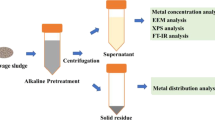
Abstract
The presence of unstable heavy metals can influence the safe recycling of sewage sludge. Since the bioleaching effects of heavy metals from pyrolysis residue were sparely studied, heavy metals speciation and its bioavailability in sewage sludge pyrolysis residue (SSPR) were investigated in this study. Pyrolysis of sewage sludge, as well as bioleaching of SSPR, was carried out successively. Besides, a four-stage sequential extraction, Community Bureau of Reference, was adopted to analyze the heavy metals speciation in SSPR and its bioleaching residue. Results indicated that pyrolysis can enrich the concentrations of Cu, Ni, Zn, and Pb in sewage sludge except for Cd. Different physical properties of heavy metals, as well as the decrease in organics and moisture in sewage sludge, would result in the enrichment phenomena of heavy metals in SSPR. Thus, the concentrations of Cu, Zn, and Ni in SSPR were higher than those of Cu, Zn, and Ni in sludge standard for agricultural use. In addition, heavy metals in SSPR could be bioleached by Acidithiobacillus ferrooxidans which is commonly grown in nature. Most of the remained heavy metals after bioleaching were distributed in F3 (oxidizable) and F4 (residual) fractions, which meant the heavy metals in SSPR were further stabilized by Acidithiobacillus ferrooxidans. Hence, the combination of pyrolysis and bioleaching can be used to stabilize heavy metals in sewage sludge, and the concentrations of heavy metals in bioleaching residue can satisfy the sludge standard for agricultural use.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
With the growth of population and rapid urbanization, the reclamation of wastewater has been treated in a standardized way. As a by-product of wastewater treatment, sewage sludge is of a wide concerned for researchers since it contains a large number of undigested inorganic and organic components with high moisture content [1, 2]. The amount of sewage sludge generated in China has exceeded 50 million tons, and 70% of them have not been managed properly [3]. In addition, based on the existing treatment methods of wastewater, the production of sewage sludge will reach more than 80 million tons in 2020 [4]. Usually, other than inorganic and organic components, sewage sludge contains a lot of pathogens which can endanger human health and the surrounding environment [5]. A transfer or landfill of wet sewage sludge produced by a simple dewatering process will cause the migrations of pollutants both in the lateral and longitudinal spaces.
The conventional treatment methods focused on the reduction of sewage sludge, mainly including drying, landfill, and incineration [6,7,8]. A considerable portion of sewage sludge was addressed with the anaerobic digestion, and the residues were applied to agriculture because of their sufficient contents of N, P, and K [9]. However, high concentrations of heavy metals in sewage sludge can pollute the groundwater, thus restricting the landfill application of sewage sludge. New technologies for the reduction of sewage sludge, as well as the resource reclamation of sewage sludge by producing the stable products, have been of concern in recent years. As an emerging technology, pyrolysis was frequently used to dispose sewage sludge [10, 11]. In general, the products of pyrolysis contain residue, pyrolytic oil, and pyrolysis gas. The optimization of pyrolysis conditions such as heating rate, final temperature, and retention time were widely investigated in order to obtain the high quality of pyrolytic products [12]. Meanwhile, the characterization of pyrolytic products was involved in the considerable researches [13, 14]. Besides, sewage sludge was pyrolyzed with other biomasses in order to produce the targeted pyrolysis residue with high content of char [15, 16]. Furthermore, the applications of sewage sludge pyrolysis residue (SSPR) were also investigated extensively [17, 18]. Usually, SSPR was developed as adsorbent, catalyst, soil conditioner, or fuel. As for the variations of heavy metals speciation, previous studies have indicated that pyrolysis can accelerate the transformation of heavy metals speciation from instability to stability to some extent [19]. Toxicity characteristic leaching procedure (TCLP) was selected to verify the variation tendency of heavy metals speciation [20]. Considering the safety re-utilization of SSPR, the bioavailability of heavy metals in SSPR must be paid much attention. However, to the best knowledge of the authors, there are no reports focusing on the bioleaching of heavy metals from SSPR. Obviously, the stabilization of heavy metals in SSPR can favor the re-utilization of SSPR. Thus, measures should be adopted to develop the further stabilization of heavy metals in SSPR.
