Abstract
Quinoa (Chenopodium quinoa Willd.) plants possess epidermal bladder cells (EBCs) on the leaf surface that accumulate excess sodium (Na+). However, whether excess cesium (Cs+) is transported from the leaf to the EBCs has not been elucidated in quinoa plants. In this study, the Cs+ concentration of EBCs and leaves of quinoa plants grown in soil treated with high concentrations of NaCl was investigated via pot experiments. Three different treatments were performed: 9.75 g plot, and 19.50 g plot, and a control (with no added NaCl). In 9.75 g plot and 19.50 g plot, 9.75 g and 19.5 g of NaCl were applied to the soil, respectively. And 0.10 g of CsCl were applied to all pots. We observed that Na+ concentration in EBCs and leaves with and without EBCs increased with increasing NaCl concentration at the vegetative and flowering stages; Na+ concentration was lower in the EBCs than in both types of leaves at both growth stages. Cs+ concentration in EBCs and both types of leaves increased with increasing NaCl concentration; Cs+ concentration was higher in EBCs than in both types of leaves at both growth stages. However, NaCl application did not affect the number of EBCs at both growth stages, but the number of EBCs in older leaves was lower than that in younger leaves at both growth stages. Moreover, EBC diameter increased with NaCl application at both growth stages; the EBCs of younger leaves (1st leaf) were larger than those of older leaves (5th leaf) at both growth stages. Therefore, NaCl increased the Cs+-accumulation capacity of quinoa plants by increasing the size of the EBCs.
Article Highlights
-
The concentration of Cs+ in the EBCs was promoted by tNaCl application and the concentrations of Cs+ were higher in the EBCs than in the leaves.
-
The size of the EBCs increased with increasing application rate of NaCl.
-
NaCl enabled the accumulation of Cs+ in EBCs of quinoa plants by increasing the size of EBCs and Cs concentration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
High levels of radioactive cesium (Cs) were released into the environment due to the Fukushima Daiichi Nuclear Power Plant accident (March 11, 2011) and the Chernobyl Nuclear Power Plant accident (April 26, 1986). The released radioactive Cs were deposited in agricultural fields, forest soils, and residential areas. Therefore, efficient and cost-effective methods for the removal of Cs from the soil are essential to manage such disasters in the future. After the Fukushima Daiichi accident, Cs were removed from the soil by stripping the soil surface, which is a costly and labor-intensive method. Phytoremediation is a cost-effective alternative for Cs removal, but earlier it was not considered feasible because of the low Cs-absorbing capacity of plants [1,2,3]. However, in 2021, a protein involved in Cs uptake was identified in Arabidopsis roots [4]. Recently, several studies have reported that phytoremediation has application potential for the removal of Cs from soil [5, 6]. However, for the phytoremediation of Cs-contaminated soils, the plants should have high Cs-absorbing ability and biomass. Quinoa plants (Chenopodium quinoa Willd.) have been reported to exhibit the highest Cs-absorbing capacity [7, 8] and biomass [9, 10]. Therefore, quinoa is one of the most commonly used plants for the phytoremediation of Cs-contaminated soils. Moreover, previous studies have reported that the growth of and absorption of Cs by quinoa plants was enhanced by the application of NaCl in soil [9,10,11,12]. Wada et al. [10] reported that the maximum proportion of Cs was accumulated in the leaves of quinoa plants, and Cs was not translocated from the leaves to the panicle after the seed-filling stage. Cs are toxic to plants when absorbed at high concentrations [13,14,15,16,17], and high concentrations of Cs accumulated in quinoa leaves should be extracellularly excreted or detoxified. Quinoa plants possess epidermal bladder cells (EBCs) on the leaf surface, and excess Na accumulates in these cells [18,19,20]. However, whether the accumulated Cs are transported from the leaves to the EBCs in quinoa plants is unclear. Thus, in this study, we investigated the concentration of Cs in EBCs and leaves of quinoa plants grown in soil treated with high concentrations of NaCl.
