Abstract
Based on the stoichiometric method and the free energy minimization method, an ideal model for the reduction of iron oxides by carbon and hydrogen under blast furnace conditions was established, and the reduction efficiency and theoretical energy consumption of the all-carbon blast furnace and the hydrogen-rich blast furnace were compared. The results show that after the reduction reaction is completed at the bottom of the blast furnace, the gas produced by reduction at 1600 °C still has a certain excessive reduction capacity, which is due to the hydrogen brought in by the hydrogen-rich blast as well as the excess carbon monoxide generated by the reaction of the coke and the oxygen brought in by the blast. During the process of the gas with excessive reduction capacity rising from the bottom of the blast furnace and gas reduction process, the excessive reduction capacity of the gas gradually decreases with the increase in the dydrogen content in the blast. In the all-carbon blast furnace, the excess gas reduction capacity is the strongest, and the total energy consumption per ton of iron reduction is the lowest. This shows that, for the current operation mode of the blast furnace, adding hydrogen in the blast furnace cannot reduce the consumption of carbon required for reduction per ton of iron, but rather increases the consumption of carbon.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
At present, the “low carbon” issue has become a hot issue in the international community, in which the “low carbon” steel revolution has been put in the first place, and much research on “low carbon” steelmaking has been carried out [1,2,3,4,5,6,7,8,9]. For example, the European Union has launched the Ultra-Low CO2 Steelmaking Project (ULCOS) for “low carbon” steelmaking and proposed a 20-year “low carbon” roadmap [10, 11]. Japan has drawn up a “low carbon” emission industrial technology roadmap and the COURSE50 project of “Cool Earth 50” [12, 13]. Among these projects, carbon reduction in the iron and steel industry has become the primary task of the “low carbon” revolution.
So far, countries around the world have not found a material that can replace steel and a method that can substantially replace carbon reduction in ironmaking and steelmaking processes. Therefore, countries around the world are looking for methods with lower CO2 emission in the field of carbon reduction ironmaking, including “low carbon” steelmaking plan in Europe (ULCOS) [11], biomass reduction [14,15,16], co-injection of natural gas or coal or hydrogen [17,18,19], etc. In terms of the ironmaking method, the HIsarna process [20,21,22] and alkaline electrolytic ironmaking process developed by ULCOS [13] undoubtedly reduce the carbon consumption in the process to the greatest extent to achieve the lowest CO2 emission. However, from a thermodynamic point of view, what is the limit of carbon reduction of iron oxides? This issue is not yet clear. Although the theoretical energy consumption and corresponding CO2 emissions of carbon reduction of iron oxide have been calculated in various textbooks, and there are also some process studies and theoretical studies [23,24,25], the research methods and conclusions are not universal. Few studies have been carried out about the quantitative relationship at different parts of the blast furnace during the coal gas rising from the bottom to the top, and the minimum energy consumption for reducing iron oxide by each reductant and the proportion of reductant and exothermic agent in the energy consumption are not clear.
In addition, the effect of artificial hydrogenation on the carbon reduction of iron oxides in blast furnace is rarely studied. Recently, some iron and steel enterprises in Germany and China added hydrogen to reduce iron oxide in blast furnace to reduce CO2 emissions [17, 26,27,28]. However, the efficiency, hydrogen utilization and CO2 emission reduction of blast furnace hydrogen-rich smelting are not clear. Therefore, it is necessary to use thermodynamic theory to study the compound reduction of carbon and hydrogen in blast furnace, so as to reveal the relationship between carbon reduction and hydrogen reduction in blast furnace, thus providing theoretical guidance for hydrogen metallurgy.
In this paper, a theoretical model for the reduction of iron oxides by carbon and hydrogen under blast furnace conditions is established based on the stoichiometric relationship of iron oxide reduction and the principle of minimum free energy of thermodynamics. The limits of theoretical carbon consumption and CO2 emission in the process of iron oxide reduction in the all-carbon blast furnace and the hydrogen-rich blast furnace are calculated, which can provide theoretical guidance for the formulation of the “low carbon” steelmaking process.
2 Thermodynamic model of reduction of iron oxide by carbon
Although the theories on the carbon reduction of iron oxide seem to be very mature in textbooks, so far, there is no ideal energy consumption data for this. The discussion and calculation of the energy consumption of ironmaking generally remain in material balance, heat balance or local thermodynamic calculation. It is necessary to use the most basic thermodynamic principle to study the carbon reduction of iron oxide from a new perspective and find some new laws in the established thermodynamic models.
2.1 Theoretical model based on stoichiometric relationship (Model-1)
The classical ideal thermodynamic principle of carbon reduction of iron oxide can be shown in Fig. 1, based on the following assumptions:
-
(1)
All chemical reactions are carried out in a closed system under the condition that the reducing agent C is sufficient and the heating agents C and O2 are also sufficient.
-
(2)
The temperature of the reactants in the initial and final states is room temperature.
-
(3)
Other energy losses in the reaction process are not considered in this ideal model.
-
(4)
Heat needed is provided by the complete combustion reaction between carbon and oxygen.
In Fig. 1, ∆H⊖ is the standard enthalpy change of the system from the initial state to the final state, and TInitial and TFinal are the temperatures of the initial and final states, respectively. The number of components (M) is \(6\) with Fe2O3, CO, CO2, O2, Fe and C, respectively. The number of elements (m) is \(3\) with Fe, C and O, respectively. Therefore, in this thermodynamic system, the number of independent chemical reactions (r) is calculated in Eq. (1). Thus, in terms of thermodynamic theory, 3 independent chemical reactions are needed to describe the initial and final states of carbon reduction of iron oxide, which can reflect the whole process.
In the closed system, the intermediate products Fe3O4 and FeO are not considered here, because this model assumes that the reductant carbon is sufficient, and thus, these two intermediate oxides do not exist when the reaction reaches the final state of thermodynamics. However, if the reductant C is not sufficient, the intermediate state will occur. After Fe3O4 and FeO appear in the system, the number of components will change to \(8\), and the number of independent chemical reactions will change to \(5\). The treatment method is the same, and thus it will not be repeated.
To complete this thermodynamic process theoretically, the carbon required for carbon reduction of iron oxide can be divided into two parts. The first kind of carbon is consumed as reducing agent, which can be calculated by stoichiometry. The second kind of carbon is used as heating agent for the endothermic reaction in the direct reduction of iron oxide by carbon. Theoretically, the carbon needed to reduce iron is the sum of these two parts.
Therefore, the Hess law can be used to design the carbon reduction of iron oxide. Taking Fe2O3 as an example, an ideal ironmaking process with limited energy utilization and minimum CO2 emission is proposed, as shown in reactions (2)–(4).
where T is the temperature; and \({\Delta H^{{ \ominus }}_{T} }\) represents the reaction enthalpy change at T under standard state. At T, the indirect reduction reaction (reaction (2)) is combined with the carbon gasification reaction (reaction (3)) to obtain reaction (5).
An ideal model for the carbon reduction of Fe2O3 (called Model-1) is constituted of reactions (4) and (5). The mass of CO2 and CO in coal gas is from the combustion reaction (reaction (4)) and the reduction reaction (reaction (5)), respectively. The needed C can be calculated from two parts as follows:
-
(1)
The mass of carbon as reducing agent is calculated from the stoichiometric relationship of reaction (5);
-
(2)
The mass of carbon as heating agent is calculated from the complete combustion reaction (reaction (4)) to meet the heat required by the reduction reaction (reaction (5)).
Thus, the iron ore, oxygen supply and carbon consumption required for the carbon reduction of Fe2O3 can be calculated.
Reactions (6)–(8) are the three most basic reactions used in the calculation.
The reaction heat of Eq. (5) equals the difference between three times of Eq. (7) and one time of Eq. (8), as shown in Eq. (9), in which x refers to the mass of carbon as reducing agent and y refers to the heat required for 1 kg Fe. 1 kg Fe (or 1 t Fe) produced is taken as the benchmark to simulate and calculate the carbon reduction of pure Fe2O3 in blast furnace.
