Abstract
The goal of this research was to evaluate the ultrasound pretreatment affect the extraction of essential oil by hydrodistillation technique. In this study, betel leaves (Piper betle L.) were subjected to ultrasonic pretreatment (frequency: 28 kHz & 40 kHz; power: 150 W; duration 15, 30 and 45 min) prior to the essential oil extraction process. The resulting essential oil was then analyzed for its yield and properties, encompassing chemical composition, functional and morphological characteristics. The findings demonstrate that ultrasonic pretreatment substantially improved the extraction yield (0.18–0.22%) of the essential oil by 22.22%, as compared to conventional extraction methods. Since the application of ultrasonic waves facilitated the breakdown of cell walls, thereby promoting the release of essential oil constituents from the betel leaf matrix. Furthermore, the analysis of the essential oil revealed that ultrasonic pretreatment had a notable influence on its chemical composition. The treated samples exhibited percentage composition change of specific bioactive compounds such as Lemairamin (39.24–42.08%), Copaene (7.38–8.45%), hydrindane (23.95–21.91%). Moreover, the antioxidant activity of essential oil increased from 81.01 to 87.02% after the ultrasonic treatment. Furthermore, the effectiveness of ultrasonic pretreatment on the betel leaves for the extraction of essential oil was checked by scanning electron microscopy (SEM) analysis. Apart from this, power use (1.48–0.82 kWh) and environmental impact of CO2 emissions (1.17–0.68 kg) were also decreased during the extraction process for the treated betel leaves. Therefore, ultrasound pretreatment is an effective and environmentally friendly method of extracting betel leaves essential oils, which may increase the quantity and quality of betel leaves oils.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Betel leaves (Piper betel L.,) are a perpetual, indigenous medicinal plant of Malaysian provenance that is a member of the Piperaceae family. In both the Southern and Northern hemispheres of the globe, approximately 700 varieties of Piper betel have been identified. Most of these species are extensively cultivated in nations like Malaysia, India, Philippines, Sri Lanka, Indonesia, East Africa, and other Southeast Asia [14, 23]. This herb, also called as Paan, is extensively grown and chewed across India [11]. The edible, stretchy leaves have a strong and delicious flavor. Some people experience oral discomfort as a result of this pungency. Hence, a unique smoke treatment known as curing is frequently used to eliminate pungency. In addition to energizing and refreshing benefits, the cured leaves create improved flavor, color (green to yellow), and other desirable organoleptic features. As a result, the vine is extensively cultivated on a massive scale across many Asian nations, producing trillions of leaves annually that are imbibed in their raw. The fresh raw form, along with various other ingredients like slaked lime, catechu, areca nut slices, scrapings of coconut, coriander seeds, aniseed, cloves, and cardamom [13, 27]. It has beneficial nutritional and bioactive components, as well as anti-inflammatory, antibacterial, antioxidant, antifungal, antidiabetic, anti-amoebic, and allied effects [15, 30]. As a result, the majority of bioactive molecules originating from plants have a diverse range of potential uses, including those in the food, flavor, fragrance, and medicinal sectors, among others [31]. Betel leaves are renowned for having a wide range of therapeutic benefits, including the treatment of abrasions, wounds, injuries, coughs, eye infections, asthma, gum swelling, and rheumatism. Because of the betel leaf's miraculous nature and essential characteristics, the betel vine is renowned as a superior cash and industrial crop. As a consequence, many investigations have been conducted to examine the bioactive substances found in essential oil [14, 17, 23, 30].
