Abstract
The kinetics of oxidation of antidiabetic drug metformin by vanadium(V) have been studied spectrophotometrically under pseudo-first order conditions in aqueous acidic and micellar medium. The observed rate of oxidation is first order with respect to both metformin and H+. The pseudo-first order rate constant is independent of ionic strength and varies inversely with dielectric constant of the medium. The effect of micelles (SDS and TX 100) on such reactions has been investigated. Sodiumdodecyl sulphate (SDS) and iso-octylphenoxy polyethoxyether (TX100) accelerate the rate of electron transfer reaction except for cationic CTAB due to the cloudiness of the reaction mixture. It is a one electron transfer process where vanadium(V)is reduced to vanadium(IV) supported by cyclic voltammetric study. The main oxidation product was identified as metformin N-oxide by FTIR and LCMS method. Activation parameters of such reaction have been calculated and reaction mechanism is suggested. Computational study based on Hartree–Fock method supports the reaction mechanism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Metformin is an oral first line anti-hyperglycaemic agent which improves glucose tolerance in patients with type-2 diabetes mellitus, lowering both basal and post prandial glucose. Metformin decreases intestinal absorption of glucose and controls insulin sensitivity by increasing peripheral glucose uptake. It belongs to a class of compound known as biguanides which is identified as N,N-dimethyl imido diacarbonimide diamide [1, 2] having two imino ( ) groups and one each of primary (–NH2), secondary (–NH) and tertiary—N (CH3)2 group as donating centres (Fig. 1).
) groups and one each of primary (–NH2), secondary (–NH) and tertiary—N (CH3)2 group as donating centres (Fig. 1).
Vanadium has been recognised as an important trace element in biological system. It is non-carcinogenic and non-toxic [3] but concentration above 1 × 10−3 M shows toxic effect due to accumulation in tissue which causes irritation of lungs, throat, eyes and nasal cavities, damage the nervous system, liver and kidney [4]. Vanadium exists in many oxidation states from + 2 to + 5 but two of which are most common, Vanadium(IV) and Vanadium(V) as VO2+ (V4+) and \(HVO_{4}^{2 - }\) (V 5+) in biological system [5]. Vanadium is present as coenzyme in human body as vanadium nitrogenase and haloperoxidase [6]. Vanadium is distributed in body tissue and bones. Bones act as storage pool for Vanadate. Vanadium(V) is more easily taken up than Vanadium(1 V) in gastro intestinal tract [7]. However Vanadium(V) is particularly reduced in stomach and precipitate in the form of strongly soluble \(VO\left( {OH} \right)_{2}^{ - }\) due to slightly alkaline nature of intestine fluid [8, 9]. Aqueous chemistry of vanadium is very interesting. Both V(IV) and V(V) undergo very complex chemistry in aqueous solution forming a range of species. In aqueous solution at very low concentration it exists as vanadyl cation (VO2+) [10]. At pH below 3, it exist in the form [VO(H2O)5]2+ which is very stable cation known from the observed spectroscopy [11, 12]. However between pH3 to 10, the concentration of monomeric vanadium is very low. At a pH above 10, it becomes [VO(OH)3]−. Depending up on the pH, vanadium can be cationic or anionic species in aqueous solution. It becomes colourless at neutral and alkaline pH [13], but yellow in acid solution. Vanadium has a wide variety of pharmacological properties. Vanadium complexes have been tested as anti-parasitic, spermicidal, anti-viral, anti-tuberculosis and anti-tumour agents [10, 14, 15]. Besides pharmaceutical properties, it is also used in redox–flow battery (VRB) system which is an electrochemical energy storage by using redox system of VO2+/VO2+ couple in acid medium [16]. A wide range of coordinate complexes with Vanadium(V) have been synthesized and known to exert insulin–enhancing effect such as pyridine-2, 6-dicarboxylate dioxo Vanadium(V) [17], Vanadium dipicolinate [13], bis (maltolato) oxovanadium(IV)[VO-(malto)2] [18], Ammonium metavanadate [19]. Recently Trivino [20,21,22] and Crans [23,24,25] reported that metformin-decavanadate has shown pharmaceutical potential as hypoglycemic, lipid lowering, metabolic regulator. It reduces the dose and time of administration. It is not only insulin mimetic agent but also an insulin enhancing agent by observing its effect in vitro and in Vivo. Metformin decavanadate showed a protective effect on pancreatic beta cells of rats suggesting possible regeneration of these cells. Therefore it acts as insulin-enhancing effect.
Because of the above importance of vanadium, Vanadium(V) is selected as oxidant in our study. Although the importance of Vanadium is well known its necessity as a fundamental element in human body is still unclear. Thus much research in the clinical, biological, physiological fields has focused on Vanadium.
Various redox reactions of Vanadium(V) have been reported by biologically active agents like EDTA [26], paracetamol [27], ascorbic acid [28], l-tryptophan, glycolic acid [29], d-Fructose [30], 2-hydroxy cyclohexanone [31], tyrosine [32], d-glucose, d-Ribose [33].
d-arabinose, d-xylose [34], NADPH [35], glutathione [36], oxalic acid [37], hydroxyl urea [38]. Both inner and outer sphere electron transfer path ways have been reported. However literature of redox reaction of Vanadium by drug is very rare. Drug–metal interaction is an emerging research to understand the drug action at molecular level. Such type of study are useful for predicting the absorption of drugs, transport of drug across the biological membrane.