The amount of reducing agent carbon required to reduce 1 kg Fe is
The amount of heat required to reduce 1 kg Fe is
The amount of heating agent carbon required to reduce 1 kg Fe is
The amount of oxygen required for complete combustion to reduce 1 kg Fe is
The amount of oxygen required for complete combustion to reduce 1 kg Fe is
Based on the above two items, the total carbon consumption for 1 kg Fe is
According to unit transfer, the theoretical carbon required for reducing 1 t Fe under ideal conditions is 457.15 kg (including 322.29 kg of reducing agent carbon and 134.86 kg of heating agent carbon, which can be regarded as the same in the later discussion). O2 required is 252 m3 or air required is 1200 m3. In this article, m3 refers to the standard state cubic meter.
The coal gas composition in Model-1 can be calculated. The volume of CO produced by carbon reduction of reaction (5) is shown in Eq. (16), namely 601.61 m3 per ton of Fe. The volume of CO2 produced by combustion reduction of reaction (4) is shown in Eq. (17), namely 251.74 m3 per ton of Fe. The ratio of reducing gas CO in (CO + CO2) can reach 70.5%.
where VCO and \(V_{{{\text{CO}}_{{2}} }}\) are the gas volume of CO and CO2, respectively; and nCO and \(n_{{{\text{CO}}_{{2}} }}\) are the gas mole of CO and CO2, respectively.
According to the calorific value of complete combustion of carbon listed in Eq. (6), ∆H ⊖kgC is used to represent the heat value of complete combustion of 1 kg of carbon, as shown in Eq. (19). kg of standard coal (symbol kgCe) is unusually used to be the unit to represent energy consumption in industry, and ∆H ⊖kgCe is used to represent the heat value of 1 kg of standard coal, as shown in Eq. (20). The calorific value of complete combustion of 1 kg carbon is equivalent to 1.12 kg standard coal, as shown in Eq. (21). Thus, the ideal theoretical energy consumption of carbon reduction of iron oxide is 512 kg standard coal per ton of Fe.
The above calculated theoretical energy consumption of carbon reduction of Fe2O3 and relevant data of its products are integrated and expressed in “g”, “mol” or “m3” respectively, which are listed in Table 1.
From the above discussion, it can be seen that the consumption and output of the carbon reduction of iron oxide are obtained by stoichiometry calculation from the thermodynamic theoretical Model-1. However, the Model-1 cannot reflect the laws under the equilibrium condition of the reduction process.
2.2 Theoretical model based on Gibbs minimum free energy method (Model-2)
For the thermodynamic equilibrium of the products of the closed reaction system under the conditions of different temperatures, pressures and changes in the number of elements, the Gibbs minimum free energy method (GMFEM) [23, 24] is used to design the theoretical model (called Model-2), as shown in Eqs. (22)–(24), to discuss the essential characteristics of carbon reduction of iron oxide.
Subjected to
Namely
where i and e represent the components and elements in the equilibrium state, respectively; n is the amount in the equilibrium state, mol; \(n_{{\text{O,iron oxide}}}\) and \(n_{{\text{O,burn}}}\) are the amount of oxygen from iron oxide and oxygen required for combustion reaction with C, respectively, mol; \(n_{{\text{C,reduction}}}\) and \(n_{{\text{C,burn}}}\) are the amount of carbon as reducing agent and combustion agent, respectively, mol; \(G\), \(G_{i}\) and \(G_{i}^\ominus\) represent the sum of the Gibbs free energy of all substance, the Gibbs free energy and the standard Gibbs free energy of the component i, respectively, J/mol; \(p_{{{\text{total}}}}\) and \(p^\ominus\) represent the total pressure of all gas and the standard atmospheric pressure, respectively, Pa, and \(p^{{\ominus }} ={1\text{ atm}} = 101,325{\;\text{Pa}}\); pi represents the partial pressure of gas component i; \(\alpha_{ie}\) represents the number of atoms in the element; T represents the thermodynamic temperature of the system, K; R is the ideal gas constant, which is equal to 8.314 J/(mol K); and \(a_{i}\) represents the activity. When i is a gaseous state, \(a_{i} = \frac{{p_{i} }}{{p^{{\ominus }} }} = \frac{{n_{i} }}{{\sum {n_{i} } }} \cdot \frac{{p_{\text{total}} }}{{p^{{\ominus }} }}\), and when i is a solid state, \(a_{i} = 1\).
According to Eq. (24), the calculation results of Model-1 would be used as the amount of elements in Model-2. Referring to Table 1, 25 groups of different constraint values were set for composition evolution in the equilibrium state of the closed system based on Eqs. (22)–(24), as shown in Table 2.
In Table 2, the columns 1–5 are constraints and the columns 6–10 are results. \(n_{{\text{O,iron oxide}}}\) is the amount of oxygen of iron oxide required for the reduction of 1 kg Fe. \(n_{{\text{C,d}}}\) is the sum of \(n_{{\text{C,reduction}}}\) and \(n_{{\text{C,burn}}}\). \(n_{{{\text{FeO}}}}\), \(n_{{{\text{Fe}}}}\), \(n_{{{\text{CO}}}}\), and \(n_{{{\text{CO}}_{{2}} }}\) are the amount of FeO, Fe, CO, and CO2 in equilibrium state, respectively, mol. The tenth column shows the proportion of CO in the gas phase system, non-dimensional.
2.3 Analysis and discussion
2.3.1 End point of carbon reduction of Fe2O3
According to the stoichiometric relationship of Model-1, the amount of carbon and oxygen required for 1 kg Fe reduction is substituted into the thermodynamic Model-2 based on GMFEM, as shown in the first row of Table 2, namely the constraint value of the No. 1 system. The corresponding results show that 1 kg iron (17.9 mol) cannot be obtained under the chemical equilibrium state under this condition, only 833 g Fe (14.92 mol) can be obtained, and 226 g FeO (3.14 mol) in the system has not been reduced. This indicates that if the reduction process is designed according to the stoichiometry relationship of 1 kg Fe, 1 kg Fe cannot be obtained at the reaction equilibrium.
In order to obtain 1 kg Fe, the amount of iron and oxygen is kept unchanged, and the value of the first column for reduction gradually increases, as shown in Nos. 2–4 systems in Table 2. It is found that when the reduction increases from 26.86 mol of No. 1 system to 29.86 mol of No. 4 system, the amount of FeO gradually decreases until it disappears in No. 4 system. When the total carbon consumption increases to 42.1 mol, 1 kg iron (17.9 mol) is completely reduced.
What are the characteristics of the system when the iron is completely reduced in theoretical Model-2?
\(\frac{{n_{{{\text{CO}}}} }}{{n_{{{\text{CO}}}} + n_{{{\text{CO}}_{{2}} }} }} = 0.787\) is a common feature in the equilibrium results of Nos. 1–3 systems, and the ratio becomes 0.799 in No. 4 system. For the following Nos. 5–7 systems, \({{n_{{{\text{CO}}}} } \mathord{\left/ {\vphantom {{n_{{{\text{CO}}}} } {\left( {n_{{{\text{CO}}}} + n_{{{\text{CO}}_{{2}} }} } \right)}}} \right. \kern-0pt} {\left( {n_{{{\text{CO}}}} + n_{{{\text{CO}}_{{2}} }} } \right)}}\) also gradually increases from 0.799 to 0.881 when the values of the second column increase and Fe is completely reduced. The reason for this phenomenon can be explained according to the “fork curve” shown in Fig. 2, which was redrawn with the selected thermodynamic data [8], and in which the reduction temperature was extended to the melting point of iron (1536 °C). φCO and φ\(_{\text{H}_{2}}\) in the vertical axis of Fig. 2 represent the equilibrium volume fraction of CO and H2 during their reaction processes, respectively.