Conventional methods for extracting essential oils (EO) from plant sources mostly include steam distillation, hydrodistillation (HD), organic solvent extraction, and cold press extraction. Conventional approaches have a few intrinsic drawbacks, such as the expression techniques of poorer extraction yield and the hazardous solvent residue in essential oils produced by organic solvent extraction. Because of its cheap cost and simplicity of usage, HD is now the most successful commercial method for obtaining essential oils from medicinal herbs and plants [13]. However, it has certain drawbacks associated with the longer extraction period, including the depletion of the volatile and thermally reactive compounds of essential oils during the extraction process. This significantly impacts the amount and quality of essential oils [6, 29]. In recent times, several innovative methods for extracting essential oils from plants have been developed, including "supercritical fluid extraction, microwave-assisted extraction (MAE), and ultrasound-assisted extraction (UAE)" [5, 6, 34]. Because of its thermal cavitation, and mechanical effects, the UAE provides impressive benefits such as time-and energy savings, a larger extraction yield, and superior essential oil quality [6]. In order to extract essential oils from various plant materials, "ultrasound-assisted hydrodistillation extraction (UAHDE)", a supplementary extraction technique that combines the benefits of UAE and HD, has become quite popular. However, to extract essential oil from the betel leaves, there are two different UAHDE methods such as simultaneous ultrasound-assisted hydrodistillation extraction and ultrasonic pretreatment followed by hydrodistillation. A study conducted by, Kumoro et al. [19] and Chen et al. [6] employed ultrasonic pretreatment with an ultrasound probe followed by the traditional HD method to extract essential oils from Cinnamomum cassia (cinnamon) bark and citronella grass leaves. They found that after the ultrasound treatment, the yield of cinnamomum cassia (cinnamon) bark essential oil enhanced by 56.94%, shorter extraction time and less energy, while in case of citronella grass leaves essential oil, yield enhanced by 27%, 60 min extraction time and less energy consumption (0.79 kWh). Similarly, Chen et al. [7] also isolated essential oil from perilla leaves using ultrasonic pretreatment prior to HD. The yield is increased 0.69% after the ultrasound treatment. Furthermore, Solanki et al. [29] and Liu et al. [21] simultaneously used HD and sonication in a Clevenger HD device fitted with an ultrasonic probe to isolate essential oils from java citronella grass and Iberis amara seeds. They found the maximum yield of citronella grass was found in the treated yield (4.11%) and energy consumption reduced by 40%, while maximum oil recovery in the Iberis amara seeds sample was found 0.42% and optimum extraction time 4 h. Both of the aforementioned UAHDE methods for extracting essential oils from natural plants were clearly distinguishable from the conventional HD method by a faster extraction rate, better product quality and less energy consumption. These methods could therefore be regarded as environmentally friendly methods.
Until now, no investigations have been published on essential oil extraction from ultrasound-pretreated betel leaves. Therefore, the purpose of this study is to assess how the ultrasonic pretreatment used before hydrodistillation and how ultrasonic treatment affected the extraction of essential oils from betel leaves. However, the suggested extraction technique contrasted with conventional HD regarding extraction yield, extraction time and environmental effect. Secondly, this study aimed to identify the primary compounds in both fresh and treated betel leaf EO, which were investigated by the creation of spectrum libraries by GC–MS and examine the essential oil's antioxidant activity by DPPH analysis. Moreover, scanning electron microscopy (SEM) was used to analyzed the extract's morphological mechanism of treated sample.
2 Materials and Methods
2.1 Collection and Selection of Plant Material
Meetha varieties of raw materials were harvested (10, March 2023) and collected from a farmer's field located at Kaktiya village near mecheda town, East Midnapore, West Bengal, India (22° 42´ N, 87° 87´ E). Meetha varieties leaves were identified by Prof. Proshanta Guha at the "Agronomy Lab of the Agricultural and Food Engineering Department, IIT Kharagpur". The leaves aged more than 60 days (i.e., commercial maturity stage) Meetha varieties were taken in the experiment, and same-day extraction was done. Petioles were removed from the harvested leaves and then cleaned in tap water. The agro-climatic conditions of the region were sub-humid.
2.2 Ultrasound Pretreatment
Prior to being placed in the ultrasonic bath, the cleaned betel leaves were sliced into little pieces, about 1 cm2 and subjected to ultrasonic bath. An ultrasound bath (LABMAN SCIENTIFIC INSTRUMENT, INDIA, LMUC-3, 240 × 140 × 100 mm) was employed to perform an ultrasound pretreatment on the betel leaves' samples with different leaf-to-water ratios such as 1:1, 1:2 and 1:3 at two different frequencies 28 kHz and 40 kHz and power 150 W, three levels of sonication time (15, 30 and 45 min) with replication. The material is transported to a Clevenger device after being sonicated to extract essential oils using the hydro distillation process.
2.3 Extraction of Essential Oil Using Hydrodistillation
100 g of each fresh and treated sample was subjected to Clevenger-type apparatus for hydrodistillation. Small size approx. 1 cm2 of leaves was prepared by the protocol described by Guha [12]. Each native and treated sample was placed into a 2 L round bottom flask with the ratio of the appropriate solvents. Distilled water was used as a solvent in the experiment. The mixture of tiny leaves and water in a round bottom flask was heated using a digital heater (temperature rang 0–450 ℃) for oil extraction. However, digital heater was taken in this experiment to control the extraction temperature at all stages. The essential oil was obtained through the vertical condenser and separated from the upper layer of the vertical receiver tube. While, some trace amount of water existing in essential oil was dried by anhydrous Na2SO4, labeled, and stored essential oil in the refrigerator at 4 ℃ for further analysis. The following equation was used to compute the essential oil yield (1).
2.4 Physical Properties of Essential Oil
The ISO 3518:2002(E) technique was used to test the specific refractive index and gravity of the essential oils extracted from betel leaves (fresh and treated). At 20 °C, the specific gravity and refractive index were determined. Moreover, the appearance of essential oil obtained from both samples using hydrodistillation technique is also analyzed. In the experiment, all of the data were collected in triplicate and provided as mean and standard deviation form.