The objective of this study was to investigate the variations of heavy metals speciation in sewage sludge before and after pyrolysis and to explore the bioavailability of heavy metals in SSPR by using Acidithiobacillus ferrooxidans. Sludge standard for agricultural use (GB4284-2018) was selected as the reference in this investigation [21]. Furthermore, the relationship of heavy metals speciation and its bioavailability in SSPR was also investigated.
2 Materials and methods
2.1 Source and characteristic of sewage sludge
Sewage sludge used in this study was collected from the Xiongda municipal wastewater treatment plant in Guangdong, China. After collection, the sample was oven-dried at 105 °C to a constant weight. An 100-mesh sewage sludge was obtained through the crushing and sieving processes. A basic analysis of sewage sludge was performed, and the results are shown in Table 1. The content of volatile in sewage sludge was higher than that of other substances. In addition, Cu, Zn, Ni, Cd, and Pb also existed in the selected sewage sludge.
Before pyrolysis, thermogravimetric (TG) analysis of sewage sludge was carried out in a TG analyzer (TGA4000). Sewage sludge was heated from 50 to 900 °C with the heating rate of 20 °C/min in a nitrogen atmosphere. Thermogravimetric-differential thermal gravity (TG-DTG) curves of the selected sewage sludge are shown in Fig. 1. The results of TG analysis indicated that the moisture and volatile losses of sewage sludge separately happened at 100–150 °C and 150–550 °C, while the coke transformation happened at 550–900 °C. For exploring the variations of heavy metals speciation and its bioavailability in sewage sludge before and after pyrolysis, 700 °C was selected as the pyrolysis temperature because of the stabilization of its products and energy saving.
According to the result of TG analysis, pyrolysis was carried out with the condition listed as follows: final temperature 700 °C, heating rate 20 °C/min, retention time 30 min, and nitrogen flow rate 500 ml/min. 10.0000 g of dried and sieved sewage sludge was placed into a tube furnace with a selected pyrolysis condition. Prior to the next use, the obtained pyrolysis residue was collected and kept in a closed vessel at room temperature.
2.2 Sequential extractions of heavy metals
BCR (Community Bureau of Reference) sequential extraction method has been frequently adopted to investigate the heavy metals speciation in sewage sludge [22]. Usually, there are four fractions presented in the heavy metals speciation, including exchangeable and acid-soluble fraction (F1), reducible fraction (F2), oxidizable fraction (F3), and residual fraction (F4). The sequential extractions of heavy metals both from sewage sludge and SSPR were carried out as described below. 1.000 g of dried sewage sludge was applied for BCR sequential extraction by mixing 40 ml of 0.11 M CH3COOH at room temperature for 16 h firstly. Then the suspension and residue were separated by using a centrifuge with the condition of 3000 rpm and 20 min. The obtained suspension was analyzed to get the F1 content of various heavy metals. The obtained residue was extracted through the addition of 40 ml of 0.5 M NH2OH·HCl, and the reaction time was 16 h. Similarly, the suspension and residue were also separated by using a centrifuge with the condition of 3000 rpm and 20 min. Then the obtained suspension was analyzed to get the F2 content of various heavy metals. The procedure of F3 fraction was relatively different from the two fractions said above. The residue obtained from F2 was firstly digested by 10 ml of 8.8 M H2O2 for 1 h and evaporated to 3 ml in water bath at 85 °C. Then 10 ml of 8.8 M H2O2 was added to react again. After this step, the residue was treated with 50 ml of 1.0 M CH3COONH4 for 16 h, and the suspension and residue were also separated by using a centrifuge with the condition of 3000 rpm and 20 min. Finally, the obtained suspension was analyzed to get the F3 content of various heavy metals. The residual fraction of heavy metals was carried out with the mixture of various acids (HNO3/HCl/HF/HClO4 = 1:3:2:2), and the suspension was detected to calculate the F4 content of various heavy metals.
2.3 Bioleaching of SSPR
The bacteria applied in this study were isolated from Dexing copper mine, China, and it was identified as Acidithiobacillus ferrooxidans [23]. The culture medium of Acidithiobacillus ferrooxidans was composed of the following (g/L): (NH4)2SO4 3.0, K2HPO4 0.5, KCl 0.1, MgSO4·7H2O 0.5, Ca(NO3)2 0.01, FeSO4·7H2O 44.7. The pH of this culture medium was adjusted to 2.0 by using H2SO4. All reagents described above were of analytical grade.
