Abstract
This comprehensive review delves into the recent advancements in the study of surfactant adsorption, a crucial factor influencing the efficiency of chemical enhanced oil recovery (cEOR) applications across various oil types. By integrating findings from the latest literature, we shed light on the dynamic interactions between surfactants and diverse adsorbents including sandstone, carbonate, and shale sandstone. This review highlights the nuanced understanding of how operational variables such as initial surfactant concentration, pH, adsorbent dose, contact time, temperature, along with adsorption kinetics, isotherm models, thermodynamics, and the presence of competing ions, contribute to the adsorption process. A novel synthesis of recent studies reveals that the Langmuir and pseudo-second-order models continue to accurately describe the equilibrium and kinetics of adsorption in most scenarios, with the adsorption process predominantly exothermic. Additionally, this review introduces cutting-edge insights into the molecular-level mechanisms underpinning surfactant adsorption, emphasizing the role of crude oil components, initial wettability of reservoir rocks, and the intrinsic properties of the rock itself. By summarizing these contemporary findings, the review aims to provide a deeper understanding of the key parameters affecting the retention of surface-active agents on various adsorbents, thereby proposing new avenues for optimizing surfactant flooding in cEOR. This enhanced focus on recent contributions to the field distinguishes our review from existing literature, offering fresh perspectives on the optimization of surfactant use in oil recovery processes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Surfactant flooding is an advanced method for enhancing oil recovery from petroleum reservoirs, exploiting the unique properties of surfactants to modify the oil–water interfacial tension (IFT) and improve oil mobilization [1,2,3]. This technique, pivotal in the domain of Enhanced Oil Recovery (EOR), relies on the intricate mechanism by which surfactants decrease IFT to ultra-low levels (as low as 10–4 dyne/cm), facilitating the release of oil entrapped by capillary forces within the reservoir's porous media. The efficacy of surfactant flooding hinges on maintaining a low IFT over extended periods [4, 5], which is challenged by the complex interactions between surfactants and the reservoir environment, including rock heterogeneity, surfactant interaction with reservoir fluids, coalescence of oil droplets, and notably, surfactant adsorption on reservoir rock surfaces [6, 7].
The interaction of surfactants with the reservoir rock and fluids is of paramount importance, as adsorption phenomena can significantly impact the efficiency of surfactant flooding operations [8, 9]. Surfactants may adsorb onto rock grains through electrostatic repulsion between charged surfactant molecules and the rock surface or via hydrogen and hydrophobic bonds in the case of non-ionic surfactants [10]. This adsorption is influenced by several factors, including surfactant concentration [8], temperature [11,12,13,14], pH [15,16,17], surfactant type, ionic strength [12, 13, 15], and adsorbent dose [18]. Of these, the surfactant type is often the only variable that can be adjusted to optimize EOR processes, given that other factors are dictated by inherent reservoir conditions.
Recent laboratory and simulation studies have shed light on the adsorption behavior of surfactants, underscoring the need for a comprehensive understanding of surfactant-reservoir interactions [9, 19,20,21]. These studies contribute to the optimization of surfactant selection and formulation, aiming to minimize adsorption losses and enhance oil recovery efficiency. Furthermore, a plethora of adsorption isotherm models—such as Langmuir, Freundlich, and Temkin—and kinetic models—like pseudo-first-order and pseudo-second-order—have been employed to elucidate the equilibrium and dynamics of surfactant adsorption, offering insights into the thermodynamics of the process, including changes in Gibbs free energy, enthalpy, and entropy [22,23,24,25,26,27,28,29,30,31].
Given the critical role of surfactants in EOR, this review aims to provide a thorough review of the surfactant flooding process, emphasizing the mechanism of action of surfactants in porous media, the challenges posed by surfactant adsorption, and the strategies to mitigate these effects. By incorporating recent findings from laboratory and simulation studies, this review endeavors to present a consolidated perspective on the state-of-the-art in surfactant technology for EOR, highlighting the significance of surfactant choice and formulation in overcoming the challenges of surfactant flooding and advancing the efficiency of oil recovery operations.
In light of the suggested references, our discussion will integrate the latest advancements and empirical data from pivotal studies, enriching our review with cutting-edge insights and contributing to the broader understanding of surfactant applications in EOR. This enhanced focus on the detailed mechanisms of surfactant action, coupled with a comprehensive survey of recent research on surfactant adsorption behavior, aims to elevate the scholarly value of our manuscript and provide a foundational resource for both academic researchers and industry practitioners in the field of petroleum engineering.
2 Surfactants
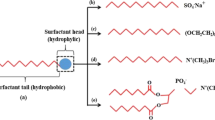
A surfactant (Abbreviation of surface-active agent) is a component which, whenever available in a system in a range of concentrations, will have a certain property of adsorbing to the surfaces or interfaces of the system and significantly modifying the surface or interfacial free energy in between these surfaces (or interfaces). The terminterface relates to the boundaries of different immiscible phases; the concept surface relates to an interaction in which one of the phases is a gas, typically air [32].It is important to note that surfactants typically amphiphilic, which means they have both hydrophilic and hydrophobic groups in their chemical structure, as seen in Fig. 1.The hydrophilic group can be dissolved in water, but the hydrophobic group cannot (oil soluble). The soluble component, or hydrophilic group, is referred as the "head," while the hydrophobic group is referred as the "tail" in conventional surfactant nomenclature. In order to reduce the IFT and change the reservoir rock preference, the head and tail surfactants attack the interface between two immiscible surfaces [33,34,35].
3 Classification of Surfactants
3.1 Based on the Source
Surfactants can be categorized in a variety of ways. The basic approach to categorize a surfactant is by its source, which can be classified as natural or synthetic.
3.1.1 Natural Surfactants
Natural surfactants, extracted from a myriad of biological sources, are increasingly sought after for their eco-friendly attributes, including biodegradability and lower toxicity, in comparison to their synthetic counterparts. These surfactants are derived from a diverse array of plant and animal materials, offering a renewable and often more sustainable option for various applications [36, 37].
3.1.1.1 Plant-Based Surfactants
Among plant-derived surfactants, saponins stand out due to their unique chemical structure and natural detergent properties [38]. Saponins are a broad class of high-molecular-weight glycosides that are characterized by their ability to form stable foams in aqueous solutions. The structural complexity of saponins is vast, with variations in their aglycone (sapogenin) core structures and sugar side chains. This structural diversity results in a wide range of physicochemical properties, influencing their solubility, surface activity, and biological activity. Saponins are found in numerous plants, including soaproot (Chlorogalumpomeridianum), soapberry (Sapindusmukorossi), soapbark (Quillajasaponaria), and yucca. Each source offers saponins with distinct structural features and, consequently, diverse functionalities. For example, Quillajasaponaria saponins are renowned for their potent emulsifying properties and are used in food and beverages, vaccines, and cosmetics. Sapindusmukorossi, commonly known as soapberry, provides saponins that are effective natural detergents, used in eco-friendly laundry and cleaning products [39,40,41,42].
3.1.1.2 Animal-Based Surfactants
In addition to plant sources, certain animal-derived materials also serve as sources of natural surfactants. For example, phospholipids from egg yolks and marine sources (such as krill and fish eggs) exhibit surfactant properties [43, 44]. These compounds, particularly phosphatidylcholine, play a crucial role in food emulsions and in pharmaceutical formulations for drug delivery systems [45].
Additionally, the applications of natural surfactants extend far beyond traditional cleaning and emulsifying roles. In agriculture, saponins serve as bio-pesticides, leveraging their natural toxicity to pests while being harmless to humans and beneficial insects. In the pharmaceutical industry, saponins are utilized as adjuvants in vaccines and for their ability to enhance the permeability of drugs across biological membranes [44, 46].
3.1.2 Synthetic Surfactants
Synthetic surfactants are chemically formulated to achieve specific properties and functionalities that are tailored to a wide array of industrial, personal care, and household applications. Unlike their natural counterparts, synthetic surfactants can be designed to exhibit enhanced stability, effectiveness, and efficiency under a variety of conditions, including extreme pH, temperature, and salinity. This flexibility in design allows for the creation of surfactants with highly specialized characteristics, such as increased solubility, targeted detergency, or specific interaction patterns with other substances [47,48,49].The production of synthetic surfactants involves the chemical modification of petroleum derivatives, fats, and oils, leading to a diverse range of surfactant molecules. Furthermore, synthetic surfactants have significantly expanded the possibilities for formulating products that meet specific consumer needs and industrial requirements. Through continuous innovation in chemical synthesis and environmental sustainability, the development of synthetic surfactants seeks to minimize potential ecological impacts while maximizing performance and biodegradability [48, 50, 51]. This ongoing advancement underscores the critical role of synthetic surfactants in modern society, driving improvements across a wide range of sectors from environmental remediation to healthcare and beyond.
3.2 Based on the Charge
Long-chain hydrocarbon residues typically contribute as the hydrophobic group's building blocks with halogenated or oxygenated hydrocarbon or siloxane chains occurs less frequently; An ionic or highly polar group also constitutes the hydrophilic group [32]. Negative, positive, or neutral charges are all possible. According to hydrophilic head's charge, a surfactant is anionic, nonionic, cationic, amphoteric (or zwitterionic), and Gemini [52, 53]. Figure 2 schematically illustrates the common types ofsurfactants.
3.2.1 Anionic Surfactants
Anionic surfactants, which constitute about half of all surfactants produced worldwide, are favored for their ease of synthesis and cost-effectiveness. These surfactants possess negatively charged head groups, such as carboxylate (e.g., sodium lauryl sulfate), sulfate (e.g., sodium dodecylbenzenesulfonate), sulfonates, and phosphate, which dissociate in water into an amphiphilic anion and a cation, typically sodium, potassium, ammonium, calcium, or various amines. These surfactants are prevalent in applications ranging from household detergents to industrial cleaning agents due to their robust cleaning performance and high lathering capabilities [42, 54].
3.2.2 Non-Anionic Surfactants
Nonionic surfactants are the second most utilized surfactant type, prized for their non-dissociating hydrophilic groups and lack of ionization in aqueous solutions, making them ideal for hard water conditions and charge-sensitive systems. These surfactants include groups such as alcohols (e.g., nonylphenol ethoxylates), phenol, ether, ester (e.g., polysorbate 80), and amide. Their environmental compatibility, especially those with sugar-based head groups like alkyl polyglucosides, makes them suitable for eco-friendly applications. Ethoxylated alcohols and sorbitan esters are key examples, widely used in personal care, household cleaning, and industrial processes.A significant advantage of nonionic surfactants is their environmental compatibility. Sugar-based head groups, for instance, have been utilized to enhance biocompatibility, reducing the environmental impact associated with surfactant use. For the lipophilic part, alkyl chains derived from fatty acids or alkylbenzene are commonly employed. The choice of these groups is often dictated by the desired solubility, toxicity, and biodegradability properties. Examples of nonionic surfactants include ethoxylated alcohols, such as C12-15 alcohols ethoxylated with an average of 7 mol of ethylene oxide per mole of alcohol, and sorbitan esters, which are derived from the reaction of sorbitol with fatty acids. These surfactants find extensive application in personal care products, household cleaners, and industrial processes where mild yet effective surface activity is required [42, 55, 56].
3.2.3 Cationic Surfactants
Cationic surfactants, with their positively charged head groups, include long-chain amines and quaternary ammonium salts (e.g., cetyltrimethylammonium bromide (CTAB)). Although their production cost is higher due to the complex manufacturing process, their unique properties, such as excellent conditioning effects in hair care products and efficacy as fabric softeners, make them indispensable in certain applications. They also play a crucial role in industrial applications like corrosion inhibition and as antimicrobial agents due to their positive charge [42, 55, 56].
3.2.4 Zwitterionic Surfactants
Zwitterionic surfactants, or amphoteric surfactants, contain both positive and negative charges within the same molecule, rendering them electrically neutral. This unique feature provides them with excellent skin compatibility and mildness, making them preferred choices in personal care formulations. Betaines (e.g., cocamidopropyl betaine) and carboxybetaines are common examples, widely used in shampoos and body washes for their gentle cleansing properties [42, 55, 56].
