Abstract
There is a pressing need to tackle carbon emissions from oil palm plantations on tropical peatland, which has garnered significant discussion and concern in recent years. In response, compaction techniques were introduced in Malaysia with the aim of mitigating CO2 emissions by improving moisture levels and reducing soil aeration. This research investigates the impact of mechanical compaction on two distinct ecosystems: a peat swamp forest (PSF) and an oil palm plantation (OPP), characterized by their unique physicochemical properties Using a specially designed compaction apparatus, significant changes in carbon emissions were observed in PSF but not in OPP, with means 1263 and 404 mg CO2-eq m−2 h−1, respectively. This disparity can be due to substrate availability between the two ecosystems. Subsequently, in the PSF, a promising pattern of a percentage ratio of approximately 1:3.5 was observed, indicating a substantial reduction in CO2 emissions (from 1295 to 468 mg m−2 h−1; 64%) alongside a corresponding increase in CH4 emissions (from −50 to 60 µg m−2 h−1; 221%). This finding suggests that compaction alters the aerobic peat horizon, bringing the peat surface closer to the groundwater level. The study underscores the importance of considering confounding factors such as decomposition degree and groundwater fluctuation when assessing the effects of compaction on tropical peat. By shedding light on these complexities, the findings contribute to a better understanding of the efficacy of compaction techniques in reducing emissions of these special case atmospheric pollutants.
Article highlights
-
The first data on in-situ compaction on tropical peatland carbon emissions.
-
Peat physicochemical properties were not affected by compaction when in contact with groundwater.
-
Appropriate clarity of mechanical compaction on tropical peatland.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Tropical peatlands are highly delicate and sensitive ecosystems, and the conversion of these areas into agricultural farms requires intensive inputs and management practices that can have detrimental effects on the ecosystem, leading to land degradation and environmental issues [1,2,3]. Intensive and appropriate management practices are crucial for ensuring sustainable crop productivity in tropical peatlands. These management practices encompass various strategies, including water table management [4], soil compaction [5], and fire prevention [6]. In their natural state, tropical peatlands maintain a high water table, which is essential for the stability and carbon storage capacity of the peat layer. However, when converted into agricultural farms, drainage is often employed to lower the water table, making the land suitable for cultivation. Drainage significantly alters the physical and chemical properties of the soil and microbial activity by introducing oxygen into the soil, triggering microbial decomposition processes, and causing the breakdown of organic matter [7]. This alteration influences the release of special case atmospheric pollutants or greenhouse gases such as CO2, CH4, organic acids, and organic particulates [8]. Climate change exacerbates the rate of carbon loss, particularly through the establishment of oil palm plantations using drainage and slash-and-burn techniques, as demonstrated in previous studies [9]. To address the rising environmental concerns, a novel technique introduced for open land practice on tropical peatland is peat mechanical compaction, which claims to effectively minimize the risk of fire by enhancing soil moisture [10]. Several studies have explored the positive effects of peat compaction on mechanical anchorage for palm stands and nutrient retention [5, 11]. However, the impact of compressed soil on the composition and decomposition of aerobic and anaerobic peat materials, as well as its ability to reduce the risk of peat fires and lower CO2 emissions, remains uncertain due to limited available data. Furthermore, the implications for CH4 emissions, which have a significantly higher global warming potential than CO2, due to induced anaerobic conditions through compaction, have yet to be fully addressed [12, 13].
In the early stages of establishing oil palm plantations on tropical peatlands, companies typically avoid using fire and instead opt for manual mechanical methods to clear the land. This approach is used whether they are clearing pristine forests, secondary forests, areas covered in Imperata sp. grass, or transitioning from other crops. The manual mechanical process involves a series of steps, including slashing, cutting, chopping, and piling/staking [14], occasionally supplemented with pesticide spraying [15]. Once the land clearing phase is completed and aligns with the intended goals, the land is prepared for planting oil palm seedlings using a compaction method. Through 2 to 3 tractor passes, compaction can increase the dry bulk density of peat by as much as 0.20 g cm−3 [16]. However, before mechanical compaction takes place, it is customary to lower the water table level to a range of 0.5 m to 1.0 m. This is done to achieve self-subsidence and improve the suitability of peat for heavy machinery usage [5]. In such situations, two interconnected factors are expected to contribute to the increase in bulk density: compaction resulting from tractor passes and drainage, which leads to the shrinkage of organic and woody materials as water is lost [17]. Furthermore, the complexity surrounding the extent of compaction arises from a range of factors, including abiotic, biotic, and anthropogenic elements (man-made compaction) [12]. Nevertheless, differentiating compaction from shrinkage as distinct phenomena can be quite challenging. Most scholarly works [5, 11, 17, 18] have defined tropical peat compaction based on the specific characteristics of the study site. For instance, in ecosystems like logged-over or drained forests, peat compaction is often attributed to variations in the water table during seasonal changes, resulting in shrinkage (leading to bulk density increase) or oxidation (caused by microbial activity), which decreases bulk density[17]. Conversely, in developed peatland ecosystems such as agricultural systems, many authors [11, 17, 19] describe peat compaction as a combined process influenced by heavy machinery and shrinkage due to drainage, both contributing to increased bulk density. This landscape-based approach to defining compaction has led to confusion among researchers regarding the precise mechanism of compaction on tropical peatlands.
Samuel and Evers [12] underscored in their comprehensive review paper the pressing need for a profound understanding of the intricacies associated with tropical peat compaction. This phenomenon involves a complex interplay of various processes, including oxidative reactions, consolidation, shrinkage, and mechanical compaction, the latter being induced by human activities. Mechanical compaction is also commonly referred to as man-made compaction. Peat compaction can be dissected into two interlinked aspects: compression and consolidation. Compression refers to the reduction in the volume of the peat material due to the displacement of the oxic phase (which lacks water). On the other hand, consolidation entails an enhancement in the mechanical strength of the material. This strengthening is a result of particle-to-particle interactions that occur when peat soil collapses under its weight within the anoxic horizon [17]. Measuring the compressibility of peat soil directly poses considerable challenges, primarily due to the multitude of interconnected factors, including peat depth, maturity, and bulk density [20]. Existing research on mechanical compaction and peat soil has predominantly viewed peat as a soil conditioner or an additive for mineral soils [21] This perspective is largely influenced by the remarkably high organic matter content found in peat, typically hovering around 99% [22]. Consequently, peat soil of this nature exhibits a compressibility level within the range of 300% to 400%. It also possesses the remarkable ability to revert to its original state [23]—unless specific hydrophobic characteristics are deliberately introduced [24].