Several metal mediated oxidative reactions were investigated in aqueous and micellar medium. In most cases micelles play a crucial role to influence the kinetics of the oxidation pathways. The most prominent role of surfactant micelles is the improvement of a reaction velocity for a particular bimolecular reaction by concentrating both the reactants at their surfaces.
Surfactant is a special kind of amphiphilic compound composed of hydrophilic part (polar head group) and hydrophobic part (nonpolar tail group). Polar head group which forms outside as surface of micelles faces to water. The hydrophobic tail are inside away from water as they are non polar. Micelles are also at work in the human body. Micelles help the absorption of complicated lipids and lipid soluble vitamins (A, D, E and K) in small intestine. The product of fat digestion are dispersed into micelles by the action of bile salt which facilitates their absorption in small intestine [39].
The remarkable ability to influence the properties of surfaces and interfaces make the surfactant accessible for numerous applications from laboratory to industry [40]. A number of reviewers and scientific reports depict the widespread application of surfactant such as pharmaceutical, detergent, cosmetic, food science, nanotechnology and drug delivery [41]. They act as carrier of drug molecules to target site. They protect the drug molecules from degradation via hydrolysis or other physiological reactions which increases their shelf life and prolongs their stability during use. The particular nature of interaction between surfactant molecules and drug molecules is highly significant with the view point of stabilisation and solubilization of drug against degradation throughout its transportation in biological system. Since most biological processes occur at interface, structure, dynamic and reactivity of bio molecules differ at the interface than those observed in the bulk [42]. It influences the kinetics of various reactions when reactions takes place at the interface. A variety of electron transfer, proton transfer, ion transfer processes occur at the interface. Keeping this in view present study was carried out both in aqueous and micellar medium to understand the mode of action of the drug in biological domain.
Micellar effect has been studied by using sodium dodecylsulfate (SDS), CTAB (cetyltrimethylammonium bromide), Triton X-100 (iso-octylphenoxy polyethoxyether) as anionic, cationic and neutral surfactant respectively (Fig. 2).
Diabetes mellitus is ranked seventh among leading cause of death. Several drugs are presently available to reduce hyperglycaemia in diabetes mellitus. But these drugs have side effects, thus searching a new compound is essential to overcome this problem. There is continuous research for alternative drug. Management of diabetes without any side effect is still a challenge to medicinal chemist. This work is an attempt to synthesize and design a new anti-diabetic drug along with study of its mechanism of action through kinetic study.
2 Experimental
2.1 Materials and methods
All chemicals used were of analar grade and double distilled water was used throughout the experiment. The metformin hydrochloride sample was procured from Orissa Drugs and Chemicals Limited, Bhubaneswar, India. The purity of the sample was found to be 99% which was checked spectrophotometrically. Analytical grade ammonium metavanadate (NH4VO3) (99% pure) was procured from Merck. The surfactants such as SDS (sodium dodecylsulfate), CTAB (cetyltrimethylammonium bromide)and Triton X-100 were also procured from Merck. The concentration of Vanadium(V) was estimated by KIO3. The pH measurements were made using pre standardised digital pH meter (Systronics, India). The pH meter was standardised using NBS buffer of pH 4.01, 6.86 and 9.20. Ionic strength was maintained at I = 0.5 mol dm−3 by using freshly prepared standardised NaClO4 solution. The strength of NaClO4 was estimated by combined ion exchange alkali metric procedure using cation exchange resin Dowex 50 X 8(Na+). The SDS (sodium dodecylsulfate), CTAB (cetyltrimethylammonium bromide), Triton X-100 were used as anionic, cationic and neutral surfactant respectively.
2.2 Kinetic measurements
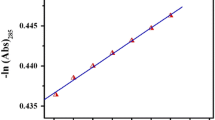
The kinetics of the reaction between metformin and ammonium metavanadate in aqueous acidic medium was investigated spectrophotometrically under pseudo-first order conditions with excess of metformin at constant ionic strength, I = 0.5 mol dm−3 using carry 100 (USA) UV–Vis spectrophotometer equipped with peltier system for temperature control. The progress of the reaction was followed spectrophotometrically by mixing the required amount of solution of metformin, ammonium metavanadate, NaClO4 (for ionic strength variation), HCl (to maintain pH) in a thermo stated cell compartment. The reaction was followed spectrophotometrically at 262 nm by monitoring the decrease of absorbance at \(\lambda_{max}\) = 262 nm as a function of time. The kobs were calculated as the gradient of plot ln (\(A_{t}\)-\(A_{\infty }\)) verses t using following Eq. (1).
where Ao, At, \(A_{\infty }\) denote optical density of reaction mixture at zero time, t time and infinite time respectively. \(A_{\infty }\) was measured after completion of the reaction (5t1/2). The correlation coefficient (R2) of the plot was found to be 0.99 in most of the cases. All calculations were made on PC using least square method. Again the kinetic measurements of the redox reaction was conducted in presence of micelle. The pseudo-first order rate constant (kobs) data represents an average of duplicate run which are reproducible to within ± 5%.
