores (e.g. W2, W3, W4, W5). This implies their water quality consistency aligning to respective rating thresholds with no category escalations, although influence of climatic drivers introduces minor variations in magnitudes. Conversely, particular stations exhibit transitions into poorer designations between humid and summer seasons, indicating deterioration (e.g. W6, W7, W8). Deterioration extent is pronounced among W9, W10, W11 and W12 wells, shifting into ‘Poor’ and ‘Very Poor’ quality brackets during monsoons. This suggests acute influence of storm water ingress under heavy precipitation or overextraction enabling polluted ingress. However, a reverse trend of water quality enhancement from rainy to dry intervals is also noted occasionally (e.g. W1), potentially due to settlement of suspended impurities over longer residence times.
Categorical analysis of the collated Water Quality Index (WQI) data reveals further intriguing seasonal patterns. During the dry season, 9 of the total 12 samples demonstrated ‘Good’ quality—aligning within the 100–200 WQI range as depicted in Table 5. This represents 75% of the tested wells exhibiting acceptable potable water chemistry. Additionally, 2 stations were classified as ‘Poor’ quality with one site graded higher at ‘Excellent’. In contrast, the wet season saw a decline in sites warranting a ‘Good’ rating to just 5, comprising 41.6% of evaluated wells. Simultaneously, counts for ‘Poor’ and ‘Very Poor’ designations escalated to 5 and 2 respectively. This indicated prevalence of deteriorating quality trends likely owed to cumulative contamination and suspended solid accruals from heavy precipitation. Essentially, the indexed rating distribution skewed towards superior quality across a greater proportion of reserves during hot and dry months. Nearly 25% of stations transitioned into below par WQI classifications in the wet season. This trend substantiates the overarching inference that summer period groundwater possesses relatively enhanced quality, while monsoonal action acutely degrades ambient reserves through surface influx and allied mechanisms. However, the sporadic variability equally underscores the need for continued WQI screening to facilitate prompt ameliorative action as essential.
The result further reveals that during the rainy season high densities of incoming solids can transform groundwater flow conditions from laminar to turbulent regimes. This disrupts travel pathways and residence times. Sediments tend to accumulate in pores spaces transforming flow geometry and progressive clogging channels. Lower rainfall seemingly enhanced residence time for chemical reactions and leaching. Large sediment loads obstruct pores, diverting influx to alternative routes and retaining contaminants like dissolved organic matter for longer durations locally. However, reduced velocities also aid particle deposition and filtration. Hence while sediment pulses from human activities may temporarily degrade quality, the associated purification mechanisms can restore groundwater reserves over seasonal cycles. Details of physiochemical analysis are shown in the next subsection.
4.1 pH
Tables 2 and 3 show the concentrations (mean standard deviation) of physicochemical characteristics in 12 well water samples collected throughout dry and rainy seasons. The pH readings of most of the water specimens captured were acidic. The pH ranged from 4.9 to 7.5 in all 12 water samples during the dry season, with a mean of 6.13, and from 4.0 to 7.20 in all 12 water samples during the wet season, with a mean of 5.40. This illustrates a decline in pH over time. This could be attributed to an increase in the intrusion of aquifers with water containing chemicals, as well as landfill and municipal trash. A physicochemical and microbiological study of six boreholes water samples within the city of Enugu at the beginning of year 2015 showed akin acidity values for pH of 2.1, 4.3, 2.0, 4.2, 4.8, and 5.0 [10]. The average values found in the current research during both seasons of the year fall beneath the maximum acceptable range of 7.00–8.50, the highest permissible level of 6.5–9.2 established by World Health Organization recommendations for the quality of drinking water [67] and the highest acceptable threshold of 6.50–8.50 advised by NIS [68] and the Nigeria standard for drinking water quality [69]. This suggests that the drinking water is unsafely acidic and prone to leaching metal ions such as Cu, Pb, and Zn from aquifers as well as plumbing and sanitary fittings.
4.2 Turbidity
The turbidity values showed high variability ranging from 3.450 to 50.00 NTU during the dry season monitoring, with an average of 9.743 ± 12.86 NTU. In the wet season, the readings spanned from 3.800 to 56.00 NTU, resulting in a marginally higher overall mean of 10.80 ± 14.37 NTU. The minor turbidity elevation from the dry to rainy season runs contrary to typical trends reported for surface waters, wherein dilution by rainfall often lowers suspended solid loads [70, 71]. However, similar observations have been documented in past groundwater studies as well, attributed to changes in subsurface flow velocities induced by recharge events [72]. High precipitation likely decreased groundwater velocities, allowing increased residence time for interactions between infiltrating water and aquifer media [73]. This facilitated additional fine particulate entrainment from the soil layers, detected as amplified turbidity [74]. Moreover, the mean turbidity levels for both seasons exceeded the 5 NTU limit defined by drinking water guidelines [63, 67, 68]. This indicates that while the aquifer showed some resilience to background suspended loads, the buffering was surpassed after surface runoff events that accelerate subsurface particulate transfer. Hence, indicative water quality parameters can exhibit yearly cycles akin to those in surface water bodies, necessitating dry–wet sampling plans for holistic contamination assessment. As excessive turbidity can directly impact human health or interfere with disinfection operations, its regular measurement alongside classical water quality variables is imperative to support groundwater management initiatives.
4.3 Temperature
The highest recorded groundwater temperature was 31.9 °C, occurring in both the dry season (at site W2) and wet season (at W3). The minimum temperatures of 29.0 °C were noted in the dry period at W9 well and twice during rainfall at W1 and W9. The mean values showed only marginal differences, with overall averages of 30.4 °C in summer and 30.3 °C during the rainy months. The rather stable high temperatures year-round indicate continuous thermal loading of the aquifers, independent of short-term ambient weather variations. Similar observations of negligible cooling despite dilution effects were seen in Quaternary aquifers elsewhere as well [75, 76]. Continuous seepage enables transfer of solar heat absorbed during shallow circulation, as evidenced by the slightly elevated dry season means. Higher temperatures promote chemical reactions [77] and stimulate microbial metabolism [78], indirectly fueling pollutant generation. More importantly, the groundwater temperature means for both seasons surpassed the guideline limit of 25 °C set by NIS [68]. Elevated temperatures can be hazardous for human consumption or alter palatability [79, 80]. Hence, mitigating thermal loading through improved groundwater governance encompassing protection zones, discharge regulations and demand management is vital for underlying quality. Overall, the minor temperature fluctuations between dry and wet periods signify that groundwater remains disconnected from short-term above ground climate, marking significant anthropogenic impact.