While some studies have investigated the effects of soil compaction on carbon emissions, soil water, and physicochemical properties [25,26,27], experimental data related to tropical peat soil-induced or artificial compaction on tropical peat physicochemical properties and carbon emissions is rare. The only available reference by Busman et al. [28] was conducted under laboratory conditions using destructive composite sub-surface samples (50 to 70 cm) and was solely based on the dry bulk density values Hence, this study aims to assess the effect of artificial compaction of tropical peat on the peat physicochemical properties and carbon emissions. The objectives of this study are, therefore: (1) to induce compaction to vary magnitudes based on compression according to peat depth using a fabricated compaction apparatus; (2) to determine the peat physicochemical properties and quantify the peat carbon emissions according to the compaction treatment and site ecosystem (PSF: secondary peat swamp forest where pre-existing compaction via drainage will be minimal) and OPP: mature oil palm plantation (where pre-existing conditions will have been impacted by the conversion process and longer-term drainage effects); (3) to establish a relationship between carbon emissions and physicochemical properties to make recommendations on the efficacy of this approach within the two varying sites types.
2 Materials and methods
2.1 Study location
The study took place in two tropical peatlands located in the peninsular region of Malaysia (Fig. 1a), specifically in Tanjong Karang in Northern Selangor (3° 41′ 49.2″ N 101° 11′ 06.0″ E) (Fig. 1b) and Sepang in Southern Selangor (2° 44′ 20.4″ N 101° 39′ 10.8″ E) (Fig. 1c). The Tanjong Karang site was characterized by a drained peat swamp forest (PSF) that had been selectively logged in the past, while the Sepang site was represented by a second-generation oil palm plantation (OPP) that was established in 1978 and had been in operation for decades. During the study period, meteorological data from Department of Meteorology Malaysia (METMalaysia) recorded similar warm and humid weather patterns in both areas. Tanjong Karang experienced an average temperature of 28.1 °C, peaking at 33.5 °C, and dropping to 23.6 °C. Concurrently, Sepang maintained an average temperature of 28.3 °C, with a high of 33.6 °C and a low of 23.9 °C. Tanjong Karang experienced mean monthly rainfall of 127.8 mm, and Sepang saw 97.4 mm. The relative humidity was slightly higher in Tanjong Karang at 81% compared to Sepang's 79%, suggesting slightly warmer and less humid conditions in Sepang.
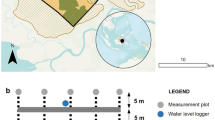
a Map of Peninsular Malaysia that shows the locations of intact peat samples collected from two different ecosystems in Selangor state that are marked by colored X: b Peat Swamp Forest with the green X and c Oil Palm Plantation with the yellow X. The map was generated using GIS software (ArcGIS Desktop 10.8, Version: 10.7.0.10450, http://www.esri.com).
The history and management of each site influenced its vegetation. The Tanjong Karang site belongs to the Raja Musa Forest Reserve (RMFR), which is part of the North Selangor Peat Swamp Forest and covers 23,000 ha. RMFR joined the Queen’s Commonwealth Canopy in 2017 and became a pioneer site for the Peat Swamp Forest Rehabilitation and Conservation Project in Southeast Asia and Malaysia. The drained PSF in Tanjong Karang had various tropical tree species common in peat swamp forests such as Shorea spp., Koompassia spp., and Dipterocarpus spp.. In contrast, the OPP in Sepang was characterized by a monoculture of oil palm trees (Elaeis guineensis), planted in orderly rows. The hydrological conditions of each site were influenced by their respective management practices. The PSF, previously drained, likely saw modifications in the natural water regime, leading to a potentially lower water table compared to its original state. The OPP's hydrological system was shaped by drainage networks and practices inherent to palm oil production, aiming to regulate water levels and soil moisture. The peat depth of the PSF was around 165.4 ± 3.9 cm (SD), while the OPP had a depth of approximately 95.2 ± 6.5 cm (SD), both considered moderate to shallow. USDA soil taxonomy classified the peat soils at both sites as Typic Fibric Tropohemist. In the drained PSF, the mechanical compaction experiment was positioned 1.0 km from the drain edge and entry point to mitigate the effects of passive compaction from corner sites due to the drainage system's development. Similarly, in the OPP, the experiment was placed between the palm planting rows, about 20 m from the main drain, to ensure data accuracy.
2.2 Fabrication of hybrid compaction apparatus
The compaction apparatus was fabricated using stainless steel based on a vertical plunger-like compression approach. This simple apparatus consisted of five key components: Fig. 2 (a) cover, (b) piston, (c) piston’s ear, (d) soil collar, and (e) perforated mesh, with a total weight of approximately 3.0 kg. A soil collar cylinder measuring 40.0 cm long with a diameter of 10.16 cm, was used to standardize compaction magnitudes and maintain compaction degree throughout the experimental period—due to the peat’s high plasticity and recovery features [23]. A piston measuring 30.0 cm high, and 10.16 cm in diameter was used to compress the peat surface to the assigned soil depth. The piston also served as a static closed chamber for capturing gas. A piston cover that fitted two gas tubing ports was placed on top of the piston to facilitate gas sampling. The size of the piston was intentionally standardized for all treatments of gas concentration calculation (6.5 L). The piston’s ears, attached at various heights following the compaction treatment, were designed as a stopper during the compaction application. Finally, to address compressibility obstructions such as available water or air from peat removed by the compaction effort, the piston surface was perforated with Ø 1.0 cm meshes. This also allowed for the free transfer of peat carbon emissions.
2.3 Artificial compaction treatment
The soil collars were then driven into the peat profile and left for two months to allow resettlement and the root necrosis and exclusion of root biomass which would otherwise contribute to additional autotrophic emissions. Since flooding inside the soil collar was observed to occur due to rainwater, the soil collars were covered loosely using a polystyrene plate to prevent direct rainfall while also preventing vapor deficits that could affect the gas carbon emission. In addition, the slope of the soil was also considered. For OPP, sites used avoided areas within 3.5 m of the palm trunk to avoid the root cycle [29] and reduce microenvironment variability. The piston was left inside the soil collar after implementing the proposed depth to emulate static compaction by heavy machinery that commonly occurs in agricultural fields. To consider the mesh-perforated surface, which may interrupt the gas diffusion from the surface soil, an integrity test was done to evaluate the compaction apparatus capability in terms of carbon emissions and degree of compactness (i.e., bulk density). The test was performed at the same time as the compaction experiment. Together, the compaction treatments were assigned as Ref (reference for control or no compaction without soil collar); T0 (no compaction with soil collar); T10 (soil compression treatment to 10.0 cm); T20 (20.0 cm); and T30 (30.0 cm), as per Fig. 3. Four replications of the five compaction treatments (including the reference samples for the integrity test) in both ecosystems were established with a total number of samples of \(n\) = 40.
2.4 Gas and groundwater level measurement
Gas sampling was undertaken for 24 weeks for PSF and 21 weeks for OPP, over three-week intervals for each sampling point after mechanical compaction treatments. Gas carbon emissions were measured using an automated gas analyzer (Ultraportable gas analyzer by Los Gatos Research, USA). Mixed gas accumulation was analyzed in real-time for six minutes and each gas concentration point represented 20 s, which were recorded and stored automatically in the analyzer’s data logger. Three concentration points for the first minute were discounted to account for the lid installation disruption.