3 Result and discussion
3.1 Stoichiometry and product analysis
The stoichiometry was determined for different reaction mixture containing various concentrations of ammonium metavanadate (V) and metformin in a molar ratio of 1:10 at I = 0.5 mol dm−3 and pH = 2.0. The reaction mixture was heated at 318 K for three hours till the reaction was completed. The unreacted Vanadium(V) concentration was estimated spectrophotometrically by measuring its absorbance at 262 nm.
The results indicated that 1 mol of metformin was consumed by 1 mol of vanadium to yield the oxidation product. The stoichiometry was found to be 1:1 as depicted in the Eq. (2).
In order to get the product, 0.2 mol of ammonium metavanadate in 20 ml of water and 0.2 mol of metformin in 20 ml of water were mixed and pH = 2.0 was maintained followed by heating the reaction till completion. From this solution metal ions were removed through cation exchange resin (Dowex 50 WX8). The solution was evaporated and concentrated slowly and was kept overnight, a brown crystalline product was formed. It was washed with ethanol several times and dried in a desiccator containing silica gel.
The FTIR spectrum of the substrate and the product was recorded by Nicolas iS 5 FTIR spectrophotometer (Thermo fisher) using KBr pellet of the sample and the spectra was presented in Fig. S1(a) and S1(b). Analysis of product [Fig. S1(b)] shows a sharp peak at 3425 cm−1 corresponds to N–H stretching in primary amine and C=NH stretching [43]. The peak at 2917 cm−1 corresponds to C–H stretching in CH3 group,1646 cm−1 corresponds to N–H stretching band and C=N stretching in amino linkage, 1556 cm−1 corresponds to secondary N–H stretching. A characteristic sharp peak at 1381 cm−1 is due to N-oxide stretching (aliphatic) which is not found in FTIR spectra of the substrate. The peak at 665 cm−1 is due to C–H out plane deformation (unsymmetrical) and peak at 804 cm−1 corresponds to N–H out plane bending. Comparison of Fig. S1(b) with Fig. S1(a) shows that metformin moiety remains unaffected during the oxidation reaction. Hence the oxidation product is metformin N-oxide. Similar product was reported by other authors for oxidation of metformin by Bromamine–T and Chloramines’–B [44].
Product of the reaction was further confirmed by mass spectra. Fig. S2 (a) and S2 (b) representing the mass spectrum of metformin and product metformin N-oxide respectively.
It shows characteristic peaks at m/z 145, 129, 64, 60, 45, 30, 16 amu. Molecular ion peak of metformin N-oxide corresponds to 145 amu and metformin ion corresponds to 129 amu, 60 amu corresponds to (CH3)2 NO+ and 30 amu corresponds to CH2=NH2+, 45amu corresponds to C2H5NH2+ whereas 16 amu corresponds to NH2+. These peaks are in accordance with the proposed product metformin N-oxide.
3.2 Mechanism and rate law
Based on the above experimental results, the probable mechanism of the redox reaction may be delineated as in Scheme 1.
Vanadium(V) is amphoteric in nature and reduction of it in acidic medium involves cationic species [45]. In acidic medium Vanadium(V) exists as bright yellow pervanadyl ion VO2+ which coexist in equilibrium with the hydrated form [V (OH)3]2+. It has been established that protonated form of oxidant is more reactive in acid medium [46]. It reacts with metformin forming intermediate complex prior to electron transfer. The intermediate complex decomposed to give the product as shown in Scheme 1. The transfer of reactant to form transition state proceeds rapidly under the experimental conditions which decomposed to give the product. From the above Eq. (2), the rate law can be derived as follows:
Substituting the value of \(\left[ {C_{1} } \right]_{e}\) in Eq. (3)
Or,
Substitute \(\left[ {VO_{2}^{ + } } \right]_{e}\) in Eq. (6)
Since \(K_{a} [H^{ + } ]_{e} \le 1.0\)Equation (11) is reduced to
Plot of \(k_{obs}\) versus \(\left[ {metformin} \right]_{T}\) keeping \(\left[ {H^{ + } } \right]\) constant (Fig. 4) shows a straight line and \(k_{obs}\) versus \(\left[ {H^{ + } } \right]_{T}\) keeping \(\left[ {metformin} \right]_{T}\) nstant also shows a straight line (Fig. 5). This supports the rate law and the proposed mechanism.
From Eq. (12)
Plot of \(\frac{{K_{obs} }}{{\left[ {metformin} \right]_{T} }}\) versus \(\left[ {H^{ + } } \right]\): is a linear line with a slope which gives composite rate constant of the above electron transfer reaction(k).
Slope = \(k_{et} K_{a} = k\) = composite rate constant
The kobs data are collected in Table 1. The composite rate constant (k) are calculated at temperature range 293.15–308.15 K and tabulated in Table 2. The activation parameters of composite rate constant are calculated and tabulated in Table 2.
The mechanism of the reaction of C1 with metformin to generate the product Metformin N-oxide is shown in Scheme 2.
This scheme of mechanism is similar to our earlier published paper [47].