2.5 Scanning Electron Microscope (SEM) Analysis
Using a "ZEISS EVO 6o (POLARON -SC7620)" scanning electron microscope, the morphological changes in fresh and treated leaves after HD extraction were examined. After being air-dried, the analyzed extracted samples were collected for SEM examination. The samples were cathode sprayed with gold before the evaluation to improve the quality of the modifications, and they were then mounted on a specimen holder to examine the morphology. Therefore, 20 kV, 1000x (A & B) and 2000x (C) were utilised as the applied voltage and magnification for the SEM investigation of the sample. The experiment was conducted to assess how the ultrasound pretreatment and extraction method affected the leaf matrix and to analyze the high-resolution morphology of the leaf surface.
2.6 Gas Chromatography-Mass Spectrometry (GC–MS) Analysis
Under different working parameters, the chemical profile of volatile oil extracted from fresh and treated leaves was investigated by GC–MS. Firstly, a gas chromatograph (GC) component to distinct the phytochemical from volatile oil before identifying those bioactive compounds at a molecular level with a mass spectrometer (MS) component. Essential oil from fresh and treated leaves underwent "GC–MS (Thermo Scientific GC (TRACETM 1300) and MS (DSQ II)" analysis that included a flame ionization detector for the purpose of characterizing volatile chemicals. To identify the chemical components contained in essential oil, 20 µl of essential oil obtained from the fresh and treated leaves were diluted in 480 µL of methanol. A GC–MS instrument was then used to receive 1 µL of this solution. The GC system included a capillary column called the Rxi-5-Sil MS (30 m, 0.25 mm, 0.25 m). The injector was kept at a constant temperature of 250 °C. The temperature of the GC chamber was retained constant at 50 °C for 2 min, subsequently elevated to 200 °C at a rate of 5 °C per minute for another 2 min, then to 250 °C at a rate of 10 °C per minute for another 2 min, and finally raised to 280 °C at a rate of 15 °C per minute and held there for 5 min. The research-grade helium (Purity > 99.9%) was transported using a steady flow of 1.70 mL/min, a split mode with a split ratio of 25, and a purge flow of 3.0 mL/min. Total time for the GC was 50 min. The MS was operated in scan mode from m/z 40 to 500 while the interface and ion source temperatures were both set to 250 °C. The GC–MS identification was performed using a 70-eV ionization energy with an electron ionization mode. Programming the sector mass analysis tool to scan between 40 and 500 amu for two seconds [23]. Using the NIST mass spectrum database, each unique quantifiable bioactive compound of each essential oil was determined by retention period, relative peak percent area and mass disintegration arrangement [8].
2.7 Antioxidant Activity
The "free radical 2,2-diphenyl-1-picrylhydrazyl radical (DPPH)" test was used to assess the antioxidant activity of extracted essential oil from the fresh and treated leaves, with a few changes to the technique used by Madhumita et al. [22]. Briefly, in 95% methanol, 2 ml of betel leaf essential oil and 2 ml of DPPH (1 × 10–4 M) were mixed to produce solutions with various concentrations of 2, 4, 6, 8, and 10 µl/ml. After 3–5 min of vigorous shaking, the samples were incubated for approximately 30 min at room temperature. After that, a UV–visible spectrophotometer (Epoch 2, BioTek, U.S.A.) was used to assess the absorbance of both essential oil at 695 nm in reference to a blank. The positive control used was ascorbic acid. The following equation was used to compute the inhibition percentage (I %):
where Ac and At represent the absorbance of the test (EO sample at different concentrations) and control (EO sample absent) samples, respectively.
The scavenging activity versus essential oil sample concentrations on a graph, the essential oil concentration that provides 50% inhibition (IC50) was determined. The results of each test were run in triplicate and are shown as mean ± SD.
2.8 Environmental Impact
The power consumption (P) and CO2 emissions of varied extraction techniques were estimated concerning the environmental effect. The following equation determined how much electricity was used to produce each gram of acquired essential oil (EO).
where m is the mass, P is the Kw, t is the time (h) of the derived essential oil, and EC is the electric consumption per gram of EO (kW/h/g/1) (g).
When fossil fuels are used to produce 1 kWh of energy, 800 g of CO2 is released into the atmosphere [10]. The following equation was employed in this calculation to estimate the CO2 emission per gram of essential oil:
where Eco2 is the CO2 emission per gram of essential oil (kg/g).
2.9 Statistical Analysis
Data analysis was performed using IBM SPSS STATISTICS 22, USA. Means and standard deviations were used to display the data. The tests were all carried out in triplicate.