Gas concentration in ppm was calculated by using ideal gas law, as given in Eq. (1).
where P = Atmospheric pressure (101,325 pa); V = Volume of headspace (m3); n = Number of moles (mol); R = Universal Gas Constant law (8.314 J. K−1 mol−1) and T = Temperature in kelvin (K) with conversion number of 1 mol of gas to gram, CO2 = 44.01 g and CH4 = 16.02 g, respectively.
The increase or decrease in the head-space concentration over time (hour, h−1) over the head-space volume (L) from peat surface area covered (m2) were fitted into linear regression (LR) [30]. The slope from LR (in this study ~ R2 > 0.90) was considered as the rate of CO2 emission or CH4 emission (+ value) / uptake (-value) and presented in, mg m−2 h−1 or µg m−2 h−1. Groundwater levels were measured manually from piezometers (1.5 m) installed around the soil collars using rulers and distance laser beam that aimed to floating polystyrene. During gas sampling, all measurements were retrieved from three perforated piezometers and the average measurements were calculated.
To assess the relative impact of different greenhouse gas emissions on climate change, the values of CO2 and CH4 emissions were converted to a GWP index using Eq. (2). The GWP index is a way for comparing the contribution of different greenhouse gases to global warming over a certain period, usually 100 years. It considers the energy absorption capacity of each gas and its atmospheric residence time. The GWP index uses CO2 as a reference, which has a GWP of 1 and assigns a GWP of 30 to CH4 from fossil sources [31]. The results were presented in CO2-eq.
2.5 Peat sampling and physicochemical properties analysis
The peat and bulk density samples were collected separately from the soil collars at the end of the compaction experiment. Considering the small inner diameter of the peat surface inside the soil collar (i.e., 0.127 m diameter; 0.051 m2 area), a bulk density core sub-sample with a volume of 50.0 cm3 at 7.0 cm peat depth was harvested from the soil collar using an open-ended syringe (50.0 ml) modified earlier by cutting off the syringe’s luer part (i.e., 0.027 m diameter with 0.0006 m2 area). Then, 30.0 g of fresh peat was scooped out using a spatula for selected further peat physicochemical property analysis. In this study, peat surface sampling was emphasized because, within a range of 0 to 10.0 cm, peat soil exerts a major control on CO2 and CH4 emissions [32] and physicochemical properties, and this range is an effective depth for soil compaction [25].
In the laboratory, the analysis of dry bulk density was conducted using the core method prescribed by Al-Shammary et al. [33]. Soil cores containing fresh peat samples were weighed to obtain wet weight (\(Ww\)). Next, the samples were oven-dried at 105 °C until constant dry weight (\(Dw\)) was attained for 48 h. The oven-dried peat samples were placed in desiccators for cooling and to prevent moisture absorption. Alongside, the moisture content parameter was obtained as well. Both parameters were calculated using Eqs. (3) and (4) and expressed in g cm−3 and percentage (%), respectively.
Loss on ignition (\(\mathrm{LOI}\)) was determined from a small fraction of composite oven-dried peat (5.0 g) (\(Dw\)) via complete combustion by adhering to the method prescribed by Marwanto et al. [34]. After that, the weighed dried peat was transferred to muffle furnace (Thermo Scientific Thermolyne Industrial Benchtop Muffle Furnaces, Fisher Scientific, USA) at 550 °C for 12 h, including a cooling process to 60 °C. Next, crucible cups containing ash were placed in the desiccator to gain weighed ash (\(DA\)) upon hitting room temperature at 30 °C. The \(LOI\) was calculated using Eqs. (5) and (6) and expressed in percentage (%).
Following the method suggested by Santos et al. [35], the value of particle density (DS) can be obtained using pycnometer or specific gravity flask (Phunque Flask). The peat particle density value was calculated using Eq. (7). Where (\(Dw\)) refers to water density (g cm−3) at the temperature; (\(Ws\)) denotes weight of peat soil sample; (\(Wsw\)) represents weight of pycnometer, peat soil, and water, while (\(Ww\)) stands for weight of pycnometer and water. The calculation of total porosity (\(TPS\)) had been based on peat particle density value using Eq. (8).
Water-filled pore space (\(WFPS\)) was determined by using gravitational moisture content based on dry weight (\(GRA\)) and volume of moisture content (\(VOL\)) parameters, as given in Eqs. (9) and (10) [36]. The calculated values were used to estimate \(WFPS\), as shown in Eq. (11).
The composite dry samples from soil core were crushed by mortar and pestle and passed through 2.0 mm sieve to determine their chemical properties [37]. The peat pH value was determined by using a benchtop pH meter model (Sartorius Benchtop Meters: pHBasic + Series, Fisher Scientific, USA) by adding 5.0 g of dried and sieved peat sample into 12.5 ml deionized water (soil–water ratio of 1:2.5) in a 25.0 ml glass beaker. To determine the total carbon, total nitrogen, hydrogen, sulfur and CN ratio, the samples were analyzed using the standard method of elemental analysis following ISO 13878:1998. This analysis was carried out using a CHNS analyzer (Vario MACRO cube, Elementary Analysis System, Germany).
2.6 Statistical analyses
A General Linear Model (GLM) was run to verify the integrity of the fabricated compaction apparatus and to assess the effect of the ecosystems and compaction treatments on the physicochemical properties and carbon emissions of peat. When statistically significant differences between parameters were observed, Tukey’s HSD multiple comparison procedure at \(p\) ≤ 0.05 was performed. To identify the relationship between physicochemical properties and water table fluctuation with carbon emissions, Pearson’s correlation coefficient method, two-tailed at the 0.05 probability level, was performed. All statistical analyses were calculated and formulated with the help of Minitab software version 18.1 (Penn, USA, 2017) while the graphs were plotted using Sigmaplot 12.5 graphing software (Systat Software Incorporation, UK, 2014).
3 Results and discussion
3.1 Impact of fabricated compaction apparatus
An integrity test was conducted alongside the compaction treatment using uncompacted peat samples without a soil collar (Reference or 'Ref') and compared to a treatment control where the soil collar was installed but without compaction ('T0'). This comparison was carried out in both ecosystems. The analysis showed that there were no significant differences in bulk density (used as an indicator for compaction), with values ranging from 0.12 to 0.15 g cm−3 for PSF and 0.18 to 0.21 g cm−3 for OPP (Fig. 4a). Additionally, there were no significant differences in carbon emissions in the form of CO2, which ranged from 1234.3 to 1294.8 mg m−2 h−1 for PSF and 289.4 to 521.6 mg m−2 h−1 for OPP (Fig. 4b), and CH4 emissions, ranging from −48.0 to −50.6 µg m−2 h−1 for PSF and −3.9 to −5.3 µg m−2 h−1 for OPP (Fig. 4c), between the Ref and T0 samples in both ecosystems. This suggests that the compaction apparatus used did not have a significant impact on bulk density or carbon emissions in the T0 (independently of any treatment), thereby allowing for subsequent analysis of compaction at varying magnitudes on physicochemical properties and carbon emissions.