2 Materials and methods
2.1 Cultivation conditions and plots
The present study was conducted in an experimental field at Nihon University (Fujisawa City, Kanagawa, Japan) in 2019, and 2020. We used the quinoa (Chenopodium quinoa Willd.) variety CICA-127 for this study. The experiments were performed in Wagner pots (1/5000a a; diameter: approximately 16 cm; height: approximately 25 cm) filled with 2.6 kg of field soil, 0.95 g of ammonium sulfate (TORAY Ind., Tokyo, Japan), 1.14 g of superphosphate (Katakura & Co-op Agri Corporation), and 0.10 g of CsCl (Wako Pure Chemical Industries, Tokyo, Japan). This field soil was used for all plots, including the control. The pots were subjected to three different plots: 9.75 g NaCl plot, 19.50 g NaCl plot [10] and control (0.00 g NaCl) treatments. In the 9.75 g and 19.50 g plots, 9.75 g and 19.5 g of NaCl were added to the field soil, respectively. NaCl and fertilizer were mixed with 2.6 kg of soil, and CsCl was added to the top 5 cm of the soil in all pots. Twenty quinoa seeds per pot were sown on May 16, 2019, and on June 22, 2020. All pots (60 pots per plot) were placed randomly and independently in unheated vinyl (roof) and net (side) houses from the sowing stage to the leaf sampling stage. Weeds, diseases, and insects were controlled during cultivation. The seedlings were thinned to three plants per pot at the third-leaf stage.
2.2 Experiment 1: Determination of Cs content in leaves and EBCs
All leaves were sampled from 15 pots in each plot on the following dates: June 24, 2019 (vegetative stage); July 22, 2019 (flowering stage); August 3, 2020 (vegetative stage); and August 24, 2020 (flowering stage). In this experiment, leaves from three pots were used as one replicate; thus, five replicates were prepared for each growth stage in a randomized block design. After removing the EBCs from the leaf surface using a brush [21], the leaves without EBCs, and EBCs were collected. Intact leaves (with EBCs) were collected from the quinoa plants in the remaining 3 pots at both stages. The EBCs and the leaves with and without EBCs were dried at 80 °C for 48 h using a drying machine. The dried leaves with and without EBCs were ground to a powder using a blender. For the measurement of Cs, K, and Na concentrations, 0.3–0.5 g of ground material and EBCs was digested in 20.0 mL of HClO4 (Kanto Chemical Co., Inc., Tokyo, Japan) for 3 h at 100 ℃ using an acid digestion system, and Cs, K, and Na concentrations were determined using atomic absorption spectrophotometry (AAS; iCE 3300 AAS Thermo Fisher Scientific, Waltham, MA, USA).
The top 5 cm of the soil from each plot (0.00 g plot, 9.75 g plot and 19.50 g plot) at the time of sowing was air-dried to measure the following physicochemical properties of the soil: pH (H2O), electrical conductivity (EC), and total Cs, K, and Na concentrations. The pH and EC were measured using a glass electrode and 1:5 water extraction method, respectively. For the measurement of total Cs, K, and Na concentration, 1.0 g of air-dried soil was digested in 20.0 mL of HClO4 (Kanto Chemical Co., Inc., Tokyo, Japan) for 3 h at 100 ℃ using an acid digestion system, and Cs, K, and Na concentrations were determined using atomic absorption spectrophotometry (iCE 3300 AAS Thermo Fisher Scientific, Waltham, MA, USA).
2.3 Experiment 2: Determination of the number and diameter of EBCs
The leaves on the upper 1st, 3rd, and 5th nodes of the main stem of six pots in each plot were sampled on June 24, 2019 (vegetative stage); July 22, 2019 (flowering stage); August 3, 2020 (vegetative stage); and August 24, 2020 (flowering stage). The leaves on the upper first node of the main stem are the youngest leaves on the main stem, and the leaves on the third and fifth nodes become older. In this experiment, leaves from one pot (three plants) were used as one replicate; thus, six replicates were prepared for each growth stage in a randomized block design. The number and diameter of EBCs were analyzed at the face and the center of the leaf surface using scanning electron microscopy (SEM) (S-3500N Hitachi Ltd., Tokyo, Japan). The number of EBCs was counted in a 0.292-mm2 leaf area (length × width: 623 µm × 468 µm) of 18 plants (3 plants × 6 replicates) in each plot. The diameter of the EBCs (μm) was measured in randomly selected nine cells per leaf of 18 plants in each leaf position and plot. The diameter was measured with a distance measurement system on the screen of an electric microscope.
2.4 Statistical analysis
In experiment 1, all values are expressed as average and their standard error. Significant differences at 5% level among plots (0.00 g, 9.75 g and 19.50 g plots) and parts (leaf, EBC and leaf without EBC) were determined using Tukey’s multiple means test and three-way (year, part, and plot) analysis of variance (ANOVA) using the Kaleida Graph ver. 4.0 software. In Table 1, the lowercase letters indicate between plots in each year. In Tables 2,3 and 4, the lowercase letters indicate between plots in each year, the capital letters indicate between parts (leaf, EBC and leaf without EBC) in each plot and year.