3.3 Spectroscopic changes
UV–Vis time scan spectra (Fig. 3a) of the reaction mixture of metformin and Vanadium(V) in HCl medium shows continuous decrease in absorption spectra of V (V) by metformin in the whole range 250–400 nm indicating reduction of metal vanadium. The spectra after completion of the oxidation reaction (24 h) is shown in Fig. 3(b). Since there is no shifting of band in the time scan spectrum during reduction, outer sphere electron transfer mechanism is predicted.
a Time scan UV–Vis spectra of oxidation of metformin by V(V) in hydrochloric acid medium. [metformin] = 5.0 × 10−5 mol dm−3, [V(V)] = 2.0 × 10−4 mol dm−3, [H+] = 0.01 mol dm−3, I = 0.5 mol dm−3, 25 °C. Scan time intervals (Δt) = 2 min (curve 1–11). b UV–Vis spectra of oxidation of metformin by V(V) in hydrochloric acid medium. [metformin] = 5.0 × 10−5 mol dm−3, [V(V)] = 2.0 × 10−4 mol dm−3,[H+] = 0.01 moldm−3, I = 0.5 mol dm−3, 25 °C after 24 h
3.4 Reaction order
The reaction order with respect to the reactants is determined from the plot of kobs versus concentration of metformin and H+ keeping other conditions constant. kobs versus metformin and HCl is a straight line Figs. 4 and 5 with a positive slope suggesting order of the reaction is unit with respect to [Metformin] and [H+].
3.5 Effect of ionic strength
The effect of ionic strength on redox reaction was studied varying I = 0.3–0.8 mol dm−3using of NaClO4as an inert electrolyte keeping the concentrations of all other reactants constant. The results are presented in Fig. S3 indicating that the redox reaction is independent of ionic strength.
3.6 Effect of dielectric constant
The effect of dielectric constant was studied at 293 K by varying 20% to 70% acetic acid keeping all other conditions constant. kobs changes from 6.35 × 10−3 s−1 to 6.97 × 10−3 s−1 when dielectric constant varies from 81.5 to 38.5 (Table S2). Plot of log kobs versus D−1 is linear (Fig. S4) with positive slope (R2 = 0.98) indicating ion–dipolar interaction in rate determining step [48].
3.7 Test of free radicals
The involvement of free radicals in the reaction was examined by polymerization test. A known quantity of acrylonitrile scavenger was added to the reaction mixture of [metformin] = 5.0 × 10−5 mol dm−3, [V (V)] = 2.0 × 10−4 mol dm−3, [H+] = 0.01 mol dm−3 in an inert atmosphere for two hours. Upon dilution with methanol no precipitate was formed indicating absence of free radical in the reaction mixture. Hence there is no intervention of free radicals in the mechanism of the reaction.
3.8 Effect of temperature
The rate of oxidation of metformin by V (V) was performed at five different temperatures in the range of 293–308 K at constant concentration of the reactants and ionic strength. The results indicate that pseudo-first order rate constant (kobs) which shows an increasing trend with rise in temperature. With increase in temperature, the composite rate constant (k) also increases. Using Arrhenius and Eyring equation by plotting log (k/T) verses 1/T, activation parameters such as activation enthalpy \(\Delta H^{ \ne } = 35.2\;{\text{kJ}}\;{\text{mol}}^{ - 1}\), activation entropy \(\Delta S^{ \ne } = - 347.9\;{\text{J}}\;{\text{K}}^{ - 1} \;{\text{mol}}^{ - 1}\) and activation free energy \(\Delta G^{ \ne } = 68.5\;{\text{kJ}}\;{\text{mol}}^{ - 1}\), activation energy Ea = 40.2 kJ mol−1 are tabulated in Table 2.
3.9 Effect of surfactants
3.9.1 Effect of SDS
Time scan spectra of the oxidation reaction of metformin by vanadium in presence of anionic micelle SDS (Fig. 6) shows a remarkable change indicating the significant effect of SDS in the redox reaction. The kinetics of oxidation of metformin by V (V) compound was studied by varying [SDS] from 0.002 to 0.014 mol dm−3 at 293 K and rate constants are tabulated in Table S1. The curve (Fig. 7) shows increase of pseudo-first order rate constant with increase of [SDS] from 0.002 mol dm−3 and reaches maximum value at 0.006 mol dm−3 which is close to CMC of SDS = 0.007 mol dm−3 [49]. On further increase beyond CMC of SDS, a decreasing trend of rate constant was observed.
The kinetic result has been explained by considering the preferential partitioning of reactants between the micellar and aqueous pseudo phase (Fig. 8) and local concentration effect. The rate enhancement can be explained in following manner.
The micellar surface of SDS is anionic nature whereas the vanadium present inside the solution is cationic species. So electrostatic attraction taking place between two opposite charge species. Metformin also remains in cationic form therefore it preferably stay in micellar stern layer region (Fig. 8) so micelle assist the reactant molecules to come closer to each other hence increase the observed pseudo-first order rate of the reaction by decreasing the energy of activation of the reaction. There is also another reason for rate enhancement. The higher collision frequency among the reactant molecules in the small volume of the micelle like confined environment known as localization effect. This increase of the rate of reaction stops at [SDS] = 0.006 mol dm−3 near the CMC of SDS which is 0.007 mol dm−3 [50], after CMC there is retardation of rate constant due to micelle–micelle repulsion. This can also be explained mathematically by using Scheme 3 and its kinetic equation.
The variation of pseudo-first order rate constant can also be explained using the assumption that the surfactant is distributed between aqueous micellar pseudophase as shown in scheme 3 where subscript W and M represents aqueous and micellar pseudo phase respectively.