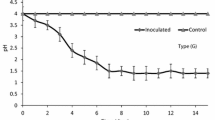
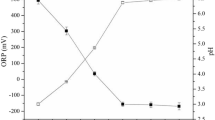
The bioleaching procedure of SSPR was listed as follows. 1.00 g of SSPR was placed into 150-ml conical flask with 50 ml culture medium. Then 10% of the inocula were added into the 150-ml conical flask, and a control group without bacteria was also set. Similarly, the remaining leaching procedure of this control group was the same compared to the experimental group with bacteria. The culture condition was set at 130 rpm, 30 °C, and sampling was conducted at the third day. Prior to the next detection, a filtration process was carried out to obtain the supernatant. Meanwhile, the bioleaching experiment said above was totally repeated for 3 times.
2.4 Analytical methods
The heavy metals ions produced by the bioleaching process were analyzed by inductively coupled plasma optical emission spectrometer (ICP-OES, ICAP7000). TG analyzer was used to confirm the condition of pyrolysis. All data obtained from the instrumental analysis were handled with Excel 2016, and the graphs existed in this study were drawn by using Origin 8.0.
3 Results and discussion
3.1 Analysis of heavy metals speciation
Table 2 shows the concentrations of heavy metals in SSPR and their standards for agricultural use (GB4284-2018). The concentrations of Cu, Zn, Ni, and Pb in SSPR were increased at different levels when compared to those in sewage sludge (shown in Table 1), while the concentration of Cd was decreased. In addition, the concentrations of Cu, Zn, and Ni in SSPR were greatly higher than those of Cu, Zn, and Ni in sludge standard for agricultural use which was promulgated in 2018. Meanwhile, the concentrations of Cd and Pb in SSPR were less than those of Cd and Pb in sludge standard for agricultural use. The enrichment factors of heavy metals after the pyrolysis of sewage sludge (concentration ratios of heavy metals between SSPR and raw sewage sludge) are shown in Fig. 2. In general, most of heavy metals in sewage sludge such as Cu, Pb, Zn, and Ni were enriched after pyrolysis. This phenomenon indicated that pyrolysis can concentrate the concentrations of Cu, Zn, Ni, and Pb except for Cd. The order of enrichment factors was: Pb > Cu > Ni > Zn. Results indicated that the concentration of Pb in SSPR was more than 3.0 times higher than that of Pb in sewage sludge, while the concentrations of Cu, Ni, and Zn in SSPR were approximately 1.0 ~ 1.5 times higher than those of Cu, Ni, and Zn in sewage sludge. However, the enrichment factor of Cd was less than 0.5, which meant the concentration of Cd in SSPR was less than that of Cd in sewage sludge accordingly.
Considering the potential forms of heavy metals compounds in sewage sludge and their physical properties, the concentrations of heavy metals can be changed in different ranges. Obviously, heavy metals in sewage sludge have different melting and boiling points because of their different compositions, such as oxides, chlorides, sulfates, sulfides, and carbonates [24]. In addition, organics and moisture in sewage sludge will be decomposed and evaporated during pyrolysis process. The pyrolysis process will lead to the decrease in organics and moisture in sewage sludge. Thus, the concentrations of heavy metals are widely increased in SSPR. Therefore, the potential forms of heavy metals compounds in sewage sludge and their physical properties, as well as the decrease in organics and moisture in SSPR, result in the different enrichment factors of heavy metals in SSPR.
The distributions of heavy metals fractions both in sewage sludge and SSPR are shown in Fig. 3. Comparing the chemical speciations of Zn and Ni in sewage sludge and SSPR, the variation tendencies of Zn and Ni were the same. These demonstrated that the distributions in F1 and F2 fractions in SSPR were decreased when compared to these in sewage sludge. Meanwhile, the distributions of F3 and F4 fractions were increased. In addition, the variation tendencies of Cu and Pb were almost the same. For Cu and Pb, the distributions of F1 and F4 fractions in SSPR were increased when compared to these in sewage sludge, while the distributions of F2 and F3 fractions were decreased. Due to the low melting and boiling points, the variation tendency of Cd was quite different when compared to other heavy metals. For Cd, there was no F2 and F4 fractions existed in SSPR, and the F1 fraction in SSPR was increased when compared to that in sewage sludge, while the F2 and F3 fractions were both decreased. In general, pyrolysis can accelerate the stabilization of Cu, Ni, Zn, and Pb in sewage sludge except for Cd.