In experiment 2, all values are expressed as average and their standard error. The significant differences at 5% level among the plots (0.00 g, 9.75 g and 19.50 g plots) and leaf position on the main stem (1st, 3rd and 5th node) were determined using Tukey’s multiple means test and two-way (leaf position and plot) ANOVA using the Kaleida Graph ver. 4.0 software. In Tables 4 and 5, the capital letters indicate between plots in each leaf position.
3 Results
3.1 Physicochemical characteristics of the soil at the sowing stage
Soil pH of 0.00 g plot was 5.60 in both years. EC (electrical conductivity) of 0.00 g plot was 0.54 dS/m in 2019, and 0.59 dS/m in 2020. Na concentration of 0.00 g plot was 1.18 mg/g in 2019, and 3.28 mg/g in 2020. Soil pH of 9.75 g and 19.50 g plots decreased with increasing NaCl application, whereas EC and Na concentration of both plots increased with increasing NaCl application in both years. The EC of the 9.75 g plot was about 3.0 times higher than that of 0.00 g plot in 2020. The EC of the 19.50 g plots in both years was about 8.0 times higher than that of 0.00 g plots. The Na concentration of the 9.75 g plot was about 2.0 times higher than that of the 0.00 g plot in 2020. The Na concentration of the 19.50 g plots in each year was about 9.0 times in 2019, about 4.5 times in 2020 higher than those of the 0.00 g plots. These differences were significant at the 5% level. However, Cs concentration in 2019 and K concentration in both years did not exhibit any change with increasing NaCl application (Table 1).
3.2 Concentration of different ions in EBCs and leaves with and without EBCs
3.2.1 Cs+ concentration
First, the Cs+ concentration of leaves, EBCs and leaves without EBCs were compared between plots each year. The Cs+ concentration in the leaves increased with increasing NaCl application in both growth stages; moreover, except for the flowering stage in 2019. In 2019, there was a significant difference at the 5% level between the 0.00 g and 19.50 g plots at the vegetative stage, in 2020, there was a significant difference at the 5% level between the 0.00 g, and 9.75 g or 19.50 g plots at both growth stages. The Cs+ concentration of the EBCs increased with increasing NaCl application except for vegetative stage in 2020. In 2019, there was a significant difference at the 5% level between the 0.00 g and 19.50 g plots at both growth stages, in 2020, there was a significant difference at the 5% level between the 0.00 g, and 9.75 g or 19.50 g plots at flowering stage. The Cs+ concentration in leaves without EBCs increased with increasing NaCl application at both growth stages. In both years, there was a significant difference at the 5% level between the 0.00 g and 19.50 g plots at both growth stages.
The Cs+ concentration of the same plot was compared between leaves, EBCs and leaves without EBCs at each growth stage in each year. The Cs+ concentration of the EBCs was higher than those of leaves and leaves without EBCs. At vegetative stage in 2019, the Cs+ concentration of leaves and leaves without EBCs of 0.00 g and 9.75 g plots were significantly lower than that of EBCs. At the flowering stage in 2019, the Cs+ concentration of leaves of 19.50 g plot was significantly lower than that of EBCs and leaves without EBC. In 2020, the Cs+ concentration of leaves and leaves without EBCs of all plots at both growth stages, except for leaf without EBCs of 19.50 g plot at vegetative stage were significantly lower than those of EBCs.
Three-way ANOVA detected the significant effect of year, part and plot on the Cs+ concentration at both growth stages (Table 2).
3.2.2 K+ concentration
First, the K+ concentration of leaves, EBCs and leaves without EBCs were compared between plots each year. The K+ concentration of leaves, EBCs and leaves without EBCs did not increase with increasing NaCl application in both growth stages, and years, except for leaves at flowering stage and leaves without EBCs at vegetative stage in 2020.
The K+ concentration of same plot was compared between leaves, EBCs and leaves without EBCs at each growth stage in each year. The K+ concentration of EBCs was significantly higher than those of leaves and leaves without EBCs at both growth stages in both years.
Three-way ANOVA detected the significant effect of year, part and plot on the K+ concentration at both growth stages, except for the plot at flowering stage (Table 3).