4.4 Electrical conductivity
The electrical conductivity (EC) values showed ten-fold variations during both dry and wet seasons among the groundwater sampling locations. In the moisture-deficit summer months, EC ranged from 10.00 to 410.00 μS/cm, while the rainy season recorded a marginally lower span of 10.00 to 360.00 μS/cm. However, contrastingly the average EC level increased in the wet period (189.6 μS/cm) relative to the dry mean of 133.9 μS/cm. Such anomalous elevations in aquatic EC levels during monsoons have been reported earlier as well [81, 82]. The higher wet season EC indicates greater mobility of weathered mineral constituents from surface layers reaching water bodies through elevated recharge [83]. This pulses ionic loads during floods, detected as soaring conductivity. Nevertheless, the EC means for both seasons were well within the 1000 μS/cm limit imposed by NIS [68] drinking water regulations. Comparable EC ranges have been noted in granitic aquifer zones of Cotmeana River Romania [41], suggesting inherent processes restricting total dissolved solids. Hence, while transient peaks might occur during rainfall, regulatory mechanisms in deeper layers aid longer-term stabilization of solute fluxes.
4.5 TDS, TSS and TS
The analysis indicates a notable seasonal variation in total dissolved solids (TDS) across water samples. Readings averaged 62.5 mg/L during summer months (dry season), ranging between 0.000 and 210.0 mg/L as per Table 2. In contrast, the rainy season exhibited higher TDS concentrations, with a mean of 127.4 mg/L within a span of 0.00–260.00 mg/L. This two-fold increase potentially stems from greater soluble species influx from environmental interfaces under wetter conditions. Runoff ingress and associated transportation of minerals from overlying soil/rock layers could be hypothesized to drive this seasonal fluctuation. Further isolation of concentration changes in constituent ionic species can better elucidate the mechanisms governing TDS dynamics. Contextualizing with local environmental findings, Ani et al. [84] analyzed industrial effluents in Enugu, Nigeria. High total dissolved solids (TDS) levels were reported across two primary sectors—beverage production and fiber-cement manufacturing facilities. While the exact concentration figures from these samples were not specified, the study indicated overall TDS presence within the prescribed drinking water guideline limits. As per the requisite quality standards set by World Health Organization [63, 67] alongside the Nigerian regulators through NIS [68], the permissible range for potable water usage is set at 500 mg/L. Comparative analysis highlights that the groundwater sources under the present investigation demonstrated seasonal TDS fluctuations contained within this threshold benchmark.
Analysis of seasonal variation indicates a prominent escalation in total suspended solids (TSS) across the wet period. Concentrations during summer season's dry phase ranged from 70.00 to 270.0 mg/L, averaging 164.2 mg/L. In contrast, the humid rainy interval exhibited elevated TSS levels of 100.0–280.0 mg/L, with mean values of 186.7 mg/L. This 13% increment could likely result from higher runoff and soil erosion rates carrying greater particulate matter fluxes into the groundwater aquifers. However, both seasonal averages fall within the prescribed limit of 2500 mg/L set by WHO guidelines for potable supplies. Granular evaluation of particle size distribution can offer further insights into the origin and transportation dynamics.
A similar escalating trend was exhibited in total solids (TS) moving from summer to humid months. Dry season levels ranged between 70.00 and 390.0 mg/L, averaging 226.7 mg/L. In contrast, elevated rainy phase concentrations were recorded spanning 110.0–560.0 mg/L with mean values reaching 311.8 mg/L. This implied 38% rise potentially stemmed from heightened particulate and dissolved species contributions from increased environmental interfaces interaction and surface runoff. While the obtained seasonal averages aligned within WHO’s upper limit of 500 mg/L, the readings exceeded NAFDAC’s more stringent potable water benchmark of 100 mg/L. The present data indicates that current water resources demonstrate episodic quality fluctuations beyond ideal quality thresholds considered over broader usage safety guidelines.
The study analysis indicated a seasonal rise in both total suspended solids (TSS) and total dissolved solids (TDS) in the groundwater samples from dry to rainy periods. The escalation indicates infiltration intensity and hydraulic gradients introduced by precipitation events that enhance erosion and entrainment rates of sediment particles. This facilitates their detachment from coal rubble piles and waste heaps within mines in the Enugu region and subsequent transfer through fractures or porous media into underlying aquifers.
4.6 Total acidity
Analysis indicates a seasonal fluctuation in total acidity concentrations across the water samples. Levels ranged between 0.100 and 10.50 mg/L during wet months, rising substantially from the 0.060 to 1.240 mg/L interval observed in dry periods. Correspondingly, average values saw an eightfold escalation from 0.347 to 2.739 mg/L. This spike potentially resulted from heightened leaching of dissolved acidic radicals into groundwater reservoirs during monsoon rainfall. Percolating fluxes introduce inorganic species into subterranean aquifers. Benchmarking against prior findings, total acidity distributions during summer align comparably with ranges reported for Enugu boreholes by Engwa et al. [10]. This suggests consistency with regional ambient water chemistry patterns. Additionally, seasonal averages remained within permissible limits for potable supplies as recommended by WHO [67] guidelines. Granular analysis of contributing acidic compounds can offer further insights into concentration dynamics. But present variability indicates largely compliant water quality in terms of acidity-induced usage risk considerations.
