Abstract
Prostatic utricle represents the caudal end of fused mullerian ducts. Untreated prostatic utricle pouch with urethro-ejaculatory duct reflux can lead to recurrent epididymo-orchitis and other complications. Recurrent urinary tract infections (UTI) may manifest in patients when it is associated with posterior urethral valve. A 13-year-old boy presented with recurrent urinary tract infections complicated by an anterior urethro-cutaneous fistula. On imaging, he was found to have posterior urethral valve, prostatic utricle cyst, and urethro-ejaculatory duct reflux. To the best of our knowledge, this is the first case report to describe the combination of these three findings in the same patient. Prostatic utricle cyst is a rare condition which should be considered in the differential of midline cysts of the lower male genitourinary tract. MCU/RGU should be performed in such cases to exclude urethro-ejaculatory duct reflux which may predispose to epididymo-orchitis. Early management of reflux by correcting the underlying anomaly will help prevent complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
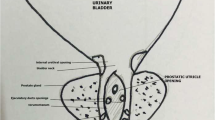
Prostatic utricle is a median 4–6-mm-long epithelium-lined sinus, opening between the two ejaculatory ducts on the verumontanum in the posterior urethra. It represents the caudal end of fused mullerian duct corresponding to the vagina and cervix in females[1]. They usually present in males less than 20 years of age. The reported incidence of utricle cysts is about 1–5% [2]. It is also seen as one of the pop-off mechanisms of PUV secondary to increased intravesical pressure [3]. We present a case of prostatic utricle cyst and urethra-ejaculatory duct reflux as pop-off mechanisms of posterior urethral valve in an adolescent with trisomy 21.
Case Presentation
A 13-year-old boy, born to non-consanguineous parents, hailing from a poor socio-economic background and previously diagnosed with trisomy 21 was brought with complaints of cry during micturition and passing pus in urine for 2 months. The child also had lower abdominal pain and fever. A firm, tender swelling was noted in the left side of the scrotum.
General examination revealed Down’s features and per abdomen findings were unremarkable. Local examination revealed tender, erythematous, left-sided scrotal swelling with serous discharge from the scrotal wall. The child was managed with intravenous antibiotics following which the swelling reduced and the child was discharged.
Few weeks later, the child returned with complaints of thin stream of urine, dribbling of urine from the under surface of scrotum and decreased urine output for 1 month. On examination, the child was found to have developed anterior urethro-cutaneous fistula. Blood investigations revealed decreased eGFR of 53.25 ml/min/1.73 m2 (grade III chronic kidney disease) and an elevated total leukocyte count. Urine culture was sterile. Elevated total count of 19,730 cells/cumm with neutrophilic leukocytosis was found. Differential counts of neutrophil, 77%; lymphocyte, 16%; and eosinophils, 3%, were noted.
Ultrasound showed bilateral hydroureteronephrosis (left > right) with mild increased renal cortical echoes, diffuse bladder wall irregularities, and wall thickening. DMSA scan showed slightly smaller left kidney with moderately impaired cortical function and no evidence of cortical scars. The right kidney was normal in size and showed good tracer uptake with no evidence of cortical scars. The child underwent micturating cystourethrogram (MCU) which revealed posterior urethral valve, a midline cystic lesion arising from the posterior urethra and urethro-ejaculatory duct reflux (Figs. 1 and 2). A small wide mouthed urachal cyst is seen in the anterosuperior aspect of the bladder. Cystoscopy showed type 1 posterior urethral valve (PUV), for which ablation was performed. Cystoscopy also revealed patulous prostatic utricle pouch orifice. In our case, PUV appeared to be contributing to the massive prostatic utricle pouch and urethro-ejaculatory duct reflux. Follow-up MCU was done which showed persistent prostatic utricle cyst. MRI pelvis was done to delineate the surrounding anatomy (Figs. 3 and 4).
Micturating cystourethrogram (MCU) shows dilated posterior urethra with abrupt narrowing (white star) due to posterior urethral valve, a smooth walled outpouching from the posterior wall of posterior urethra-prostatic utricle cyst (solid white arrow), bilateral urethro-ejaculatory duct reflux (solid black arrows), and urachal cyst (red arrow)
No abnormality of anterior abdominal wall musculature or cryptoorchidism were noted to suggest Prune-Belly syndrome. Prostatic utricle pouch was removed by robotic transperitoneal excision (Fig. 5). The child was on regular monthly follow-up for a period of 3 months, after which the child came for 6 monthly follow-up visits for a period of 2 years. The child was symptom-free with normal serum creatinine values.
Discussion
Utricle cysts may manifest with urinary tract infections, pain, post-voiding incontinence, recurrent epididymitis, and hematospermia [4]. Retrograde urethrogram (RGU) is the gold standard investigation to detect utricle cyst and urethra-ejaculatory duct reflux, but in this case, due to PUV, the findings are clearly seen on MCU. A grading system was proposed by Ikoma as seen on MCU [5]: grade 0, opening located on the urethra, but the utricle does not extend over the verumontanum; grade 1, larger than grade 0, but it does not reach bladder neck; and grade II, more enlarged, and the dome extends over the bladder neck. The prostatic utricle opens into the central area of the verumontanum in the prostatic urethra in grades 0, I, and II. In grade III, the opening is situated in the bulbous urethra just distal to the external sphincter. According to this grading system, our patient had grade 1 prostatic utricle cyst (Fig. 2). In this case, giant utricle cyst and urethra-ejaculatory duct reflux are the first pop-off mechanisms followed by bilateral hydroureteronephrosis and dysplasia of the kidneys with reduced function due to recurrent UTI.