3.2.3 Na+ concentration
First, the Na+ concentration of leaves, EBCs and leaves without EBCs were compared between plots each year.
The Na+ concentration in the leaves with EBCs increased with increasing NaCl application at both growth stages in both years. At vegetative stage, the Na+ concentrations of the leaves, EBCs and leaves without EBC of 9.75 g and 19.50 g plots were significantly higher than those of 0.00 g plots in both years. At flowering stage, the Na+ concentrations of the leaves, EBCs and leaves without EBCs of 19.50 g plot were significantly higher than that of 0.00 g plot in both years.
The Na+ concentration of same plot was compared between leaves, EBCs and leaves without EBCs at each growth stage in each year.
At vegetative stage in 2019, the Na+ concentration of leaves and leaves without EBCs of 9.75 g and 19.50 g plots were significantly higher than that of EBCs. At flowering stage in 2019, the Na+ concentration of leaves and leaves without EBCs of 19.50 g plot was significantly higher than that of EBCs. At vegetative stage in 2020, the Na+ concentration of leaves and leaves without EBCs of 9.75 g plots were significantly higher than that of EBCs. At flowering stage in 2020, the Na+ concentration of leaves of 19.50 g plot and leaves without EBCs of 9.75 g and 19.50 g plots were significantly higher than that of EBCs.
Three-way ANOVA detected the significant effect of year, part and plot on the Na+ concentration at both growth stages (Table 4).
3.3 Number and diameter of EBCs
3.3.1 Number
In this table, the first leaf is the youngest among all leaves, and the fifth leaf is older than the first and third leaves. At vegetative stage, the number of EBCs of first, third and fifth leaves of 0.00 g plot were 266.1 cells/mm2, 126.7 cells/mm2 and 60.9 cells/mm2, respectively. At flowering stage, the number of EBCs of first, third and fifth leaves of 0.00 g plot was 285.6 cells/mm2, 146.0 cells/mm2 and 81.9 cells/mm2, respectively. The number of EBCs did not change with increasing NaCl application at both growth stages.
The number of EBCs of same plot was compared between leaf positions at each growth stage.
The number of EBCs of the older leaves was lower than that of the younger leaves at both growth stages. At vegetative stage, the number of EBCs of first leaves of all plots was significantly higher than those of third and fifth leaves, and at flowering stage, the number of EBCs of first leaves of 0.00 g and 19.50 g plots was significantly higher than those of third and fifth leaves.
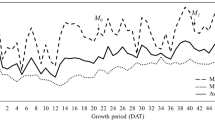
Two-way ANOVA detected the significant effect of leaf position on the number of EBCs at both growth stages, and the significant effect of a plot on the number of EBCs at flowering stage (Table 5, Fig. 1).
3.3.2 Diameter
At vegetative stage, the diameter of EBCs of first, third and fifth leaves of 0.00 g plot was 69.8 μm, 57.9 μm and 50.2 μm, respectively. At flowering stage, the diameter of EBCs of first, third and fifth leaves of 0.00 g plot was 61.4 μm, 57.3 μm and 41.9 μm, respectively. The diameter of the EBCs of first and third leaves increased with increasing NaCl application at vegetative stage. However, the diameter of the EBCs of fifth leaf did not increase with increasing NaCl Application at vegetative stage. In first leaf at vegetative stage, the diameter of EBCs of 9.75 g and 19.50 g plots was significantly bigger than that of 0.00 g plot. In third leaf at vegetative stage, the diameter of EBCs of 9.75 g plot was significantly bigger than that of 0.00 g plot. And at flowering stage, the diameter of the EBCs of first leaf increased with increasing NaCl application. The diameter of EBCs of the third and fifth leaves did not increase with increasing NaCl at flowering stage. In first leaf at flowering stage, the diameter of EBCs of 19.50 g plots was significantly bigger than those of 0.00 g and 9.75 g plots.
The diameter of EBCs of same plot was compared between leaf positions at each growth stage. At vegetative stage, the diameter of EBCs of 9.75 g and 19.50 g plots of first leaf were bigger than those of third and fifth leaves. At flowering stage, the diameter of EBCs of 19.50 g plots of first and third leaves was bigger than that of fifth leaf.