3 Results and discussion
3.1 Effects of Ultrasound Duration, Frequency, and Duration of Extraction on Essential Oil Yield
Investigating the extraction conditions of betel leaves essential oils was done since several operational parameters may possibly alter the extraction process. Using the hydrodistillation method, the essential oil obtained from fresh betel leaves is 0.18% with 2.5 h of extraction time. The impact of ultrasound duration on essential oil output is seen in Fig. 1. The essential oil was extracted from the treated betel leaves for two hours after they had been exposed to various ultrasonic treatments ranging from 15, 30 and 45 min at 28 kHz and 40 kHz of a power level of 150 W. It is evident that the yield of betel leaves oil improves when the ultrasound duration is increased from 15 to 30 min and progressively reduces as the ultrasound duration is increased further. An increment in the ultrasonic period might result in a higher degree of cell disruption in the piece of betel leaves, which would facilitate the release of essential oils into the solution and enhance the yield of the essential oil of betel leaf. Since, higher oil macromolecular species were converted into small macromolecular species when the higher ultrasound period was applied [20].
Moreover, when the ultrasound duration increased, the localized temperatures might escalate as well, resulting in the reduction of potentially volatile and damaged compounds [2, 6]. Additionally, betel leaves essential oil yield was slightly reduced after more than 30 min of ultrasonic treatment. Hence, a 30-min ultrasound duration was used for the following optimization. However, no significant change was observed in the both frequencies at 30 and 45 min of treatment. While the ultrasonic power and duration were raised by more than 150 W and 45 min, respectively, a decrease in yield was seen. The impact of ultrasonic frequency on essential oil output is seen in Fig. 2. The leaves samples that had been exposed to ultrasound treatment were extracted of their essential oils for two hours after being exposed to varied ultrasound frequencies of 28 kHz and 40 kHz for 30 min. As can be observed from Fig. 2, at the ultrasonic frequency of 28 kHz, the extraction yield (0.22%) rises continuously, while as the ultrasonic frequency increases up to 40 kHz, the extraction yield (0.20%) falls as the ultrasonic power is raised further. Therefore, the destruction of plant cells occurred by the concentrated pressures and shear stresses produced when the cavitation bubbles collapsed encircling the betel leaf surfaces. Because rate of ultrasonic treatment rises, facilitating more cytoplasmic components to be released into the mixture and increasing the yield of essential oils. [28]. The desired components might degrade at excessively high ultrasonic frequency due to the overheated ambient temperatures and much more free radicals produced when more cavitation bubbles collapsed; as a result, a minimal reduction was found in the yield of essential oils [24]. Similar behaviour was seen when the essential oil from cinnamomum cassia bark was extracted using ultrasound [6]. So, it was concluded that 150 W would be the proper ultrasound power for the ensuing experiment.
Therefore, ultrasound-assisted extraction (UAE) offers remarkable advantages including time and energy savings, a higher extraction yield, and greater essential oil quality. These advantages are mostly attributable to the thermal cavitation and mechanical effects of UAE. Because thermal cavitation, which includes the production and implosive collapse of tiny gas bubbles in a liquid media, is used in ultrasound-assisted extraction. When compared to conventional extraction techniques, this quick and effective heating dramatically shortens the extraction time. Despite this, the mechanical effects of UAE, in particular the acoustic streaming and microturbulence produced by cavitation, improve the mass transfer and mixing efficiency within the extraction medium. More target chemicals are extracted from the plant material as a consequence of the increased agitation and enhanced mass transfer as compared to traditional techniques, increasing the extraction yield. Due to the combination of mechanical and thermal cavitation effects, UAE also provides improved essential oil quality.
3.2 Physical Properties of Essential Oil
The standard protocol recommended by "ISO 3126: 1977 (E) and ISO 3524: 2003 (E)" was employed to evaluate the physical properties of betel leaves essential oil (optical rotation specific gravity and refractive index). The analysis's findings were contrasted against information from quality standards to assess the quality of the betel leaf essential oils extracted by HD from both samples. Table 1 demonstrates no appreciable differences in the physical properties of essential oils extracted using the HD techniques from the fresh and treated betel leaves sample. Therefore, it is evident that the obtained betel leaf essential oils' physical characteristics adhere to quality criteria.
3.3 Morphology Study by SEM Analysis
The morphological alteration in the surface structure of the betel leaf samples before and after the ultrasonic pretreatment was analyzed after HD extraction through SEM analysis. Figure 4 showed the ultrasonic pretreatment mechanism to help better understand how it works and how it affects the extraction process of betel leaf essential oil. As shown in Fig. 3A (untreated sample), there has been no discernible disturbance to the sample's surface structure, which is smooth and undamaged. As can be observed from Fig. 3B (treated at 28 kHz) and 3C (treated at 40 kHz), the betel leaves samples have cellular breakage after the ultrasonic processing, and their surface exhibits many cracks and pores. Additionally, the scattered nature of the sample leaves is enhanced after ultrasonic treatment. Therefore, the SEM findings clarified the extraction mechanism for ultrasound pretreatment on hydrodistillation process [4, 6, 16], as shown in Fig. 4. Although, cavitation bubbles are generated at the surfaces of the betel leaves during the ultrasonic irradiation and increase over time. As the bubbles attain the critical value, they will dramatically implode, creating micro-jets that are flowing at speeds of more than 400 km/h and exerting a localized high pressure of 200 MPa on the surfaces of the tissue of betel leaves [6, 18, 32, 33]. On the one hand, sonication dispersed and flattened the surface area of leaves, and then betel leaves rupture surface area, facilitating the interaction of the tissues with water. Therefore, during the ultrasonication, the cellular membranes of the betel leaves' tissues were damaged, and physical contact was made between the intracellular active ingredients and the external water, allowing water to enter the interior cells and disperse the active compounds. The betel leaves tissues were more severely destroyed by pretreatment by ultrasound irradiation than by conventional HD, which improved mass transfer and caused a more lavish and faster release of active ingredients. So, ultrasonic pretreatment led to a shorter extraction time, better essential oil quality and a higher essential oil yield.