Prenatal diagnosis of PUV and associated pop-off mechanisms are very difficult because the urachus is patent till 26–28 weeks, and hence, it is normally not manifested during anomaly scan at 20 weeks. In trisomy 21, a delayed scan at 30–32 weeks can pick these findings once the closure of urachus begins. In undetected cases of trisomy 21, postnatal screening of all associated cardiac and genitourinary anomalies can easily detect this anomaly leading to the prevention of long-term morbidity and end-stage renal disease.
The associations of utricle cysts with various genitourinary abnormalities have been described including hypospadias, intersex disorders, cryptorchidism, ipsilateral renal agenesis, anorectal malformation, and syndromes like Prune-Belly syndrome and Down’s syndrome [6]. However, a combination of findings involving prostatic utricle cyst, urethro-ejaculatory duct reflux, and posterior urethral valve in the same patient has not been described in literature. The posterior urethral valve has been proposed to be due to an abnormal anterior integration of the Wolffian duct into the posterior urethra [7]. This opens up opportunities for establishing an embryological and genetic basis for the rare combination of findings with disturbances involving both the Mullerian and Wolffian ducts. Liu et al. stated that it is rare to have prostatic utricle cyst in the absence of external genital abnormalities like hypospadias [8]. However, our patient did not have congenital abnormalities of the urethra. Two reasons are considered for the low number of reported cases. One is that the majority of prostatic utricles are asymptomatic and escape diagnosis. Another is that symptoms of the prostatic utricle are varied and nonspecific, and such patients are often treated symptomatically for urinary tract infection without further examination to determine the underlying cause.
Surgical excision is considered the treatment of choice. Perineal, suprapubic, extravesical, transperitoneal, parasacral, transvesical, transtrigonal, retropubic, transanorectal posterior, or anterior sagittal approaches have been described [8]. Endoscopic procedures were found to improve or cure 82% of the patients in one study [9]. Fertility concerns and recurrence are best minimized with robotic surgery.
Conclusion
Prostatic utricle cyst is a rare condition which should be considered in the differential of midline cysts of the lower male genitourinary tract. MCU/RGU should be performed in such cases to exclude urethro-ejaculatory duct reflux which may predispose to epididymo-orchitis. Early management of reflux by correcting the underlying anomaly where possible will help to prevent complications.
Data Avalability
Not applicable.
Code Availability
Not applicable.
References
Oh C-S, Chung I-H, Won H-S, Kim JH, Nam K-I. Morphologic variations of the prostatic utricle. Clin Anat. 2009;22(3):358–64.
Liu B, He D, Zhang D, et al. Prostatic utricles without external genital anomalies in children: our experience, literature review, and pooling analysis. BMC Urol. 2019;19:21.
Godwin OI, Ayotunde OO. Posterior urethral valves with severe unilateral vesicoureteral reflux in a 3-year-old boy. Ann Ib Postgrad Med. 2007;5(2):73–6.
Momin YA, Dhende NP, Ghodke BA, Ansari SAH, D’Costa GF, Mahajan VR. An abnormally large prostatic utricle cyst associated with unilateral renal agenesis. Urol Ann. 2013;5(2):129.
Ikoma F, Shima H, Yabumoto H. Classification of enlarged prostatic utricle in patients with hypospadias. Br J Urol. 1985;57(3):334–7.
Shebel HM, Farg HM, Kolokythas O, El-Diasty T. Cysts of the lower male genitourinary tract: embryologic and anatomic considerations and differential diagnosis. Radiographics. 2013;33(4):1125–43.
Krishnan A, de Souza A, Konijeti R, Baskin LS. The anatomy and embryology of posterior urethral valves. J Urol. 2006;175:1214.
Liu B, He D, Zhang D, et al. Prostatic utricles without external genital anomalies in children: our experience, literature review, and pooling analysis. BMC Urol. 2019;19(1):21.
Coppens L, Bonnet P, Andrianne R, de Leval J. Adult mullerian duct or utricle cyst: clinical significance and therapeutic management of 65 cases. J Urol. 2002;167(4):1740–4.
Author information
Authors and Affiliations
Contributions
RK and SA conceived and designed the case report. RK performed the imaging studies, and the images were reviewed by SA. KS was the operating surgeon and analyzed the data. RK wrote the manuscript. All the authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable, consent obtained for publication.
Consent to Participate
Not applicable, because the study was done on a patient who was referred to MCU and MRI scan to our department.
Consent for Publication
Obtained — written consent from mother.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Imaging
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kashyap, R., Adithan, S. & Sambandan, K. Late Presentation of Posterior Urethral Valve with Giant Prostatic Utricle Cyst and Urethro-Ejaculatory Duct Reflux in an Adolescent Trisomy 21 Patient: Case Report. SN Compr. Clin. Med. 4, 194 (2022). https://doi.org/10.1007/s42399-022-01276-0
Accepted:
Published:
DOI: https://doi.org/10.1007/s42399-022-01276-0