4.7 Total alkalinity
Analysis of total alkalinity indicates a reverse trend to acidity, with levels reducing from summer to monsoon months. Dry season concentrations ranged between 28.00 and 52.10 mg/L, averaging 36.48 ± 7.398 mg/L. In contrast, a narrower span of 10.00–22.10 mg/L was observed during humid conditions, with mean values declining to 29.07 mg/L. The 29% drop could potentially result from agricultural runoff influencing groundwater chemistry. However, distributions across both periods aligned within permissible limits for drinking water as prescribed by World Health Organization [67]and Nigerian Industrial Standards [68]. Prior local findings in Enugu boreholes by Edeonovo [9] and broader south-eastern region spikes by Okoye and Adiele [85] demonstrated similar compliance, substantiating consistency with ambient water quality patterns. Comparable dry and wet seasonal fluctuations have been reported in other Nigerian states too by researchers like Amangabara and Ejenma [86] alongside Essien and Bassey [87]. The current variability indicates largely safe potable water reserves in terms of alkaline-induced usage risk considerations.
4.8 Total hardness
Through both seasons, the overall hardness levels were relatively consistent. While the results varied between 100.0 and 110.0 mg/L during the season of rainfall, with an average reading of 118.9 mg/L, the range recorded during the dry season was 110.1–100.2 mg/L. The region's typical soil properties hydrogeology, and geochemistry could explain the uniformity in total hardness across all samples. All water samples collected during both seasons showed total hardness concentrations that fell less of the recommended guideline limit of 150 mg/L by Nigerian Industrial Standards [68], both in terms of range and mean values. Data from previous surveys also indicated reduced hardness percentages in the water from boreholes in Enugu state and other areas of southeast Nigeria in relation to the established standard threshold [88], but some water samples collected in borehole from the environs of Ebonyi, Nigeria were over the specified reference value [89].
4.9 BOD5
The biochemical oxygen demand (BOD5) concentration showed a widespread during both dry and wet monitoring periods across the 12 groundwater sources. In the hot seasons, BOD5 ranged between 3.140 mg/L (site W10) up to 6.370 mg/L (site W3), with an overall average of 5.155 mg/L over all locations. For the rainy measurement phase, the minimum and maximum BOD5 values recorded were 2.880 mg/L and 6.100 mg/L respectively at site W7, culminating in a slightly lower mean of 4.288 mg/L. The decline in average BOD5 from the dry to wet seasons ties in with earlier findings on shallow tropical aquifers by Olajire and Imeokparia [90], who reported an analogous drop from 4.21 mg/L in February–April to 3.18 mg/L in June–August attributable to dilution. However, both seasonal means exceeded the 3 mg/L limit imposed by water quality guidelines [67, 69], indicating significant organic pollution regardless of moisture conditions. This could be ascribed to the extensive permeation of degrading organic matter from pit latrines, farms, and municipal waste dumps prevalent in the area [3, 91]. The longer residence times in dry periods seemingly permitted greater leaching of byproducts from these decaying surface sources or from anoxic reactions with aquifer mineral phases [92, 93]. Hence while dilution by recharge is temporarily beneficial, addressing contaminant infiltration pathways is imperative to restore potability in the long-term.
4.10 COD
The average chemical oxygen demand (COD) levels in the groundwater samples collected over two distinct seasonal cycles showed a differential distribution. During the dry period, COD ranged from 5.840 mg/L (site W8) up to 10.51 mg/L (site W7). Comparatively in the rainy season, the concentration fluctuation was lower, varying between 3.490 mg/L (W7) and 7.450 mg/L (W5). Computing mean values for the two monitoring periods indicated significantly elevated COD in the dry season (5.207 mg/L) relative to the wet season. Such seasonal discrepancies in contaminant indicators have been documented in prior groundwater studies as well. A median COD concentration of 4.50 mg/L in post-monsoon compared to 8.60 mg/L in the pre-monsoon season was reported by Kumar et al. [94] across India. Likewise, greater organic matter influx during summer vs rainfall was observed in an alluvial aquifer in Ganges basin [11]. The likely rationale for this seasonal difference is the continuous buildup of permeating organic solvents and degrading natural organic matter in the aquifers over prolonged dry periods [95]. Their extended residence time and constrained flushing facilitates increased leaching from overlying landfills, industrial zones, or agricultural lands [5]. In contrast, the dilution and sweeping effect of groundwater recharge from precipitation lowers organic strength in the rainy season [96].
4.11 Fe
Fe concentrations in water samples ranged from 0.000 to 11.03 mg/L in the course of the dry season and from 0.000 to 12.29 mg/L in the wet season, respectively. There was a little increase in iron content throughout the period of rainfall, as indicated by both dry and wet season Fe average concentrations of 1.244 mg/L and 1.499 mg/L, respectively, which were both over the acceptable guideline range of 0.30 mg/L [68]. Nwachukwu et al. [64] and Uzoije et al. [97] conducted independent studies on the iron concentration levels in several water sources in the region of south-eastern Nigeria. In the first, elevated iron concentrations were identified in shallow hand-dug wells, lakes/pools, and tributaries, while in the latter, high iron levels were observed in shallow water sources (hand-dug wells and springs) inside the farming settlement as well as in some deep-water reserves like boreholes in Nsukka.
4.12 Zn
The zinc (Zn) concentrations showed variability spanning two orders of magnitude during both dry and wet period monitoring. During the months without rain, Zn ranged from 0.010 to 3.000 mg/L across the groundwater sources. For the rainy season, the recorded values showed a similar spread between 0.020 and 3.610 mg/L. The mean Zn level displayed a subtle rise from 1.317 mg/L in dry periods to 1.437 mg/L during wet conditions. However, the average Zn content for both seasons was found to comply with the maximum contaminant limit of 3.00 mg/L set by international water quality guidelines [67,68,69]. Analogous low quantities have been previously reported from regional aquifers as well by Okoye et al. [88] and Edeonovo [9] in Imo and Enugu urban respectively, attributed to poor mobility in subsurface conditions. The positive correlation between precipitation and Zn availability noted here has also been documented in past assessments of temperate karstic systems [98]. Enhanced infiltration through preferential flow paths seemingly facilitates greater dissolution and mass transfer of zinc salts applied as agricultural fertilizers or accumulated in surface soils [99].