The comparison of a bulk density b CO2 and c CH4 emissions between the reference and T0 samples at the PSF and the OPP. The error bars indicate the standard error of means. The different letters indicate significant differences between treatments and land uses. The means were separated using Tukey’s HSD post-hoc test (α = 0.05)
3.2 Peat resilience in the presence of groundwater fluctuation
Notably, despite the depth-based compression applied by the compaction apparatus, there was no discernible relationship between the treatment and bulk density within each ecosystem, as indicated in Table 1. Similarly, it is evident that other physicochemical properties such as total porosity, gravimetric water content, total carbon, hydrogen, sulphur, and CN ratio remained unaffected by the compaction treatments. However, a significant disparity in bulk density between the two ecosystems is apparent, with the OPP showing a mean of 0.232 g cm−3 compared to that of the PSF, at 0.171 g cm−3. Correspondingly, peat porosity in OPP was lower than in PSF, with mean values of 85.5% and 89.7%, respectively. Furthermore, gravimetric water content was higher in PSF compared to OPP, with mean values of 225.3% and 175.3%, respectively. In OPP, total carbon, hydrogen, and CN ratios were higher than in PSF, with mean values of 42.5%, 13.8%, and 31.16, respectively. Conversely, the sulphur content in PSF was higher than in OPP, with mean values of 4.26%, respectively. Notably, properties such as loss on ignition, water-filled pore space, volumetric water content, pH, and total nitrogen were not affected by either the ecosystem type or the compaction treatment.
The lack of response of peat properties to the compaction treatments was unexpected and suggested that at the forces applied here at least, the peat in both ecosystems was resilient towards mechanical compaction. There are several reasons why the peat from both ecosystems exhibited resilience. First, this effect is a result of groundwater fluctuation interaction, especially at maximum compaction, T30 (− 30.0 cm compression), where the modified soil surface level was closest to the water table level. The water table level at maximum compaction during the study of PSF and OPP fluctuated from −6.26 ± 7.35 cm (SD) and −26.8 ± 8.59 cm (SD) when compaction went above −30.0 cm (Table 2). Groundwater level interference was also observed by Baker et al. [38], who suggested that groundwater level fluctuation in wetting and drying cycles could cause looseness or impaired pore structure or swelling. In addition, Pires et al. [39] also analyzed the changes in a pore system using gamma-ray computed tomography (CT) and found that wetting and drying cycles could result in a repair mechanism in the compacted soil structure. This is important when considering potential rebound post compaction. but does not account for the continuous compaction which occurred throughout the experimental period.
Second, the ineffectiveness of mechanical compaction on peat may be further enhanced by newly decomposed peat, or available labile C from above the peat surface, which has translocated to a deeper depth by vertical compaction and readily has sponge-like features [40]. Recent dead plant biomass is known as a source of labile C [41]. During compaction treatment, labile C from the peat surface could be relocated to the deeper horizon to augment consolidation. Achieving instantaneously in this case is highly challenging due to the presence of labile C, which consists of intricate components derived from recently deceased plants with a sturdy structure that discourages herbivore-like traits [42, 43]. Hence, peat will return to its former state, as the peat pores could still be active and have elastic and plastic properties [22, 23]. Unless the peat pores are inactive due to excessive drying, which will manifest as hydrophobicity [24].
Third, peat failure to compress could be a result of a lack of mineral or clay content [44, 45], which serves as a cohesive or macropores filler to stabilize the anisotropic structure of peat. This is because mineral content such as fly ash, gypsum, and lime with peat admixtures can promote peat stabilization and could possibly support a more effective compaction [44,45,46,47,48]. If this were the situation, a more pronounced reaction to compaction treatments could be anticipated at the OPP sites, where lime is applied. The most plausible explanation may be that the compaction effort was insufficient to elicit a response by the physiochemical variables measured. According to Mutert et al. [5], the changes in bulk density may occur when the compaction of peat is drained to 0.50 m to 0.80 cm from the peat surface, allowing self-subsidence to 1.0 m prior to heavy machine passes. Conversely, the induced compaction in this study was kept at a maximum of 30.0 cm depth, and the water table level was not controlled, which might not have been sufficient to initiate changes in physicochemical properties of peat, especially bulk density. Nevertheless, the prolonged drainage of the OPP site accounts for the consistently elevated bulk density values observed there.
Overall, while not expected, this result points to the impressive resilience of intact peatlands to compaction, and their ability to maintain intact pore structure, water-holding capacity, and porosity. These features are key to the hydrological ecosystem services provided by peatlands in terms of flood protection and water storage capacity [49]. This result also reinforces the direct role of drainage as the key driver of compaction and consolidation within peatlands [24], with the mechanical compaction only compounding the effect. The significant difference between ecosystems corroborate with the findings of several authors [50,51,52,53,54], who assessed the physicochemical changes of converted land from a peat swamp forest to an oil palm plantation. These typical changes are based on the origin ecosystem attributes and the different management involving the lowering of water table level by drainage, agricultural input (e.g., fertilizer, pesticide, and agri-implements), and litter input from surrounding plant biomass. Consequently, the distinct traits exhibited at the OPP sites will also be associated with the degree of decomposition (e.g., bulk density, total porosity, water-filled pore space, gravimetric, and volumetric water content, and CN ratio) [36, 50].
Therefore, a key question to emerge from this work however is if drainage-based compaction is recoverable with rewetting. While the work from Pires et al. [39] suggests that the recovery of natural wet and dry cycles is key to the rehabilitation of pore structure, in-situ assessments of bulk density and pore structure recovery over time will need to be assessed in line with rehabilitation activities if key hydrological ecosystem services are to be recovered at previously drained and converted OPP sites.
3.3 Carbon emission changes due to mechanical compaction
Despite physicochemical properties being unaffected by site-specific mechanical compaction, carbon emissions in the form of CO2 and CH4 from the peat surface showed inverse patterns due to compaction, especially in the PSF ecosystem. During compaction, CO2 emissions decreased according to the treatment, with the greatest reduction measured at T30, by 827.2 mg m−2 h−1 (64%) and followed by 465.1 mg m−2 h−1 (36%) at T20 (Fig. 5a). When combined, the average emissions decrease was 2% or 27.6 mg m−2 h−1 per cm of compression (when compressed beyond 20 cm). Various studies have reported CO2 emissions from forest and oil palm plantations. The CO2 emissions in this study ranged from 467.5 to 1294.8 mg m−2 h−1 and 290.18 to 521.6 mg CO2 m−2 h−1 at SF and OP, respectively, which are similar to Dariah et al. [55], Husnain et al. [56], and Melling et al. [53] (Table 3). However, compared to many other studies, the value for OPP emissions is generally low. For example, Matysek et al. [29] obtained a higher value in their study of the same oil palm plantation during dry season condition (means up to 1244.7 ± 149.2 (SE) mg m−2 h−1; Table 3) and found the heterotrophic emissions to be dependent on the age of the plantation, with those converted the longest (2nd generation) producing emissions comparable to this study (663.8 ± 102.2 (SE) mg m−2 h−1.