The observed first order rate constant of the overall reaction in micellar medium is expressed by the Eq. (14)
[V]m → concentration of vanadium in micellar medium, kw → second order rate constant in aqueous phase, k M2 → second order rate constant in micellar medium, [Dn] = [SDS]T–CMC, [Dn] → micellized surfactant, KSB → substrate binding constant
Sm and Sw denotes substrate in aqueous and micellar pseudophase respectively. As micellar pseudo phase occupies only a small fraction (2-3%) of total solution volume it may be assumed that
This shows linear increase of observe rate constant with an increase of [surfactant]. This explains the enhancement of pseudo first order (kobs) rate constant as in Fig. 7. By assuming the rate in micellar pseudo-phase is zero that is k M2 = 0 Eq. (14) is reduced to Eq. (17)
The above Eq. (17) explains the decrease of pseudo first order rate constant with increase in the substrate concentration beyond [surfactant] > 0.006 mol dm−3.
Similar micellar effect has been reported by different research groups Buntan [51], Richardson [52], for oxidation of organic sulfides with peroxo mono sulphate and bicarbonate–activated hydrogen peroxide respectively. Metal catalyzed Ce(IV) oxidation of formaldehyde to formic acid in aqueous medium [53]. Oxidation of propanol to propionaldehyde in vanadium(V) in aqueous medium [54], oxidation of methanol to formaldehyde in aqueous medium [55]. Oxidation of ascorbic acid by vanadium(V) in aqueous medium [56].
3.9.2 Effect of CTAB
The rate measurements in presence of cationic micelle CTAB could not be made even at very low concentration (0.001–0.005) mol dm−3 due to cloudiness of the reaction mixture.
3.9.3 Effect of Triton X-100
The rate constant measurements in presence of neutral micelle Triton X-100 was carried out at 303 K by varying [Triton X-100] = 0.002–0.014 mol dm−3 as displayed in Fig. S5 and reflected in Table S1. The rate enhancement effect was observed up to 0.012 mol dm−3 and then it reaches a limiting value at 0.014 mol dm−3. This may be due to hydrophobic effect which assists the micellar binding of the substrate. The micellar environment has an effect of rate magnification which is governed by both electrostatic and non-electrostatic effects. The substrate bound to the neutral micelle undergoes with a faster oxidation reaction (km/kw = 1.5–2.0) as compared to aqueous phase.
3.10 Cyclic voltammetry study
Voltammetry measurements were made on a CHI661 workstation with a normal three-electrode configuration, consisting of a glassy carbon electrode, an Ag/AgCl reference electrode and a Pt-wire auxiliary electrode. Prior to the measurement, the working electrode was polished with decreasing grades of alumina (1.0–0.1 μm) to achieve a mirror smooth surface, then it was ultrasonic washed in acetone and ultra-purified water in succession.
The cyclic voltammeter study of the redox reaction between Vanadium(V) and metformin containing 0.1 M KCl as supporting electrolyte was carried out using a glassy carbon as electrode and Ag/AgCl as reference electrode. CV results of the redox reaction are presented in Table S3. The representative cyclic voltammogram is shown in Fig. 9. A negative sweep initiated at 1.0 V in the potential range + 1.5 V to − 1.5 V with scan rate of 100 mV/s yields an irreversible couple corresponding V (V)/V (IV) redox process. The complex shows an irreversible reduction (peak) at Epc = 0.15 V corresponding V(V)–V(IV) redox reaction. Oxidation wave at Epa1 at 0.61 V corresponding to VIV–VV oxidation reaction and Epa 2 0.3 V corresponds to VV. The peak current ratio Ipa1/Ipc = 0.6 which is less than unity showing one electron transfer process followed by chemical reduction.
3.11 Computational study
Quantum mechanical calculation of thermodynamic parameters such as free energy, entropy, enthalpy change and change in molecular parameters such as bond angle, bond length, point group of the reaction between [V(OH)3]2+ and metformin is based on Hartree–Fock theory with no imaginary frequency. The calculations are based on several basis sets for the reactants and products. They are 6-31G (d,p) (for a), LAN2DZ (for b), 6-31 + G (df,p) (for c) and 6-31G (d,p) (for d) where, d, f, p stands for polarization function, + indicates diffused function and G represents Gaussian type orbital’s (GTO). The geometry of the reactants(a, b) and the products(c, d) are fully optimized which is shown in Fig. 10. The respective points group of a, b, c, d are C1, C1, D2h and C1. All calculations are implemented using Gaussian-16 software package and the parameters are represented in Tables S4, S5and S6.
From the data in the above table it is observed that the bond angle N(1)–C (2)–N (5) and N (5)–C (7)–N (8) is almost constant in reactants and products. The structural moiety of metformin N-oxide almost remains same as metformin. There is a slight increase of bond length (N1–C2 = 1.506 Å) in metformin N-oxide as compared to metformin (1.395 Å) due to co-ordination of N with more electronegative oxygen but all other bond lengths remains constant. Dipole moment of metformin is found to be 3.091D where as in metformin N-oxide it is 1.383D. Hartree–Fock calculation of the above reaction shows \({{\Delta }}H = - 7. 9 \times 10^{ 5}\) kJ mol−1, \(\Delta S = 14.75 \times 10^{{ - 2}}\) JK−1 mol−1, \(\Delta G = -7.92 \times 10^{5}\) = kJ mol−1. Low value of \({{\Delta }}H\) and \({{\Delta }}S\) favours the electron transfer process. Negative value of \({{\Delta }}G\) indicates the feasibility of the reaction of metformin as in Eq. (1) and negative value of ∆H indicates exothermic reaction. All these data from Hartree–Fock calculation supports the predicted reaction mechanism as indicated in Scheme 1.