3.3 Other Type of Surfactant
3.3.1 Gemini Surfactants
Gemini surfactants, distinguished by their unique molecular architecture featuring dual hydrophilic heads and hydrophobic tails connected via a spacer, markedly outperform traditional surfactants in terms of surface activity. This superior performance is attributed to their structural configuration, which enables more efficient surface tension reduction and micelle formation at significantly lower concentrations than their single-headed counterparts. Quaternary ammonium Gemini surfactants, typified by the notation 16-5-16—where '16' denotes the length of the hydrophobic alkyl chains and '5' the number of carbon atoms in the spacer—exemplify this group's effectiveness. Their remarkable efficiency is not only crucial in applications such as enhanced oil recovery, where they improve oil mobilization by altering the wetting properties and reducing the interfacial tension between oil and water, but also in antimicrobial formulations, where their ability to disrupt microbial cell membranes makes them potent biocidal agents [56,57,58,59]. Figure 3 vividly illustrates the schematic structure of a Gemini surfactant, providing a visual representation of the molecular arrangement that underpins their enhanced performance. The figure underscores the dual-head and tail configuration, bridged by a spacer, which is central to the Gemini surfactants' functionality.
Moreover, the Gemini surfactants' efficacy is significantly influenced by the balance between hydrophilicity and hydrophobicity, enhanced by the presence of two polar heads. This dual-head structure promotes a higher degree of interaction with water and oil phases, respectively, facilitating more effective surface activity. The type m-s-m Gemini surfactants, where 'm' indicates the alkyl chain length and 's' the spacer length, represent the most extensively researched subclass within this category [56, 57].
3.3.2 Polymer Surfactants
A polymeric surfactant is a macromolecule with both hydrophilic and hydrophobic components. Introduce a novel surfactant with a wide range of various applications. These include classical applications as emulsion stabilizers and more "modern" applications as thickeners for EOR, where they can be employed in place of traditional surfactants mixed with polymers [60, 61]. Therefore, these macromolecular systems allow a significantly wider configuration than other surfactants. Based on hydrophilic and lipophilic component distribution, consider two primary forms structurally. Polysoaps are macromolecules composed of the polymerization of intrinsically amphiphilic monomers or oligomers. While macrosurfactants are polymers with clear separation among the two groups, these are made by copolymerizing a hydrophobic monomer with a hydrophilic monomer. Thus, these copolymer structures could be random, gradient, or block [62,63,64]. The forms of typical complex polymer surfactants are shown in Fig. 4.
4 Surfactant Adsorption: Influence of Various Variables and Interactions
Surfactant adsorption on reservoir rocks is a multifaceted process influenced by a range of operational variables [1, 65]. The comprehensive review of such variables, including the pH, salt concentration/ionic strength, initial surfactant concentration, adsorbent dose, contact time, and temperature, is crucial for the optimization of cEOR strategies. Herein, we systematically investigate these variables to elucidate their collective impact on the adsorption efficiency, as illustrated in Table 1. Upon careful examination of the data presented in Table 1, the adsorptive capacity of surfactants on rock reservoirs exhibits significant variation across different rock types, from sandstone to carbonate and shale. This variation emphasizes the critical role played by the inherent mineralogy and surface chemistry intrinsic to each rock type. These fundamental properties govern the interactions with various surfactant classes, thereby dictating the adsorption dynamics. It is critical to note that the initial wettability of the reservoir rock markedly influences surfactant adsorption. This wettability, dictated by the rock's exposure to crude oil components, prescribes how surfactants will interact with the rock surface. Differences in adsorption mechanisms on water-wet versus oil-wet surfaces necessitate a thorough characterization of the rock's initial state [66,67,68].Moreover, the spectrum of surfactant structures, from natural glycosides like saponins to synthetic anionic surfactants, manifests unique adsorption behaviors. Saponins, owing to their amphipathic structure, engage with reservoir rocks differently compared to synthetic surfactants, which are tailored to establish specific surface interactions.The mineralogical composition and surface chemistry of reservoir rocks—encompassing sandstone, carbonate, and shale—determine the nature and extent of surfactant adsorption. The variability in rock properties such as porosity, permeability, and surface area affect the adsorption process. For example, carbonate rocks with a higher calcite content may have distinct interactions with anionic surfactants due to the divalent cations present, which can bridge surfactant molecules to the rock surface [69, 70].
Furthermore, the physicochemical milieu underpinning the adsorption process significantly alters its efficiency and kinetics. Factors such as pH and ionic strength pivotally influence the electrostatic interactions between surfactants and the rock surfaces. Simultaneously, the surfactant concentration and the amount of adsorbent are intrinsically linked to the adsorption magnitude. Additionally, parameters like contact time and ambient temperature play crucial roles in achieving adsorption equilibrium, with temperature specifically influencing the surfactant's physical state and its propensity for the adsorbent surface. Through this rigorous analytical endeavor, our objective is to elucidate a comprehensive, methodically structured understanding of the numerous factors that influence surfactant adsorption. By systematically categorizing and correlating empirical data from extensive studies, we articulate the operational conditions that enhance surfactant adsorption efficacy, thereby advancing the development of more efficient cEOR methodologies. The insights gathered from the systematic examination of data within Table 1 not only highlight the strategic imperative in surfactant selection and application within oil recovery operations but also facilitate a coherent, logically structured narrative that enhances the clarity and impact of the subsequent discussion.
4.1 pH Effect
The pH significantly impacts surfactant adsorption since the charge of solid surfaces changes with pH. One of the most critical variables in any process is the pH level, which may affect adsorbents' adsorption of surface-active compounds (the rock reservoir) and is also the subject of this investigation. Lebouacheraet al. [84]studied the influence of a variety of parameters on SDS adsorption on Algerian rock reservoirs. The pH range has an effect on the degree of SDS adsorption on the surface of Algerian rock in Fig. 5.
pH effect on the SDS adsorption for all concentrations [84]
The sand particles revealed a significant capacity for adsorption (15.9, 12.365, 9.7896, and 7.165) for the concentration range of (200- 800ppm) respectively in the pH between (3–7) for anionic SDS surfactant. Then, adsorption rapidly decreases within a pH range of (7–12). When pH increases, anionic surfactant adsorption decreases. The adsorption capacity decreases in alkaline solution due to decreased positively charged sites on the adsorbent and competition for adsorption sites with both OH & anionic surfactant. These findings confirmed the previous studies, indicating that as the reservoir's pH increased, surfactant adsorption on the solid matrix decreased [15, 74, 89, 90].
Several surface-active agents have been studied onto rock reservoir adsorption. Bera et al. [74] examined the effect of pH on the efficacy of different surfactants (anionic, non-ionic, and cationic) adsorbed on a sand surface that is clean. At low pH, sand particles demonstrated a significant capacity for anionic and nonionic surfactant adsorption. However, with cationic surfactants, the reverse tendency was seen. When the pH is low, sand has a high capacity for adsorption of SDS owing to the solution's acidic composition, this contributes to the positive charge on the sand surface, increasing the surface's interaction with the anionic surfactant SDS. Tergitol 15-S-7 adsorption decreases up to neutral pH and stays virtually constant in the alkaline pH range (non-ionic). This may be shown by the presence of an electron pair on the oxygens in the ethylene oxide group of a non-ionic ethoxylated surfactant that is attracted to the positively charged surfaces of sand particles at pH values higher than 7. The alkaline area has a decreased adsorption capacity owing to a variety of hydrophobic interactions, not simply the surfactant itself. Increased adsorbent capacity when pH rises owing to the fact that cationic surfactant (CTAB) is highly attracted to negatively charged sand surfaces due to its positively charged head groups. According to the results of the research, it is feasible to minimize or change surfactant adsorption on rock surfaces by modifying the pH of the solution for non-ionic and ionic surfactants, which is a significant aspect in evaluating the economic feasibility of surfactant flooding [74].
Additionally, Bryant et al. evaluated induced wettability modifications in cEOR applications by adsorbing and removing amine sulfates with well-characterized molecular structures from mica surfaces exposed to decane solutions of the surfactant brine. Protonation of the surface amine groups is improved, resulting in the greatest increase in wettability at low PH levels. When the PH was elevated to 8 or 9, no adsorbed surfactant molecules remained on the mica surface [91]. Mushtaq et al. [92] found that adding alkali inhibited the adsorption of both anionic surfactants (FS-1 and FS-2) on sandstone. At pH 6, FS-1 and FS-2 adsorption were 4.32 and 4.94 mg/g, respectively. Additionally, at pH 10, FS-1 and FS-2 were found to be 0.51 and 0.89 mg/g, respectively, indicating that the charges on the surface of the rocks had switched from mostly positive to predominantly negative. The negative charges on the surface form repulsive interactions with the negative charges on the surfactants, significantly reducing adsorption. Ishiguro and Li [93],They analyzed the adsorption of SDS onto pore silicon dioxide powder gels and found that owing to its hydrophobic surface on siloxane silica can adsorbs SDS. SDS adsorption reduces when the pH of the silica rises due to higher electrostatic repulsion. Additionally, they reported that when the repulsion increases, SDS adsorption becomes undetectable. The influence of pH on the adsorption of SDS was determined using the modified Langmuir equation and the 1-pK basic Stern model. Tagavifar et al. [83]. The pH effect was investigated using the adsorption of alkali (Na2CO3) anionic surfactant on Indiana limestone across a pH and surfactant-to-solid ratio range. They found that the anionic surfactantsadsorption is 2.4 mg/m2 at pH ~ 8 and practically linearly decreases with pH levels higher than 9. In low pH conditions, the dominant mode of adsorption on calcite and clay is charge-regulated. Although hydrogen—bonded adsorption is significant in calcite, it is the dominating mode around pH levels ~ 10. Jian et al. [85] investigated the adsorption among each component and surfactant mix on various mineral surfaces with varying charges. The key discovery was that when LB was combined with IOS and also L38, its adsorption increased as a result of surfactant complexation on the pure calcite surface at a lower pH.These systems' adsorption was influenced by at least three factors. The first element is surfactant complex, or more precisely, anionic/cationic surfactant complexes; the second factor is surfactant electrostatic interaction with solid surfaces; and the third factor is the distribution of surfactant complexes between the surface and the micelles.
More recently, Liu et al., Liu et al. determined the anionic surfactant the adsorption behavior, alcohol alkoxy sulfate (AAS). With 9.5 pH, the surface of silica is highly charged negatively, providing an abundance of sites in order to bind AAS through calcium ion bridging. As a result, they revealed that decreasing the pH of the fluid (making it less alkaline) reduces AAS adsorption. The increased pH results in an increment in the amount of anionic surface sites present for interacting with such an anionic surfactant through a bridging agent such as calcium cation [94]. At pH values ranging from 1–14, the impact of pH on the adsorption of MB using traditional TiO2, synthetic TiO2, and NS/TiO2 was examined. It was established that as the pH level increased, thus increased the adsorption efficiency. TiO2 adsorbents acquire a positive charge at the surface when the pH is low. Since adsorbents have such a positively charged surface, electrostatic repulsion exists among the adsorbent and also the MB cationic fragments, resulting in a decrease in adsorption efficiency. Since many H + ions compete for MB at low pH, adsorption is decreased under acidic conditions. Whenever the solution pH decreased due the deprotonation, the charge also on adsorbent surface became progressively negative [95]. In light of the findings of this study and the data in Table 1, it is obvious that active surface adsorption has been affected throughout a broad pH range, (i.e., 3–11).