An illustration of a potential ultrasound pretreatment with a hydrodistillation process mechanism [6]
3.4 Bioactive Compounds by GC–MS Analysis
Strongly scented betel leaves essential oil produced a lot of yellow and white liquid volatile EO when isolated from fresh and ultrasonic treated leaves. The GC–MS was used to determine the volatile chemical composition and relative percentage values of fresh and treated betel leaf essential oil produced by the HD technique under optimal circumstances. The GC–MS data of fresh and treated samples have been represented in Table 2. Figure 5A–C showed 39–45 bioactive compounds with different percentage compositions, corresponding to 97.78–98.88% of the fresh and treated betel leaf essential oil that had been optimized and may have contributed to the plant's diverse aromatic and therapeutic qualities. Whereas, 6–8 bioactive compounds were exhibited as the major bioactive compounds in the fresh and treated (28 and 40 kHz) leaves.
Moreover, Table 2 displays the major bioactive compound with various compositions. The detected chemical components, their retention times (Rt), and their proportions in the whole oil are shown in Table 2. As can be observed, the detected bioactive compound molecules have remarkably similar chemical compositions but different percentages. The major bioactive compounds found in the essential oil of the fresh and ultrasonic-treated betel leaves essential oil leaves are hydrindane (21.91–23.95%), lemairamin (39.24–42.08%), copaene (7.38–8.45%), eugenol acetate (3.78–3.82%) and indane (9.41–11.38%). As can be observed, after the ultrasonic pretreatment on the leaves, some major bioactive compounds of treated betel leaves have significantly increased with increasing the treatment frequency. Therefore, the differences in essential oil chemical profiles are caused by several variables, including extraction methods, plant type, growth stage, harvesting period, meteorological circumstances, a nation of origin, and ecological and topographical regions [1]. As oxygenated and bicyclic compound monoterpenes, linalool and Indane give out a strong and distinctive scent that is utilized in perfumes, shampoos, cosmetics, pharmaceuticals, detergents, and other products. Moreover, these substances have antibacterial, antifungal, and insecticidal effects, making them useful as a natural disinfectants [1, 14].
3.5 Antioxidant Activity of Essential Oil
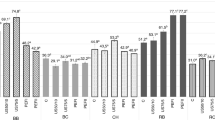
The DPPH assay was used to assess the antioxidant activity of fresh and treated betel leaves essential oil at various doses and compare them to ascorbic acid. The capacity of essential oils to scavenge DPPH radicals is strongly correlated with their concentration, as seen in Fig. 6. As the sample concentration was raised from 2 to 10 µl/ml, So, the percentage inhibition of betel leaf essential oil obtained from fresh and treated samples rose from 41.40 to 81.01% (fresh sample), 43.95 to 86.87% (28 kHz) and 44.50 to 87.02% (40 kHz), respectively. In fresh and treated betel leaf essential oil sample solutions, the readings of the DPPH radical by 10 µl/ml were observed to be 81.01%, 86.87% and 87.02%, respectively. Compared to fresh leaves, ultrasound-treated leaves showed high antioxidant activity against DPPH radical activity. As per concentration observations, the IC50 values for essential oil obtained from fresh and treated leaves were 4.87 µl/ml (fresh), 3.67 µl/ml (28 kHz) and 3.01 (40 kHz) µl/ml, respectively. A similar observation was noticed for old age leaf by Madhumita et al. [22].