4.13 SO4 2−
According to the findings, the sulphate concentration varied between 0.000 and 100.0 mg/L during the period of rainfall and vary from 20.10 to 24.34 mg/L during the dry time of the year. Additionally, the average sulphate composition during both the dry and wet seasons, respectively, was found to be 21.78 mg/L and 31.27 mg/L, demonstrating a higher concentration of sulphates during the rainy season. Both during the dry and wet seasons, it was discovered that the SO42− content in the groundwater source was below the 200 mg/L [63, 69] regulatory agency mandated permitted level. These findings are consistent with previous studies conducted in Enugu and some regions of Nigeria’s Southeast, which have also reported that the SO42− concentration in deep well water sources are below permissible limit [9, 88].
4.14 Na
The Sodium (Na) levels showed a wide range from 0 to 22.44 mg/L across the groundwater sampling sites during the moisture-deficit summer seasons. In the rainy measurement phase, the concentration span was somewhat similar (0–21.28 mg/L). Computing mean values for both periods indicated average Na contents of 11.81 mg/L and 11.97 mg/L for the dry and wet seasons respectively. The rather consistent sodium values between seasons have also been documented in granitic aquifer assessments by Rădulescu et al. [41], who hypothesized buffering mechanisms regulating subsurface sodium fluxes. Although rainfall dilution can temporarily lower solute levels, continued seepage from overlying soils facilitates regeneration [100]. The means stay well below the allowable limit of 200 mg/L in drinking water standards by NIS [68] and the EU [101]. Analogous low-sodium groundwater has been previously reported from the region as well, including 11.28–19.56 mg/L in Imo state [85] and 5.02–13.8 mg/L in Nsukka [97]. This implies a certain resilience in deeper aquifers against sodium contamination, attributable to strong base cation exchange regulation retaining infiltrating sodium. Monitoring of sodium remains vital considering its implications on hardness, taste, and human health.
4.15 K
Analysis indicates a significant elevation in potassium content from dry to humid seasonal phases across sampled groundwater reserves. Concentration in summer months spanned a range of 0.000–12.03 mg/L, averaging 6.408 mg/L. In contrast, wet period levels were notably higher—spanning 1.320–15.11 mg/L with mean values climbing to 10.17 mg/L. This 58% surge could potentially be explained by the intake of dissolved fertilizer-derived potassium species leached via infiltration and recharged into subterranean aquifers during months corresponding to peak cropping activities. Percolating fluxes introduce minerals into reservoirs underlying farmlands. Findings of comparable studies in southeast Nigeria provide contextual alignment—Uzoije et al. [97] reported presence across hand-dug wells and springs in Nsukka farmland settlements up to 10.0 mg/L, while [85] noted borehole water ranges of 1.00–12.00 mg/L in Imo state.
5 Conclusions and recommendation
In line with the aim of this study, which is to evaluate the effect of precipitation seasonal patterns on groundwater quality in a coal-enriched environment in Enugu Urban, the findings reveal variations in groundwater quality across the 12 clusters analyzed during both the dry and rainy seasons. The Water Quality Index (WQI) was employed to quantify these differences, providing a comprehensive assessment of the overall groundwater quality. During the dry season, the WQI values of the water samples ranged from 26.31 to 150.08, indicating a wide spectrum of groundwater quality, from excellent to poor. In contrast, during the rainy season, the WQI values exhibited a notable increase, ranging from 87.56 to 234.58 highlighting good to very poor quality. This shift in WQI values suggests that precipitation patterns have a substantial impact on groundwater quality in the study area. Among the different clusters Achara layout exhibited the most suitable groundwater quality compared to other areas such as Independence Layout and Abakpa. This finding highlights the importance of considering local hydrogeological conditions and land use patterns when assessing groundwater quality. The groundwater samples analyzed in both seasons demonstrated acidic characteristics, with a notable decrease in pH levels during the rainy season. This trend suggests that precipitation influences the geochemical processes governing groundwater acidity. Some samples exhibited turbidity levels exceeding the recommended standards, indicating the presence of suspended particles that can affect water clarity and overall quality. The study also revealed an increase in total hardness and sulphate levels during the rainy season. These changes are attributed to the enhanced dissolution and transport of minerals and contaminants from the surrounding geological formations during periods of increased precipitation. Considering the study's results, the following recommendations are proposed:
-
As the biochemical oxygen demand (BOD) levels of all 12 well water samples exceeded the standard limits, it is possible that contamination may have originated from biological or microbial sources. Therefore, a bacteriological assessment in Enugu Urban is recommended to identify potential sources of contamination.
-
Prior to utilizing well water sources in the study area for human consumption, a thorough evaluation and/or treatment should be performed to determine their amounts of nitrate, potassium, iron, phosphate and pH as well as possible bacterial parameter concentration and BOD levels
-
In view of the observed seasonal variability of groundwater quality parameters in Enugu Urban, it is recommended that regular water quality tests be conducted to monitor the trends of variation in these parameters. These tests will also help identify the geochemical properties of the region and shed light on the reasons behind the persistence of geographical variance in borehole quality of water.
-
Appropriate adaptation measures like proper water management practices should be implemented to foster resilience to future climate change, thereby enhancing water security.
-
To ensure the provision of safe and clean water, it is imperative for governments to establish collaborative efforts with appropriate institutes to carryout regular and seasonal investigations on water quality across several sources, with a particular focus on ground water. Such studies are crucial for effective monitoring and aid in the identification of potential hazards and the implementation of appropriate interventions.
The implication of the findings of this study underscores the need for regular monitoring and management of groundwater resources in response to changing precipitation patterns. The variations in groundwater quality between the dry and rainy seasons emphasize the importance of considering seasonal factors in groundwater assessment and protection strategies. Furthermore, the spatial differences in groundwater quality among the studied clusters highlight the necessity of tailored approaches based on local hydrogeological conditions. Future research should focus on long-term monitoring of groundwater quality in the region, taking into account the potential impacts of climate change on precipitation patterns and groundwater recharge. Additionally, investigating the specific geological and anthropogenic factors contributing to the observed variations in groundwater quality would provide valuable insights for sustainable water resource management in Enugu Urban.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials, available at the behest of the first author.