4 Conclusion
The kinetics of oxidation of metformin by Vanadium(V) was studied in aqueous acidic and micellar medium. Followings are the conclusions from the above study.
-
1.
The stoichiometry of metformin and Vanadium(V) reaction is found to be 1:1.
-
2.
The reaction is found to be first order with respect to metformin and H+.
-
3.
The reaction is independent of ionic strength and varies inversely with dielectric constant of the medium.
-
4.
SDS and Triton X100 anionic and neutral surfactants accelerate the rate of electron transfer reaction.
-
5.
The main product of the oxidation reaction is identified as metformin N-oxide through outer sphere electron transfer pathway which is supported by mechanism of the reaction.
-
6.
Positive value of activation free energy and activation enthalpy indicates that the transition state is highly solvated and negative entropy of activation suggests the formation of compact activated complexes.
-
7.
The lower activation enthalpy 35.2 kJmol−1 obtained in this study and CV study suggest that it is a one electron transfer process.
-
8.
The geometry optimization and calculated thermo chemical parameters of reactants and products by Hartree–fock method using Gaussian-16 software also supports the reaction mechanism.
-
9.
This kinetic study is helpful in determining the drug potency and mode of action of drug towards the biological targets.
-
10.
Thermodynamic and kinetic signature, in combination with structural biology helps to elucidate the driving mechanism of target-drug binding.
-
11.
Metal bound drug also increases the serum half life of the drug than the free drug.
It is a model reaction between the drug metformin and the trace element, insulin mimic vanadium in biological system. More research is to be carried out for interaction of drug with other metal ions present in biological system.
The present study would definitely forecast new dimensions in understanding the interaction of drug with metal ions in biological system. We hope the present study will not only help in academic sphere but also in biological and pharmaceutical premises.
References
Adikwu MU, Yoshikawa Y, Takada K (2004) Pharmacodynamic–pharmacokinetic profiles of metformin hydrochloride from a mucoadhesive formulation of a polysaccharide with antidiabetic property in streptozotocin-induced diabetic rat models. Biomaterials 25(15):3041–3048. https://doi.org/10.1016/j.biomaterials.2003.09.073
Nell O, Editor MJ (2001) Metformin the merck index, 13th edn. Merck and Co Inc, Rahway, p 5966
Ghosh SK, Saha R, Saha B (2015) Toxicity of inorganic vanadium compounds. Res Chem Intermed 41(7):4873–4897
Trevino S, Díaz A, Sánchez-Lara E, Sanchez-Gaytan BL, Perez-Aguilar JM, Gonzalez-Vergara E (2019) Vanadium in biological action: chemical, pharmacological aspects, and metabolic implications in diabetes mellitus. Biol Trace Elem Res 188:68–98. https://doi.org/10.1007/s12011-018-1540
Thompson H, Orvig C (2006) Vanadium in diabetes: 100 years from phase 0 to phase 1. J Inorg Biochem 100:1925–1935. https://doi.org/10.1016/j.jinorgbio.2006.08.016
Bogden JD, Higashino H, Lavenhar MA, Bauman JW, Kemp FW, Aviv A (1982) Balance and tissue distribution of vanadium after short-term ingestion of vanadate. J Nutr 112:2279–2285. https://doi.org/10.1093/jn/112.12.2279
Wiegmann TB, Day HD, Patak RV (1982) Intestinal absorption and secretion of radioactive Vanadium(48VO3−) in rats and effect of Al(OH)3. J Toxicol Environ Health 10:233–245. https://doi.org/10.1080/15287398209530246
Rehder D (2013) The future of/for vanadium. Dalton Trans 42:11749–11761. https://doi.org/10.1039/c3dt50457c
Rehder D (2015) The role of vanadium in biology. Metallomics 7:730–742. https://doi.org/10.1039/c4mt00304g
Crans DC, Smee JJ, Goidamakskes E, Yang L (2004) The chemistry and biochemistry of vanadium and the biological activities exerted by vanadium compounds. Chem Rev 104:849–902. https://doi.org/10.1021/cr020607t
Jakusch T, Jin W, Yang L, Crans DC, Kiss T (2003) Chemical speciation of insulin mimeticVO(IV) complexes of pyridine-N-oxide derivative: binary and ternary system. J Inorg Biochem 95(2–3):69–76
Chasteen ND, Clarke MJ et al (1983) The biochemistry of vanadium. In: Structure and bonding, vol 53. Springer, Berlin, pp 105–138
Sostarecz AG, Gaidamauskas E, Distin S, Bonetti SJ, Levinger NE, Crans DC (2014) Correlation of insulin-enhancing properties of vanadium-dipicolinate complexes in model membrane systems: phospholipid langmuir monolayers and AOT reverse micelles. Chem Eur J 20:1–12