For different types of sludge such as municipal sludge, paper mill sludge, and sewage sludge, the distributions of heavy metals speciation were quite different [10]. Due to the high temperature of pyrolysis, the organics and moisture will be decomposed and evaporated. Thus, the heavy metals speciation will be changed subsequently. In general, due to the integrative actions of acid rain and domestic sewage, sewage sludge contains a certain amount of Cl−, SO42−, and NO3−. Furthermore, research indicated that the presence of anions such as Cl−, SO42−, and NO3− will affect the variations of heavy metals speciation in sewage sludge [25].
3.2 Bioavailability of heavy metals in SSPR
Usually, TCLP is chosen to evaluate the migration of heavy metals in samples, such as soil, ore, and sludge. There is no exception for the pyrolysis residue, and reports have already investigated that some heavy metals in pyrolysis residue will be leached through TCLP process [26, 27]. These results indicated that using pyrolysis residue as a raw material is not safe without any measures to eliminate the heavy metals inside. Considering the wide distributions of Acidithiobacillus ferrooxidans in nature, as well as the frequent investigations to the recycling of SSPR, the effects of Acidithiobacillus ferrooxidans on the heavy metals in SSPR are very important. Figure 4 shows the leaching rates of heavy metals in SSPR at the third day of reaction with and without bacteria. The leaching rates of Cu, Zn, Ni, Pb, and Cd were quite different. For Cu and Zn, nearly 70% of them were bioleached under the action of Acidithiobacillus ferrooxidans, while less than 20% and 10% were leached without Acidithiobacillus ferrooxidans, respectively. As for Ni, Pb, and Cd, nearly 25%, 15%, and 35%, respectively, were bioleached from SSPR under the action of Acidithiobacillus ferrooxidans, while only 5%, 2%, and 10%, respectively, were leached without Acidithiobacillus ferrooxidans. These phenomena indicated that heavy metals in SSPR can still be bioleached by Acidithiobacillus ferrooxidans, and the environmental risks of heavy metals in SSPR should be taken into consideration, especially for the recycling process of SSPR.
The concentrations of each heavy metal speciation in bioleaching residue and their standards for agricultural use are shown in Table 3. As for Cu, Zn, and Pb, there was no distributions in F1 and F2 fractions, while there were relatively low distributions in F3 and F4 fractions. In fact, the F3 and F4 fractions are relatively stable. Therefore, even though the Cu distributions in F3 and F4 fractions were higher than those of Cu in sludge standard for agricultural use, the environmental risk of Cu in the bioleaching residue was extremely low. Although the distribution of Cd in F1 fraction was still present, the concentration of this fraction was lower than that of Cd in sludge standard for agricultural use. For Ni, the distributions in F2, F3, and F4 existed, but the concentrations of F2 and F4 fractions were lower than those of Ni in sludge standard for agricultural use. In addition, the F3 fraction was relatively stable, so even though the distribution of Ni in F3 fraction exceeded the standard described before, the environmental risk of Ni was negligible.
In general, the results of the leaching rates of heavy metals were related to the heavy metals speciation in SSPR. As shown in Fig. 3, most of heavy metals in sewage sludge were stabilized through pyrolysis process, especially for Zn, Cu, Ni, and Pb. This result could explain the phenomenon shown in Fig. 4. Previous studies have investigated that Acidithiobacillus ferrooxidans can oxidize Fe2+ to Fe3+ in the culture medium and produce H+ to create a acidic environment simultaneously [28, 29]. As described before, F1 represents the exchangeable and acid-soluble fraction, and F2 represents the reducible fraction. The F1 and F2 fractions of heavy metals were relatively unstable. Combining the information shown in Table 3, the F1 and F2 fractions of heavy metals in SSPR were removed furthest by using Acidithiobacillus ferrooxidans. The heavy metals presented in the bioleaching residue were satisfied with the sludge standard for agricultural use.