The means of cumulative a CO2 and b CH4 emissions between compaction treatments at PSF (\(n\) = 32) and OP (\(n\)= 28). The error bars indicate the standard error of means. The different letters indicate significant differences between treatments and land uses. The means were separated using Tukey’s HSD post-hoc test (\(\alpha\)= 0.05)
Contrary to CO2, CH4 emission showed a significant increase in T20 (from −49.7 to −7.5 µg m−2 h−1; 85%) and T30 (from −49.7 to 60.0 µg m−2 h−1; 224%), respectively, (by an average 150%) within the PSF ecosystem (Fig. 5b). Our results found uncompacted (T0) PSF to be a strong sink for CH4 and OPP T0 to be a weak CH4 sink, similar to Sakabe et al. [57] but opposed to the CH4 emissions reported by Melling et al. [54] for both ecosystems (Table 4). This may have been due to their OPP site being newly converted (4 years) and thus potentially having a higher labile C surface peat content, a source of the substrate which has a CH4 production priming effect [58, 59], as compared to our matured OPP. The effect of the compaction treatments worked to reduce the sink effect in both ecosystems. However, only PSF T30 became a mean source of CH4 in this study. Given that there was no change in the physicochemical variables with treatments, the only significant change between treatments was not the compaction (as indicated by bulk density value), but rather the increase in relative water table level (−36.21 at T0 and −6.21 at T30).
For this reason, we can use the data here to explore the direct relationship between emissions and groundwater levels from in-situ data. For CO2 emission, a significant relationship was found in PSF (p < 0.001; R2 = 0.610) (Fig. 6a). The CO2 predicted emission from PSF is equal to – 24.9*GWL + 363.05 when CO2 emission is measured in mg m−2 h−1. From this established model, the CO2 emission can be reduced by 6.7 mg m−2 h−1 for each centimeter of groundwater level (GWL) increased. On the other hand, a significant regression between CH4 emission and GWL was also found at PSF (p < 0.001; R2 = 0.700) (Fig. 6b) after eliminated pulse emissions (before was CH4 = 1.981*GWL + 35.98 with p = 0.008; R2 = 0.212). The predicted CH4 emission in PSF is equal to 1.24*GWL + 4.27 in centimeter when CH4 emission is measured in µg CH4 m−2 h−1. Thereby, CH4 emission increased by 1.03 µg CH4 m−2 h−1 for each centimeter of GWL increased.
Therefore, to further explain the carbon reduction from peat in secondary peat swamp forests, a model illustrating the carbon emission exchange and GWL (Fig. 6) was employed. Thus, high, and low GWL scenarios (extreme inter-seasonal effect) were used representing high (−6.21 cm) and low (−66.21 cm) GWLs from the initial soil surface height (see Table 2 for water table mean at T0, control). The 30.0 cm value was derived from the effective change in carbon emission due to mechanical compaction, which affects peat depth. From the estimation presented in Table 5, 59.1% change in carbon emissions was observed when the water table was raised or lowered (e.g., inter-seasonal) by 30.0 cm.
Interestingly, this estimation is similar to that of Dhandapani et al. [60], which measured CO2 and CH4 in a primary peat swamp forest in Setiu, Terengganu, and in a secondary peat swamp forest in Tanjung Karang, North Selangor. They observed a difference of more than 50% in CO2 emissions. Thus, there is evidence to suggest that the secondary peat swamp forest in Tanjung Karang shares similar traits with the primary peat swamp forest in Terengganu, Peninsular Malaysia, which has been a protected area since the 1990s (27 years), particularly if the GWL has been raised to + 30.0 cm from the initial level. Besides, this is also indicating that rehabilitation of secondary tropical peat forest is progressing splendidly and in agreement with [61] that mentioned more than half of the tree species from primary forest can be found after 25 years of logging operations are ceased.
Hence, this study proposed that the GWL in the PSF of Tanjung Karang be maintained at 10.0 cm or higher to stabilise or reduce carbon emissions by 51.6%. However, it should be noted that the peat surface of this PSF will always produce carbon emissions due to the decomposition process of organic materials that are supplied continuously from available trees [41], besides root respiration [60]. Therefore, it is expected that approximately 53.7 t CO2-eq ha−1 yr−1 will be emitted naturally by this forest, indicating a 10.0 cm GWL depth from the peat surface.
Yet importantly, this relationship only stands for relatively intact (i.e., PSF) peatlands. As illustrated, lower water table depths/greater drainage per se as found at all the OPP treatments (Fig. 7f) did not result in a greater sink potential for CH4. Indeed, all OPP treatments were low CH4 sinks despite having lower water level depths ranging from −56.45 (T0) to −26.83 (T30). In the OPP ecosystem, the litterfall input is undoubtedly limited [36] because of its monoculture system [62]. Despite ample oxic and anoxic conditions due to water table fluctuation, insufficient litter input could limit the source of substrate availability that promotes microbial activity [62]. Moreover, the effect of excessive periodical drainage in the matured OPP may also affect the water holding capacity resulting from irreversible drying condition [63] and an enhanced hydrophobic trait [24] due to disconnecting peat pores from water table level in long period [52]. This phenomenon could explain why CO2 and CH4 at OPP (Fig. 7b, d) remain unchanged although the water table fluctuates and through depth alteration by compaction treatment.
Consideration of temporal water table fluctuations, of particular interest, is in the event of water table exceeds the peat surface on WAC 6 and WAC 9 in PSF with a water level difference of 20.1 cm (Fig. 7a, c, e) specifically on both carbon emissions. This may reflect the temporary water retention induced by anisotropic deformation on deeper peat [23]. In turn, the condition is likely to trigger temporary carbon emissions. Similarly, the CO2 and CH4 production was also found by Brouns et al. [64] during oxygenation treatment of deep peat layers that had not previously been exposed to air. This pulse and resulting emissions are often discounted in the long-term calculations (IPCC) [27, 52]. Therefore, the mechanism of changes in water tables above the ground level accompanied by mechanical compaction is important when undertaking a full C cost accounting for the process of oil palm conversion (as opposed to just a comparison of oil palm and forest). This pulse in emissions in association with the process of conversion agrees with that described by Hooijer et al. [17], but is crucially not accounted for in the IPCC 2014 accounting [65]. Nevertheless, the frequency is limited, and further study is needed to verify the reproducibility of the results reported here.