Moreover, ascorbic acid (control) had an IC50 value of 8.42 µl/ml. Ultrasonic-treated betel leaf essential oil has been shown to have good antioxidant activity with a reduced IC50 value. The stronger capacity of the essential oil to serve as a DPPH scavenger and vice versa, which were necessary to produce a 50% scavenging response, is shown by the lower IC50 value, which also provides a superior protective activity [26]. As a result of this work, the antioxidant activity of ultrasonically treated (both 28 kHz and 40 kHz) betel leaf essential oil was expressed with a low IC50 value, demonstrating the oil's potent and efficient DPPH activity. The essential oil obtained from treated leaves is more effective than the control since its IC50 value was lower than that shown for ascorbic acid. whereas, treated leaves essential oil was found to have an almost similar concentration of eugenol. Therefore, the capacity of betel leaf essential oil to donate hydrogen atoms may be a modulator of its propensity to scavenge DPPH radicals. The maximum value of betel leaf essential oil DPPH radical scavenging activity is due to hydrodistillation extraction and the effect of ultrasound pretreatment, which is associated with larger concentrations of monoterpenes and sesquiterpenes [3, 9, 25]. The findings of the DPPH experiment indicated that treated leaves essential oil had strong antioxidant activities, while essential oil obtained from treated leaves (40 kHz) mainly displayed significant antioxidant activities as compared to fresh betel leaves essential oil.
3.6 Environmental Impact
The CO2 emissions and energy consumption produced by this extraction process under ideal extraction conditions were also contrasted with those produced by the conventional HD method in order to highlight the advantages of the ultrasound pretreatment followed by the hydrodistillation method. The results were summarised in Fig. 7. The ultrasound pretreatment approach showed a clear reduction in energy use. 1 g of betel leaf essential oil requires 1.48 kWh of electrical energy for untreated leaves using HD but only 0.80–0.82 kWh for treated samples by HD. This difference was attributed to the ultrasonic irradiation of the betel leaves' tissues, which led to a quick and effective extraction for ultrasonically treated leaves. Considering the effects of pollution on the environment, the computed quantity of CO2 expelled into the atmosphere per gram of betel leaves oil extracted by HD (1.17 kg) is much larger than that of treated samples (0.65–0.68 kg). Consequently, ultrasound pretreatment may be recommended as a cost-effective and environmentally friendly method for obtaining essential oil from betel leaves.
4 Conclusion
The ultrasound pretreatment method was effectively used to extract betel leaf essential oil. In this study, ultrasound pretreatment prior to the hydrodistillation method was used to extraction of essential oil from the betel leaves. The ideal extraction settings for the sonication were 30 min. at 150 watts and an extraction performed on HD for 120 min. The maximum yield of treated betel leaves essential oil is 0.22%, which is about 22.22% greater than the fresh leaf's (0.18%) yield. Despite this, the treated leaf's extraction time (120 min) is much less than fresh leaves (180 min), proving that it is a quicker extraction method. Around 39 and 45 bioactive compounds were confirmed using GC–MS analysis in the essential of fresh and treated betel leaves. Ultrasound treatment would not change the kinds of primary active ingredients found in betel leaves essential oil like hydrindane, indane and copaene. Moreover, compared to untreated betel leaves, oil extracted by the ultrasonically treated leaves had a greater concentration of the essential Lemairamin component (42.08%). The examination of physical constants indicates that the physical qualities of the produced betel leaf oil satisfy quality criteria. The SEM examination reveals that the ultrasonic irradiation-generated structural degradation of the betel leaves tissues is the main driving factor behind the effective extraction. Moreover, compared to fresh leaves oil extraction using HD, utilizing ultrasonically treated leaves for HD might save energy use and CO2 emissions. Therefore, ultrasound pretreatment is an effective method for obtaining the essential oil of betel leaves and may also be utilized for getting the oil from many other plant sources.
Data availability
The data will be made available on request.
References
Abdelli M, Moghrani H, Aboun A, Maachi R (2016) Algerian Mentha pulegium L. leaves essential oil: chemical composition, antimicrobial, insecticidal and antioxidant activities. Ind Crops Prod 94:197–205. https://doi.org/10.1016/j.indcrop.2016.08.042