4.2 Initial Surfactant Concentration Effect
To quantify the amount of surfactant adsorption and the proportion of surfactant removed from such a solution, it is necessary to investigate the initial surface agent concentration effect. Increasing the initial surfactant concentration often results in an increase in the percentage of static adsorption, according to previous research [28, 96, 97]. It has been attributed by several authors to the adsorbate species' saturation of sorbent surface adsorption sites. The percentage of surfactant adsorption increases with increasing contact time at all starting surface agent concentrations [96]. Surface-active concentration differences between the aqueous and solid phases function as a driving factor to overcome mass transfer barrier from surfactant molecules. Arabloo et al. [28] investigated various initial concentrations of Glycyrrhiza Glabra onto local sandstone formation. At several contact times, increasing the Glycyrrhiza Glabra concentration increased adsorption density. This behavior could be seen in equilibrium adsorption data too. Glycyrrhiza Glabra adsorption is rapid at the beginning of the experiment and slows until equilibrium is attained. The trend becomes more obvious when concentrations increase. Glycyrrhiza Glabra equilibrium was reached more rapidly at a lower initial concentration. There is less competition between surfactant molecules and binding sites due to the low surfactant molecule to available adsorption site ratio [28, 98]. TFG adsorption on sandstone rock was investigated by Barati-Harooni et al. [96]. The IFT between water and restricted oil may be greatly reduced with this new natural surfactant. A variety of adsorption models were used to simulate this process of surfactant adsorption on the minerals in sandstones. According to the authors, increasing the surfactant concentration (5000–80000 mg/L) results in an adsorption amount due to the concentration difference among the crushed rock surface and the bulk solution increases. Additionally, the surfactant's adsorption gradient on crushed rock is high from starting concentrations up to 40,000 mg/L, and continues with a low gradient above this concentration. E.g., at 5000 mg/L, adsorption occurs at 2.5943 mg/g rate, while at 40,000 mg/L, adsorption occurs at 12.021 mg/g rate. Adsorption reaches 14.763 mg/g when 80,000 mg/L is included. This demonstrates that the adsorption process involves a brief period of high adsorption followed by a longer period of reduced adsorption [96]. Barati et al. [78], when they used Trigoonellafoenum-graceum as a newly introduced non-ionic surfactant on mineral phases, they demonstrate the importance of equilibrium adsorption in determining the quantity of surfactant lost onto the absorbent. When surfactant concentrations were increased from (0.5–8 wt %), adsorption was found to increase due to the difference concentration between the bulk solution and solid–liquid interface. Moreover, it is critical to note that the adsorption percentage decreases and achieves saturation. Ahmadi et al. [73] evaluated the adsorption behaviors (respectively dynamic and static) of such a natural non-ionic surfactant at concentrations ranging from (0.1wt % to 8%), derived from Zyziphus Spina Christi leaves and applied to the real shale-sandstone reservoir sample. It has been reported that as the surfactant concentration increases, the capacity of the surfactant to adsorb on the sandstone samples increases significantly. This is likely due to the increase in the concentration gradient between the bulk and surface of the sandstone rocks as the surfactant solution's basic concentration increases. Bera et al. [74] explain the performance of adsorption of three different surfactants on clean sand particles, namely anionic, cationic, and non-ionic. The concentrations evaluated were between 0 and 1200 mg/L. The SDS adsorbed on adsorbent was found to be the lowest when compared to some others. Tergitol 15-S-7 and CTAB are considerably more adsorbed on particles of sand at equilibrium than SDS. The adsorption isotherms of all surfactants were observed to quickly increase with surfactant concentration. The sudden increase in the adsorption isotherm can be explained by the surfactant aggregates surface formation known as the "hemi micelles" on the surfacesanddue to lateral interaction between different hydrocarbon chains. This interaction produces a second driving force that balances out the electrostatic attraction that is already there, resulting in a considerable increase in adsorption. Throughout all the cases, the authors found an increase in adsorption with increasing concentration up to a certain point, after which no increase was seen. For CTAB, micelles are formed and remain in bulk solution, acting as a potential chemical sponge for any extra surfactant that is added to the system after CMC has been reached. When the concentration of surfactants increased, the adsorption of the surfactant was characterized by low or no increases in the surfactant adsorption isotherm. As a consequence, the SDS concentration increases. Due to the SDS surfactant's negative head groups, sand and surfactant molecules are strongly attracted to each other. As a result, before using CMC, increasing the concentration of surfactant had no effect on adsorption.Tergitol 15-S-7, on the other hand, shows a little increase in adsorption after CMC treatment, owing to the existence of weak hydrophobic and hydrogen bond interactions [74]. Yekeen et al. [80] studied the adsorption and foaming capabilities of SDS surfactants at various concentration from 0.01 to 1%. They measured the rates of SDS adsorption on kaolinite by determining the surfactant's CMC (using surface tension) during kaolinite equilibration. The results suggest that while the CMC of the surfactants at 0 wt% NaCl and surface tension are comparable to those of pure SDS, the CMC and surface tension of the surfactants increased slightly during equilibration with kaolinite. Meanwhile, the CMC's surface tension was essentially comparable throughout adsorption. SDS's CMC increases to 0.25 wt% following equilibration. When the CMC value is normalized to the mass of kaolinite, the change in CMC results in an estimated adsorption approximately 0.005 g-SDS/g-Kaolinite or 5 g-SDS. Park et al. [75] tested different flooding adsorption characteristics of four surfactant compounds at concentrations of 0.001 to 1.0 wt%. DBS was the most adsorbent, followed by PONP, PS20, and LS. According to the literature (e.g., 936.10–6 mol/g for DBS and 066.10–6 mol/g for LS), these adsorption concentrations are extremely constant. For a better understanding from how anionic surfactants attach to kaolinite's surface, consider: At low surfactant concentrations, hydrophobic contact between the hydrocarbon chains of the surfactant and the kaolinite surface under neutral pH is the major mechanism of adsorption. Smaller surfactant aggregates (called'soloids' or 'hemimicelles') emerge on the kaolinite surface as surfactant concentration increases, Because of alkyl–alkyl hydrophobic interactions between the bulk and the adsorbed molecules. At some point, the CMC is reached, and then surfactant molecules begin adsorbing above the CMC, creating bilayers and multilayers. If low concentrations of non-ionic surfactants are used, weak molecules are attracted to the monomer form of the surfactant. When the polyoxyethylene component comes into contact with hydroxyl groups on the surface, hydrogen bonds are formed between them. There is no indication of significant hydrophilic groups between hydrophobic groups in the lateral direction. At increasing concentrations, hemimicelles form and molecules prefer to float evenly on the surface. As a result, the amount of adsorption significantly increases due to cooperative interactions between adsorbates in this setting. As the adsorption process accelerates, the orientation of the adsorbed molecules changes, and surface aggregates form as such a result of lateral alkyl–alkyl interactions [12, 75, 99, 100].
Recent research by Lebouachera et al. [84] analyses the effect of several parameters on SDS adsorption on the Algerian rock Hassi Messaoud. At equilibrium, concentrations of SDS surfactant of 200, 400, 600, and 800 ppm exhibited adsorption capacities of 2.05, 2.29, 2.90, and 4.21 mg/g, respectively. Fast adsorption is a result of SDS molecules coming into touch with widely available surface adsorption sites. In comparison, progressive adsorption may be attributed to SDS molecules adsorbing into the rock reservoir's pores. A single/Gemini cationic surfactant and a single/Gemini zwitterionic surfactant were produced as test quaternary ammonium surfactants by Mao et al. [101]. At concentrations between 0.01 and 0.1%, several types of viscoelastic surfactants were tested for their capacity to attach to carbonate rocks and sandstones and their ability to moisten them. Weight percentages range from 0.2 and 1.2%. The experimental findings show that the adsorption initially increased and became stable as the surfactant concentration increased. That's because when concentrations increase, the surfactant produces monomolecular layers on the adsorbent surface. Furthermore, Increased surfactant concentrations are no longer effective at increasing adsorption. Additionally, rocks had no noticeable effect on surfactant adsorption, despite the surfactants being opposite. Owing to the existence of two hydrophobic chains and two hydrophilic groups connected by intervals through chemical interactions, Gemini zwitterionic surfactants have the highest adsorption capacity and greater hydrophobicity than standard single-tailed surfactants. It may minimize the attractive force between hydrophilic groups and also the hydration layers' barrier and increasing the charge density of the individual surfactant's molecular heads. This increases the adsorption of Gemini zwitterionic surfactant on charged rock surfaces and concentrates them at the water surface. As a consequence, the Gemini zwitterionic surfactant is optimized for enhanced adsorption on the rock surface [101]. Ahmadi et al. discuss the adsorption equilibrium of various types of nanosilica and Zyziphus Spina Christi, a novel surfactant, in aqueous solutions for the aim of enhanced oil recovery (EOR) and reservoir stimulation. They performed batch adsorption experiments on Zyziphus Spina Christi and different stages of Nanosilica. The initial concentrations of surfactant and nanosilica are different, ranging from 1000 to 80,000 ppm for surfactant and 500 to 2000 ppm for nanosilica, respectively. Increased surfactant concentration increases the surfactant's ability for adsorption on carbonate samples. This is because the concentration gradient between the bulk and the surface of the carbonate rock increases as the initial concentration of the surfactant solution increases [72]. Muherei et al. investigated the adsorption characteristics surfactants (Triton X100 and SDS) and their mixtures (1:2; 1:1; 2:1 SDS: TX100 by weight) in the combination of different naturally occurring adsorbents, sandstone and shale. The findings shows that the anionic surfactant, SDS, has only a minimal capacity for adsorption to both shale and sandstone. The maximum adsorption quantities of SDS on both shale and sandstone were lower than those of TX100. This was related to repulsive interactions between negatively charged SDS and negatively charged shale/sandstone [71]. Rashid et al. recently evaluated the impact concentration solution on methylene blue (MB) adsorption used traditional and synthetic TiO2, and NS/TiO2 at different concentrations from 10 to 40 mg/L (30 ppm Co; adsorbent0.06 g; pH 10; temperature of 298K; agitation 100 rpm). It was found that the initial dye concentration has a significant influence on the amount of solute molecules that can be pushed to overcome the solid-to-liquid mass transfer barrier through adsorption [95]. Thus, increasing the surfactant concentration results in an increase in surface adsorption [102].
4.3 Adsorbent Dosage Effect
Adsorption of a solute generally increases with adsorbent concentration, since an increase in adsorbent concentration results in an increase in active exchangeable adsorption sites [74, 103]. As seen in Fig. 6, SDS sand adsorption is dose-dependent. At 25 °C, the adsorption process with SDS at 200 and 800 ppm concentrations were investigated. As shown in the figure, adsorption increased on the ranging 3.294–7.96 mg/g when the dosage of rock risen from 1 to 9 g and then stayed nearly constant until the final amount of adsorbent. For each surfactant concentration, it was shown in Fig. 6 that after a certain adsorbent dosage, it remains constant [84]. This was due to the reason that with increased adsorbent dosage, there are more adsorption sites. At high adsorbent doses, increasing the number of adsorption sites had little influence on surfactant adsorption because equilibrium was established at a low concentration of surfactant with in solution before saturation. Adsorbent dose was changed from 11 to 18 mg, which significantly improved static adsorption efficiency from 8.75 to 9.1756 mg/g. This result is due mainly to the reason that SDS surfactant saturated the adsorption sites at low adsorbent concentrations (11 mg) but abandoned them unsaturated at high dosages (> 18 mg). These findings were confirmed by previous studies [104].
Influence of on the adsorption of SDS at 200 and 800 ppm [84]
Bera et al. [74] published in the literature on the impact of adsorbent amounts ranging from 2 to 16 g. The findings reveal that for SDS surfactant at a concentration of 1000ppm, adsorption increases as adsorbent dosage and subsequently stabilizes at a certain dose. As the number of adsorbent increases, the number of active adsorption sites increases accordingly, and the adsorption process accelerates as the order proceeds. After a dose of adsorbent equal to 8g, no further adsorption occurs due to the accumulation of significant adsorption sites and the resulting particle interactions between the system's sand particles. At high adsorbent concentrations, particle interaction occurs, this results in a reduction in the overall surface area of the adsorbent and an increase in the diffused distances. According to Shukla et al., another possible explanation for this behavior is that as the adsorbent dosage increases, adsorption sites capable of absorbing surfactant ions become more available. The adsorption rate is high when the adsorbent dose is low due to effectively available active sites; however, when the adsorbent dosage is high, the surfactant ions cannot rapidly access the adsorption sites until equilibrium is reached [105, 106]. Li et al. [107] established a systematic relationship between static adsorption and the effect of the combined flooding system's solid-to-liquid wt% on the quartz sand surface. As the solid–liquid weight ratio decreases, the number of THSB and DPG particles adsorbed on the sand surface increases. The adsorption process might very well be characterized by the quartz sand's interaction with chemical agents. The following two factors were directly accountable for adsorption: (1) Electrostatic force applied by the reservoir rocks' negative charge; (2) covalent chemical bonds, such as hydrogen bonds and hydrophobic covalent bonds. The dosage effect on the adsorption of MB was studied using commercial and synthetic TiO2, and NS/TiO2 at different dosage: 0.03 g, 0.06 g, and 0.09 g. The findings indicate that increasing the quantity of adsorbent resulted in an increase in the early stage of dye (MB) removal due to the availability of additional active charge-containing sites for adsorption. Equilibrium is reached and the maximum adsorption occurs at this point in the adsorption process. As a result, no additional improvement in adsorption was seen after the saturation, even if the amount of adsorbent increased [95]. As a result, it is critical to control the dose of an adsorbent to obtain reduced surfactant adsorption on rock reservoirs.