At vegetative stage, the average diameter of EBCs of first, third and fifth leaves was 94.5 μm, 71.7 μm and 58.3 μm, respectively. The average diameter of EBC of 0.00 g, 9.75 g and 19.50 g plots were 59.6 μm, 89.4 μm and 75.5 μm, respectively. At flowering stage, the average diameter of EBCs of first, third and fifth leaves was 68.1 μm, 61.3 μm and 47.4 μm, respectively. The average diameter of EBCs of 0.00 g, 9.75 g and 19.50 g plots were 53.6 μm, 55.6 μm and 67.6 μm, respectively. Two-way ANOVA detected the significant effect of leaf position plot on the diameter of EBCs at both growth stages (Table 6, Fig. 1).
4 Discussion
4.1 Effects of NaCl on the concentration of Na+, K+ and Cs+ ions in leaves and EBCs
The presence of toxic compounds in the cytoplasm or around the nucleus of plant cells impairs their physiological functions and can lead to cell death. However, some plants translocate toxic compounds to the vacuoles to maintain cellular physiology and metabolic functions [22,23,24]. EBCs with large vacuoles are larger than epidermal cells; thus, they push the nucleus to the edge of the cell [25]. Therefore, EBCs accumulate large amounts of toxic substances and ions. Na+ is an ion that inhibits plant growth [26]. In the present study, the average Na+ concentration of the soil and the leaves from both NaCl application (9.75 g and 19.50 g) plots increased (Tables 1 and 4). However, the average Na+ concentration in the EBCs was lower than those in the leaves and leaf without EBCs at both growth stages (Table 4); this result does not follow those of previous studies [18,19,20]. Quinoa is characterized by the presence of EBCs on the surface of leaves, stem, and panicles, and the EBCs accumulate high proportion of Na+, thereby enhancing the salt tolerance of the plants [20, 27,28,29,30,31]. However, Wada et al. [10] reported that in quinoa plants, the highest concentration of Na+ was accumulated in the stems (leaf, stem, and panicle), indicating that quinoa plants can accumulate Na+ in the stem. Thus, quinoa plants accumulate excess Na+ in the EBCs present on the stem surface but not in those on the leaf surface. Moreover, we determined the Na+ concentration of only leaves and EBCs on the leaf surface but did not investigate that of the stems and EBCs on the stem surface. Therefore, to identify the most functional EBCs for Na+ accumulation, it is essential to compare the Na+ concentrations in the EBCs of the leaves and in those of the stems of quinoa plants.
Many plants absorb K+ ions from the soil to maintain cellular osmolality under high salinity conditions [32, 33]. In the present study, the K+ concentration in the leaves and EBCs did not increase with NaCl application except for the leaf at flowering stage and leaf without EBC at vegetative stage in 2020. And the results of two-way ANOVA showed that K+ concentration was not affected by the plot (NaCl application) at flowering stages. Moreover, at average K+ concentration of 0.00 g plot was 92.7 mg/g, and that of 19.50 g plot was 91.9 mg/g. The value of 19.50 g plot was not higher than that of 0.00 g plot (Table 3). One reason for this result could be that K+ was not added to the soil. Moreover, NaCl application increased the absorption of Cs, a Group I alkali metal, instead of K+ (Table 2), and average Cs+ concentration of the EBCs of all plots at both growth stages (2.45 mg/g at vegetative stage, 3.59 mg/g at flowering stage) was higher than that of the leaves (1.62 mg/g and 2.76 mg/g) (Table 2). This suggested that the Cs+ absorbed by the leaves was transferred to the ECBs. At high concentrations, Cs+ is toxic to plants [4, 5, 34]. Moreover, as Cs+ concentration in the cells increases, cytotoxicity increases due to the decrease in or the inhibition of enzyme activity [6]. Therefore, upon absorption of high concentrations of Cs+ by the leaves, it is actively transferred to EBCs, which possess large vacuoles, to avoid damage. Each EBCs complex consists of a leaf epidermal cell, a stalk cell, and a bladder; the stalk cells function as both a selectivity filter and flux controller of ions and other metabolites [35], suggesting that the stalk cells also play an important role in the transport of Cs+ to EBCs. However, further studies are needed to verify this hypothesis. Therefore, most of the absorbed Cs+ in quinoa plants is accumulated in the EBCs on the leaf surface to discharge Cs+ from the leaves.