References
Behailu TW, Badessa TS, Tewodros BA. Analysis of physical and chemical parameters in ground water used for drinking around Konso Area, Southwestern Ethiopia. J Anal Bioanal Techn. 2017;8(5):379. https://doi.org/10.4172/2155-9872.1000379.
UNESCO. The United Nations World Water Development Report 2021: Valuing Water. UNESCO, Paris.
Adelana SMA, Abiye TA, Nkhuwa DCW, Tindimugaya C, Oga MS. Urban groundwater management and protection in Sub-Saharan Africa. Appl Groundw Stud Afr. 2008. https://doi.org/10.1201/9780203889497-1.
Taylor RG, Scanlon B, Döll P, Rodell M, van Beek R, Wada Y, et al. Ground water and climate change. Nat Clim Chang. 2013;3(4):322–9. https://doi.org/10.1038/nclimate1744.
Lapworth DJ, MacDonald AM, Krishan G, Rao MS, Gooddy DC, Darling WG. Groundwater recharge and age-depth profiles of intensively exploited groundwater resources in northwest India. Geophys Res Lett. 2015;42(18):7554–62. https://doi.org/10.1002/2015gl065798.
Nnaemeka-Okeke RC. Urban sprawl and sustainable city development in Nigeria. J Ecol Eng. 2016;17(2):1–11. https://doi.org/10.12911/22998993/.
Okeke FO, Eziyi IO, Udeh CA, Ezema EC. City as habitat; assembling the fragile city. Civ Eng J. 2020;6(6):1143–54. https://doi.org/10.28991/cej-2020-03091536.
Okeke FO, Ezema EC, Nnaemeka-Okeke RC, Okosun AE, Okeke CA. Architectural design response to population issue in sub-Saharan cities. E3S Web Conf. 2023;434:02005. https://doi.org/10.1051/e3sconf/202343402005.
Edeonovo MJ. Quality assessment of borehole waters in Enugu urban [master’s thesis]. Anambra: Nnamdi Azikiwe University; 2010.
Engwa AG, Tagbo NR, Iyala CP, Unaegbu M. Physicochemical and microbial analysis of portable water sources in Enugu Metropolis. J Public Health Epidemiol. 2015;7(3):65–70. https://doi.org/10.5897/jphe2014.0680.
Datta PS, Tyagi SK. Major ion chemistry of groundwater in Delhi area: chemical weathering processes and groundwater flow regime. J Geol Soc India. 1996;47(2):179–88.
Sawyerr HO, Adedotun AT, Abiodun SA, Salami OO. Impact of dumpsites on the quality of soil and groundwater in satellite towns of the Federal Capital Territory, Abuja, Nigeria. J Health Pollut. 2017;7(14):15–21. https://doi.org/10.5696/2156-9614-7.14.15.
World Bank. Why we need to close the infrastructure gap in Sub-Saharan Africa 2017. https://www.worldbank.org/en/region/afr/publication/why-we-need-to-close-the-infrastructure-gap-in-sub-saharan-africa. Accessed 26 Feb 2023.
Vivien F, Nataliya P. Nigeria’s infrastructure: a continental perspective. World Bank policy research working paper. 2011. https://doi.org/10.1596/1813-9450-5686.
Estache A. Infrastructure and development: a survey of recent and upcoming issues. Rethink Infrastruct Dev. 2008;2:47.
Oyelami AC, Ojo OA, Aladejana JA, Agbede OO. Assessing the effect of a dumpsite on groundwater quality: a case study of Aduramigba estate within Osogbo metropolis. J Environ Sci Earth Sci. 2013;3:120–31.
Edet A. Hydrogeology and groundwater evaluation of a shallow coastal aquifer, southern Akwa Ibom State (Nigeria). Appl Water Sci. 2016;7:2397–412. https://doi.org/10.1007/s13201-016-0432-1.
Barbieri M, Barberio MD, Banzato F, Billi A, Boschetti T, Franchini S, et al. Climate change and its effect on groundwater quality. Environ Geochem Health. 2021. https://doi.org/10.1007/s10653-021-01140-5.
Eneh EO, Oluigbo SN. Mitigating the impact of climate change through waste recycling. Res J Environ Earth Sci. 2012;4(8):776–81.
IPCC. The science of climate change. Cambridge: Cambridge University Press; 1995. p. 53.
Ojolowo S, Wahab B. Municipal solid waste and flooding in Lagos metropolis, Nigeria: deconstructing the evil nexus. J Geogr Reg Plan. 2017;10:174.
Whitehead PG, Wilby RL, Battarbee RW, Kernan M, Wade AJ. A review of the potential impacts of climate change on surface water quality. Hydrol Sci J. 2009;54(1):101–23. https://doi.org/10.1623/hysj.54.1.101.
Guasch H, Serra A, Corcoll N, Bonet B, Leira M. Metal ecotoxicology in fluvial biofilms: potential influence of water scarcity. In: Hutzinger O, editor. The handbook of environmental chemistry. Berlin: Springer; 2010. p. 41–53.
Ezekwe CI, Edoghotu MI. Water quality and environmental health indicators in the Andoni River estuary, Eastern Niger Delta of Nigeria. Environ Earth Sci. 2015;74:6123–36. https://doi.org/10.1007/s12665-015-4635-9.
Mallick J, Singh C, AlMesfer M, Kumar A, Khan R, Islam S, Rahman A. Hydro-geochemical assessment of groundwater quality in Aseer Region, Saudi Arabia. Water. 2018;10:1847. https://doi.org/10.3390/w10121847.
Jamiu A, Aladejana R, Kalin M, Philippe S, Ibrahim H. Assessing the impact of climate change on groundwater quality of the shallow coastal aquifer of eastern Dahomey Basin, Southwestern Nigeria. Water. 2020;12:224. https://doi.org/10.3390/w12010224.