4.4 Contact Time Effect
The researchers performed contact time testing to evaluate the correlation among thesurfactant quantity adsorbed onto contact time/agitation and the quantity of adsorbent. Contact time affects the process's economic efficiency, particularly in cEOR applications, and also the kinetics of adsorption. As a consequence, contact time is a critical and important factor in the process of adsorption [108, 109]. Barati et al. studied the TrigoonellaFoenum-Graecum adsorption andthey reported that after 24 h of contact time, they had reached equilibrium adsorption, or maximum adsorption. Interestingly, the initial adsorption rate is high because the solid has a high specific surface area available for surfactant adsorption and surfactant adsorbs largely through electrostatic attraction between the surfactant head group and the sites charged on the solid surface. Eventually, the rate of adsorption decreases when the surface gets electrically neutral and forms a monolayer, and adsorption occurs by the interactions of hydrophobic chains. Over extended durations, activity of monomer stays almost constant, while surfactant diffuses into the adsorbent very slowly, resulting in a prolonged rate of adsorption [78]. In another investigation, Park et al. determined that after 1200 min (20 h) of measuring the quantity of adsorption of five different surfactants as time function, all surfactants reached the adsorption equilibrium. Thus, when a surfactant's adsorption process was quantified in terms of the quantity of adsorption per unit time (mol/g min), it was found that the timing of start to equilibration is DBS > PONP > PS20 > LS [75]. Ahmadi et al. investigated the contact timeeffect on new surfactant adsorption onto carbonate reservoir rock. Once the system's concentration varies over time, which also is in equilibrium, and the test is considered complete. According to preliminary findings, equilibrium adsorption occurred within 2 days [73]. Ahmadi et al. also conducted an experimental analysis of natural surfactant adsorption in a shale-sandstone oil resource and it was determined that the majority of adsorption occurred after the first six days for the initial surfactant doses. Because the shale sandstone samples have a greater surface area at the onset of surfactant adsorption, the rate of adsorption is also increased. Once the capacity of the adsorbent is reduced owing to the development of a monolayer through the adsorbed material, the rate for surfactant transmission between the outer and inner surfaces of adsorbent particles is highly depend on the uptake rate [77]. Barati-Harooni et al. investigated the experimental and numerical modelling of the adsorption0f non-ionic surfactantupon sandstone minerals in the enhanced oil recovery process as a function of time. It was reported that the initial stages of surfactant adsorption on solid rock are high, but the adsorption increases slowly and eventually approaches equilibration. At the first process, the surface area attainable for surfactant adsorption is large, and the surfactant ratio to available for adsorption surface is high, resulting in rapid surfactant adsorption. Further in, as the available surface area is covered, this ratio decreases and adsorption occurs slowly and eventually reaches equilibration [96, 98].
Arabloo et al. [28] investigated the influence of contact time on Glycyrrhiza Glabra adsorption on reservoir rock samples with varied initial concentrations. The findings show that during the first three hours, density adsorption rapidly increases. Following after, the adsorption density increased gradually, and after approximately one day, complete adsorption equilibrium was reached. Moslemizadeh et al. measured the surfactant rate adsorption during the contact time for various initial surfactant concentrations ranging from 0.1 to 5%wt. The equilibrium adsorption was achieved in 3 h or less for most of the initial concentrations. The adsorption process occurs with a high rate of adsorption owing to the significant amount of specific surface area available for surfactant adsorption on MMT. Surfactant adsorption ability of MMT decreases significantly after monolayer formation. Furthermore, the quantity of surfactant adsorption increases as the surfactant's initial concentration increases [79]. A similar trend of results was found by Lebouachera et al., who used the same approach to analyze SDS adsorption on an Algerian sandstone reservoir over time for cEOR applications. After 11 h of adsorption, equilibrium conditions were determined for crushed rock samples ranging in concentration from 200 to 800ppm [84].
In recent study, Rashid et al. investigated the effect of time interval on the adsorption, of MB, the time intervals used were 20, 40, 60, 80, 100, 120, and 140 min. The findings indicate that the early stage of MB adsorption is very rapid owing to the availability of free active sites in conventional, synthetic, and NS/TiO2 particles. During time, the adsorption process slows to the point that the rate adsorption becomes parallel to the x-axis, this is almost stable at 100 min [95].
4.5 Temperature and Thermodynamic Parameters Effect
Temperature is another critical parameter that affects the adsorption process. Energy and entropy factors must be addressed when determining if an adsorption process will proceed in any particular adsorption mechanism [110, 111]. The fundamental thermodynamic parameters entropy, enthalpy, and Gibbs free energy may be approximated using the Langmuir isotherm constant, the value of which is temperature dependent. Gibbs free energy probability can be determined by calculating the following formula:
where R indicates the gas constant standard (8.314 J mol/K), T represents the temperature in degrees Kelvin (K), and KL indicates the Langmuir constant.
Additional parameters associated with \({\Delta {\text{G}}}^{^\circ }\) that are critical for a process's feasibility and spontaneity are entropy (ΔS◦) and enthalpy (ΔH◦), as indicated by the equation below:
Equations (1) and (2) are added to obtain:
The gradient and intercept of a plot of 1/T against ln KL can be used to calculate the values of ΔH◦ and ΔS◦ [112,113,114,115,116]. Negative values of ΔG◦ indicate a spontaneous adsorption process. Similarly, positive values of ΔH◦ indicate an endothermic reaction. Additionally, the amount of ΔH◦ appears to be related to such sorption, namely physisorption (ΔH◦ < 50 kJ/mol) and chemisorption (ΔH◦ > 50 kJ/mol) [109]. As a result, numerous researchers investigated the temperature effect on the surface-active agent’sadsorption by various adsorbents from different reservoirs; these studies are summarized in Table 2.
According to Table 2, surfactant adsorption on the majority of the adsorbents reported was exothermic. The TrigoonellaFoenum-Graceum adsorption (TFG) on the Iranian Aghajari oil field, SDS and Titriplex III on the Assam reservoir, SDS CTAB, and Tergitol 15-S-17 on the Indian reservoir was shown as exothermic, as the negative value of ΔH◦. As a result of the presence of negative ΔG◦ and positive ΔS◦ in the case of Trigoonellafoenum-graceum (TFG) retention, the adsorption process appears to be spontaneous and favorable. The positive ΔS◦was attributed to the affinity of the Persian oil field to ZSC, the most likely explanation is that during the adsorption process, the variability at the solid–solution interface increased, as well as certain structural changes in the surfactant and adsorbent. Recently, Lebouachera et al. found that when the temperature is changed from 293 to 253K, ΔG◦ negative values have been shown to decrease from (− 5.4982 kJ/mol to − 3.3767 kJ/mol),This might be related to the process's spontaneity and practicality resulting in a reduced adsorption capacity [84]. In the 303–323 and 303-343K temperature ranges, Bera et al., and Saha et al., made similar observation [74, 81]. According to Zendehboudiet al., Ahmadi et al. and Barati et al. investigations, the negative values of ΔG◦ increased when the temperature increases, showing that the process of adsorption is spontaneous [73, 78, 117].
Almost numerous researchers reported that the adsorption of surfactants was exothermic, with the exception of a few cases. As said by Mao et al., the capacity of Gemini cationic and Gemini zwitterionic adsorption on sandstone and carbonate reservoirs can be affected by temperature changes from 298 to 373 K. The experimental findings indicate that as the temperature increased, the adsorption of surfactants on various rocks decreased, and the degree of reduction became more gradually, that can be attributed to the fact that temperature may affect the adsorption process in two ways. Firstly, due to the exothermic nature of adsorption, it will be reduced by changing the temperature. Secondly, the temperature changes increases the surfactant's solubility in water, hence lowering surfactant adsorption on the adsorbent surface [101]. Increasing temperature between 298 to 353 K had such a significant effect on the effectiveness of a non-ionic surfactant's adsorption (TFG), on carbonate minerals in the Iranian south west oil fields (Aghajari oil field). Additionally, it was demonstrated in the authors' research that temperature had such a significant effect on the process of adsorption in comparison to the Algerian adsorption process on anionic SDS surfactant. Higher adsorption with increasing temperature is then attributed to the development of additional adsorption sites, pore expansions, diffusion over the energy barrier, increased sorption and transport efficiency against the energy barrier, or a combination of these factors [118]. Shamsi Jazeyi et al. examined the effect of temperature on sodium polyacrylate's effectiveness as a catalytic agent on a variety of minerals/rocks, including NI-blend on Carlpool dolomite. Their findings indicated that Polyacrylate can significantly decrease NI-blend adsorption on Carlpool dolomite at the varying temperatures examined [119]. Lebouachera et al. have also investigated the change of temperature between 25 and 80 °C for different concentrations on Algerian sandstone reservoir adsorption by Sodium Dodecyl Sulphate. The analysis revealed that equilibrium was obtained at 353K (25 min) compared to 323K (45 min) and 298K (6 h). Which might be a result of the composition of the rock samples. that also makes the surface of sand negatively charged [120, 121]. Haloi et al. analyzed the temperature effect on the adsorption of rhamnolipid at constant agitation rates of 150 rpm.They reported that as temperature increases, rhamnolipid adsorption decreases. The adsorptionmaximum occurs at low temperatures because the adsorption interaction is exothermic [13].
4.6 Competing Ions Effect
The solution's ionic strength has a considerable effect on the aqueous phase equilibrium of adsorption onto rock reservoirs. Numerous scientists have conducted extensive research on this impact. Adsorption generally decreases when theaqueous ionic strength increases [122,123,124,125]. The availability of coexisting ions in solutions (e.g., Cl, NO3, SO42−, PO43−, and other metal cations) results in competing adsorption, which may affect the interaction between surfactant solutions with rock reservoirs [126]. Several studies have revealed that flooding a reservoir with low salinity increases oil recovery. The concentrations of salt must be in the range of 500 to 3000 ppm for low salinity flooding [127].Yekeen et al. [80]evaluate the SDS adsorptionon kaolinite mostly as function of the surfactant concentration and electrolyte concentrations (NaCl, CaCl2, and AlCl3). The findings shows that when the concentrations of NaCl and CaCl2 increase, the SDS adsorption on kaolinite increases. Therefore, adsorption in the presence of AlCl3 exhibits a distinct appearance. The adsorption is constant as the AlCl3 concentration increases. The electrostatic double layer (EDL) screening effect of salts and SDS's propensity to form complexes with divalent (Ca2+) and trivalent (Al3+) cations are significant factors impacting SDS adsorption and foaming behaviors in the presence of AlCl3, CaCl2, and NaCl salts. The author proposes that kaolinite's high adsorption capacity at high salinity is due to the compressing of electrical double layer on the adsorbent's surface and a decrease in electrostatic repulsion between the adsorbent's surface and the adsorbed surfactant species. By lowering the electrical double layers and zeta potential of the kaolinite surface, the additional salt increases adsorption. Interestingly, SDS adsorption on kaolinite in the presence of AlCl3 has a distinct behavior. The adsorption is unaffected by the presence or absence of AlCl3. Adsorption of SDS on kaolinite remains constant at a concentration of AlCl3 of 0.025–0.05 wt.% and 0.1 wt.% at a rate of 0.02 g-SDS/g-Kaolinite. When AlCl3 and CaCl2 were present, the average adsorption of SDS on kaolinite was significantly higher than when NaCl was present. Another study by Lv et al. [128] investigated the adsorption of dodecyl benzene sulfonate and amphoteric surfactants (betaine) on kaolinite for several systems (potassium chloride, sodium carbonate, sodium metaborate, and sodium tetraborate) with the same salinity (10 g/l). When the sodium chloride solution was replaced with alkali, the isotherm's maximal adsorption reduced considerably. When the alkyl benzene sulfonate and dodecyl benzene sulfonateanionic surfactants were blended with alkali, the adsorption peak and subsequent decline disappeared due to the alkali consuming multivalent cations, despite the fact that neither precipitation or dissolution actually happened. By comparing the results of surfactant adsorption in three different types of alkali, it is possible to determine that sodium tetraborate lowers the surfactant adsorption more effectively than sodium metaborate and the conventional alkali, sodium carbonate, at a certain concentration. Budhathoki et al. [129] proposed polystyrene sulfonates (PSSs) as a sacrificial agent to minimize adsorption of anionic surfactant in a sand-stone reservoir that has saline with total TDS of over 300,000 mg/l and total hardness (Ca2+and Mg2+) of over 13,000 mg/l. That's mostly due to the adsorption of cations, particularly Ca2+ and Mg2+, onto the negatively charged sand surface, creating additional positively charged sites for the anionic surfactant to adsorb. Similarly, similar behavior is observed in the case of Ottawa sand, although the variation is not as obvious as it is with Berea sandstone. Ahmadi et al. [130] did another investigation in which they included several salts in the test mixture, including KCl, NaCl, and MgCl2, in understanding the influence of salt on the Zyziphus Spina Christi (ZSC) adsorption process. The results indicate that raising the salt concentration has a substantial influence on the magnitude of the surfactant's adsorption density, especially when MgCl2 and NaCl are present in the solution, whereas KCl has the least effect on the maximum adsorption density of ZSC surfactant.