4.2 Effects of NaCl on the number and size of EBCs
Many halophytes have developed salt glands as an evolutionary mechanism to store and exclude salt [36, 37]. Various types of salt glands are present in plants, including EBCs, multicellular glands, bicellular hair, and unicellular hair [38]. Several studies have reported that EBCs play an important role in enhancing the salt tolerance of quinoa plants [25, 30, 31, 39, 40]. Therefore, we hypothesized that the size and density of EBCs are important factors in conferring the salt-tolerance ability of quinoa plants. In the present study, the average density of EBCs on the leaf surface in younger leaves of all plots was higher than that in mature leaves at both growth stages (244.5 cells/mm2 in first leaf, 118.8 cells/mm2 in third leaf and 55.1 cells/mm2 in fifth leaf at vegetative stage, 243.0 cells/mm2 in first leaf, 140.9 cells/mm2 in third leaf and 73.5 cells/mm2 in fifth leaf at flowering stage) (Table 5, Fig. 1), which is similar to that reported by Orsini et al. (2011). Moreover, the density and size of the leaf epidermal salt glands vary in response to various environmental conditions, such as salinity, light intensity, and aeration. For example, the size of EBCs and ploidy level of M. crystallinum increase under salinity stress [29]. In contrast, Orsine et al. [41] did not report any significant differences in EBCs density between untreated and salt-treated plants. Karimi and Ungar [42] reported that an increase in salinity led to a decrease in salt hair density and an increase in EBCs size. Thus, the effects of salt stress on the size and density of EBC may vary among plant species and experimental conditions. In the present study, the density of the EBC did not increase; however, the results of two-way ANOVA showed that the size of the EBCs was affected with NaCl application at both growth stages. The average EBCs diameter of the 9.75 g (89.4 μm at vegetative stage, 55.6 μm at flowering stage) and 19.50 g (75.5 μm at vegetative stage, 67.6 μm at flowering stage) plots were bigger than that of the 0.00 g (59.6 μm at vegetative stage, 53.6 μm at flowering stage) plots at both growth stages (Table 6, Fig. 1). Therefore, the accumulation capacity of toxic ions, i.e., Cs+ and Na+, increased by the application of NaCl. Isobe et al. [9] and Wada et al. [10] reported that the growth of quinoa plants and their Cs-absorbing increased with the application of NaCl. Moreover, the present study elucidates that NaCl promoted the growth, Cs-absorbing capacity, and size of the EBC to increase Cs accumulation. Thus, quinoa can be used for the phytoremediation of Cs-contaminated soil.
5 Conclusions
Quinoa plants possess EBCs on the leaf surface, and excess Na accumulates in these cells. We observed that NaCl application increased the growth of the aboveground parts of Cs absorption by quinoa plants in a previous study. However, whether the accumulated Cs+ are transported from the leaves to the EBCs in quinoa plants is unclear. Thus, in this study, we investigated the concentration of Cs+ in EBCs and leaves of quinoa plants grown in soil treated with high concentrations of NaCl. We observed that the concentrations of Cs+ were higher in the EBCs than in the leaves and the accumulation of Cs+ in the EBC was promoted by the application of NaCl. NaCl application did not affect the number of EBCs, but, the diameter of EBC increased with NaCl application. The accumulation of Cs+ was also supported by the increase in the size of EBC. Therefore, the optimal conditions for increasing both these parameters should be investigated in future studies. The results of this study suggest that quinoa can be used for the phytoremediation of Cs-contaminated soils.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Soudek P, Valenova S, Vavrikova Z, Vanek T. 137 Cs and 90 Sr uptake by sunflower cultivated under hydroponic condition. J Environ Radioact. 2006;88:236–50.
Hasegawa H. Cesium and higher plants. J Crop Res. 2012;57:1–6.
Ogata N, Fujii T, Kato M. Phytoremediation of radioactive cesium contaminated soil by cultivation of Amaranthus spp. Jpn J Crop Sci. 2015;84:9–16.
Ashraf MA, Akihiro T, Ito K, Kumagai S, Sugita R, Tanoi K, Rahman A. ATP binding cassette proteins ABCG37 and ABCG33 function as potassium-independent cesium uptake carriers in Arabidopsis roots. Mol Plant. 2021;14:664–78.
Adams E, Miyazaki T, Saito S, Uozumi N, Shin R. Cesium inhibits plant growth primarily through reduction of potassium influx and accumulation in Arabidopsis. Plant Cell Physiol. 2019;60:63–76.
Rai H, Kawabata M. The dynamic of radio-cesium in soils and mechanism of cesium uptake into higher plants: newly elucidated mechanism of cesium uptake into rice plants. Plant Sci. 2020. https://doi.org/10.3389/fpls.2020.00528.