Bahmani L, Aboonajmi M, Arabhosseini A, Mirsaeedghazi H (2018) Effects of ultrasound pre-treatment on quantity and quality of essential oil of tarragon (Artemisia dracunculus L.) leaves. J Appl Res Med Arom Plants 8:47–52. https://doi.org/10.1016/j.jarmap.2017.10.002
Bartikova H, Hanusova V, Skalova L, Ambroz M, Bousova I (2014) Antioxidant, pro-oxidant and other biological activities of sesquiterpenes. Curr Top Med Chem 14(22):2478–2494. https://doi.org/10.2174/1568026614666141203120833
Benmoussa H, Béchohra I, He S, Elfalleh W, Chawech R (2023) Optimization of sonohydrodistillation and microwave assisted hydrodistillation by response surface methodology for extraction of essential oils from Cinnamomum cassia barks. Ind Crops Prod 192:115995. https://doi.org/10.1016/j.indcrop.2022.115995
BeyechaHundie K, Aga Bullo T, MekonnenBayisa Y, AbdissaAkuma D, SeidBultum M (2023) Optimization of microwave-assisted hydro-distillation essential oil extracted from Rumex Crispus leaves using definitive screening design. Arab J Chem 16(5):104665. https://doi.org/10.1016/j.arabjc.2023.104665
Chen G, Sun F, Wang S, Wang W, Dong J, Gao F (2021) Enhanced extraction of essential oil from Cinnamomum cassia bark by ultrasound assisted hydrodistillation. Chin J Chem Eng 36:38–46. https://doi.org/10.1016/j.cjche.2020.08.007
Chen Z, Wu K, Zhu W, Wang Y, Su C, Yi F (2022) Chemical compositions and bioactivities of essential oil from perilla leaf (Perillae folium) obtained by ultrasonic-assisted hydro-distillation with natural deep eutectic solvents. Food Chem 375:131834. https://doi.org/10.1016/j.foodchem.2021.131834
de AlencarFilho JMT, Araújo LDC, Oliveira AP, Guimarães AL, Pacheco AGM, Silva FS, Cavalcanti LS, Lucchese AM, Almeida JRGds, Araújo ECdc (2017) Chemical composition and antibacterial activity of essential oil from leaves of croton heliotropiifolius in different seasons of the year. Rev Bras 27(4):440–444. https://doi.org/10.1016/j.bjp.2017.02.004
Farhadi N, Babaei K, Farsaraei S, Moghaddam M, GhasemiPirbalouti A (2020) Changes in essential oil compositions, total phenol, flavonoids and antioxidant capacity of Achillea millefolium at different growth stages. Ind Crops Prod 152:112570. https://doi.org/10.1016/j.indcrop.2020.112570
Ferhat MA, Meklati BY, Smadja J, Chemat F (2006) An improved microwave Clevenger apparatus for distillation of essential oils from orange peel. J Chromatogr A 1112(1–2):121–126. https://doi.org/10.1016/J.CHROMA.2005.12.030
Guha P (2006) Betel leaf: the neglected green gold of India. J Hum Ecol 19(2):87–93. https://doi.org/10.1080/09709274.2006.11905861
Guha P (2007) Extraction of essential oil: an appropriate RuralTech-nologyfor minimizing wastage of surplus betel leaves. Agric Mech Asia, Afr Latin Am 38(4):47–50
Guha P, Nandi S (2019) essential oil of betel leaf (Piper betle L.): a novel addition to the world food sector. In: Malik S (ed) Essential oil research. Springer Nature Switzerland, pp 149–196. https://doi.org/10.1007/978-3-030-16546-8_5
Gupta RK, Guha P, Srivastav PP (2022) Phytochemical and biological studies of betel leaf (Piper betle L.): review on paradigm and its potential benefits in human health. Acta Ecolog Sin. https://doi.org/10.1016/j.chnaes.2022.09.006
Gupta RK, Guha P, Srivastav PP (2022) Natural polymers in bio-degradable/edible film: a review on environmental concerns, cold plasma technology and nanotechnology application on food packaging—a recent trends. Food Chem Adv 1(November):100135. https://doi.org/10.1016/j.focha.2022.100135
Jafari R, Zandi M, Ganjloo A (2022) Effect of ultrasound and microwave pretreatments on extraction of anise (Pimpinella anisum L.) seed essential oil by ohmic-assisted hydrodistillation. J Appl Res Med Arom Plants 31:100418. https://doi.org/10.1016/j.jarmap.2022.100418
Karak S, Das S, Biswas M, Choudhury A, Dutta M, Chaudhury K, De B (2019) Phytochemical composition, β-glucuronidase inhibition, and antioxidant properties of two fractions of Piper betle leaf aqueous extract. J Food Biochem 43(12):1–12. https://doi.org/10.1111/jfbc.13048
Khadhraoui B, Turk M, Fabiano-Tixier AS, Petitcolas E, Robinet P, Imbert R, Maâtaoui ME, Chemat F (2018) Histo-cytochemistry and scanning electron microscopy for studying spatial and temporal extraction of metabolites induced by ultrasound. Towards chain detexturation mechanism. Ultrason Sonochem 42:482–492. https://doi.org/10.1016/j.ultsonch.2017.11.029
Kumoro AC, Wardhani DH, Retnowati DS, Haryani K, Yustika S, Fajar TA (2021) Extraction of essential oil from ultrasound pre-treated citronella grass (Cymbopogon nardus) leaves by hydrodistillation method. Chem Eng Trans 87:643–648. https://doi.org/10.3303/CET2187108
Liu J, Yang F, Xia J, Wu F, Pu C (2021) Mechanism of ultrasonic physical-chemical viscosity reduction for different heavy oils. ACS Omega 6(3):2276–2283. https://doi.org/10.1021/acsomega.0c05585