Jian et al. [85]studied thesurfactant blends adsorption behavior of able to create complexes based on an anionic C15-18 internal olefin sulfonate (IOS), a zwitterionic lauryl betaine (LB), and an anionic C13-alcohol polyethylene glycol ether carboxylic acid (L38). The LB and L38 adsorption as single surfactant solutions was determined to be 0.33 mg/m2 and 1.61 mg/m2 on calcite, respectively, and 0.43 mg/m2 and 2.20 mg/m2 on dolomite, respectively. This value varies from those seen in DI water because L38 exhibits significant attraction due to its high surface potential and surface charge, In contrast, the increased ionic strength of LB results in less attraction. Additionally, when the surfactant was combined with the salt solution, the adsorption of L38 was significantly reduced compared to when the surfactant was used alone. L38 is another anionic surfactant that, like IOS, exhibits the greatest adsorption on the calcite and dolomite surfaces owing to the electrostatic attraction. Meanwhile, in a solution of 5% CaCl2, the adsorption of L38 increased significantly (for all minerals studied), which was attributed to the mineral surface's enhanced positive zeta potential. To facilitate adsorption at higher temperatures, all surfactant solutions were produced in a Na2SO3 solution (0.2 M), which efficiently inhibits the L38degradation at higher temperatures, as previously described. The adsorption of L38 on various mineral surfaces was significantly low when the adsorption was performed out at higher temperatures in the presence of sodium sulphite. The explanation for this decrease in adsorption was that sulphite can react with oxygen to form sulphate, because that's the potential controlling ion and also has the ability to lower the surface potential of positive binding sites to negative values. This results through an electrostatic attraction among the mineral surface and the L38 surfactant's negative hydrophilic head group, which contributes for the surfactant's low adsorption at high temperatures [85, 131].
5 Adsorption Mechanism
Surfactant molecules are transported from the bulk solution phase to the reservoir rock surface during the surfactant adsorption process [128]. As illustrated in Fig. 7, the adsorption process is separated into four stages. The first stage, surfactant concentrations were lowered for this procedure and it is possible to identify the adsorption process by obtaining a slope of 1. In this stage, when non-ionic surfactants are adsorbing from an aqueous solution to a solid surface, molecular electrostatic interactions occur, have included chemical interactions, hydrophobic bonding, and hydrogen bonding. Furthermore, electrostatic interactions control the adsorption process in systems with charged ionic surfactants and solid particles [132,133,134]. Surfactant adsorption on solid particles is influenced by chemical interactions that occur only in specific conditions in which covalent bonding among the surfactant and the solid is possible compared to other driving forces [135]. Fatty acid adsorption on fluorite and hematite are examples of chemisorption in which the surfactant interacts with the mineral surface [133, 136, 137].
Four-stage adsorption mechanisms schematic diagram. Figure inspired from Adak et al. [142]
Hydrophobic interaction is also another significant contributor to surfactant adsorption on solid particles. The adsorption process occurs once the attraction exists among a hydrophobic group of adsorbed molecules and an active molecule in the solution. Hydrogen bonding among surfactant species and surface species could perhaps occur in many ways where the surfactant contains hydroxyl, amine, phenolic, or carboxylic groups. Hydrogen bonding affects the non-ionic surfactants and saponins adsorption. When a bond is formed between the functional groups of the surfactant and the mineral surfaces, adsorption occurs according to hydrogen bonding. Moreover, non-ionic surfactants are not electrostatically or chemically adsorbed but are also physically adsorbed. In the case of saponins, Hydrogen bonds among hydroxyl groups and the solid surface species are noticeable [12, 138,139,140].
The association of adsorbed surfactants in patches at the solid–liquid interface controls adsorption in stage 2; because of this, electrostatic attraction and lateral interactions are the primary driving forces here. Additionally, the critical hemimicelle concentration (HMC) is related to the transition between stages 1 and 2 [141]. Stage 3 shows a decrease in slope as compared to stage 2. Because the ions of surfactant had covered all of the surface sites by the ending of stage 2, it’s been linked to this phenomenon with further adsorption occurs in stage 3 as a result of interaction between first- and second-layer hydrocarbon chains. Also, it was associated to a charge reversal caused by the adsorbed surfactant ions. Stage 4 is the adsorption plateau stage, which appears at or around the CMC point and is indicated by almost no increase in adsorption when surfactant concentration is increased [8].
The CMC is a significant factor for surfactants since it impacts their ability to thicken. Lower CMC will result in lower amounts of surfactant being used, which will result in considerable economic advantage under the concept of achieving the structure criteria. The surfactant concentration and arrangement correlation are clearly illustrated in Fig. 8. Surfactant CMC research can have a significant impact on the functionality of surfactants. Hence more specific study is needed.
6 Adsorption Equilibrium and Kinetic
Adsorption equilibrium knowledge is an essential aspect in the applicable design and analysis of the system of adsorbent-adsorbate [126]. It highlights surfactant molecules diffusing from the bulk of the solution toward the liquid–solid interface, as well as the kinetics occurring at the interface. Adsorption kinetics is used to determine the adsorption rate over time and provides valuable information on adsorption processes, which is useful for understanding the mechanisms. Toward this purpose, numerous models have been developed over the years to define the adsorption equilibrium of surfactants on diverse rock reservoirs, covering isotherm models with two and three parameters such as Langmuir [30, 143], Frendlich [28], Temkin [29, 144], Jovanovic [24], Hasley [26, 145], Redlich–Peterson [146, 147], Sips [148], Khan [23], Toth [149], and Brouers–Sotolongo [27]. Those certain isotherm models reveal accurate information on an adsorption process's adsorbing property and the exchangeable sites arrangement on the surface of adsorbent [28]. Table 3 summarizes a variety of previously published models. To express equilibrium adsorption in cEOR applications and compared to the other models, FreundlichandLangmuir models are mostly used. Freundlich and Langmuir models were established significantly in research studies to define equilibrium adsorption in cEOR applications [109].
New natural surfactant Saponins was evaluated for adsorption upon the shale-sandstone reservoir [77]. The carbonate reservoir was controlled with the same surfactant [73]. Additionally, Montmorillonite (MMT) was evaluated by including a new bio-based surfactant called Mulberry leaf extract [79]. Different forms of Nanosilica and ZyziphusSpinacea Christi were used to adsorb onto a carbonate reservoir by the same authors [72].
Barati-Harooni et al. [96] were investigated the equilibrium data of Saponins on the sandstone samples from an Iranian southwest oil field. Using 12 models by two or three parameters to provide it, they found that experimental data fit well to the Jovanovic/Brouers-Sotolongo models with two or three parameters, respectively, when compared to other models. Additionally, in the Freundlich model, it was discovered that the majority of the materials also adopted the Langmuir isotherm for surfactant adsorption performance [109]. The performance of SDS adsorption on Algerian rock reservoirs was demonstrated by Lebouachera et al. using five equilibrium models (Langmuir, Freundlich, Jovanovic, Elovich, and Temkin). Langmuir's maximal adsorption capacity of 4.1754 mg/g was well explained by higher R2 values [84]. Other rock reservoirs from other places, such as sandstone from the Indian oil field, Assam reservoir, and Middle East reservoir, were lesser than other adsorbents, for instance, and shown a low capacity for adsorption in comparison to Algerian rock reservoir. Maximum adsorption capabilities reported in the literature were 0.74; 0.771, 16.89 mg/g respectively [74, 81, 120].
As previously mentioned, a comprehensive knowledge of adsorption kinetics and rate-controlling processes is required. Additionally, kinetics understanding is necessary for designing full-scale batch processes [109, 133]. In recent times, researchers successfully provided a range of kinetic models to predict surfactant adsorption, including the pseudo-first-order (PFO), pseudo-second-order (PSO), and intra-particle diffusion models (IPD). The Elovic model (EM), the Avrami model (AM), and others have been reported with all of these three most often used models. All of the above-mentioned models are clearly listed in Table 4.
The fitting models are summarized in Table 5. In several cases, the pseudo-second-order model well fitted the experimental data for a higher R2 (99%), proving that all surfactants chemisorb on the various rock reservoirs mentioned.
Lebouachera et al. [84] adapted the kinetic parameters for SDS adsorption on sandstone type rock using PFO and PSO. The concordance between experimental data and model predictions, as indicated by a higher R2from 0.975 to 0.99, a low root mean square error (RMSE) result within the range (0.14–1.02), and an appropriatevalue of Chi-squared test, it demonstrates the validity of the pseudo-second order model's application. Similarly, Bera et al. [74] tested PFO, PSO and intraparticle diffusion kinetics. They revealed that qe,cal significantly close to qe,expestimated from the PSO equation, with a significant R2 and a low MSE for the 3 models, suggesting that the data on the adsorption kinetics of surfactants on surfaces are represented using a second-order kinetic model [102]. In accordance to the kinetics discussed above, a finding was reached in another research by Saha et al. [81], who used the Elovic model to illustrate the kinetics of SDS adsorption onto the Assam oil field (EM). Elovich and intraparticle diffusion have low fitting values (0.76 and 34.67 percentage error for intraparticle diffusion model, respectively, and 0.84 and 10.32% error for the Elovich model, respectively). Within all models, the pseudo-second-order model fits perfectly the reported adsorption kinetics data. According to Arabloo et al. [28], the pseudo-second order model fits the experimental data for the adsorption of Glycyrrhiza Glabra on Iranian oil field sandstone formations. Ahmadi et al. [73] found the similar tendency for non-ionic surfactant adsorption on carbonate from an Iranian oil field in the south west (Aghajari oil field) The pseudo-second order model is used to obtain as similar to the experimental findings as possible while preserving error rates of less than 10%. The above indicates that chemisorption and diffusion had an effect on adsorption formed by the interaction with the rock surface.
7 Strategies for Optimizing Surfactant Adsorption in Enhanced Oil Recovery
As aforementioned, the efficiency of cEOR is critically impacted by surfactant adsorption onto reservoir rocks, leading to potential losses in surfactant efficacy and increased project costs. With the aim of maximizing surfactant utility in cEOR, recent advancements have introduced several strategies to minimize surfactant adsorption. This section integrates insights from contemporary research, delineating effective measures to curtail surfactant adsorption. The utilization of sacrificial agents is a prevalent strategy. These substances, which adsorb onto potential surfactant binding sites on rock surfaces prior to surfactant introduction, safeguard the more valuable surfactants from loss. Recent investigations into organic polymers and inorganic salts have underscored their effectiveness as sacrificial agents, enhancing surfactant availability for oil mobilization [164]. Equally crucial is the molecular design of surfactants. Adjustments in surfactant structures, such as the hydrophile-lipophile balance (HLB), have led to formulations that exhibit reduced affinity for rock surfaces. Gemini surfactants, characterized by their dual hydrophilic heads and hydrophobic tails, display diminished adsorption owing to their unique architecture that facilitates efficient micellization and lowers the requisite surfactant concentration for effective oil displacement [165]. Manipulating the pH and salinity of the injection fluid can significantly affect electrostatic interactions between surfactants and reservoir rocks. Specific surfactant types show minimal adsorption under particular pH and salinity conditions, advocating for precise adjustment of these parameters to mitigate adsorption. This strategy demands an in-depth understanding of both the reservoir rock chemistry and the surfactant's physicochemical attributes [3, 166, 167]. The integration of nanoparticles into surfactant formulations emerges as a novel approach to minimize adsorption. Nanoparticles serve as protective carriers for surfactant molecules, preventing their direct contact with adsorptive rock surfaces. This innovation not only reduces surfactant adsorption but also enhances EOR by improving surfactant transport through the reservoir [168]. Meanwhile, Surfactant partitioning significantly influences the efficacy of EOR techniques by affecting surfactant retention in the reservoir [169]. This process, which involves the distribution of surfactants between the oil and water phases, can markedly alter the success of surfactant flooding methods. Therefore, effectively managing surfactant partitioning is crucial for enhancing the efficiency of recovery processes and reducing surfactant losses. Belhaj et al. [169] emphasize the necessity of incorporating strategies to address surfactant partitioning within EOR frameworks to optimize overall recovery efficacy. A recent study by Belhaj et al. [4] conducts comprehensive experimental evaluations of surfactant systems across a variety of reservoir conditions, underscoring how partitioning directly affects surfactant performance. Furthermore, Belhaj et al. [3] introduce innovative surfactant formulations engineered to exhibit lower partitioning rates. These formulations have demonstrated the potential to significantly enhance oil recovery rates by ensuring a higher concentration of surfactants remains active in the aqueous phase, directly contributing to more effective EOR operations.