Priyantha RS, Kazama S, Sawamoto M. Effects of climate and land use changes on groundwater resources in coastal aquifers. J Environ Manage. 2006;80(1):25–35. https://doi.org/10.1016/j.jenvman.2005.08.008.
Linzey D. Atlantic climate adaptation solutions association saltwater intrusion and climate change 2011. http://www.gov.pe.ca/photos/original/cle_WA1.pdf. Accessed 15 Jan 2021.
Hiyama T, Babiker IS, Mohamed MA. Groundwater as a Key for adaptation to changing climate and society. Tokyo: Springer; 2014.
Unsal B, Yagbasan O, Yazicigil H. Assessing the impacts of climate change on sustainable management of coastal aquifers. Environ Earth Sci. 2014;72(7):2183–93. https://doi.org/10.1007/s12665-014-3130-z.
Nistor MM, Dezsi S, Cheval S, Baciu M. Climate change effects on groundwater resources: a new assessment method through climate indices and effective precipitation in Beli ̧s district, Western Carpathians. Meteorol Appl. 2016;23(3):554–61. https://doi.org/10.1002/met.1578.
Serdeczny O, Adams S, Baarsch F, Coumou D, Robinson A, Hare W, Schaeffer M, Perrette M, Reinhardt J. Climate change impacts in Sub-Saharan Africa: from physical changes to their social repercussions. Reg Environ Change. 2017;17(6):1585–600. https://doi.org/10.1007/s10113-015-0910-2.
Adegbola R, Oseni S, Majolagbe A. The impact assessment of oil spillage and trace metal assessment on groundwater in Baruwa, Lagos Southwest and Nigeria. Int Sci Investig J. 2013;2:12–32.
Ayolabi EA, Folorunso AF, Kayode OT. Integrated geophysical and geochemical methods for environmental assessment of municipal dumpsite system. Int J Geosci. 2013;4:850–62.
Aslam RA, Shrestha S, Pandey VP. Groundwater vulnerability to climate change: a review of the assessment methodology. Sci Total Environ. 2017;612:853–75. https://doi.org/10.1016/j.scitotenv.2017.08.237.
Buffoni L, Brunetti M, Mangianti F, Maugeri M, Nanni T. Variations in climate in Italy in the last 130 years. Bollettino Geofisico Year. 2002;XXIII.
Labat D, Godderis Y, Probst JL, Guyot JL. Evidence for global runoff increase related to climate warming. Adv Water Resour. 2004;27:631–42. https://doi.org/10.1016/j.advwatres.2004.02.020.
Huntington TG. Evidence for intensification of the global water cycle: review and synthesis. J Hydrol. 2006;319:83–95. https://doi.org/10.1016/j.jhydrol.2005.07.003.
IPCC. The physical science basis—summary for Policymakers. Contribution of WG1 to the Fourth assessment report of the Intergovernmental Panel on Climate Change. 2007. http://www.ipcc.ch/ipccreports/ar4-wg1.htm. Accessed 26 Feb 2021.
Dragoni W, Sukhija BS. Climate change and groundwater: a short review. Geol Soc Lond Spec Publ. 2008;288:1–12. https://doi.org/10.1144/SP288.1.
Rădulescu A, Lungu M, Păunescu G, Ghinea C, Rădulescu D. Hydrochemical evaluation of groundwater and the mechanisms controlling dissolved constituents in the Cotmeana River Granitic Bedrock Aquifer (Romania). Expo Health. 2020;12(4):611–26.
Dixon LH, Leighton LH. Assessment of coal processing in Nigeria. Int J Eng Res Dev. 1997;40–5.
Martin ZP, Angermeier PL, Ciparis S, Orth DJ. Coal-mining intensity influences species and trait distributions of stream fishes in two Central Appalachian watersheds. Ecol Freshw Fish. 2020;30(3):347–65. https://doi.org/10.1111/eff.12588.
Martin JD, Duwelius RF, Crawford CG. Effects of surface coal mining and reclamation on the geohydrology of six small watersheds in West-Central Indiana. Reston: US Geological Survey; 1990. https://doi.org/10.3133/wsp2368b.
Adelagun ROA, Etim EE, Godwin OE. Application of water quality index for the assessment of water from different sources in Nigeria. In: Moujdin IA, Summers JK, editors. Promising techniques for wastewater treatment and water quality assessment. London: IntechOpen; 2021. https://doi.org/10.5772/intechopen.98696.
Nemerow NL, Sumitomo H. Benefits of water quality enhancement. Report No. 16110 DAJ, prepared for the U.S. Environmental Protection Agency. Syracuse: Syracuse University; 1970.
Chauhan A, Singh S. Evaluation of ganga water for drinking purpose by water quality index at Rishikesh, Uttarakhand, India. Rep Opin. 2010;2(9):53–61.
Balan IN, Shivakumar M, Kumar PDM. An assessment of groundwater quality using water quality index in Chennai, Tamil Nadu, India. Chron Young Sci. 2012;3:146–50.
Asaah AV. Geology and mineral resources of Nigeria. Econ Geol. 2011;106(3):523. https://doi.org/10.2113/econgeo.106.3.523.
Maina B, Kachalla A, Comfort CA. Impact of coal mining on the environment in Mainganga Community of Akko Local Government, Gombe State, Nigeria. Glob J Hum-Soc Sci. 2016;16(3):11–24.
Okeke FO, Echendu IF, Nnaemeka-Okeke RC. Determinants of intra-urban travel in southeast Nigeria; evidence from city of Enugu. Transp Probl. 2021;16(4):185–98. https://doi.org/10.21307/tp-2021-070.
Echendu IF, Okeke FO, Nnaemeka-Okeke RC. An investigation of challenges in the existing pattern of intra-city traffic in Enugu metropolis. TEMA J Land Use Mobil Environ. 2020;13(2):191–208. https://doi.org/10.6092/1970-9870/6797.