Liu X-Y, Ou H, Xiang Z-B, Gregersen H (2019) Optimization, chemical constituents and bioactivity of essential oil from Iberis amara seeds extracted by ultrasound-assisted hydro-distillation compared to conventional techniques. J Appl Res Med Arom Plants 13:100204. https://doi.org/10.1016/j.jarmap.2019.100204
Madhumita M, Guha P, Nag A (2019) Optimization of the exhaustive hydrodistillation method in the recovery of essential oil from fresh and cured betel leaves (Piper betle L.) using the Box-Behnken design. J Food Process Preserv 43(11):1–14. https://doi.org/10.1111/jfpp.14196
Madhumita M, Guha P, Nag A (2019) Extraction of betel leaves (Piper betle L.) essential oil and its bio-actives identification: Process optimization, GC-MS analysis and anti-microbial activity. Ind Crops Prod 138:111578. https://doi.org/10.1016/j.indcrop.2019.111578
Mollaei S, Mamizadeh Z, Hazrati S, Hashempour H (2021) The effect of ultrasonic pre-treatment on the yield, chemical composition and biological activity of essential oil in Oliveria decumbens flowers. J Appl Res Med Arom Plants 24:100313. https://doi.org/10.1016/j.jarmap.2021.100313
Morshedloo MR, Craker LE, Salami A, Nazeri V, Sang H, Maggi F (2017) Effect of prolonged water stress on essential oil content, compositions and gene expression patterns of mono- and sesquiterpene synthesis in two oregano (Origanum vulgare L.) subspecies. Plant Physiol Biochem 111:119–128. https://doi.org/10.1016/j.plaphy.2016.11.023
Ray A, Jena S, Dash B, Kar B, Halder T, Chatterjee T, Ghosh B, Panda PC, Nayak S, Mahapatra N (2018) Chemical diversity, antioxidant and antimicrobial activities of the essential oils from Indian populations of Hedychium coronarium Koen. Ind Crops Prod 112:353–362. https://doi.org/10.1016/j.indcrop.2017.12.033
Roy A, Guha P (2021) Traditional and functional uses of betel leaf (Piper betle L.) pertaining to food sector: a review. J Postharvest Technol 9(1):72–85
Shirsath SR, Sable SS, Gaikwad SG, Sonawane SH, Saini DR, Gogate PR (2017) Intensification of extraction of curcumin from Curcuma amada using ultrasound assisted approach: effect of different operating parameters. Ultrason Sonochem 38:437–445. https://doi.org/10.1016/j.ultsonch.2017.03.040
Solanki KP, Desai MA, Parikh JK (2018) Sono hydrodistillation for isolation of citronella oil: a symbiotic effect of sonication and hydrodistillation towards energy efficiency and environment friendliness. Ultrason Sonochem 49:145–153. https://doi.org/10.1016/j.ultsonch.2018.07.038
Suri MA, Azizah Z, Asra R (2021) A review: traditional use, phytochemical and pharmacological review of red betel leaves (Piper Crocatum Ruiz & Pav). Asian J Pharm Res Dev 9(1):159–163. https://doi.org/10.22270/ajprd.v9i1.926
Thakker MR, Parikh JK, Desai MA (2018) Ultrasound assisted hydrotropic extraction: a greener approach for the isolation of geraniol from the leaves of Cymbopogon martinii. ACS Sustain Chem Eng 6(3):3215–3224. https://doi.org/10.1021/acssuschemeng.7b03374
Vinatoru M, Mason TJ, Calinescu I (2017) Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. In TrAC Trends Anal Chem 97:159–178. https://doi.org/10.1016/j.trac.2017.09.002
Xing P, Wang J, Lyu T, Zhuang Y, Du X, Luo X (2015) Ultrasound-assisted impurity removal from petroleum coke. Sep Purif Technol 151:251–255. https://doi.org/10.1016/j.seppur.2015.07.036
Zhang X, Zhang L, Zhang Y, Xiong T, Niu Y, Huang Y (2023) Extracting myricetin and dihydromyricetin simultaneously from Hovenia acerba seed by Ultrasound-Assisted extraction on a lab and small Pilot-Scale. Ultrasonics Sonochem 93:106304. https://doi.org/10.1016/j.ultsonch.2023.106304
Acknowledgements
The Ministry of Education (formerly, the Ministry of Human Resource Development), Government of India, and the Agricultural and Food Engineering Department, Indian Institute of Technology Kharagpur, are acknowledged by the author, for their support of this work. They are also thankful to Dr. Sujosh Nandi and Dr. Jagan Sugumar Karthik, scholars of the Agricultural and Food Engineering Department, IIT Kharagpur, India, for their immense support
Author information
Authors and Affiliations
Contributions
RKG: investigation, resources, conceptualization, methodology, writing—original draft, and writing—review and editing. PG: supervision, resources, funding acquisition, and writing—review and editing.
Corresponding author
Ethics declarations
Conflict of Interest
The authors conclude they have no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gupta, R.K., Guha, P. Effect of Ultrasonic Pretreatment on Yield and Properties of Essential Oil of Betel Leaf (Piper betle L.). Chemistry Africa 7, 79–92 (2024). https://doi.org/10.1007/s42250-023-00756-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-023-00756-7