Based on No. 4 system, when the amount of C element in the system remains unchanged of (29.86 + 11.24) mol, and the amount of O element increases from (26.86 + 22.5) to (26.86 + 23.5) mol, namely No. 8 system, the equilibrium calculation results show that FeO appears again and the corresponding ratio \({{n_{{{\text{CO}}}} } \mathord{\left/ {\vphantom {{n_{{{\text{CO}}}} } {\left( {n_{{{\text{CO}}}} + n_{{{\text{CO}}_{{2}} }} } \right)}}} \right. \kern-0pt} {\left( {n_{{{\text{CO}}}} + n_{{{\text{CO}}_{{2}} }} } \right)}}\) returns from 0.799 to 0.787 again. Based on No. 8 system, the amount of C and O elements in the system is increased alternately until No. 16 system. As long as \({{n_{{{\text{CO}}}} } \mathord{\left/ {\vphantom {{n_{{{\text{CO}}}} } {\left( {n_{{{\text{CO}}}} + n_{{{\text{CO}}_{{2}} }} } \right)}}} \right. \kern-0pt} {\left( {n_{{{\text{CO}}}} + n_{{{\text{CO}}_{{2}} }} } \right)}}\) is back to 0.787, there will be unreduced FeO in the system. Based on No. 8 system, the system changes to No. 9 system when 1 mol C is added, and the results show that \({{n_{{{\text{CO}}}} } \mathord{\left/ {\vphantom {{n_{{{\text{CO}}}} } {\left( {n_{{{\text{CO}}}} + n_{{{\text{CO}}_{{2}} }} } \right)}}} \right. \kern-0pt} {\left( {n_{{{\text{CO}}}} + n_{{{\text{CO}}_{{2}} }} } \right)}}\) in No. 9 system increases to 0.804, and FeO disappears. This phenomenon repeats until to No. 16 system.
At 1873 K, the condition for complete reduction of iron oxide is that the ratio \({{n_{{{\text{CO}}}} } \mathord{\left/ {\vphantom {{n_{{{\text{CO}}}} } {\left( {n_{{{\text{CO}}}} + n_{{{\text{CO}}_{{2}} }} } \right)}}} \right. \kern-0pt} {\left( {n_{{{\text{CO}}}} + n_{{{\text{CO}}_{{2}} }} } \right)}}\) is 0.790, as shown in No. 14 system in Table 2 and the uppermost curve of CO reduction in Fig. 2, namely reaction (25).
If the temperature of the system decreases, the reduction process of iron oxide by CO will move along the top curve in Fig. 2. This thermodynamic equilibrium movement phenomenon has certain practical significance for iron oxides in the upper part of the blast furnace.
2.3.2 Minimum energy consumption for carbon reduction of iron oxide in blast furnace
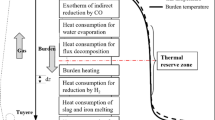
The temperature distribution diagram of the modern blast furnace is shown in Fig. 3. The reduction gas from the bottom of the blast furnace will undergo indirect reduction of iron oxide in the process of rising to the top of the blast furnace. Under the combined effect of direct reduction at the bottom and indirect reduction in the gas rising process, the minimum energy consumption and system gas state of 1 t iron produced by carbon reduction of iron oxide will be discussed in this section.
The thermodynamic equilibrium of the reduction reaction of iron oxide at the bottom temperature of 1600 °C of the blast furnace has been calculated by GMFEM in the theoretical Model-2. The results show that \(\frac{{n_{{{\text{CO}}}} }}{{n_{{{\text{CO}}}} + n_{{{\text{CO}}_{{2}} }} }} \ge 0.790\) is the characteristic of the complete reduction of iron oxide. Taking No. 4 system as an example, Fe2O3 is reduced with 41.10 mol C (493.20 g) to obtain 1 kg Fe, and \({{n_{{{\text{CO}}}} } \mathord{\left/ {\vphantom {{n_{{{\text{CO}}}} } {\left( {n_{{{\text{CO}}}} + n_{{{\text{CO}}_{{2}} }} } \right)}}} \right. \kern-0pt} {\left( {n_{{{\text{CO}}}} + n_{{{\text{CO}}_{{2}} }} } \right)}}\) is 0.799 when 32.84 mol CO and 8.26 mol CO2 are produced.
The indirect reduction reactions would occur at the process of gas rising from the bottom to the top of the blast furnace, when \({{n_{{{\text{CO}}}} } \mathord{\left/ {\vphantom {{n_{{{\text{CO}}}} } {\left( {n_{{{\text{CO}}}} + n_{{{\text{CO}}_{{2}} }} } \right)}}} \right. \kern-0pt} {\left( {n_{{{\text{CO}}}} + n_{{{\text{CO}}_{{2}} }} } \right)}}\) is greater than the curve value of the reaction \({\text{CO}} + {\text{FeO}} = {\text{Fe}} + {\text{CO}}_{{2}}\) at different temperatures. It can be seen from Fig. 2 that the curve value \({{n_{{{\text{CO}}}} } \mathord{\left/ {\vphantom {{n_{{{\text{CO}}}} } {\left( {n_{{{\text{CO}}}} + n_{{{\text{CO}}_{{2}} }} } \right)}}} \right. \kern-0pt} {\left( {n_{{{\text{CO}}}} + n_{{{\text{CO}}_{{2}} }} } \right)}}\) is decreasing gradually from 0.790 at 1600 °C to a smaller value at the lower temperature. The smallest value depends on the temperature at the top of the blast furnace, which is slightly different in the different blast furnaces.
Taking a 5000 m3 blast furnace of a Chinese enterprise as an example, the statistical value of blast furnace gas in June 2014 is shown in Table 3.
It can be seen from Table 3 that the average value of \({{n_{{{\text{CO}}}} } \mathord{\left/ {\vphantom {{n_{{{\text{CO}}}} } {\left( {n_{{{\text{CO}}}} + n_{{{\text{CO}}_{{2}} }} } \right)}}} \right. \kern-0pt} {\left( {n_{{{\text{CO}}}} + n_{{{\text{CO}}_{{2}} }} } \right)}}\) of blast furnace gas is 52.38%, and the corresponding temperature in Fig. 2 is 600 °C. It can be considered that the temperature at the top of the blast furnace is 600 °C. \({{n_{{{\text{CO}}}} } \mathord{\left/ {\vphantom {{n_{{{\text{CO}}}} } {\left( {n_{{{\text{CO}}}} + n_{{{\text{CO}}_{{2}} }} } \right)}}} \right. \kern-0pt} {\left( {n_{{{\text{CO}}}} + n_{{{\text{CO}}_{{2}} }} } \right)}}\) is 0.79 at 1600 °C at the bottom of the blast furnace and decreases to 0.52 at 600 °C at the top. How much iron can be produced by indirect reduction?
The indirect reduction reactions are calculated in Eq. (26).
Under the condition of indirect reduction, the amount of CO required for the reduction of 1000 g Fe is 0.60 m3 or 26.86 mol, as shown in Eqs. (27) and (28), and its heat effect is − 168.79 kJ, as shown in Eq. (29). It can be seen that the above indirect reduction reaction is a slightly exothermic reaction, which shows that there is no need to supplement energy in the indirect reduction process of CO.
Or
Or
The excessive reduction capacity of the gas of the blast furnace is defined as the amount of CO participating in indirect reduction reaction in the process from the bottom to the top of the blast furnace gas. At the top of the blast furnace at 600 °C, the CO ratio is shown in Eq. (30), so that the excessive reduction capacity of the gas can be expressed in Eq. (31).
Taking No. 4 system as an example, the indirect reduced iron produced by the excess reduction gas is 0.43 kg, as shown in Eq. (32). Thus, the total amount of iron obtained in No. 4 system is 1.43 kg, which is the sum of 1 kg Fe from 1600 °C reduction and 0.43 kg Fe from the indirect reduction in the temperature decreasing process from 1600 to 600 °C. In other words, 41.1 mol C (493.2 g) can be used to obtain 1.43 kg Fe, namely the carbon consumption per ton of iron is changed from 493.2 to 344.90 kg, and the composition and amount of CO and CO2 in the top gas are also changed accordingly.
In addition, it can be seen that the indirect reduction ratio (ηindirect) is 0.3, as shown in Eq. (33) and the direct reduction ratio (ηdirect) is 0.7, as shown in Eq. (34).