3.4 Implication of mechanical compaction to current OPP management practice
No correlation between carbon emission and physicochemical properties was found when the data within the ecosystem was analyzed. Interestingly, the pooled data of physicochemical properties from both ecosystems showed several associations with carbon emissions (Table 6). These relationships can be interpreted in regard to the degree of decomposition resulting from oxidation and shrinkage. However, increases in bulk density and its decomposition proxies owing to ecosystem shifting, from PSF to OPP should not be interpreted as a result of mechanical compaction, but rather due to oxidation and shrinkage from periodic drainage alone [17, 66]. These coupled components have given rise to an important question regarding the actual definition of mechanical peat compaction. This issue could result in the misconception of mechanical compaction based on the use of heavy machinery during the opening of the plantation from a forest area. Here, through this study also, it is believed that mechanical compaction effectiveness could be deteriorated by the standard water table level from − 40.0 cm to − 60.0 cm [67], provided that the peat has a higher proportion of labile carbon at the early stages of the land opening period.
4 Conclusions
The current research marks an initial exploration into the mechanical compaction of tropical peatlands, which is particularly insightful for two distinctive ecosystems: PSF and OPP. Our findings reveal that mechanical compaction significantly affects the levels of greenhouse gases emitted from these ecosystems, driving a decrease in CO2 and an increase in CH4, specifically during periods of high-water tables as noted within the PSF sites. Despite these changes, other physiochemical aspects in both ecosystems remained unaffected, likely due to the slacking effect attributed to the high compressibility and low clay content of the peatlands. Noteworthy is the establishment of a strong correlation between water table depth and emission levels. This relation could prove invaluable for predicting future emissions from peat swamp forests. Although additional compaction in areas that have been naturally compacted due to drainage for agricultural conversion (like the OPP sites) showed no significant changes in physiochemical parameters or carbon emissions, it hints towards a potential bulk density threshold in compaction effects. While it is essential to distinguish the effects of compaction from those of groundwater levels, the undetectable in-situ effect of compaction in already converted areas is worth acknowledging. Further compaction is inadvisable when it has a tangible impact. More comprehensive research is required to delineate these effects and to explore the limitations of compaction's impact on tropical peatlands. These findings contribute to our understanding of the environmental implications of peatland management and can guide future research and mitigation efforts in these critical ecosystems.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Dhandapani S, Girkin NT, Evers S (2022) Spatial variability of surface peat properties and carbon emissions in a tropical peatland oil palm monoculture during a dry season. Soil Use Manag 38:381–395. https://doi.org/10.1111/sum.12741
Roucoux KH, Lawson IT, Baker TR, Del Castillo TD, Draper FC, Lähteenoja O et al (2017) Threats to intact tropical peatlands and opportunities for their conservation. Conserv Biol 31:1283–1292. https://doi.org/10.1111/cobi.12925
Cole LES, Willis KJ, Bhagwat SA (2021) The future of Southeast Asia’s tropical peatlands: Local and global perspectives. Anthropocene 34:100292. https://doi.org/10.1016/j.ancene.2021.100292
Evans CD, Peacock M, Baird AJ, Artz RRE, Burden A, Callaghan N et al (2021) Overriding water table control on managed peatland greenhouse gas emissions. Nature 593:548–552. https://doi.org/10.1038/s41586-021-03523-1
Mutert E, Fairhurst TH, von Uexkull HR (1998) Agronomic management of oil palms on deep peat. Better Crops Int 13:22–27
Sutikno S, Rinaldi R, Saputra E, Kusairi M, Saharjo BH, Putra EI (2020) Water management for hydrological restoration and fire prevention in tropical peatland. IOP Conf Ser: Mater Sci Eng 933:012053. https://doi.org/10.1088/1757-899X/933/1/012053
Xu Z, Wang S, Wang Z, Dong Y, Zhang Y, Liu S et al (2021) Effect of drainage on microbial enzyme activities and communities dependent on depth in peatland soil. Biogeochemistry 155:323–341. https://doi.org/10.1007/s10533-021-00828-1
Hadipoor M, Keivanimehr F, Baghban A, Ganjali MR, Habibzadeh S (2021) Chapter 24—Carbon dioxide as a main source of air pollution: prospective and current trends to control. In: Núñez-Delgado A (ed) Sorbents materials for controlling environmental pollution. Elsevier, pp 623–688
Raharjo JT, Fauzi R, Hadiyan Y, Andriyani EP, Mulyono BT (2023) Feasibility study of zero burning peatland agriculture in Mempawah District, West Kalimantan. Iop Conference Series Earth and Environmental Science. https://doi.org/10.1088/1755-1315/1168/1/012006
Melling L, Tang ACI (2018) Water table and soil moisture management. Haze and biomass burning in Asia—A systems perspective to reveal opportunities with benefits for long-term transformations bandung, Indonesia: International Institute for Applied Systems Analysis
Melling L, Henson IE (2011) Greenhouse gas exchange of tropical peatlands-A review. J Oil Palm Res 23:1087–1095
Samuel MK, Evers SL (2023) The role of compaction on physicochemical properties and carbon emissions of tropical peat soils: A review. Jurnal Teknologi (Sci Eng). 85:83–96. https://doi.org/10.11113/jurnalteknologi.v85.18340
Wijedasa LS, Jauhiainen J, Könönen M, Lampela M, Vasander H, Leblanc MC et al (2017) Denial of long-term issues with agriculture on tropical peatlands will have devastating consequences. Glob Change Biol 23:977–982. https://doi.org/10.1111/gcb.13516
Ismail AB, Jamaludin J (2007) Land clearing techniques employedd at MARDI Peat Research Station, Sessang, Sarawak, and their immediate impacts. In: Ismail AB, Ong HK, Hanif MJM, Kalsom MSU (eds) A case study at MARDI Peat Research Station, Sessang, Sarawak, Malaysia. MARDI, Malaysia, pp 1–8
Samuel MK (2020) Assessing the role of artificial compaction of physicochemical properties and carbon emissions of tropical peat soils. Dissertation, The University of Nottingham
Melling L, Chua KH, Lim KH (2008) Managing peat soils under oil palm. https://toolsfortransformation.net/wp-content/uploads/2017/01/Melling-et-al.-Managing-Peat-Soils-Under-Oil-Palm.pdf. Accessed 14 Nov 2017
Hooijer A, Page SE, Jauhiainen J, Lee WA, Lu X, Idris A et al (2012) Subsidence and carbon loss in drained tropical peatlands. Biogeosciences 9:1053–1071. https://doi.org/10.5194/bg-9-1053-2012
Othman H, Mohammed AT, Darus FM, Harun MH, Zambri MP (2011) Best management practices for oil palm cultivation on peat: Ground water-table maintenance in relation to peat subsidence and estimation of CO2 emissions at Sessang, Sarawak MPOB. http://jopr.mpob.gov.my/wp-content/uploads/2013/09/joprv23aug2011-Ahmad1.pdf. Accessed 8 August 2018
Othman H, Darus FM, Tarmizi A, Mohammed (2009) Experiences in Peat Development for Oil Palm Planting in the MPOB Research Station at Sessang , Sarawak. Malaysian Palm Oil Board (MPOB). http://palmoilis.mpob.gov.my/OPB/index.php/2020/03/29/experiences-in-peat-development-for-oil-palm-planting-in-the-mpob-research-station-at-sessang-sarawak/. Accessed 5 September 2016
Price JS, Cagampan J, Kellner E (2005) Assessment of peat compressibility: Is there an easy way? Hydrol Process 19:3469–3475. https://doi.org/10.1002/hyp.6068
Ekwue EI, Harrilal A (2010) Effect of soil type, peat, slope, compaction effort and their interactions on infiltration, runoff and raindrop erosion of some Trinidadian soils. Biosys Eng 105:112–118. https://doi.org/10.1016/j.biosystemseng.2009.10.001
Huat BBK, Kazemian S, Prasad A, Barghchi M (2011) State of an art review of peat: general perspective. Int J Phys Sci 6:1988–1996. https://doi.org/10.5897/IJPS11.192
Rezanezhad F, Price JS, Quinton WL, Lennartz B, Milojevic T, Cappellen PV (2016) Structure of peat soils and implications for water storage, flow and solute transport: a review update for geochemists. Chem Geol 429:75–84. https://doi.org/10.1016/j.chemgeo.2016.03.010
Perdana LR, Ratnasari NG, Ramadhan ML, Palamba P, Nasruddin, Nugroho YS (2017) Hydrophilic and hydrophobic characteristics of dry peat. 2nd international Tropical Renewable Energy Conference (i-TREC) 105. https://doi.org/10.1088/1755-1315/105/1/012083.