Broadley MR, Willey NJ. Difference in root uptake of radiocaesium by 30 plant taxa. Environ Pollut. 1997;97:11–5.
Broadley MR, Willey NJ, Mead A. A method to assess taxonomic variation in shoot caesium concentration among flowering plants. Environ Pollut. 1999;106:341–9.
Isobe K, Nakajima E, Morita M, Kawakura S, Higo M. Effects of NaCl on growth and cesium absorption in Quinoa (Chenopodium quinoa Willd.). Water Air Soil Pollut. 2019;230:66.
Wada K, Takagi R, Horikoshi M, Higo M, Isobe K. Effects of NaCl application on cesium accumulation in the aboveground parts of Quinoa (Chenopodium quinoa Willd.). Water Air Soil Pollut. 2020;231:552.
Wilson C, Read JJ, Abo-Kassem E. Effect of mixed-salt salinity on growth and ion relations of a quinoa and wheat variety. J Plant Nutr. 2002;25:2689–704.
Isobe K, Ogisima E, Sato R, Sugiyama H, Higo M, Torigoe Y. Varietal and specific differences in salinity tolerance of quinoa (Chenopodium quinoa Willd.) for germination and initial growth. Jpn J Crop Sci. 2014;83:9–14.
Sheahan JJ, Ribeiro-Neto L, Sussman MR. Cesium-insensitive mutants of Arabidopsis thaliana. Plant J. 1993;3:647–56.
Hasegawa H. Selection for mutants with low nitrate uptake ability in rice (Oryza sativa). Physiol Plant. 1996;96:199–204.
Hampton CR, Bowen HC, Broadley MR, Hammond JP, Mead A, Payne KA, Pritchard J, White PJ. Cesium toxicity in Arabidopsis. Plant Physiol. 2004;136:3824–37.
Burger A, Lichtscheidl I. Stable and radioactive cesium: a review about distribution in the environment, uptake and translocation in plants, plant reactions and plants’ potential for bioremediation. Sci Total Environ. 2018;618:1459–85.
Moon JY, Adams E, Miyazaki T, Kondoh Y, Muroi M, Watanabe N, Osada H, Shin R. Cesium tolerance is enhanced by a chemical which binds to BETA-GLUCOSIDASE 23 in Arabidopsis thaliana. Sci Rep. 2021;11:21109.
Adams P, Nelson DE, Yamada S, Chmara W, Jensen RG, Bohnert HJ, Griffiths H. Growth and development of Mesembryanthemum crystallinum (Aizoaceae). New Phytol. 1998;138:171–90.
Agarie S, Shimoda T, Shimizu Y, Baumann K, Sunagawa H, Kondo A, Cushman JC. Salt tolerance, salt accumulation, and ionic homeostasis in an epidermal bladder-cell-less mutant of the common ice plant Mesembryanthemum crystallinum. J Experimental Botany. 2007;58:1957–67.
Adolf VI, Jacobsen SE, Shabala S. Salt tolerance mechanisms in quinoa (Chenopodium quinoa Willd.). Environ Exp Bot. 2013;92:43–54.
Zhang X, Oppenheimer DG. A simple and efficient method for isolating trichomes for downstream analyses. Plant Cell Physiol. 2004;45:221–4.
Marty F. Plant vacuoles. Plant Cell. 1999;11:587–600.
Andreev IM. Functions of the vacuole in higher plant cells. Russ J Plant Phy. 2001;48:672–80.
Jiang YT, Yang LH, Ferjani A, Lin WH. Multiple functions of the vacuole in plant growth and fruit quality. Mol Hortic. 2021;1:4.
Zhang Y, Mutailifu A, Lan H. Structure, development, and the salt response of salt bladders in Chenopodium album L. Front Plant Sci. 2022;13: 989946.
Turhan A, Asik BB, Kuscu H. The influence of irrigation water salinity and humic acid on nutrient contents of onion (Allium cepa L.). J Agric Sci. 2020;26:147–53.
Alatorre EB, Pottosin I, Shabala L, Chen ZH, Zeng F, Jacobsen SE, Shabala S. Differential activity of plasma and vacuolar membrane transporters contributes to genotypic differences in salinity tolerance in a halophyte species, Chenopodium quinoa. Int J Mol Sci. 2013;14:9267–85.
Oh DH, Barkla BJ, Vera-Estrella R, Pantoja O, Lee SY, Bohnert HJ, Dassanayake M. Cell type-specific responses to salinity—the epidermal bladder cell transcriptome of Mesembryanthemum crystallinum. New Phytol. 2015;207:627–44.