Interestingly, the exploration of surfactant mixtures introduces a complex layer of interactions not present in single surfactant systems, potentially improving or hindering EOR effectiveness through synergistic or antagonistic behaviors. Surfactant mixtures can exhibit improved surface activity and lower CMC, enhancing oil displacement. However, understanding the dynamic interplay between surfactant molecules within mixtures is essential to leverage these benefits fully [133, 170, 171].
Recently, a significant advancement in refining surfactant adsorption strategies is employing Design of Experiments (DoE) techniques [172, 173]. DoE offers a structured approach to systematically study multiple variables' impacts on surfactant adsorption, facilitating the identification of conditions that optimize adsorption and maximize oil recovery. This methodical investigation deepens our comprehension of surfactant-reservoir interactions, allowing for the precise adjustment of surfactant formulations and injection protocols. Recent studies underscore DoE's value in surfactant science, particularly for cEOR, by rigorously evaluating novel surfactant compositions and their interplay with reservoir conditions. This focus on DoE not only mirrors the ongoing innovation in the field but also heralds the development of cost-effective and environmentally sustainable solutions for surfactant adsorption challenges [84, 102, 168, 170, 174, 175].
In summary, optimizing surfactant adsorption transcends mere technical adjustments, demanding a strategic approach that embraces recent scientific advancements and innovative methodologies like DoE. Supported by current research, the outlined strategies offer promising avenues to reinforce surfactant efficiency in oil recovery processes, emphasizing the necessity for continual innovation and refinement within the field.
8 Conclusion and Future Perspectives
Surfactants have emerged as pivotal agents in the petroleum industry, largely due to their unparalleled efficacy in reducing oil–water interfacial tension and modulating wettability, thus significantly enhancing oil recovery. The extensive body of research conducted thus far underscores the critical role of surfactant adsorption on rock surfaces in EOR processes. Given the substantial economic impact of surfactant costs, the efficient adsorption of surfactants has garnered considerable attention, prompting rigorous investigation into various adsorbents and adsorbates. Experiments have explored a range of rock types, including sandstone, carbonate, shale, montmorillonite, kaolinite, and limestone, assessing the influence of factors such as surfactant concentration, pH, contact time, dose, salinity, and temperature on adsorption efficiency.
Empirical findings frequently align with the Langmuir isotherm and pseudo-second-order kinetic model, indicating that these models adeptly capture the adsorption dynamics of surfactants. Thermodynamic studies further reveal that surfactant adsorption is predominantly exothermic and spontaneous, highlighting the process's energetic favorability. Despite the extensive knowledge base surrounding adsorption science, it remains a compelling option for its potential cost-effectiveness in production. The integration of this knowledge with efforts to reduce surfactant adsorption in applications like drilling fluids and chemical EOR opens a new frontier for exploration and innovation in the petroleum industry.
As we stand on the point of leveraging surfactant-based EOR to its fullest potential, addressing the existing technological and environmental challenges becomes paramount. There is a pressing need for further research, particularly kinetic adsorption studies, to deepen our understanding of the underlying mechanisms and interactions between adsorbents and adsorbates. Although adsorption technology has been a cornerstone in the industry for decades, the pursuit of more selective, stable, and economically viable adsorption processes remains a vibrant area of interest.
Looking forward, the transition from laboratory-scale research to pilot and industrial-scale applications presents a significant challenge, underscored by economic considerations. As we navigate these complexities, the goal of optimizing adsorption processes for cost-effective production looms large. The journey from foundational research to the practical application of surfactant-based EOR strategies promises to unlock unprecedented opportunities in the petroleum sector, heralding a new era of exploration and technological advancement. Embracing these challenges and opportunities with a collaborative and innovative spirit will undoubtedly shape the future of oil recovery technologies in the decades to come.
References
Belhaj AF, Elraies KA, Mahmood SM et al (2020) The effect of surfactant concentration, salinity, temperature, and pH on surfactant adsorption for chemical enhanced oil recovery: a review. J Pet Explor Prod Technol 10:125–137
Laben AB, Kayiem HHA, Alameen MA et al (2022) Experimental study on the performance of emulsions produced during ASP flooding. J Pet Explor Prod Technol 12:1797–1809
Belhaj AF, Elraies KA, Mahmood SM, et al (2020) A comprehensive surfactant performance assessment in Harsh Malaysian reservoir conditions. In: Offshore Technology Conference Asia. OTC, p D012S001R067
Belhaj AF, Elraies KA, Sarma HK, et al (2021) Surfactant partitioning and adsorption in chemical EOR: the neglected phenomenon in porous media. In: SPE Asia Pacific Oil and Gas Conference and Exhibition. SPE, p D031S023R006
Belhaj A, Singh N, Sarma H (2022) Critical Assessment of the Hybrid Impact of Surfactants on Modified Salinity Water Flooding. In: SPE Canadian Energy Technology Conference. OnePetro
Curbelo FDS, Santanna VC, Neto ELB et al (2007) Adsorption of nonionic surfactants in sandstones. Colloids Surf A Physicochem Eng Asp 293:1–4
Amirianshoja T, Junin R, Idris AK, Rahmani O (2013) A comparative study of surfactant adsorption by clay minerals. J Pet Sci Eng 101:21–27
Schramm LL (2000) Surfactants: fundamentals and applications in the petroleum industry. Cambridge University Press
Mwangi PM (2010) An experimental study of surfactant enhanced waterflooding
Hama SM, Manshad AK, Ali JA (2023) Review of the application of natural surfactants in enhanced oil recovery: state-of-the-art and perspectives. Energy Fuels 37:10061–10086
Ball B, Fuerstenau DW (1971) Thermodynamics and adsorption behaviour in the quartz/aqueous surfactant system. Discuss Faraday Soc 52:361–371
Paria S, Khilar KC (2004) A review on experimental studies of surfactant adsorption at the hydrophilic solid–water interface. Adv Colloid Interface Sci 110:75–95
Haloi S, Saikia MD, Gogoi SB, et al (2021) Aggregation and static adsorption behaviour of Achromobacter sp. TMB1 produced rhamnolipids on sandstone core in relation to microbial enhanced oil recovery. J Pet Sci Eng 205:108831
Belhaj AF, Elraies KA, Shuhili JA, et al (2020) Surfactant adsorption evaluation in the presence of crude oil at high reservoir temperature condition. In: Offshore Technology Conference Asia. OTC, p D011S008R001
Baviere M, Ruaux E, Defives D (1993) Sulfonate retention by kaolinite at high pH-effect of inorganic anions. SPE Reserv Eng 8:123–127
Dick SG, Fuerstenau DW, Healy TW (1971) Adsorption of alkylbenzene sulfonate (ABS) surfactants at the alumina-water interface. J Colloid Interface Sci 37:595–602
Fuerstenau DW, Wakamatsu T (1975) Effect of pH on the adsorption of sodium dodecane-sulphonate at the alumina/water interface. Faraday Discuss Chem Soc 59:157–168
Wu SH, Pendleton P (2001) Adsorption of anionic surfactant by activated carbon: effect of surface chemistry, ionic strength, and hydrophobicity. J Colloid Interface Sci 243:306–315
Belhaj AF, Fakir SH, Singh N, Sarma HK (2023) A comparative enhanced oil recovery study between low-salinity water and hybrid surfactant process for a carbonate reservoir. In: SPE Western Regional Meeting. SPE, p D041S013R007
Fakir SH, Belhaj AF, Singh N, Sarma HK (2023) Beneficial advantages of nanoparticle-enhanced surfactant-assisted low salinity waterflooding process. In: SPE Western Regional Meeting. SPE, p D031S012R004
Hussien OS, Elraies KA, Almansour A et al (2019) Experimental study on the use of surfactant as a fracking fluid additive for improving shale gas productivity. J Pet Sci Eng 183:106426
Daryasafar N, Borazjani O, Daryasafar A (2018) Simulation of kinetic behavior of natural surfactants adsorption using a new robust approach. J Chemom:e3031
Khan AR, Al-Waheab IR, Al-Haddad A (1996) A generalized equation for adsorption isotherms for multi-component organic pollutants in dilute aqueous solution. Environ Technol 17:13–23
Jovanovic DS (1969) Physical adsorption of gases. I. Isotherms for monolayer and multilayer adsorption. Kolloid-Zeitschrift Zeitschrift Fur Polym 235:1203
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. Part I. Solids J Am Chem Soc 38:2221–2295
Halsey G (1948) Physical adsorption on non-uniform surfaces. J Chem Phys 16:931–937
Hossain MA, Ngo HH, Guo WS, Nguyen TV (2012) Palm oil fruit shells as biosorbent for copper removal from water and wastewater: experiments and sorption models. Bioresour Technol 113:97–101
Arabloo M, Ghazanfari MH, Rashtchian D (2015) Spotlight on kinetic and equilibrium adsorption of a new surfactant onto sandstone minerals: a comparative study. J Taiwan Inst Chem Eng 50:12–23
Temkin MJ, Pyzhev V (1940) Recent modifications to Langmuir isotherms
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Elboughdiri N, Ferkous H, Rouibah K, et al (2024) Comprehensive investigation of cu2+ adsorption from wastewater using olive-waste-derived adsorbents: experimental and molecular insights. Int J Mol Sci 25
Rosen MJ, Kunjappu JT (2012) Surfactants and interfacial phenomena. Wiley
Green DW, Willhite GP (1998) Enhanced oil recovery. In: Henry L (ed) Doherty Memorial Fund of AIME. Society of Petroleum Engineers Richardson, TX
Behrens EJ (2013) Investigation of loss of surfactants during enhanced oil recovery applications-adsorption of surfactants onto clay materials
Atta DY, Negash BM, Yekeen N, Habte AD (2021) A state-of-the-art review on the application of natural surfactants in enhanced oil recovery. J Mol Liq 321:114888
Sofla SJD, Sharifi M, Sarapardeh AH (2016) Toward mechanistic understanding of natural surfactant flooding in enhanced oil recovery processes: the role of salinity, surfactant concentration and rock type. J Mol Liq 222:632–639
Holmberg K (2001) Natural surfactants. Curr Opin Colloid Interface Sci 6:148–159
Yusuf M, Wathon MH, Thanasaksukthawee V et al (2021) Adsorption of saponin natural surfactant on carbonate rock and comparison to synthetic surfactants: an enhanced oil recovery prospective. Energy Fuels 35:11193–11202
Siva M, Ramamurthy K, Dhamodharan R (2017) Development of a green foaming agent and its performance evaluation. Cem Concr Compos 80:245–257
Oleszek W, Hamed A (2010) Saponin-based surfactants. Surfact Renew Resour 1:239–251
Hostettmann K, Marston A (2005) Saponins. Cambridge University Press
Sahu SS, Gandhi ISR, Khwairakpam S (2018) State-of-the-art review on the characteristics of surfactants and foam from foam concrete perspective. J Inst Eng Ser A 99:391–405
Singh N, Halliday HL, Stevens TP, et al (2015) Comparison of animal‐derived surfactants for the prevention and treatment of respiratory distress syndrome in preterm infants. Cochrane Database Syst Rev