Wang D, Blumfield TJ, Xu Z (2020) Long-term impacts of soil compaction and cultivation on soil carbon and nitrogen pools, foliar δ13C and δ15N as well as tree growth in a hoop pine plantation of subtropical Australia. J Soils Sediments 20:2829–2842. https://doi.org/10.1007/s11368-020-02654-8
Ishikura K, Hirano T, Okimoto Y, Hirata R, Kiew F, Melling L et al (2018) Soil carbon dioxide emissions due to oxidative peat decomposition in an oil palm plantation on tropical peat. Agr Ecosyst Environ 254:202–212. https://doi.org/10.1016/j.agee.2017.11.025
Wakhid N, Hirano T, Okimoto Y, Nurzakiah S, Nursyamsi D (2017) Soil carbon dioxide emissions from a rubber plantation on tropical peat. Sci Total Environ 581–582:857–865. https://doi.org/10.1016/j.scitotenv.2017.01.035
Busman NA, Maie N, Ishak CF, Sulaiman MF, Melling L (2021) Effect of compaction on soil CO2 and CH4 fluxes from tropical peatland in Sarawak, Malaysia. Environ Dev Sustain 23:11646–11659. https://doi.org/10.1007/s10668-020-01132-y
Matysek M, Evers SL, Samuel MK, Sjogersten S (2018) High heterotrophic CO2 emissions from a Malaysian oil palm plantations during dry-season. Wetlands Ecol Manage 26:415–424. https://doi.org/10.1007/s11273-017-9583-6
Parkin VR, Hargreaves S (2012) Calculating the detection limits of chamber-based soil greenhouse gas flux measurements. J Environ Qual 41:705–715. https://doi.org/10.2134/jeq2011.0394
Arias PA, Bellouin N, Coppola E, Jones RG, Krinner G, Marotzke J et al (2021) Climate change 2021: the physical science basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press. https://doi.org/10.1017/9781009157896.002. Accessed 6 April 2023
Moore TR, Dalva M (1997) Methane and carbon dioxide exchange potentials of peat soils in aerobic and anaerobic laboratory incubations. Soil Biol Biochem 29:1157–1164
Al-Shammary AAG, Kouzani AZ, Kaynak AB, Khoo SY, Norton M, Gates WP (2018) Soil bulk density estimation methods: a review. Pedosphere. https://doi.org/10.1016/S1002-0160(18)60034-7
Marwanto S, Sabiham S, Funakawa S (2019) Importance of CO2 production in subsoil layers of drained tropical peatland under mature oil palm plantation. Soil Tillage Res. https://doi.org/10.1016/j.still.2018.10.021
Santos CF, Ribeiro ICA, Pelegrino MHP, Carneiro JP, Silva BM (2022) A simple gravimetric methodology to determine soil particle density. Commun Soil Sci Plant Anal 53:1623–1629. https://doi.org/10.1080/00103624.2022.2063310
Tonks AJ, Aplin P, Beriro DJ, Cooper HV, Evers SL, Vane CH et al (2017) Impacts of conversion of tropical peat swamp forest to oil palm plantation on peat organic chemistry, physical properties and carbon stocks. Geoderma 289:36–45. https://doi.org/10.1016/j.geoderma.2016.11.018
Sakata R, Shimada S, Arai H, Yoshioka N, Yoshioka R, Aoki H et al (2015) Effect of soil types and nitrogen fertilizer on nitrous oxide and carbon dioxide emissions in oil palm plantations. Soil Sci Plant Nutrit 61:48–60. https://doi.org/10.1080/00380768.2014.960355
Baker BJ, Fausey NR, Islam KR (2004) Comparison of soil physical properties under two different water table management regimes. Soil Sci Soc Am J 68:1973–1981
Pires LF, Bacchi OOS, Reichardt K (2007) Assessment of soil structure repair due to wetting and drying cycles through 2D tomographic image analysis. Soil Tillage Res 94:537–545. https://doi.org/10.1016/j.still.2006.10.008
Campos JRdR, Silva AC, Fernandes JSC, Ferreira MM, Silva DV (2011) Water retention in a peatland with organic matter in different decomposition stages. Revista Brasileira De Ciencia Do Solo 35:1217–1227. https://doi.org/10.1590/S0100-06832011000400015
Jauhiainen J, Page SE, Vasander H (2016) Greenhouse gas dynamics in degraded and restored tropical peatlands. Mires and Peat 17:1–12. https://doi.org/10.19189/MAP.2016.OMB.229
Gregorich E, Janzen HH, Ellert BH, Helgason B, Qian B, Zebarth BJ et al (2017) Litter decay controlled by temperature, not soil properties, affecting future soil carbon. Glob Change Biol 23:1725–1734. https://doi.org/10.1111/gcb.13502
Yule CM, Gomez L (2009) Leaf litter decomposition in a tropical peat swamp forest in Peninsular Malaysia. Wetlands Ecol Manage 17:231–241. https://doi.org/10.1007/s11273-008-9103-9
Alakukku L (1996) Persistence of soil compaction due to high axle load traffic. I. Short-term effects on the properties of clay and organic soils. Soil Tillage Res 37:211–222. https://doi.org/10.1016/0167-1987(96)01016-1
Alakukku L (1996) Persistence of soil compaction due to high axle load traffic. II. Long-term effects on the properties of fine-textured and organic soils. Soil Tillage Res 37:223–238. https://doi.org/10.1016/0167-1987(96)01017-3
Kolay P, Aminur M (2011) Physical and geotechnical characteristics of stabilized and unstabilized tropical peat soil. World J Eng 8:223–230. https://doi.org/10.1260/1708-5284.8.3.223
Kolay P, Siti Noor Linda T (2018) Physical and Geotechnical Properties of Tropical Peat and Its Stabilization. IntechOpen. https://doi.org/10.5772/intechopen.74173.Accessed9November2020
Kolay PK, Pui M (2010) Peat Stabilization using Gypsum and Fly Ash. J Civ Eng Sci Technol 1:1–5. https://doi.org/10.33736/jcest.75.2010