Okeke FO, Okosun AE, Udeh CA, Okekeogbu CJ. Cities for people: the dependency & impact of automobile in the life of city dwellers. Eur J Sustain Dev. 2020;9(3):157–78. https://doi.org/10.14207/ejsd.2020.v9n3p157.
Okeke FO, Gyoh L, Echendu FI. Impact of land use morphology on urban transportation. Civ Eng J. 2021;7(10):1753–73. https://doi.org/10.28991/cej-2021-03091758.
Ikem A, Osibanjo O, Sridhar MKC, Sobande A. Evaluation of groundwater quality characteristics near two waste sites in Ibadan and Lagos, Nigeria. Water Air Soil Pollut. 2002;140:307–33. https://doi.org/10.1023/A:1020165403531.
NIMET. Nigerian Meteorological Agency weather review bulletin 2017. Abuja: Nigerian Meteorological Agency; 2017.
APHA. Standard methods for the examination of water and wastewater. Washington, DC: American Public Health Association; 1998.
Meranger JC, Subramanian KS, Chalifoux CA. National survey of cadmium, copper, lead, zinc, Calcium and magnesium in Canadian Drinking Water Supplies. Environ Sci Tech. 1997;13:707–11.
Helrich K. Official methods of analysis of the association of analytical chemists. 15th ed. Arlington: Association of Analytical Communities; 1990.
Lenore SC, Arnold EG, Andrew DE. Standard methods for the examination of water and wastewater. Arlington: American Public Health Association (APHA); 1999.
Edwards GP, Molof AH, Schneeman RW. Determination of orthophosphate in fresh and saline waters. J Am Water Works Assoc. 1965;57:917. https://doi.org/10.1002/j.1551-8833.1965.tb01479.x.
Murphy J, Riley J. A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta. 1962;27:31. https://doi.org/10.1016/S0003-2670(00)88444-5.
World Health Organization. Guidelines for drinking-water quality. Third Edition, Incorporating 1st and 2nd Addenda, vol. 1, Recommendations. Geneva: WHO Press; 2008.
Nwachukwu RE, Agbazue VE, Ihedioha JN, Ifeanyi ES. Health- risk assessment in relation to heavy metals in water sources in rural regions of Southeast Nigeria. Int J Phys Sci. 2014;9(6):109–16.
Cooray T, Wei Y, Zhong H, Zheng L, Weragoda SK, Weerasooriya R. Assessment of groundwater quality in CKDu affected areas of sri lanka: implications for drinking water treatment. In: Moujdin IA, Summers JK, editors. Promising techniques for wastewater treatment and water quality assessment. London: IntechOpen; 2021. p. 1698. https://doi.org/10.3390/ijerph16101698.
World Health Organization. Chronic kidney disease of unknown origin (CKDu): national research programme for chronic kidney disease of unknown origin (CKDu) in Sri Lanka. Geneva: World Health Organization; 2013.
World Health Organization. Guidelines for drinking-water quality: fourth edition incorporating the first addendum, vol. 4.1.2. Geneva: WHO Press; 2017. p. 631.
Nigerian Industrial Standards. Nigerian standard for drinking water quality. Nigerian Industrial Standard NIS, 554; 2007.
National Agency for Food and Drug Administration and Control. Guidelines for registration and production of packaged water in Nigeria. Abuja: NAFDAC; 1999.
Hudson PF, Heitmuller FT, Leitch MB. Hydrologic connectivity of oxbow lakes along the lower Guadalupe River, Texas: the influence of geomorphic and climatic controls on the ‘flood pulse concept.’ J Hydrol. 2012;414–415:174–83. https://doi.org/10.1016/j.jhydrol.2011.10.029.
Maulé C, Stein J. Hydrologic flow path definition and partitioning of spring meltwater. Water Resour Res. 1990;26:2959–70.
Molénat J, Gascuel-Odoux C, Aquilina L, Ruiz L. Use of gaseous tracers (CFCs and SF6) and transit-time distribution spectrum to validate a shallow groundwater transport model. J Hydrol. 2013;480:1–9. https://doi.org/10.1016/j.jhydrol.2012.11.043.
Güler C, Thyne GD, McCray JE, Turner KA. Evaluation of graphical and multivariate statistical methods for classification of water chemistry data. Hydrogeol J. 2002;10(4):455–74. https://doi.org/10.1007/s10040-002-0196-6.
Bahir M, El Mountassir O, Chehbouni A, Dhiba D, El Jiar H, Carreira PM. Hydrogeochemical and isotopic assessment for characterizing groundwater quality and recharge processes in the Essaouira Basin, Northwestern Morocco. Arab J Geosci. 2022. https://doi.org/10.1007/s12517-022-09817-6.
Ramngaihawma B, Kumar S, Bharti VK, Tluanga M, Blick J. Physico-chemical analysis of selected potable water in parts of Mamit District, Mizoram. Sci Technol J. 2021;9(1):77–82. https://doi.org/10.22232/stj.2021.09.01.13.
Rao SE, Ray L, Khan T, Ravi G. Thermal conductivity, density and porosity of sedimentary and metamorphic rocks from the Lower and Higher Himalaya, Western Himalaya, India. Geophys J Int. 2022;231(1):459–73. https://doi.org/10.1093/gji/ggac176.
Kadlec RH, Reddy KR. Temperature effects in treatment wetlands. Water Environ Res. 2001;73(5):543–57. https://doi.org/10.2175/106143001x139614.
Mattison RG, Taki H, Harayama S. The bacterivorous soil flagellate Heteromita globosa reduces bacterial clogging under denitrifying conditions in sand-filled aquifer columns. Appl Environ Microbiol. 2002;68(9):4539–45. https://doi.org/10.1128/AEM.68.9.4539-4545.2002.
Barbosa AE, Fernandes JNS, David LM. Key issues for sustainable urban stormwater management. Water Res. 2012;46(20):6787–98. https://doi.org/10.1016/j.watres.2012.05.029.