Similarly, the calculation results of the other 24 systems “direct reduction at furnace bottom + indirect reduction during gas rising process” are listed in Table 4. WC,d, WFe,i and WFe,all show the carbon consumption from direct reduction at the bottom of the blast furnace, iron from indirect reduction and total iron from direct reduction and indirect reduction, respectively, kg. VCO and \(V_{\text {CO}_2}\) show the standard state volume amount of CO and CO2 formed at the bottom per ton of iron, respectively, m3. VCO,3 and \(V_{\text {CO}_2,}{_3}\) show the standard state volume amount of CO and CO2 formed at the top of the blast furnace per ton of iron, respectively, m3. WC shows the actual consumption of carbon per ton of iron after comprehensive direct reduction and indirect reduction, kg. \(V_{{\text {O}_2,}}{_3}\) and \(V_{{\text {N}_2}}\) show the standard state volume of oxygen and incidental nitrogen when the blast furnace is injected with air, respectively, m3. Columns 12, 13 and 14 in Table 4 show the volume content of CO, CO2 and N2 in the top gas of furnace, respectively. WC,deposited shows the amount of deposited carbon per ton of iron, kg.
It can be seen that except that the reduction of Nos. 1, 2, 3, 8, 10, 12 and 15 systems is not completed, and the comprehensive carbon consumption of No. 7 system is the lowest relatively, which is 332.36 kg/t. For Nos. 4, 5, 6, and 7 systems, under the condition that the amount of oxygen added to the system remains unchanged and the amount of carbon continues to increase, comprehensive carbon consumption decreases. The characteristic of this phenomenon is that the value \({{n_{{{\text{CO}}}} } \mathord{\left/ {\vphantom {{n_{{{\text{CO}}}} } {\left( {n_{{{\text{CO}}}} + n_{{{\text{CO}}_{{2}} }} } \right)}}} \right. \kern-0pt} {\left( {n_{{{\text{CO}}}} + n_{{{\text{CO}}_{{2}} }} } \right)}}\) is the highest in stages, which is 0.881. Thus, when the oxygen content remains the same and the amount of C continues to increase, is it possible to continue to reduce the total carbon consumption?
Based on No. 7 system, Nos. 19–25 systems are designed. It is found that with the increase in the amount of carbon, the carbon consumption per ton of iron continues to decrease. However, when the amount of carbon at the bottom of the blast furnace is 589.2 kg, No. 23 system is exactly 1, and the comprehensive carbon consumption per ton of iron is 314.72 kg. After that, if carbon continues to increase, such as in Nos. 24 and 25 systems, although the \({{n_{{{\text{CO}}}} } \mathord{\left/ {\vphantom {{n_{{{\text{CO}}}} } {\left( {n_{{{\text{CO}}}} + n_{{{\text{CO}}_{{2}} }} } \right)}}} \right. \kern-0pt} {\left( {n_{{{\text{CO}}}} + n_{{{\text{CO}}_{{2}} }} } \right)}}\) value at the bottom of the furnace remains 1, the system will show carbon deposition, that is, the carbon has reached saturation.
In other words, No. 23 system corresponds to the minimum carbon consumption of blast furnace operation, which is 314.73 kg per ton of iron.
Based on the above discussion, it can be concluded that when the reducing agent of iron oxide is carbon, to reach the lowest carbon consumption per ton of iron, it has the following characteristics:
-
(1)
In the gas formed at the bottom of the blast furnace, \({{n_{{{\text{CO}}}} } \mathord{\left/ {\vphantom {{n_{{{\text{CO}}}} } {\left( {n_{{{\text{CO}}}} + n_{{{\text{CO}}_{{2}} }} } \right)}}} \right. \kern-0pt} {\left( {n_{{{\text{CO}}}} + n_{{{\text{CO}}_{{2}} }} } \right)}} = 1\);
-
(2)
Indirect reduction accounted for 46.8% of the total reduction;
-
(3)
The comprehensive carbon consumption per ton of iron is 314.72 kg, and the oxygen required for reduction per ton of iron is 267.79 m3. In the reaction system with the lowest carbon consumption of pure iron, \({{\text{C}} \mathord{\left/ {\vphantom {{\text{C}} {{\text{O}}_{{2}} }}} \right. \kern-0pt} {{\text{O}}_{{2}} }} = 1.175{\text{ kg/m}}^{{3}}\).
3 Reduction principle of hydrogen-rich blast furnace
In the blast furnace, if the iron oxide is reduced by two reductants, C and H, two thermodynamic models can be established, as the reduction of iron oxide by carbon in the previous case. The assumptions of the models are the same as those described above and will not be repeated here.
3.1 Thermodynamic model of reduction of iron oxide by carbon and hydrogen based on stoichiometric relationship (Model-1′)
For the co-reduction of iron oxide by carbon and hydrogen, the thermodynamic Model-1′ can be described in Fig. 4.
In the system, the components are Fe2O3, H2, H2O, CO, CO2, O2, Fe and C, and the quantity is \(M = 8\); the elements are Fe, C, O and H, and the quantity is \(m = 4\). The number of independent chemical reactions in this system is \(r = M - m = 8 - 4 = 4\).
In thermodynamic theory, only the following four independent chemical reactions are required to describe the reduction of iron oxide by carbon and hydrogen, as shown in Eqs. (3), (4), (35) and (36).
Reaction (37) can be obtained by combining reactions (3) and (35). Therefore, reactions (4), (36) and (37) can be used to describe the reaction process of the whole system. This is the thermodynamic Model-1′.
For Eq. (37), if 1 kg Fe is obtained by reduction, it can be calculated from the stoichiometric relationship of the reaction:
(1) The amount of reductant carbon required to reduce 1 kg Fe is
(2) The heat to be provided is
For Eq. (36),
If 1 kg Fe is obtained by reduction, it can be calculated from the stoichiometric relationship of the reaction:
(1) The amount of reductant H2 required to reduce 1 kg Fe is
(2) The heat to be provided is
These heats are all provided by the complete combustion reaction of carbon as Eq. (43).
According to the above two items, it can be seen that when carbon and hydrogen are used as reductants to reduce 1 kg Fe, respectively, the consumption of reductants and the energies required are very different, that is, the reduction of 1 kg Fe by carbon requires 322.3 g carbon and 4422.56 kJ of heat, while reduction of 1 kg Fe by hydrogen requires only 53.7 g H2 and 878.42 kJ of heat. The heat required for the reduction reaction by carbon and hydrogen is provided by the carbon oxidation reaction. If two reductants jointly reduce Fe2O3 to obtain 1 kg of Fe, setting that the fraction of carbon in the reductants is x, the fraction of hydrogen is 1 − x, and the consumption and output of other materials are calculated based on this fraction.
-
1.
Carbon consumption of each reaction and total carbon participating in reaction in system
-
(1)
The carbon that provides heat for the reduction reaction is divided into two parts:
The carbon that provides heat for carbon reduction is:
$$W_{{{\text{C}},2}} = \frac{4422.56}{{393.52}} \times 12x = 134.86x{\text{ g}}$$(44)The carbon that provides heat for hydrogen reduction is:
$$W_{{{\text{H}}_{2} ,2}} = \frac{878.42}{{393.52}} \times 12(1 - x) = 26.78(1 - x){\text{ g}}$$(45)It should be noted that if all are reduced by hydrogen (\(x = 0\)), \(W_{{{\text{H}}_{2} ,2}} = 26.78{\text{ g}}\), and the total carbon required for heating is \(W_{{\text{C}}} = W_{{{\text{C}},2}} + W_{{{\text{H}}_{2} ,2}} = 134.86x + 26.78(1 - x)\).
-
(2)
The carbon consumption for carbon reduction is WC,1 = 322.3x g.