Moya F, Maturana A (2007) Animal-derived surfactants versus past and current synthetic surfactants: current status. Clin Perinatol 34:145–177
Kahn MC, Anderson GJ, Anyan WR, Hall SB (1995) Phosphatidylcholine molecular species of calf lung surfactant. Am J Physiol Cell Mol Physiol 269:L567–L573
Ramanathan R (2009) Animal-derived surfactants: where are we? The evidence from randomized, controlled clinical trials. J Perinatol 29:S38–S43
Halliday HL (1997) Synthetic or natural surfactants. Acta Pædiatrica 86:233–237
Curstedt T, Calkovska A, Johansson J (2013) New generation synthetic surfactants. Neonatology 103:327–330
Johnson P, Trybala A, Starov V, Pinfield VJ (2021) Effect of synthetic surfactants on the environment and the potential for substitution by biosurfactants. Adv Colloid Interface Sci 288:102340
Jahan R, Bodratti AM, Tsianou M, Alexandridis P (2020) Biosurfactants, natural alternatives to synthetic surfactants: physicochemical properties and applications. Adv Colloid Interface Sci 275:102061
Tmáková L, Sekretár S, Schmidt Š (2016) Plant-derived surfactants as an alternative to synthetic surfactants: surface and antioxidant activities. Chem Pap 70:188–196
(2020) The Classification of Surfactants. In: Surfactant Science and Technology. pp 17–59
Myers D (2020) Surfactant science and technology. Wiley
Yokoi T, Yoshitake H, Tatsumi T (2004) Synthesis of mesoporous silica by using anionic surfactant. In: Studies in Surface Science and Catalysis. Elsevier, pp 519–527
Salager J-L (2002) Surfactants types and uses. FIRP Bookl 300
Saxena N, Mandal A (2022) Surfactants and their types. In: Natural Surfactants. Springer, pp 3–10
Menger FM, Littau CA (1993) Gemini surfactants: a new class of self-assembling molecules. J Am Chem Soc 115:10083–10090
Bhadani A, Shrestha RG, Koura S et al (2014) Self-aggregation properties of new ester-based gemini surfactants and their rheological behavior in the presence of cosurfactant—monolaurin. Colloids Surfaces A Physicochem Eng Asp 461:258–266
Li Y, Wang X, Wang Y (2006) Comparative studies on interactions of bovine serum albumin with cationic gemini and single-chain surfactants. J Phys Chem B 110:8499–8505
Boublia A, Guezzout Z, Haddaoui N, et al (2023) The curious case of polyaniline-graphene nanocomposites: a review on their application as exceptionally conductive and gas sensitive materials. Crit Rev Solid State Mater Sci:1–25
Guezzout Z, Boublia A, Haddaoui N (2023) Enhancing thermal and mechanical properties of polypropylene—nitrile butadiene rubber nanocomposites through graphene oxide functionalization. J Polym Res. https://doi.org/10.1007/s10965-023-03585-x
Raffa P, Wever DAZ, Picchioni F, Broekhuis AA (2015) Polymeric surfactants: synthesis, properties, and links to applications. Chem Rev 115:8504–8563
Raffa P, Broekhuis AA, Picchioni F (2016) Polymeric surfactants for enhanced oil recovery: A review. J Pet Sci Eng 145:723–733
Cao Y, Li H (2002) Interfacial activity of a novel family of polymeric surfactants. Eur Polym J 38:1457–1463
Liu Z, Zhao G, Brewer M et al (2021) Comprehensive review on surfactant adsorption on mineral surfaces in chemical enhanced oil recovery. Adv Colloid Interface Sci 294:102467. https://doi.org/10.1016/j.cis.2021.102467
Somasundaran P, Zhang L (2006) Adsorption of surfactants on minerals for wettability control in improved oil recovery processes. J Pet Sci Eng 52:198–212
Joonaki E, Gahrooei HRE, Ghanaatian S (2016) Experimental study on adsorption and wettability alteration aspects of a new chemical using for enhanced oil recovery in carbonate oil reservoirs. J Unconv Oil Gas Resour 15:11–21
Yan J, Plancher H, Morrow NR (1997) Wettability changes induced by adsorption of asphaltenes. SPE Prod Facil 12:259–266
Perera MSA (2023) A review of underground hydrogen storage in depleted gas reservoirs: Insights into various rock-fluid interaction mechanisms and their impact on the process integrity. Fuel 334:126677
Liu Z, Zhao G, Brewer M et al (2021) Comprehensive review on surfactant adsorption on mineral surfaces in chemical enhanced oil recovery. Adv Colloid Interface Sci. https://doi.org/10.1016/j.cis.2021.102467
Muherei MA, Junin R (2009) Equilibrium adsorption isotherms of anionic, nonionic surfactants and their mixtures to shale and sandstone. Mod Appl Sci 3:158
Ahmadi MA, Shadizadeh SR (2012) Adsorption of novel nonionic surfactant and particles mixture in carbonates: enhanced oil recovery implication. Energy Fuels 26:4655–4663
Ahmadi MA, Shadizadeh SR (2013) Experimental investigation of adsorption of a new nonionic surfactant on carbonate minerals. Fuel 104:462–467
Bera A, Kumar T, Ojha K, Mandal A (2013) Adsorption of surfactants on sand surface in enhanced oil recovery: isotherms, kinetics and thermodynamic studies. Appl Surf Sci 284:87–99
Park S, Lee ES, Sulaiman WRW (2015) Adsorption behaviors of surfactants for chemical flooding in enhanced oil recovery. J Ind Eng Chem 21:1239–1245
Zargartalebi M, Kharrat R, Barati N (2015) Enhancement of surfactant flooding performance by the use of silica nanoparticles. Fuel 143:21–27
Ahmadi MA, Shadizadeh SR (2015) Experimental investigation of a natural surfactant adsorption on shale-sandstone reservoir rocks: static and dynamic conditions. Fuel 159:15–26
Barati A, Najafi A, Daryasafar A et al (2016) Adsorption of a new nonionic surfactant on carbonate minerals in enhanced oil recovery: experimental and modeling study. Chem Eng Res Des 105:55–63
Moslemizadeh A, Dehkordi AF, Barnaji MJ et al (2016) Novel bio-based surfactant for chemical enhanced oil recovery in montmorillonite rich reservoirs: adsorption behavior, interaction impact, and oil recovery studies. Chem Eng Res Des 109:18–31
Yekeen N, Manan MA, Idris AK, Samin AM (2017) Influence of surfactant and electrolyte concentrations on surfactant Adsorption and foaming characteristics. J Pet Sci Eng 149:612–622
Saha R, Uppaluri RVS, Tiwari P (2017) Effect of mineralogy on the adsorption characteristics of surfactant—reservoir rock system. Colloids Surf A Physicochem Eng Asp 531:121–132
Radnia H, Nazar ARS, Rashidi A (2017) Experimental assessment of graphene oxide adsorption onto sandstone reservoir rocks through response surface methodology. J Taiwan Inst Chem Eng
Tagavifar M, Jang SH, Sharma H et al (2018) Effect of pH on adsorption of anionic surfactants on limestone: experimental study and surface complexation modeling. Colloids Surf A Physicochem Eng Asp 538:549–558
Lebouachera SEI, Chemini R, Khodja M et al (2018) Experimental investigations of SDS adsorption on the Algerian rock reservoir: chemical enhanced oil recovery case. Res Chem Intermed 44:7665–7690
Jian G, Puerto M, Wehowsky A et al (2018) Characterizing adsorption of associating surfactants on carbonates surfaces. J Colloid Interface Sci 513:684–692
Belhaj AF, Elraies KA, Alnarabiji MS et al (2021) Experimental investigation, binary modelling and artificial neural network prediction of surfactant adsorption for enhanced oil recovery application. Chem Eng J 406:127081. https://doi.org/10.1016/j.cej.2020.127081
Kalam S, Abu-Khamsin SA, Patil S et al (2023) Adsorption reduction of a gemini surfactant on carbonate rocks using formic acid: static and dynamic conditions. Fuel 345:128166
Abbas AH, Abd Alsaheb RA, Abdullah JK (2023) Comparative study of natural chemical for enhanced oil recovery: focus on extraction and adsorption at quartz sand surface. Petroleum 9:83–93. https://doi.org/10.1016/j.petlm.2022.01.007
Dang CTQ, Chen ZJ, Nguyen NTB, et al (2011) Development of isotherm polymer/surfactant adsorption models in chemical flooding. In: SPE Asia Pacific oil and gas conference and exhibition. Society of Petroleum Engineers
Somasundaran P, Hanna HS (1985) Adsorption/desorption of sulfonate by reservoir rock minerals in solutions of varying sulfonate concentrations. Soc Pet Eng J 25:343–350
ElMofty O (2012) Surfactant enhanced oil recovery by wettability alteration in sandstone reservoirs
Mushtaq M, Tan IM, Rashid U et al (2015) Effect of pH on the static adsorption of foaming surfactants on Malaysian sandstone. Arab J Geosci 8:8539–8548
Li P, Ishiguro M (2016) Adsorption of anionic surfactant (sodium dodecyl sulfate) on silica. Soil Sci plant Nutr 62:223–229
Liu Z, Hedayati P, Sudhölter EJR et al (2020) Adsorption behavior of anionic surfactants to silica surfaces in the presence of calcium ion and polystyrene sulfonate. Colloids Surf A Physicochem Eng Asp 602:125074
Rashid R, Shafiq I, Iqbal MJ et al (2021) Synergistic effect of NS co-doped TiO2 adsorbent for removal of cationic dyes. J Environ Chem Eng 9:105480
Barati-Harooni A, Najafi-Marghmaleki A, Tatar A, Mohammadi AH (2016) Experimental and modeling studies on adsorption of a nonionic surfactant on sandstone minerals in enhanced oil recovery process with surfactant flooding. J Mol Liq 220:1022–1032
Elias SD, Rabiu AM, Oyekola O, Seima B (2016) Adsorption characteristics of surfactants on different petroleum reservoir materials
Sen GS, Bhattacharyya KG (2008) Immobilization of Pb (II), Cd (II) and Ni (II) ions on kaolinite and montmorillonite surfaces from aqueous medium. J Environ Manage 87:46–58
Hanna HS, Somasundaran P (1979) Equilibration of kaolinite in aqueous inorganic and surfactant solutions. J Colloid Interface Sci 70:181–191
Sastry NV, Dave PN (1999) Adsorption behavior of surfactant-polyacrylamide mixtures with kaolin. J Surfactants Deterg 2:459–472
Mao J, Wang D, Yang X, et al (2018) Adsorption of surfactant on stratum rocks: Exploration of low adsorption surfactants for reservoir stimulation. J Taiwan Inst Chem Eng
Lebouachera SEI, Chemini R, Khodja M et al (2019) Experimental design methodology as a tool to optimize the adsorption of new surfactant on the Algerian rock reservoir: cEOR applications. Eur Phys J Plus 134:436. https://doi.org/10.1140/epjp/i2019-12821-9
Belhaj AF, Elraies KA, Shuhili JA et al (2022) Static adsorption evaluation for anionic-nonionic surfactant mixture on sandstone in the presence of crude oil at high reservoir temperature condition. SPE Reserv Eval Eng 25:261–272
Abbad B, Lounis A (2014) Removal of methylene blue from colored effluents by adsorption onto ZnAPSO-34 nanoporous material. Desalin Water Treat 52:7766–7775
Shukla A, Zhang Y-H, Dubey P et al (2002) The role of sawdust in the removal of unwanted materials from water. J Hazard Mater 95:137–152
Sarma GK, Sen GS, Bhattacharyya KG (2016) Adsorption of crystal violet on raw and acid-treated montmorillonite, K10, in aqueous suspension. J Environ Manage 171:1–10
Li W, Dai C, Ouyang J et al (2018) Adsorption and retention behaviors of heterogeneous combination flooding system composed of dispersed particle gel and surfactant. Colloids Surf Physicochem Eng Asp 538:250–261
Srivastava V, Sharma YC, Sillanpää M (2015) Green synthesis of magnesium oxide nanoflower and its application for the removal of divalent metallic species from synthetic wastewater. Ceram Int 41:6702–6709
Iftekhar S, Ramasamy DL, Srivastava V, et al (2018) Understanding the factors affecting the adsorption of Lanthanum using different adsorbents: a critical review. Chemosphere
Moumeni O, Mehri M, Kerkour R et al (2023) Experimental and detailed DFT/MD simulation of α-aminophosphonates as promising corrosion inhibitor for XC48 carbon steel in HCl environment. J Taiwan Inst Chem Eng 147:104918. https://doi.org/10.1016/j.jtice.2023.104918