World Health Organization. Guidelines for drinking-water quality, vol. 1. 3rd ed. Geneva: World Health Organization; 2004.
Gupta DP, Sunita, Saharan JP. Physiochemical analysis of ground water of selected area of Kaithal City (Haryana) India. Researcher. 2009;1(2):1–5.
Pareta K, Pareta U. Study of physico-chemical characteristics of Yamuna River around Hamirpur (UP), Bundelkhand region central India. Int J Appl Biol Pharm Technol. 2011;2(1):398–403.
Jain CK, Bandyopadhyay A, Bhadra A. Assessment of ground water quality for drinking purpose, District Nainital, Uttarakhand, India. Environ Monit Assess. 2010;166(1):663–76. https://doi.org/10.1007/s10661-009-1031-5.
Ani JU, Asegbeloyin JN, Melkiti MC. Physicochemical characterization of industrial effluents: case studies of beverage and fibre cement plants in Enugu, Nigeria. N Y Sci J. 2011;4(4):114–7.
Okoye COB, Adiele GC. Physicochemical and bacteriological qualities of groundwater resources in Ezinihitte Mbaise Local Government Area of Imo State, Nigeria. Int J Chem Sci. 2014;12(1):23–38.
Amangabara GT, Ejenma E. Groundwater quality assessment of Yenagoa environs, Bayelsa State, Nigeria between 2010 and 2011. Resour Environ. 2011;2(2):20–9. https://doi.org/10.5923/j.re.20120202.04.
Essien OE, Bassey ED. Spatial variation of borehole water in Uyo Municipality, Nigeria. Int J Environ Sci Manag Eng Res. 2012;1(1):1–9.
Okoye COB, Ugwu JN, Ibeto C. Characterization of rural water resources for potable water supply in some parts of south eastern Nigeria. J Chem Soc Nigeria. 2010;35(1):83–8.
Omaka ON, Offor IF, Onwe IM. Hydrogeochemical attributes and groundwater quality in Ngbo community of Ohaukwu area council, Ebonyi State, Nigeria. Int J Appl Environ Sci. 2015;10(1):35–47. https://doi.org/10.4136/ambi-agua.1453.
Olajire AA, Imeokparia FE. Water quality assessment of Osun River: studies on inorganic nutrients. Environ Monit Assess. 2001;69(1):17–28. https://doi.org/10.1023/a:1010796410829.
Nwankwoala HO, Udom GJ. Hydrochemical facies and ionic ratios of groundwater in Port Harcourt, Southern Nigeria. Res J Environ Earth Sci. 2011;3(6):601–8.
Wakida FT, Lerner DN. Non-agricultural sources of groundwater nitrate: a review and case study. Water Res. 2005;39(1):3–16. https://doi.org/10.1016/j.watres.2004.07.026.
Soladoye O, Ajibade LT. A groundwater quality study of Lagos State, Nigeria. Int J Appl Sci Technol. 2014;4(4):271.
Kumar M, Ramanathan A, Rao MS, Kumar B. Identification and evaluation of hydrogeochemical processes in the groundwater environment of Delhi. India Environ Geol. 2006;50(7):1025–39. https://doi.org/10.1007/s00254-006-0275-4.
Zhang H, Sun H, Bian K, Niu Y, Yang H, Yang J, Balaji P. Seasonal variation characteristics of karst groundwater chemistry and water quality assessment in the northern part of the Baiquan Spring Area. J Water Chem Technol. 2024;46:80–92. https://doi.org/10.3103/S1063455X24010119.
Li P, Qian H, Howard KWF, Wu J, Lyu X. Anthropogenic pollution and variability of manganese in alluvial sediments of the Yellow River, Ningxia, northwest China. Environ Monit Assess. 2014;186(3):1385–98. https://doi.org/10.1007/s10661-013-3461-3.
Uzoije AP, Onunkwo AA, Ibeneme SI, Obioha EY. Hydrogeology of Nsukka South-east—a preliminary approach to water resources development. Am J Eng Res. 2014;3(1):150–62.
Vesper DJ, White WB. Metal transport to karst springs during storm flow: an example from Fort Campbell, Kentucky/Tennessee, USA. J Hydrol. 2003;276(1–4):20–36. https://doi.org/10.1016/S0022-1694(03)00023-4.
Bradl HB. Adsorption of heavy metal ions on soils and soils constituents. J Colloid Interface Sci. 2004;277(1):1–18. https://doi.org/10.1016/j.jcis.2004.04.005.
Jalali M. Salinization of groundwater in arid and semi-arid zones: an example from Tajarak, western Iran. Environ Geol. 2007;52:1133–49. https://doi.org/10.1007/s00254-006-0551-3.
Union E. Drinking water regulations 2014: statutory instruments. Dublin: EU Government Publications Stationary Office; 2014.
Acknowledgements
The authors acknowledge the support of Peter Eze-Steven of Enugu state university of science and technology Nigeria, for the laboratory and technical assistance of the manuscript when it was first presented in a conference.
Funding
This research did not have the courtesy of any funding from any organization.
Author information
Authors and Affiliations
Contributions
Rosemary Chidinma Nnaemeka-Okeke: Conceptualization, method, Analysis, Writing- Original draft and Data collection. Francis Ogochuwku Okeke: Conceptualization, literature review, validation of results, method, analysis, discussion, and final editing. All authors have read, understood, and have complied as applicable with the statement on “Ethical responsibilities of Authors” as found in the Instructions for Authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study adhered to the ethics guidelines of the University of Nigeria and followed the Declaration of Helsinki—principles of informed consent, voluntary participation and withdrawal, confidentiality, and privacy of the participants. The authors confirm they sought and got consent from all participants in the study.
Consent for publication
All named authors in the paper have agreed that the manuscript be submitted for publication.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nnaemeka-Okeke, R.C., Okeke, F.O. Assessing the influence of seasonal precipitation patterns on groundwater quality in the coal rich environment of Enugu, Nigeria. Discov Appl Sci 6, 208 (2024). https://doi.org/10.1007/s42452-024-05837-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-024-05837-x