Thus, the total carbon consumption is \(w_{{\text{C}}} = (322.3 + 134.86)x + (1 - x)26.78,{\text{ g}}\) or \(\sum {n_{\text C} = \frac{{322.3 + 134.86}}{{12}}x + \frac{{26.78}}{{12}}(1 - x) = 38.1x + 2.23(1 - x),{\text{ mol}}}.\)
-
(1)
-
(2)
Oxygen consumption and total oxygen in system
-
(1)
The oxygen that provides heat for carbon reduction is:
$$V_{{{\text{O}}_{2} ,{\text{C}}}} = \frac{134.86}{{12}} \times 0.0224x = 0.252x{\text{ m}}^{{3}}$$(46)$${\text{Or}}\;n_{{\text{O, C}}} = \frac{0.252x}{{0.0224}} \times 2 = 22.5x{\text{ mol}}$$(47) -
(2)
The oxygen that provides heat for hydrogen reduction is:
$$V_{{{\text{O}}_{2} ,{\text{H}}_{2} }} = \frac{26.78}{{12}} \times 0.0224(1 - x) = 0.05(1 - x){\text{ m}}^{{3}} $$(48)$${\text{Or}}\;n_{{\text {O,H}_{2} }} = \frac{{0.05(1 - x)}}{{0.0224}} \times 2 = 4.46(1 - x)\;{\text{mol}}$$(49) -
(3)
Oxygen from Fe2O3 reduced to 1 kg Fe
$$n_{{\text O,{\text{Fe}}_{2} {\text{O}}_{3} }} = \frac{1}{{0.05585}} \times 1.5 = 26.86{\text{ mol}}$$(50)The total oxygen in the system is
$$\sum {n_{{\text{O}}} = 26.86 + 22.5x + 4.46(1 - x)}{\text{ mol}}$$(51)
-
3.
Hydrogen consumption and total hydrogen in system
The hydrogen required for reduction is the total hydrogen in the system:
$$V_{{{\text{H}}_{2} }} = \frac{53.7}{2} \times 0.0224(1 - x) = 0.6(1 - x){\text{ m}}^{{3}} $$(52)$${\text{Or}}\;\sum {n_{{\text{H}}} = \frac{53.7}{2} \times 2(1 - x) = 53.7(1 - x){\text{ mol}}}$$(53) -
4.
Thermodynamic model of material balance equation. The total Fe in the system is 1 kg or 17.9 mol. Thus, the thermodynamic model-2′ of the material balance equation of Fe, O, C and H in the carbon and hydrogen co-reduction system based on the Gibbs free energy minimization principle is obtained, as shown in Eqs. (54) and (55).
$$\min G = \sum\limits_{{i = 1}}^{M} {n_{i} G_{i} } = \sum\limits_{{i = 1}}^{M} {n_{i} } (G_{i}^{{\ominus }} + RT\ln a_{i} )= \quad n_{\text C} G_{{\text{C}}}^{{\ominus }} + n_{{{\text{Fe}}_{{\text{2}}} {\text{O}}_{{\text{3}}} }} \times G_{{{\text{Fe}}_{{\text{2}}} {\text{O}}_{{\text{3}}} }}^{{\ominus }} + n_{{{\text{Fe}}}} \times G_{{{\text{Fe}}}}^{{\ominus }} + \quad n_{{{\text{FeO}}}} \times G_{{{\text{FeO}}}}^{{\ominus }} + n_{{{\text{Fe}}_{{\text{3}}} {\text{O}}_{{\text{4}}} }} \times G_{{{\text{Fe}}_{{\text{3}}} {\text{O}}_{{\text{4}}} }}^{{\ominus }}+ \quad n_{{{\text{CO}}}} \times (G_{{{\text{CO}}}}^{{\ominus }} + RT\ln \frac{{n_{{{\text{CO}}}} \times p_{{{\text{total}}}} }}{{n_{{{\text{H}}_{{\text{2}}} }} + n_{{{\text{H}}_{{\text{2}}} {\text{O}}}} {\text{ + }}n_{{{\text{CO}}}} + n_{{{\text{CO}}_{{\text{2}}} }} }})+ \quad n_{{{\text{CO}}_{{\text{2}}} }} \times (G_{{{\text{CO}}_{{\text{2}}} }}^{{\ominus }} + RT\ln \frac{{n_{{{\text{CO}}_{{\text{2}}} }} \times p_{{{\text{total}}}} }}{{n_{{{\text{H}}_{{\text{2}}} }} + n_{{{\text{H}}_{{\text{2}}} {\text{O}}}} {\text{ + }}n_{{{\text{CO}}}} + n_{{{\text{CO}}_{{\text{2}}} }} }})+ \quad n_{{{\text{H}}_{{\text{2}}} {\text{O}}}} \times (G_{{{\text{H}}_{{\text{2}}} {\text{O}}}}^{{\ominus }} + RT\ln \frac{{n_{{{\text{H}}_{{\text{2}}} {\text{O}}}} \times p_{{{\text{total}}}} }}{{n_{{{\text{H}}_{{\text{2}}} }} + n_{{{\text{H}}_{{\text{2}}} {\text{O}}}} {\text{ + }}n_{{{\text{CO}}}} + n_{{{\text{CO}}_{{\text{2}}} }} }}) {\text{ + }} \quad n_{{{\text{H}}_{{\text{2}}} }} \times (G_{{{\text{H}}_{{\text{2}}} }}^{{\ominus }} + RT\ln \frac{{n_{{{\text{H}}_{{\text{2}}} }} \times p_{{{\text{total}}}} }}{{n_{{{\text{H}}_{{\text{2}}} }} + n_{{{\text{H}}_{{\text{2}}} }} {\text{ + }}n_{{{\text{CO}}}} + n_{{{\text{CO}}_{{\text{2}}} }} }}) $$(54)$$\begin{gathered} 2n_{{{\text{Fe}}_{{2}} {\text{O}}_{{3}} }} + n_{{{\text{Fe}}}} + n_{{{\text{FeO}}}} + 3n_{{{\text{Fe}}_{{3}} {\text{O}}_{{4}} }} = 17.9 \hfill \\ \!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!3n_{{{\text{Fe}}_{{2}} {\text{O}}_{{3}} }} + n_{{{\text{FeO}}}} + 4n_{{{\text{Fe}}_{{3}} {\text{O}}_{{4}} }} + n_{{{\text{CO}}}} + 2n_{{{\text{CO}}_{{2}} }} = \\ 26.86 + 22.48x + 4.46(1 - x) \hfill \\ n_{{{\text{CO}}}} + n_{{{\text{CO}}_{{2}} }} + n_{{\text{C}}} = {26.86x + 11.24} x + 2.23(1 - x) \hfill \\ 2n_{{{\text{H}}_{2} }} + 2n_{{{\text{H}}_{2} {\text{O}}}} = 53.7(1 - x) \hfill \\ \end{gathered}$$(55)
3.2 Thermodynamic model of reduction of iron oxide by carbon and hydrogen based on Gibbs minimum free energy method (Model-2′)
In the system of reduction of iron oxide by carbon and hydrogen, the amount of carbon and hydrogen required for the reduction to 1 kg Fe can be obtained by setting the reduction fraction x of carbon and the total amount of each element of C, O, Fe and H in the system. Without losing generality, assuming that the reduction ratio borne by carbon is \(x = 0.8\), the initial conditions are substituted into Eq. (55), and the calculation results of various cases can be calculated according to thermodynamic Model-2′. A total of 23 calculation systems are designed, as shown in Tables 5 and 6.
In Table 5, \(n_{{{\text{O}},{\text{Fe}}_{2} {\text{O}}_{3} }}\) is the amount of oxygen from iron oxide; \(n_{{{\text{O}},{\text{ Heat - C}}}}\) and \(n_{{{\text{O}},{\text{ Heat - H}}_{{2}} }}\) are the amount of oxygen consumed to provide heat for the reduction by carbon and hydrogen, respectively; \(\sum {n_{{\text{O}}} }\) is the total amount of oxygen in the system; \(n_{{{\text{FeO}}}}\) and \(n_{{{\text{Fe}}}}\) are the amount of FeO and the amount of Fe obtained by reduction at the equilibrium of the system, respectively; \(n_{{\text{C, reduc}}}\), \(n_{{{\text{C}},{\text{ Heat - C}}}}\), \(n_{{{\text{C}},{\text{ Heat - H}}_{{2}} }}\) and \(\sum {n_{{\text{C}}} }\) are the amount of carbon consumed as a reductant, the carbon that provides heat for carbon reduction, the carbon that provides heat for hydrogen reduction, and the total carbon in the system, respectively; and \(\sum {n_{{\text{H}}} }\) is the total amount of hydrogen in the system.
In Table 6, \(n_{{{\text{CO}}}}\), \(n_{{{\text{CO}}_{2} }}\), \(n_{{{\text{H}}_{2} }}\) and \(n_{{{\text{H}}_{2} {\text{O}}}}\) are the amounts of CO, CO2, H2, and H2O at the equilibrium of each reaction in the system, respectively; and \(V_{{{\text{H}}_{{2}} }}\) is the total amount of H2 required to reduce 1 ton of iron at 1873 K.