Maurya MR, Kumar A, Bhat AR, Bader C, Rehder D (2006) Dioxo- and oxovanadium(V) complexes of thiohydrazone ONS donor ligands: synthesis, characterization, reactivity, and antiamoebic activity. Inorg Chem 45:1260–1269
Thompson KH, Batra CA, Orvig C (2006) Metal complexes of maltol and close analogues in medicinal inorganic chemistry. Chem Soc Rev 35:545–556
Jihye Lee, Muya JT, Chung H, Chang J (2019) Unraveling V(V)–V(IV)–V(III)–V(II) redox electrochemistry in highly concentrated mixed acidic media for a vanadium redox flow battery: origin of the parasitic hydrogen evolution reaction. ACS Appl Mater Interfaces 11:42066–42077
Crans DC, Tahir M, Johnson MD, Wilkins PC, Yang L, Robbins K, Johnson A, Alafano JA, Godzala ME, Austin LT, Willsky GR (2003) Vanadium(IV) and Vanadium(V) complexes of dipicolinic acid and derivatives. Synthesis, X-ray structure, solution state properties: and effect in rats with STZ-induced diabetes. Inorg Chim Acta 356:365–378
Thompson KH, Orvig C (2006) Metal complexes in medicinal chemistry: new vistas and challenges in drug design. Dalton Trans 6:761–764
Crans DC, Zhang B, Gaidamauskas E, Keramidas AD, Willsky GR, Roberts CR (2010) Is vanadate reduced by thiols under biological conditions? changing the redoxpotential of V(V)/V(IV) by complexation in aqueous solution. Inorg Chem 49:4245–4256. https://doi.org/10.1021/ic100080k
Trevino S, Díaz A (2020) Vanadiumandinsulin: partnersinmetabolicregulation. J Inorg Bio 208:111094. https://doi.org/10.1016/j.jinorgbio.2020.111094
Treviño S, Velazquez-vazquez D, Sanchez-lara E, Diaz A, Flores-hernandez JA, Pérez-benítez A, Brambila E, González-vergara E (2016) Metforminium decavanadate as a potential metallopharmaceutical drug for the treatment of diabetes mellitus. Oxidative Med Cell Longev. https://doi.org/10.1155/2016/6058705
Trevino S, Sánchez-Lara E, Sarmiento-Ortega VE, Sánchez-Lombardo I, Flores-Hernández JA, Pérez-Benítez A, Brambila-Colombres E, González-Vergara E (2015) Hypoglycemic, lipid-lowering and metabolic regulation activities of metforminium decavanadate (H2Metf)3[V10O28]·8H2Ousing hypercaloric-induced carbohydrate and lipid deregulation in Wistar rats as biological model. J Inorg Biochem 147:85–92. https://doi.org/10.1016/j.jinorgbio.2015.04.002
Chakton A, Chatterjee PB, Sedgwick MA, Haller KJ, Crans DC(2013) Counterion affects interaction with interfaces: the antidiabetic drugs metformin and decavanadate. Eur J Inorg Chem 1859–1868
Crans DC, Yang L, Haase A, Yang X (2018) Health benefits of vanadium and its potential as an anticancer agent. Met Ions Life Sci 18:437–467. https://doi.org/10.1515/9783110470734-015
Crans DC, Henry LR, Cardiff G, Posner BI (2019) Developing vanadium as an anti-diabetic or anticancer drug: a clinical and historical perspective. Met Ions Life Sci 19:203–230. https://doi.org/10.1515/9783110527872-014
Mishra AP, Khan R, Pandey RR (2009) Kinetic studies on effects of EDTA and surfactants on reduction of vanadium(V) to vanadium(IV) in sulphuric acid medium. Indian J Chem 48A:1228–1234
Yiase SG, Adejo SO, Ukoha PO, Iorhuna BT, Gbertyo JA (2014) Kinetics and mechanism of oxidation-reduction reaction of vanadium(V) ion with paracetamol. Int J Inorg Bioinorg Chem 4(2):40–44
Wilkins PC, Johnsen MD, Holder AA, Crans DC (2006) Reduction of vanadium(V) by l-ascorbic acid at low and neutral pH: kinetic, mechanistic and spectroscopic characterization. Inorg Chem 45:1471–1479
Bakore GV, Shanker R (1966) Kinetics of the oxidization of glycolic acid by Vanadium(V). Can J Chem 44:1717–1720
Khan Z, Babu PSS, Kabir-ud-In (2004) Kinetics and mechanism of the oxidation of d-fructose by vanadium(V) in H2SO4 medium. Carbohydr Res 339:133–140
Panigrahi GP, Swain R (2000) kinetics and mechanism of oxidation of 2-hydroxycyclohexanol by vanadium(V). Indian J Chem Sect A Inorg Phys Theor Anal Chem 39:775–778
Onkar AS, Naik PN, Gunagi SD, Nandibewoor ST, Chimatadar SA (2012) Kinetics and oxidation of tyrosine by vanadium(V) by aqueous hydrochloric acid and ethanoic acid medium. Indian J Chem 51A:1574–1579
Sengupta KK, Basu SN (1980) Kinetics and mechanism of oxidation of d-glucose and d-ribose by chromium (w) and vanadium(V) in perchloric acid medium. Carbohydr Res 80:223–232
Odebunmia EO, Ogunlaja AS, Owaludea SO (2010) Kinetics of oxidation of d-arabinose and d-xylose by vanadium(V) in the presence of manganese II as homogeneous catalyst. Orbit Electr J Chem 2(2):127–139