4 Conclusions
Pyrolysis can enrich the concentrations of Cu, Ni, Zn, and Pb in sewage sludge except for Cd. The decrease in organics and moisture in sewage sludge, as well as the physical properties of heavy metals, would result in the enrichment phenomena of heavy metals in SSPR. The concentrations of Cu, Zn, and Ni in SSPR were higher than those of Cu, Zn, and Ni in sludge standard for agricultural use. In addition, heavy metals in SSPR can be bioleached by using Acidithiobacillus ferrooxidans which is commonly grown in nature. Most of the remained heavy metals were distributed in F3 and F4 fractions, which meant the heavy metals were further stabilized by Acidithiobacillus ferrooxidans. Therefore, the combination of pyrolysis and bioleaching can be used to stabilize heavy metals in sewage sludge, and the concentrations of heavy metals in the bioleaching residue can fulfill the sludge standard for agricultural use.
References
Westerhoff P, Lee S, Yang Y, Gordon GW, Hristovski K, Halden RU, Herckes P (2015) Characterization, recovery opportunities, and valuation of metals in municipal sludges from U.S. wastewater treatment plants nationwide. Environ Sci Technol 49(16): 9479–9488
Zhang J, Lu F, Zhang H, Shao L, Chen D, He P (2015) Multiscale visualization of the structural and characteristic changes of sewage sludge biochar oriented towards potential agronomic and environmental implication. Sci Rep. https://doi.org/10.1038/srep09406
Dai XH (2018) Analysis of current situation and demand of sludge treatment and disposal chain technology. https://smehen.gov.cn/jscx/xxcy/jnhb/05/1863207.shtml. Accessed 23 May 2018
Liu T (2018) In-depth investigation and investment prospect evaluation report of China's sludge treatment and disposal industry in 2018–2024. https://www.chyxx.com/research/201803/624759.html. Accessed 1 March 2018
Hossain MK, Strezov V, Chan KY, Ziolkowski A, Nelson PF (2011) Influence of pyrolysis temperature on production and nutrient properties of wastewater sludge biochar. J Environ Manage 92:223–228
Hernandez AB, Ferrasse JH, Chaurand P, Saveyn H, Borschneck D, Roche N (2011) Mineralogy and leachability of gasified sewage sludge solid residues. J Hazard Mater 191(1–3):219–227
Lee U, Chung JN, Ingley HA (2014) High-temperature steam gasification of municipal solid waste, rubber, plastic and wood. Energy Fuels 28(7):4573–4587
Christodoulou A, Stamatelatou K (2016) Overview of legislation on sewage sludge management in developed countries worldwide. Water Sci Technol 73(3):453–462
Withers PJA, Elser JJ, Hilton J, Ohtake H, Schipper WJ, Van-Dijkf KC (2015) Greening the global phosphorus cycle: how green chemistry can help achieve planetary P sustainability. Green Chem 17(4):2087–2099
Shao JG, Yuan XZ, Leng LJ, Huang HJ, Jiang LB, Wang H, Chen XH, Zeng GM (2015) The comparison of the migration and transformation behavior of heavy metals during pyrolysis and liquefaction of municipal sewage sludge, paper mill sludge, and slaughterhouse sludge. Bioresource Technol 198:16–22
Feng YF, Sun HJ, Xue LH, Liu Y, Gao Q, Lu KP (2017) Biochar applied at an appropriate rate can avoid increasing NH3 volatilization dramatically in rice paddy soil. Chemosphere 168:1277–1284
Cícero F, Heyder L, Thais C, Ailton V, Jader B, Natália A, Etelvino N, Luciano C (2017) Influence of pyrolysis temperature on chemical and physical properties of biochar from sewage sludge. Arch Agron Soil Sci. https://doi.org/10.1080/03650340.2017.1407870
Novotny EH, Maia CMBF, Carvalho MTM, Madari BE (2015) Biochar: pyrogenic carbon for agricultural use - a critical review. Rev Bras Cienc Solo 39:321–344