3.3 Analysis and discussion
3.3.1 Analysis of reduction at bottom of blast furnace
For No. 1 system, the total amounts of C, O, H and Fe in the system are set according to the calculation results of theoretical Model-1, i.e., stoichiometry, and then this condition is substituted into thermodynamic Model-2 for calculation. The results show that the reduction of iron oxide is only 71.56%, and the remaining 5.37 mol exists in the form of FeO.
Starting from No. 1 system, the conditions for the completion of iron oxide reduction are studied from the following two paths.
Based on No. 1 system, the amount of reductant hydrogen is fixed at 10.74 mol. When the amount of reductant carbon increases from 21.49 mol of No. 1 system to 26.29 mol of No. 7 system, it can be seen from the calculation results of Nos. 1–7 systems in Table 5 that iron oxide will be completely reduced at this time. In the calculation results of Nos. 1–7 systems, \(\frac{{n_{{{\text{H}}_{{2}} }} }}{{n_{{{\text{H}}_{{2}} }} + n_{{{\text{H}}_{{2}} {\text{O}}}} }}\) is always 0.47, indicating that the reduction capacity of H2 is greater than that of CO at high temperatures, and H2 reduction reaction reaches equilibrium. When the carbon increases to 26.29 mol of No. 7 system, the value \(\frac{{n_{{{\text{H}}_{{2}} }} }}{{n_{{{\text{H}}_{{2}} }} + n_{{{\text{H}}_{{2}} {\text{O}}}} }}\) is 0.797, and FeO in the system is completely reduced to 1 kg iron (17.9 mol). It can be seen from the fork curve as shown in Fig. 5 that the value \(\frac{{n_{{{\text{H}}_{{2}} }} }}{{n_{{{\text{H}}_{{2}} }} + n_{{{\text{H}}_{{2}} {\text{O}}}} }}\) of 0.797 is the characteristic of CO and CO2 when FeO in the system is completely reduced.
Equilibrium diagram of iron oxide reduction by CO and H2 [5]
Another path is that on the basis of No. 1 system, when the amount of reductant carbon is fixed at 21.49 mol, and the amount of reductant hydrogen increases from 10.74 mol of No. 1 system to 31.74 mol of No. 14 system, the iron oxide can be completely reduced, as shown in the calculation results of Nos. 1 and 8–14 systems in Table 5. When the reductant hydrogen increases continuously, the value of \(\frac{{n_{{{\text{CO}}}} }}{{n_{{{\text{CO}}}} + n_{{{\text{CO}}_{{2}} }} }}\) is always the non-equilibrium value of 0.787, and the value of \(\frac{{n_{{{\text{H}}_{{2}} }} }}{{n_{{{\text{H}}_{{2}} }} + n_{{{\text{H}}_{{2}} {\text{O}}}} }}\) is also unchanged, which is always the equilibrium value of 0.47. However, at this time, the amount of unreduced FeO continues to decrease, and the amount of Fe continues to increase. When the reductant hydrogen increased from 10.74 to 30.74 mol, FeO was completely reduced.
It can be seen from Table 6 that when the reductant hydrogen in the system continues to increase, the amount of \(n_{{{\text{CO}}}}\) and \(n_{{{\text{CO}}_{{2}} }}\) in Nos. 8–14 systems remains unchanged, while the amount of \(n_{{{\text{H}}_{{2}} }}\) and \(n_{{{\text{H}}_{{2}} {\text{O}}}}\) continues to increase, but the value \(\frac{{n_{{{\text{H}}_{{2}} }} }}{{n_{{{\text{H}}_{{2}} }} + n_{{{\text{H}}_{{2}} {\text{O}}}} }}\) remains unchanged, indicating that the indirect reduction process dominated by H2 has been moving in equilibrium under the condition of the value \(\frac{{n_{{{\text{H}}_{{2}} }} }}{{n_{{{\text{H}}_{{2}} }} + n_{{{\text{H}}_{{2}} {\text{O}}}} }}\) of 0.47 at 1600 °C, and leads the reduction process until FeO is completely reduced to Fe.
3.3.2 Variation of gas composition for continuing to increase carbon after complete reduction of iron oxides at bottom of blast furnace
From the carbon increasing path of Nos. 1–7 systems and the hydrogen increasing path of Nos. 8–14 systems, no matter carbon and hydrogen reducing agent is added, iron oxide can be completely reduced. If the iron oxides are completely reduced, what effect does the addition of carbon or hydrogen in the system have on the gas composition formed at the bottom of the blast furnace? How will the characteristics of the indirect reduction reactions that occur with these gases change during the ascent? What is the most reasonable hydrogen addition amount in the blast furnace? And on what basis? What is the minimum energy consumption for carbon and hydrogen reduction? All these questions need to be answered by the calculation of thermodynamic Model-2′.
From the calculation of No. 14 system, it can be inferred that on the premise that the hydrogen-dominated indirect reduction of iron oxides has been completed, the total amount of reducing agent element hydrogen in the fixed system is 30.74 mol, and the amount of reducing agent carbon is increased from 21.49 to 31.69 mol. It can be found that the ratios of \(\frac{{n_{{{\text{CO}}}} }}{{n_{{{\text{CO}}}} + n_{{{\text{CO}}_{{2}} }} }}\) and \(\frac{{n_{{{\text{H}}_{{2}} }} }}{{n_{{{\text{H}}_{{2}} }} + n_{{{\text{H}}_{{2}} {\text{O}}}} }}\) increase simultaneously.
It can be seen from Figs. 5 and 6 that under the condition of 1873 K, when the iron oxide is completely reduced, the increased carbon reacts with CO2 and H2O as shown in Eq. (3). The vertical coordinates pCO and p\(_{\text{H}_{2}}\) in Fig. 6 represent the relative partial pressures of CO and H2, respectively.
Reaction equilibrium curve of CO2, H2O and C [9]. a Gasification reaction of CO2 and C; b gasification reaction of H2O and C
If there is excess carbon at the temperature greater than 1300 K, CO2 and H2O cannot exist stably and will be completely gasified into CO and H2. This causes the values of \(\frac{{n_{{{\text{CO}}}} }}{{n_{{{\text{CO}}}} + n_{{{\text{CO}}_{{2}} }} }}\) and \(\frac{{n_{{{\text{H}}_{{2}} }} }}{{n_{{{\text{H}}_{{2}} }} + n_{{{\text{H}}_{{2}} {\text{O}}}} }}\) to move away from the equilibrium value of the “fork curve” and move toward 1. When the amount of carbon in No. 23 system increases to 46.13 mol, the values of \(\frac{{n_{{{\text{CO}}}} }}{{n_{{{\text{CO}}}} + n_{{{\text{CO}}_{{2}} }} }}\) and \(\frac{{n_{{{\text{H}}_{{2}} }} }}{{n_{{{\text{H}}_{{2}} }} + n_{{{\text{H}}_{{2}} {\text{O}}}} }}\) are both equal to 1. At this time, the smelting at the bottom of the blast furnace enters a new stage, that is, the carbon is saturated. If carbon is added, the carbon will precipitate from the iron to form carbides.
3.3.3 Excessive reduction capacity of CO and H2 and mechanism of indirect reduction at bottom of blast furnace
Based on the analysis of the reduction process of iron oxides at the bottom of the blast furnace, it can be found that iron can be completely reduced regardless of the path of carbon increase and hydrogen increase, but the composition and excessive reduction capacity of the gas produced by the reduction are different. For different systems, the comprehensive energy consumption after indirect reduction in the bottom reduction and ascending processes, the gas composition and the excessive reduction capacity at the bottom of the blast furnace were calculated respectively, and the results are listed in Table 3.