Kanamori K, Sakurai M, Kinoshita T, Uyama T, Ueki T, Michibata H (1999) Direct reduction from vanadium(V) to vanadium(IV) by NADPH in the presence of EDTA. A consideration of the reduction and accumulation of vanadium in the ascidian blood cells. J Inorg Biochem 77:157–161
Song B, Aebischer N, Orvig C (2002) Reduction of [VO2(ma)2]− and [VO2(ema)2]− by ascorbic acid and glutathione: kinetic studies of pro-drugs for the enhancement of insulin action. Inorg Chem 41:1357–1364
Bruyere VE, Rodenas LA, Morode PI, Belsa MA (2001) Reduction of vanadium(V) by oxalic acid in aqueous acid solutions. J Chem Soc Dalton Trans 3593–3597
Gabricevic M, Besic E, Birus M, Zahl A, Eldik RV (2006) Oxidation of hydroxyurea with oxovanadium(V) ions in acidic aqueous solution. J Inorg Biochem 100:1606–1613
Hanafy NAN, El-Kemary M, Leporatti S (2018) Micelles structure development as a strategy to improve smart. Cancer Therapy 10(7):238. https://doi.org/10.3390/cancers10070238
Sar P, Ghosh A, Scarso A, Saha B (2019) Surfactant for better tomorrow: applied aspect of surfactant aggregates from laboratory to industry. https://doi.org/10.1007/s11164-019-04017-6
Kwon G, Yokoyama M, Okano T, Sakurai Y, Kataoka K (1997) Block copolymer micelles for drug delivery: loading and release od deoxorubicin. J Controll Rel 48(2–3):195–201
Ghosh A, Das P, Saha D, Sar P, Ghosh SK, Saha B (2015) Rate enhancement via sodium dodecyl sulfate (SDS)encapsulation of metal-mediated cerium(IV) oxidation of d-mannitol to d-mannose at room temperatureand pressure: a kinetic and mechanistic approach. Res Chem Intermed 42:2619–2639
Sar P, Roy SG, De P, Ghosh S (2020) Synthesis of glutamic acid derived organogels and their applications in dye removal from aqueous medium. Macromol Mater Eng. https://doi.org/10.1002/mame.201900809
Vaz N, Manjunath AS, Puttasyswamy, (2015) Oxidation of met formin with alkaline chloramine-B: delineation of reaction mechanism and kinetic modeling. Indian J Chem 54A:484–488
Rosseinsky DR (1971) Ionic-strength independence of the vanadium(V)-iodide reaction rate, and the structure of aqueous vanadium(V). J Inorg Nuclear Chem 33(11):3976–3978
Littles JS, Water WA (1965) In: Wibers KB (ed) Oxidation of inorganic chemistry, part A. Academic Press, New York, p 185
Panda J, Patnaik AK, PradhanGC Mohanty P (2015) Electron transfer reaction between pyridinium chlorochromate(VI) and metformin, an antidiabetic drug in aqueous acid medium. World Journal of Pharmaceutical Research 4(11):1093–1108
Amies VES (1966) Solvent effect on reaction rates and mechanism. Academic press, New York/London
Cordes EH, Gilter C (1973) Progress in bio inorganic chemistry Kaiser ET, Kejdy FJ. Wiley, New York
Sar P, Ghosh A, Saha B (2014) The influence of SDS micelle on the oxidativetransformation of propanol to propionaldehyde by quinquivalent vanadium in aqueous mediumat room temperature. Res Chem Intermed. https://doi.org/10.1007/s11164-014-1858-4
Bunton CA (1979) In: Mittal KL, Lindman B (eds) Solution chemistry of surfactants. Plenum, New York
Yao H, Richardson DE (2003) Bicarbonate surfoxidants: micellar oxidations of aryl sulphides with bicarbonate-activated hydrogen peroxide. J Am Chem Soc 125:6211–6221
Ghosh A, Saha R, Sar P, Saha B (2013) Rate enhancement via micelle encapsulation for room temperature metal catalyzed Ce(IV) oxidation of formaldehyde to formic acid in aqueous medium at atmospheric pressure: a kinetic approach. J Mol Liq 186:122–130. https://doi.org/10.1016/j.molliq.2013.07.003
Sar P, Ghosh A, Saha B (2014) The influence of SDS micelle on oxidative transformation of propanol to propionaldehyde by quinquivalent vanadium in aqueous medium at room temperature. Res Chem Intermed. https://doi.org/10.1007/s11164-014-1858-4
Sar P, Ghosh A, Ghosh D, Saha B (2014) Micellar catalysis of quinquivalent vanadium oxidation of methanol to formaldehyde in aqueous medium. Res Chem Intermed. https://doi.org/10.1007/s11164-014-1682-x
Wen XL, Liu ZL, Han ZX, Rieker A (1997) Significant micellar effect on the oxidative electrochemistry of ascorbic acid. J Chem Res(s) 3:108–109
Acknowledgements
One of the authors S. K. Pradhan thankful to UGC for grant of Teacher fellowship and Department of Higher Education, Govt. of Odisha for grant of study leave. We sincerely thank Mr. Kamalakanta Sahoo for computational study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pradhan, S.K., Sahoo, A.K., Patnaik, A.K. et al. Kinetics of oxidation of antidiabetic drug metformin hydrochloride by vanadium(V) in acidic and micellar medium. SN Appl. Sci. 2, 2058 (2020). https://doi.org/10.1007/s42452-020-03664-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-03664-4