Barkla JB, Vera-Estrella R, Raymond C. Single-cell-type quantitative proteomic and ionomic analysis of epidermal bladder cells from the halophyte model plant Mesembryanthemum crystallinum to identify salt-responsive proteins. BMC Plant Biol. 2016;16:110.
Kiani-Pouya A, Rasouli F, Shabala L, Tahir AT, Zhou M, Shabala S. Understanding the role of root-related traits in salinity tolerance of quinoa accessions with contrasting epidermal bladder cell patterning. Plant. 2020;251:103.
Böhm J, Messerer M, Muller HM, Scholz-Starke J, Gradogna A, Scherzer S, Maierhofer T, Bazihizina N, Zhang H, Stigloher C, Ache P, Al-Rasheid KAS, Mayer KFX, Shabala S, Carpaneto A, Haberer G, Zhu JK, Hedrich R. Understanding the molecular basis of salt sequestration in epidermal bladder cells of Chenopodium quinoa. Curr Biol. 2018;28:3075–85.
Fish DC, Dobbs JP, Elliott JM. Effect of osmotic pressure, Na+/K+ ratio and medium concentration on the enzyme activity and growth of L cells in suspension culture. In Vitro. 1973;9:108–13.
Mengel K, Arneke WW. Effect of potassium on the water potential, the pressure potential, the osmotic potential and cell elongation in leaves of Phaseolus vulgaris. Physiol Plant. 1982;54:402–8.
White PJ, Broadley MR. Mechanisms of caesium uptake by plants. New Phytol. 2000;147:241–56.
Bazihizina N, Böhm J, Messerer M, Stigloher C, Müller HM, Cuin TA, Maierhofer T, Cabot J, Mayer KFX, Fella C, Huang S, Al-Rasheid KAS, Alquraishi S, Breadmore M, Mancuso S, Shabala S, Ache P, Zhang H, Zhu JK, Hedrich R, Scherzer S. Stalk cell polar ion transport provide for bladder-based salinity tolerance in Chenopodium quinoa. New Phytol. 2022;235:1822–35.
Flower TJ, Colmer TD. Plant salt tolerance: adaptations in halophytes. Ann Bot. 2015;115:327–31.
Santos J, Al-Azzawi M, Aronson J, Flower TJ. eHALOPH a database of salt-tolerant plants: helping put halophytes to work. Plant Cell Physiol. 2016;57: e10. https://doi.org/10.1093/pcp/pcv155.
Dassanayake M, Larkin JC. Making plants break a sweat: the structure, function, and evolution of plant salt glands. Front Plant Sci. 2017;8:1–20.
Kiani-Pouya A, Roessner U, Jayasinghe NS, Lutz A, Rupasinghe T, Bazihizina N, Bohm J, Alharbi S, Hedrich R, Shabala S. Epidermal bladder cells confer salinity stress tolerance in the halophyte quinoa and Atriplex species. Plant Cell Environ. 2017;40:1900–15.
Otterbach S, Khoury H, Rupasinghe H, Mendis H, Kwan KH, Lui V, Natera S, Klaiber I, Allen NM, Jarvis DE, Tester M, Roessner U, Schmöckel SM. Characterization of epidermal bladder cells in Chenopodium quinoa. Plant Cell Environ. 2021;44:3836–52.
Orsini F, Accorsi M, Gianquinto G, Dinelli G, Antognoni F, Carrasco KBR, Martinez EA, Alnayef M, Marotti I, Bosi S, Biondi S. Beyond the ionic and osmotic response to salinity in Chenopodium quinoa: functional elements of successful halophytism. Funct Plant Biol. 2011;38:818–31.
Karimi SH, Ungar IA. Development of epidermal salt hairs in Atriplex triangularis Willd. in response to salinity light intensity, and aeration. Bot Gaz. 1989;150:68–71.
Acknowledgements
This research was supported by the College of Bioresource Sciences, Nihon University.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
KI: Total coordination of experiment. KW and SO: Quinoa cultivation, analyzed ion concentration, size, and number of epidermal bladder cells. MH: Statistical analysis and measuring soil chemistry.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Isobe, K., Wada, K., Oishi, S. et al. Effects of NaCl application on cesium concentration, number, and size of epidermal bladder cells in quinoa plants. Discov Appl Sci 6, 75 (2024). https://doi.org/10.1007/s42452-024-05713-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-024-05713-8