Tables 5 and 6 are the gas components produced by the reduction reaction at the bottom of the blast furnace at 1600 °C for the different systems designed. For different systems, the values of \(\frac{{n_{{{\text{CO}}}} }}{{n_{{{\text{CO}}}} + n_{{{\text{CO}}_{2} }} }}\) and \(\frac{{n_{{{\text{H}}_{2} }} }}{{n_{{{\text{H}}_{2} }} + n_{{{\text{H}}_{2} {\text{O}}}} }}\) are different. When these gases rise to 600 °C in the upper part of the blast furnace, the characteristics of the system equilibrium after indirect reduction are \(\frac{{n_{{{\text{CO}}}} }}{{n_{{{\text{CO}}}} + n_{{{\text{CO}}_{2} }} }} = 0.52\) and \(\frac{{n_{{{\text{H}}_{2} }} }}{{n_{{{\text{H}}_{2} }} + n_{{{\text{H}}_{2} {\text{O}}}} }} = 0.75\), respectively.
Similar to Eq. (56), the excessive reduction capacity of hydrogen is defined as Eq. (57)
Equations (56) and (57) are the moles of CO and H2 that can be used for reduction by the gas rising from the bottom of the blast furnace (1600 °C) to the top (600 °C), respectively.
The sum of the excessive reduction capacity of CO and H2 represents the comprehensive excess value of the reducing capacity in the bottom system, and the total number of moles of gas that can be used for reduction in the process of the gas rising from the bottom of the blast furnace to the top.
From the stoichiometric relationship of Eq. (58) for the indirect reduction of iron oxides by CO and H2, it can be seen that 1 mol of CO (or H2) is reduced to 2/3 mol (or 0.667 mol) of Fe.
In addition, the comprehensive residual excessive reduction capacity of the gas components at the bottom of the blast furnace of each system in Table 5 can be calculated when the iron oxide is reduced in equilibrium by carbon and hydrogen. However, for different reducing agent increasing paths, the comprehensive excessive reduction capacity changes in different laws, and the energy consumption per ton of steel based on reducing agent is also different, which is manifested in four aspects:
-
(1)
Starting from No. 1 system, the amount of fixed reducing agent hydrogen remains unchanged and gradually increases to the amount of reducing agent carbon in No. 7 system. The iron is completely reduced, and the excessive reduction capacity of CO and H2 are \(\Delta n_{{{\text{CO}}}} = 9.91{\text{ mol}}\) and \(\Delta n_{{{\text{H}}_{{2}} }} = - 1.51{\text{ mol}}\), respectively. The excessive reduction capacity of H2 is always negative, so that the comprehensive excessive reduction capacity is \(\Delta n = 8.41{\text{ mol}}\). When the gas at the bottom of the blast furnace rises to the top, the amount of iron reduced by the excessive reduction is
$$w_{{\text{Fe,g}}} = 8.41 \times \frac{2}{3} \times 0.05585 \times 1000 = 313{\text{ kg}}$$(60)In No. 7 system, under the condition that the bottom of the blast furnace has been reduced to produce 1 ton of iron, 313 kg of iron is obtained through indirect reduction during the process of gas rising from the bottom to the top, and the amount of reduced iron in the blast furnace is increased from 1 to 1.31 t. The consumption of reducing agent carbon per ton of iron is 326.59 kg, and the consumption of hydrogen is 91.63 kg.
-
(2)
For Nos. 8–14 systems, when the amount of reducing agent carbon is kept at 21.49 mol and the amount of reducing agent hydrogen gradually increases from 10.74 to 31.73 mol, 1 ton of iron at the bottom of the blast furnace is completely reduced, but \(\Delta n_{{{\text{CO}}}}\) is always 8.26, and \(\Delta n_{{{\text{H}}_{2} }}\) continues to decrease from − 1.79 to − 4.37, resulting in the reduction in the comprehensive excessive reduction capacity from 6.46 to 3.88, which is lower than 8.40 of No. 7 system in the carburization path. The consumption of carbon reductant per ton of iron in No. 14 system is 324.3 kg, which is 2.29 kg less than that in No. 7 system, but the consumption of hydrogen is 310.64 m3, which is 210.01 m3 more than that in No. 7 system.
-
(3)
On the basis of No. 14 system, when the amount of hydrogen in the reducing agent remained at 31.73 mol, and the carbon in the reducing agent continued to increase from 21.49 to 35.89 mol in No. 22 system, both \(\Delta n_{{{\text{CO}}}}\) and \(\Delta n_{{{\text{H}}_{2} }}\) continued to increase, and the excessive reduction of H2 gradually became positive value, which resulted in a continuous increase in the combined excessive reduction value to 25.32 mol. In terms of total energy consumption per ton of iron, the consumptions of carbon and hydrogen in No. 22 system were reduced to the lowest value (i.e., 279.77 kg/t and 182.85 m3/t). However, at this time, \(\frac{{n_{{{\text{CO}}}} }}{{n_{{{\text{CO}}}} + n_{{{\text{CO}}_{2} }} }} = 0.{996}\) and \(\frac{{n_{{{\text{H}}_{2} }} }}{{n_{{{\text{H}}_{2} }} + n_{{{\text{H}}_{2} {\text{O}}}} }} = 0.{98}\) are close to 1.
-
(4)
When the amount of carbon increased from 35.89 mol in No. 22 system to 36.69 mol in No. 23 system, both \({{n_{{{\text{CO}}}} } \mathord{\left/ {\vphantom {{n_{{{\text{CO}}}} } {\left( {n_{{{\text{CO}}}} + n_{{{\text{CO}}_{{2}} }} } \right)}}} \right. \kern-0pt} {\left( {n_{{{\text{CO}}}} + n_{{{\text{CO}}_{{2}} }} } \right)}}\) and \({{n_{{{\text{H}}_{{2}} }} } \mathord{\left/ {\vphantom {{n_{{{\text{H}}_{{2}} }} } {\left( {n_{{{\text{H}}_{{2}} }} + n_{{{\text{H}}_{{2}} {\text{O}}}} } \right)}}} \right. \kern-0pt} {\left( {n_{{{\text{H}}_{{2}} }} + n_{{{\text{H}}_{{2}} {\text{O}}}} } \right)}}\) increased to 1. The energy consumption structure of 1 t iron changed, and the carbon consumption increased from 279.77 kg/t in No. 22 system to 281.47 kg/t, while hydrogen consumption decreased from 182.85 to 180.77 m3/t. This shows that with the increase in carbon, the total energy consumption of iron oxide reduction is basically unchanged, and the ratio of "carbon/hydrogen" in energy consumption increases.
Based on the above analysis, two important conclusions can be drawn. First, adding hydrogen in the blast furnace will reduce the excessive reduction capacity of the bottom gas, which leads to a decrease in the indirect reduction capacity of the reducing gas in the process of rising from the bottom to the top. Second, if hydrogen is added from the bottom of the blast furnace when the reducing gas rises to the top of the blast furnace, the limit of the hydrogen utilization rate is \({1} - \frac{{n_{{{\text{H}}_{2} }} }}{{n_{{{\text{H}}_{2} }} + n_{{{\text{H}}_{2} {\text{O}}}} }} = 1 - 0.75 = 25\%\), which is also the maximum value of its replacement carbon reduction. This is an important parameter for carbon and hydrogen reduction based on the thermodynamic theoretical model under the background of active research on adding hydrogen to blast furnaces in various countries around the world.
Under the condition of blast furnace air injection, the amount of oxygen used in Table 5 is converted into the amount of air based on the replacement of oxygen by air. The data with unit of mol of gas composition CO, CO2, H2, H2O, and N2 in Tables 6 and 7 are converted to those in the unit of m3 based on 1 t iron obtained by reduction, as shown in Table 8. Then, the volume fraction of each gas component is obtained. V\(_{\text{H}_{2}\text{O}}\), V\(_{{{\text{O}}_{{2}} }}\), and ∑Vall in Table 8 are the gas volume amount of H2O, O2, and all gas when reduction reaction reaches equilibrium at the bottom of the blast furnace under condition of blast furnace air injection, respectively, m3.
It can be seen from Table 8 that in a blast furnace with an upper temperature of 600 °C, when the upper reaction is completed, the concentrations of CO, CO2, H2 and H2O in the system are basically the same, but the amount of N2 is slightly different. The reason is that if the total consumption of carbon in the system is small, the oxygen demand is small, and the nitrogen brought into the blast furnace from the air is small. Therefore, the proportion of N2 in blast furnace gas can be used as an important indicator of blast furnace operation efficiency.