Wang XD, Li CX, Li ZW, Yu GW, Wang Y (2019) Effect of pyrolysis temperature on characteristics, chemical speciation and risk evaluation of heavy metals in biochar derived from textile dyeing sludge. Ecotoxicology Environ Saf 168:45–52
Ren NN, Tang YY, Li M (2017) Mineral additive enhanced carbon retention and stabilization in sewage sludge-derived biochar. Process Saf Environ. https://doi.org/10.1016/j.psep.2017.11.006
Hu YY, Yang F, Chen FF, Feng YH, Chen DZ, Dai XH (2018) Pyrolysis of the mixture of MSWI fly ash and sewage sludge for co-disposal: Effect of ferrous/ferric sulfate additives. Waste Manage 75:340–351
Sousa AATC, Figueiredo CC (2016) Sewage sludge biochar: effects on soil fertility and growth of radish. Biol Agric Hortic 32:127–138
Faria WM, Figueiredo CC, Coser TR, Vale AT, Schneider BG (2018) Is sewage sludge biochar capable of replacing inorganic fertilizers for corn production? Evidence from a two-year field experiment. Arch Agron Soil Sci. https://doi.org/10.1080/03650340.2017.1360488
Huang RX, Zhang B, Saad EM, Ingall ED, Tang YZ (2018) Speciation evolution of zinc and copper during pyrolysis and hydrothermal carbonization treatments of sewage sludge. Water Res 132:260–269
Zhou JJ, Ma HR, Gao M, Sun WY, Zhu C, Chen XP (2017) Changes of chromium speciation and organic matter during low-temperature pyrolysis of tannery sludge. Environ Sci Pollut Res 25(3):2495–2505
Standardization Administration of the P. R. C. (2018) Control standards of pollutants in sludge for agricultural use. https://www.doc88.com/p-2304833315530.html. Accessed 24 May 2018
Chanaka Udayanga WD, Veksha A, Giannis A, Lisak G, Chang VWC, Lim TT (2018) Fate and distribution of heavy metals during thermal processing of sewage sludge. Fuel 226:721–744
Gu WH, Bai JF, Dai J, Zhang CL, Yuan WY, Wang JW, Wang PC, Zhao X (2014) Characterization of extreme acidophile bacteria (Acidithiobacillus ferrooxidans) bioleaching copper from flexible PCB by ICP-AES. J Spectrosc. https://doi.org/10.1155/2014/269351
Rumble-John RCRC (2018) Handbook of Chemistry and Physics (98th ed.) (Internet Version). CRC Press/Taylor & Francis, Boca Raton, FL
Liu J, Fu J, Ning X, Sun S, Wang Y, Xie W (2015) An experimental and thermodynamic equilibrium investigation of the Pb, Zn, Cr, Cu, Mn and Ni partitioning during sewage sludge incineration. J Environ Sci 35:43–54
Shen Z, Zhang J, Hou D, Tsang DCW, Ok YS, Alessi DS (2019) Synthesis of MgO-coated corncob biochar and its application in lead stabilization in a soil washing residue. Environ Int 122:357–362
Salam A, Bashir S, Khan I, Rizwan MS, Chhajro MA, Feng X, Zhu J, Hu H (2019) Biochars immobilize lead and copper in naturally contaminated soil. Environ Eng Sci 35(12):1349–1360
Gu WH, Bai JF, Dong B, Zhuang XN, Zhao J, Zhang CL, Wang JW, Shih KM (2017) Enhanced bioleaching efficiency of copper from waste printed circuit board driven by nitrogen-doped carbon nanotubes modified electrode. Chem Eng J 324:122–129
Yang YK, Chen S, Yang DS, Zhang W, Wang HJ, Zeng RJ (2019) Anaerobic reductive bio-dissolution of jarosites by Acidithiobacillus ferrooxidans using hydrogen as electron donor. Sci Total Environ 686:869–877
Acknowledgements
This work was financially supported by the Gaoyuan Discipline of Shanghai-Environmental Science and Engineering (Resource Recycling Science and Engineering), the Key Discipline of Shanghai Polytechnic University (XXKZD1602), the Natural Science Foundation of China (51578397), and the program of Shanghai Technology Research Leader (17XD1420500).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gu, W., Bai, J., Dong, B. et al. Heavy metals speciation and its bioavailability in sewage sludge pyrolysis residue. SN Appl. Sci. 1, 1107 (2019). https://doi.org/10.1007/s42452-019-1132-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-1132-x