Boulechfar C, Ferkous H, Delimi A, et al (2023) Corrosion Inhibition of Schiff Base and their Metal Complexes with [Mn (II), Co (II) and Zn (II)]: Experimental and Quantum Chemical Studies. J Mol Liq:121637
Schwuger MJ (1990) M. Rosen: Surfactants and Interfacial Phenomena, John Wiley & Sons Ltd, New York, Chichester, Brisbane, Toronto, Singapore 1989. 431 Seiten, Preis:£ 36.85. Berichte der Bunsengesellschaft für Phys Chemie 94:796
Sheng J (2011) Modern chemical enhance oil recovery: theory and practice. Gulf Professional
Ruthven DM (1984) Principles of adsorption and adsorption processes. John Wiley & Sons
Wiśniewska M (2012) The temperature effect on the adsorption mechanism of polyacrylamide on the silica surface and its stability. Appl Surf Sci 258:3094–3101
Juang L-C, Wang C-C, Lee C-K (2006) Adsorption of basic dyes onto MCM-41. Chemosphere 64:1920–1928
Zendehboudi S, Ahmadi MA, Rajabzadeh AR et al (2013) Experimental study on adsorption of a new surfactant onto carbonate reservoir samples—application to EOR. Can J Chem Eng 91:1439–1449
Hashemian S, Ardakani MK, Salehifar H (2013) Kinetics and thermodynamics of adsorption methylene blue onto tea waste/CuFe2O4 composite. Am J Anal Chem 4:1
ShamsiJazeyi H, Verduzco R, Hirasaki GJ (2014) Reducing adsorption of anionic surfactant for enhanced oil recovery: Part II. Applied aspects. Colloids Surf A Physicochem Eng Asp 453:168–175
Lebouachera SEI, Chemini R, Khodja M, et al Experimental investigations of SDS adsorption on the Algerian rock reservoir: chemical enhanced oil recovery case. Res Chem Intermed:1–26
Drach M, Jabłoński J, Narkiewicz-Michałek J, Szymula M (2010) Co-adsorption of surfactants and propyl gallate on the hydrophilic oxide surfaces. Appl Surf Sci 256:5444–5448
Yu B, Zhang Y, Shukla A et al (2001) The removal of heavy metals from aqueous solutions by sawdust adsorption—removal of lead and comparison of its adsorption with copper. J Hazard Mater 84:83–94
Ajmal M, Khan AH, Ahmad S, Ahmad A (1998) Role of sawdust in the removal of copper (II) from industrial wastes. Water Res 32:3085–3091
Anirudhan TS, Sreedhar MK (1998) Adsorption thermodynamics of Co (II) on polysulphide treated sawdust
Raji C, Shubha KP, Anirudhan TS (1997) Use of chemically modified sawdust in the removal of Pb (II) ions from aqueous media. Indian J Environ Health 39:230–238
Zhang L, Zeng Y, Cheng Z (2016) Removal of heavy metal ions using chitosan and modified chitosan: a review. J Mol Liq 214:175–191
Austad T, RezaeiDoust A, Puntervold T (2010) Chemical mechanism of low salinity water flooding in sandstone reservoirs. In: SPE improved oil recovery symposium. Society of Petroleum Engineers
Lv W, Bazin B, Ma D et al (2011) Static and dynamic adsorption of anionic and amphoteric surfactants with and without the presence of alkali. J Pet Sci Eng 77:209–218
Budhathoki M, Barnee SHR, Shiau B-J, Harwell JH (2016) Improved oil recovery by reducing surfactant adsorption with polyelectrolyte in high saline brine. Colloids Surf Physicochem Eng Asp 498:66–73
Ahmadi MA, Shadizadeh SR (2018) Spotlight on the new natural surfactant flooding in carbonate rock samples in low salinity condition. Sci Rep 8:10985
Ferkous H, Sedik A, Delimi A et al (2023) A comparative study of novel synthesized sulfamide compounds: electrochemical, morphological, XPS, and theoretical investigations on copper corrosion inhibition in 1.0 M HCl. J Mol Liq. https://doi.org/10.1016/j.molliq.2023.123781
Somasundaran P, Grieves RB (1975) Advances in interfacial phenomena of particulate/solution/gas systems; applications to flotation research. Aiche Symp Ser 71(150):191
Zhang R, Somasundaran P (2006) Advances in adsorption of surfactants and their mixtures at solid/solution interfaces. Adv Colloid Interface Sci 123:213–229
Somasundaran P, Huang L (2000) Adsorption/aggregation of surfactants and their mixtures at solid–liquid interfaces. Adv Colloid Interface Sci 88:179–208
Peck AS, Wadsworth ME (1964) Infrared study of the depression effect of fluoride, sulphate and chloride on the chemisorption of oleate on fluorite and barite. In: 7th International Mineral Processing Congress, New York. pp 259–267
French RO, Wadsworth ME, Cook MA, Cutler IB (1954) The quantitative application of infrared spectroscopy to studies in surface chemistry. J Phys Chem 58:805–811
Gaudin AM, Fuerstenau DW (1955) Streaming potential studies. Quartz flotation with anionic collectors. Min Eng 7
Zhang L, Somasundaran P, Mielczarski J, Mielczarski E (2002) Adsorption mechanism of n-dodecyl-β-d-maltoside on alumina. J Colloid Interface Sci 256:16–22
James RO, Healy TW (1972) Adsorption of hydrolyzable metal ions at the oxide—water interface. III. A thermodynamic model of adsorption. J Colloid Interface Sci 40:65–81
Das D, Panigrahi S, Misra PK, Nayak A (2008) Effect of organized assemblies. Part 4. Formulation of highly concentrated coal−water slurry using a natural surfactant. Energy Fuels 22:1865–1872
Kalam S, Abu-Khamsin SA, Kamal MS, Patil S (2021) Surfactant adsorption isotherms: a review. ACS Omega 6:32342–32348. https://doi.org/10.1021/acsomega.1c04661
Adak A, Bandyopadhyay M, Pal A (2005) Adsorption of anionic surfactant on alumina and reuse of the surfactant-modified alumina for the removal of crystal violet from aquatic environment. J Environ Sci Heal 40:167–182
Langmuir I (1917) The constitution and fundamental properties of solids and liquids. II Liquids J Am Chem Soc 39:1848–1906
Temkin MI (1941) Adsorption equilibrium and the kinetics of processes on nonhomogeneous surfaces and in the interaction between adsorbed molecules. Zh Fiz Chim 15:296–332
Hill K, Rhode O (1999) Sugar-based surfactants for consumer products and technical applications. Eur J Lipid Sci Technol 101:25–33
Redlich O, Peterson DL (1959) A useful adsorption isotherm. J Phys Chem 63:1024
Hameed BH, Mahmoud DK, Ahmad AL (2008) Equilibrium modeling and kinetic studies on the adsorption of basic dye by a low-cost adsorbent: Coconut (Cocos nucifera) bunch waste. J Hazard Mater 158:65–72
Sips R (1948) Combined form of Langmuir and Freundlich equations. J Chem Phys 16:490–495
Toth J (1971) State equation of the solid-gas interface layers. Acta chim hung 69:311–328
Kapoor A, Yang RT (1989) Correlation of equilibrium adsorption data of condensible vapours on porous adsorbents. Gas Sep Purif 3:187–192
Freundlich H (1907) Über die adsorption in lösungen. Zeitschrift für Phys Chemie 57:385–470
Schmitt M, Fernandes CP, Wolf FG et al (2015) Characterization of Brazilian tight gas sandstones relating permeability and Angstrom-to micron-scale pore structures. J Nat Gas Sci Eng 27:785–807
Sing KSW (1985) Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl Chem 57:603–619
Jovanović DS (1969) Physical adsorption of gases. Kolloid-Zeitschrift und Zeitschrift für Polym 235:1214–1225
Hassan HZ, Mohamad AA, Alyousef Y, Al-Ansary HA (2015) A review on the equations of state for the working pairs used in adsorption cooling systems. Renew Sustain Energy Rev 45:600–609
Ahmadi MA, Shadizadeh SR (2013) Induced effect of adding nano silica on adsorption of a natural surfactant onto sandstone rock: experimental and theoretical study. J Pet Sci Eng 112:239–247
Kamal MS, Sultan AS, Hussein IA (2015) Screening of amphoteric and anionic surfactants for cEOR applications using a novel approach. Colloids Surf A Physicochem Eng Asp 476:17–23
Lagergren S (1898) Zur theorie der sogenannten adsorption geloster stoffe. K Sven Vetenskapsakademiens Handl 24:1–39
Ho Y-S (2006) Second-order kinetic model for the sorption of cadmium onto tree fern: a comparison of linear and non-linear methods. Water Res 40:119–125
Chien SH, Clayton WR (1980) Application of Elovich equation to the kinetics of phosphate release and sorption in soils. Soil Sci Soc Am J 44:265–268
Pérez-Marín AB, Zapata VM, Ortuno JF et al (2007) Removal of cadmium from aqueous solutions by adsorption onto orange waste. J Hazard Mater 139:122–131
Cestari AR, Vieira EFS, Pinto AA, Lopes ECN (2005) Multistep adsorption of anionic dyes on silica/chitosan hybrid: 1. Comparative kinetic data from liquid-and solid-phase models. J Colloid Interface Sci 292:363–372
Weber TW, Chakravorti RK (1974) Pore and solid diffusion models for fixed-bed adsorbers. AIChE J 20:228–238
Kalam S, Abu-Khamsin SA, Kamal MS, Patil S (2021) A review on surfactant retention on rocks: mechanisms, measurements, and influencing factors. Fuel 293:120459
Ojinnaka CM, Ajienka JA, Abayeh OJ et al (2016) Formulation of best-fit hydrophile/lipophile balance-dielectric permittivity demulsifiers for treatment of crude oil emulsions. Egypt J Pet 25:565–574
Belhaj AF, Aris BM, Shuhli J, Elraies KA et al (2020) Partitioning behaviour of novel surfactant mixture for high reservoir temperature and high salinity conditions. Energy 198:117319. https://doi.org/10.1016/j.energy.2020.117319
Al-Shalabi EW, Sepehrnoori K (2016) A comprehensive review of low salinity/engineered water injections and their applications in sandstone and carbonate rocks. J Pet Sci Eng 139:137–161
ShamsiJazeyi H, Miller CA, Wong MS et al (2014) Polymer-coated nanoparticles for enhanced oil recovery. J Appl Polym Sci. https://doi.org/10.1002/app.40576
Belhaj AF, Elraies KA, Alnarabiji MS et al (2019) Experimental investigation of surfactant partitioning in Pre-CMC and Post-CMC regimes for enhanced oil recovery application. Energies. https://doi.org/10.3390/en12122319
Kesarwani H, Saxena A, Mandal A, Sharma S (2021) Anionic/nonionic surfactant mixture for enhanced oil recovery through the investigation of adsorption, interfacial, rheological, and rock wetting characteristics. Energy Fuels 35:3065–3078
Mao J, Wang D, Yang X et al (2019) Adsorption of surfactant on stratum rocks: exploration of low adsorption surfactants for reservoir stimulation. J Taiwan Inst Chem Eng 95:424–431
Isah A, Arif M, Hassan A et al (2022) Fluid–rock interactions and its implications on EOR: critical analysis, experimental techniques and knowledge gaps. Energy Rep 8:6355–6395
Boublia A, El S, Lebouachera I, Haddaoui N (2022) State - of - the - art review on recent advances in polymer engineering : modeling and optimization through response surface methodology approach. Springer, Berlin Heidelberg
Belhaj AF, Elraies KA, Alnarabiji MS et al (2019) Experimental investigation of surfactant partitioning in pre-CMC and post-CMC regimes for enhanced oil recovery application. Energies 12:2319
Sander R, Pan Z, Connell LD (2017) Laboratory measurement of low permeability unconventional gas reservoir rocks: a review of experimental methods. J Nat Gas Sci Eng 37:248–279
Acknowledgements
This work is part of a project supported by petroleum company SONATRACH-Algeria that we would to thank its contribution for providing additives and reservoirs rocks.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Ethical Approval
Not applicable.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lebouachera, S.E.I., Balamane-Zizi, O., Boublia, A. et al. Understanding the Factors Affecting the Adsorption of Surface-Active Agents onto Reservoir Rock in Chemical Enhanced Oil Recovery Applications: A Comprehensive Review. Chemistry Africa 7, 2283–2306 (2024). https://doi.org/10.1007/s42250-024-00931-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-024-00931-4