Bacon KL, Baird AJ, Blundell A, Bourgault MA, Chapman P, Dargie GC et al (2017) Questioning ten common assumptions about peatlands. Mires and Peat. 19:1–23. https://doi.org/10.19189/MaP.2016.OMB.253
Firdaus MS, Gandaseca S, Ahmed OH (2011) Effect of drainage and land clearing on selected peat soil physical properties of secondary peat swamp forest. Int J Phys Sci 6:5462–5466. https://doi.org/10.5897/IJPS11.598
Firdaus MS, Gandaseca S, Ahmed OH, Majid NMA (2010) Effect of converting secondary tropical peat swamp forest into oil palm plantation on selected peat soil physical properties. Am J Environ Sci 6:402–405. https://doi.org/10.3844/ajessp.2010.402.405
Ishikura K, Yamada H, Toma Y, Takakai F, Morishita T, Darung U et al (2017) Effect of groundwater level fluctuation on soil respiration rate of tropical peatland in Central Kalimantan. Indonesia Soil Sci Plant Nutrit 63:1–13. https://doi.org/10.1080/00380768.2016.1244652
Melling L, Hatano R, Goh KJ (2005) Soil CO2 flux from three ecosystems in tropical peatland of Sarawak, Malaysia. Tellus B: Chem Phys Meteorol 57:1–11. https://doi.org/10.1111/j.1600-0889.2005.00129.x
Melling L, Hatano R, Goh KJ (2005) Methane fluxes from three ecosystems in tropical peatland of Sarawak. Malays Soil Biol Biochem 37:1445–1453. https://doi.org/10.1016/j.soilbio.2005.01.001
Dariah A, Marwanto S, Agus F (2014) Root- and peat-based CO2 emissions from oil palm plantations. Mitig Adapt Strat Glob Change 19:831–843. https://doi.org/10.1007/s11027-013-9515-6
Husnain H, Wigena IGP, Dariah A, Marwanto S, Setyanto P, Agus F (2014) CO2 emissions from tropical drained peat in Sumatra, Indonesia. Mitig Adapt Strat Glob Change 19:845–862. https://doi.org/10.1007/s11027-014-9550-y
Sakabe A, Itoh M, Hirano T, Kusin K (2018) Ecosystem-scale methane flux in tropical peat swamp forest in Indonesia. Glob Change Biol 24:5123–5136. https://doi.org/10.1111/gcb.14410
Arai H, Hadi A, Darung U, Limin SH, Hatano R, Inubushi K (2014) A methanotrophic community in a tropical peatland is unaffected by drainage and forest fires in a tropical peat soil. Soil Sci Plant Nutrit 60:577–585. https://doi.org/10.1080/00380768.2014.922034
Girkin NT, Turner BL, Ostle N, Craigon J, Sjögersten S (2018) Root exudate analogues accelerate CO2 and CH4 production in tropical peat. Soil Biol Biochem 117:48–55. https://doi.org/10.1016/j.soilbio.2018.09.033
Dhandapani S, Ritz K, Evers SL, Yule CM, Sjögersten S (2019) Are secondary forests second-rate? Comparing peatland greenhouse gas emissions, chemical and microbial community properties between primary and secondary forests in Peninsular Malaysia. The Science of the total environment 655:220–231. https://doi.org/10.1016/j.scitotenv.2018.11.046
Brühl CA (2001) Leaf litter ant communities in tropical lowland rain forests in Sabah, Malaysia. Dissertation, Universität Würzburg
Dhandapani S, Ritz K, Evers SL, Sjögersten S (2019) Environmental impacts as affected by different oil palm cropping systems in tropical peatlands. Agr Ecosyst Environ 276:8–20. https://doi.org/10.1016/j.agee.2019.02.012
Leng LY, Ahmed OH, Jalloh MB (2019) Brief review on climate change and tropical peatlands. Geosci Front 10:373–380. https://doi.org/10.1016/j.gsf.2017.12.018
Brouns K, Verhoeven JTA, Hefting MM (2014) Short period of oxygenation releases latch on peat decomposition. Sci Total Environ 481:61–68. https://doi.org/10.1016/j.scitotenv.2014.02.030
IPCC (2014) Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II, and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Intergovermental Panel on Climate Change (IPCC). https://archive.ipcc.ch/pdf/assessment-report/ar5/syr/SYR_AR5_FINAL_full_wcover.pdf. Accessed 27 June 2016
Rhymes J, Jones L, Wallace H, Jones TW, Dunn C, Fenner N (2016) Small changes in water levels and groundwater nutrients alter nitrogen and carbon processing in dune slack soils. Soil Biol Biochem. https://doi.org/10.1016/j.soilbio.2016.04.018
RSPO (2018) Principles and criteria for the production of sustainable palm oil Roundtable on Sustainable Palm Oil (RSPO). https://rspo.org/resources?category=rspo-principles-and-criteria-2018-pc-standards&id=6025. Accessed 22 February 2019
Funding
Open access funding provided by Malaysian Agricultural Research & Development Institute (MARD) under 12th Malaysia Plan with Grant No. PRP515-1001-P29999. The research leading to these results received funding from 2015 -MARDI Ph.D. Scholarship under Agreement No. MDI/SM/PL/312/001Jld.14 at The University of Nottingham.
Author information
Authors and Affiliations
Contributions
All the authors contributed to the study conception and design. Material preparation, investigation, data collection, and analysis were performed by M.K.S. The first draft of the manuscript was written by M.K.S, and all authors (M.K.S and S.L.E) commented on the previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Samuel, M.K., Evers, S.L. Assessing the potential of compaction techniques in tropical peatlands for effective carbon reduction and climate change mitigation. SN Appl. Sci. 5, 347 (2023). https://doi.org/10.1007/s42452-023-05548-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-023-05548-9