4 Operation evaluation of full carbon blast furnace and hydrogen-rich blast furnace
In Sects. 2 and 3, 48 different systems were simulated for the ironmaking process of full carbon blast furnace and hydrogen-rich blast furnace by using the principle of multivariate and multiphase equilibrium minimum free energy. According to the principle of reaction stoichiometry and heat balance, the material balance constraint for minimum free energy was designed. Under the conditions of the carbon reductant and the carbon and hydrogen composite reductant, the composition of the gas phase produced by the reduction of iron oxides in the lower part of the blast furnace was calculated, and the excessive reduction capacity of the gas at the bottom of the blast furnace was defined. Under the condition that the top temperature of the blast furnace is 600 °C, the indirect reduction of the gas at the bottom of the blast furnace when it rises to the top is calculated. Combined with the bottom reduction and the indirect reduction in the gas rising process, the theoretical minimum energy consumption per ton of iron of full carbon blast furnace and hydrogen-rich blast furnace is 314.72 kg C and 279.77 kg C, 182.85 m3 H2, respectively. The influence of hydrogenation on the energy consumption of blast furnace is obtained from the perspective of thermodynamic theory. According to the theoretical calculation results, the following will comprehensively evaluate the energy consumption and economic benefits of full carbon blast furnace and hydrogen-rich blast furnace.
For the full carbon blast furnace, Nos. 1–3, 8, 10, 12 and 15 systems in Tables 1–4 have not yet reached the reduction limitation, that is, the reduction has not been completed, and the following discussion will not be considered for the time being. In No. 4 system, FeO disappears, and the carbon consumption per ton of iron is 345.63 kg, which is characterized by \(\frac{{n_{{{\text{CO}}}} }}{{n_{{{\text{CO}}}} + n_{{{\text{CO}}_{{2}} }} }} = 0.797\). The corresponding utilization rate of CO is \(0.797 - \left( {\frac{{n_{{{\text{CO}}}} }}{{n_{{{\text{CO}}}} + n_{{{\text{CO}}_{{2}} }} }}} \right)_{{\text{furnace top}}} = 0.797 - 0.520 = 27.7\%\) Under the condition that the total oxygen content of the system remains unchanged, the carbon consumption was decreased with the increase in the carbon content in the system. When the carbon content increased to No. 23 system from No. 4 system, the carbon consumption per ton of iron decreased to 314.72 kg, which is characterized by \(\frac{{n_{{{\text{CO}}}} }}{{n_{{{\text{CO}}}} + n_{{{\text{CO}}_{{2}} }} }} = 1\). And then, as the carbon content in the system continues to increase, the value \(\frac{{n_{{{\text{CO}}}} }}{{n_{{{\text{CO}}}} + n_{{{\text{CO}}_{{2}} }} }}\) is equal to 1, indicating that carbon cannot be added effectively, and the added carbon is manifested as carbon precipitation. This shows that in the process of the gas rising from the bottom of the blast furnace to the top, the maximum utilization rate of CO can be increased to 48%, and the energy consumption per ton of iron can be as low as 314.72 kg.
The completion of the reduction reaction at the bottom of the hydrogen-rich blast furnace should satisfy the conditions of \(\frac{{n_{{{\text{CO}}}} }}{{n_{{{\text{CO}}}} + n_{{{\text{CO}}_{{2}} }} }} = 0.797\) and \(\frac{{n_{{{\text{H}}_{{2}} }} }}{{n_{{{\text{H}}_{{2}} }} + n_{{{\text{H}}_{{2}} {\text{O}}}} }} = 0.460\). In the progress of the gas rising from the bottom of the blast furnace to the top, due to \(\left( {\frac{{n_{{{\text{CO}}}} }}{{n_{{{\text{CO}}}} + n_{{{\text{CO}}_{{2}} }} }}} \right)_{{\text{furnace top}}} = 0.520\) and \(\left( {\frac{{n_{{{\text{H}}_{{2}} }} }}{{n_{{{\text{H}}_{{2}} }} + n_{{{\text{H}}_{{2}} {\text{O}}}} }}} \right)_{{\text{furnace top}}} = 0.750\), the utilization rates of CO and H2 are \(0.797 - 0.520 = 27.7\%\) and \(0.460 - 0.750 = - 29.0\%\), respectively.
The two types of blast furnaces are evaluated from the perspective of energy consumption and economy.
(1) Full carbon blast furnace
The theoretical minimum of energy consumption per ton of iron is 314.72 kg C, and the oxygen per ton of iron is 133.9 m3.
(2) 80% C and 20% H2 for hydrogen-rich blast furnace
The theoretical minimum energy consumption is 279.77 kg C and 182.85 m3 H2, respectively, and the oxygen per ton of iron is 108.71 m3.
Under the condition that the reducing agent composition at the bottom of the blast furnace is 80% C and 20% H2, the used amount of H2 per ton of iron is 182.85 m3, and the amount of H2 actually participating in the indirect reduction is only \(182.85 \times 25\% = 45.7{\text{ m}}^{{3}}\). Taking reactions (2) and (3) as an example, the amount of H2 reducing agent required to reduce 1 kg of iron is
The mass of iron reduced by the added H2 is \(\frac{45.7}{{0.6}} = 76.2{\text{ kg}}\).
The above analysis results show that when 182.85 m3 H2 is added in the hydrogen-rich blast furnace with 20% hydrogen reducing agent, only 45.7 m3 H2 participates in the reduction, and the remaining 137.15 m3 H2 enters the blast furnace gas. Meanwhile, the reduction efficiency of carbon per kilogram of carbon is \(\frac{1000 - 76.2}{{279.77}} = 3.3{\text{ kg Fe}}\).
From the comparison results of the economic benefits of the full-carbon reductant blast furnace and the carbon and hydrogen reductant blast furnaces, it can be seen that at the theoretical minimum energy consumption, 182.85 m3 H2 in the hydrogen-rich blast furnace only replaces \(\left( {314.72 - 279.77} \right) = 34.95{\text{ kg C}}\). Thus, it can be seen that hydrogen-rich blast furnace increases reduction costs, unless access to hydrogen is very easy or free.
5 Conclusions
-
1.
Sufficient carbon content at the bottom of the blast furnace is the basic condition to ensure the lowest energy consumption in all-carbon blast furnaces and hydrogen-rich blast furnaces. Under the condition that the bottom temperature is 1600 °C, sufficient excess carbon can make CO2 and H2O have a complete chemical reaction at the bottom of the furnace, and convert them into CO and H2, so as to obtain the maximum excessive reduction capacity of thermodynamics. When the excessive reduction capacity of the reductant based on carbon at the bottom of the blast furnace is at its maximum, the CO utilization rate is 48% and the hydrogen utilization rate is only 25% during the process of the reducing gas rising from the bottom to the top.
-
2.
Theoretically, the minimum energy consumption per ton of Fe during full carbon blast furnace reduction is 314.72 kg C. The minimum consumption of H2 per ton of iron is 182.85 m3 in hydrogen-rich blast furnace which has a ratio of carbon and hydrogen reducing agents of 4/1. If the theoretical minimum energy consumption is considered, compared with the full carbon blast furnace, the hydrogen-rich blast furnace only replaces 34.95 kg C with 182.85 m3 H2.
References
S. Mishra, J. Sustain. Metall. 6 (2020) 541–556.
R.R. Wang, Y.Q. Zhao, A. Babich, D. Senk, X.Y. Fan, J. Clean. Prod. 329 (2021) 129797.
K. Ma, J. Deng, G. Wang, Q. Zhou, J. Xu, Int. J. Hydrogen Energy 46 (2021) 26646–26664.
L. Ren, S. Zhou, T. Peng, X. Ou, Renew. Sustain. Energy Rev. 143 (2021) 110846.
Q.S. Zhu, Chemical Industry and Engineering Progress 41 (2022) 1391–1398.
T.J. Yang, J.L. Zhang, Z.J. Liu, K.J. Li, Ironmaking 40 (2021) No. 4, 1–11.
G. Wang, J.S. Wang, H.B. Zuo, Q.G. Xue, China Metallurgy 29 (2019) No. 10, 1–6.
F.M. Zhang, X.F. Cheng, G.Y. Yin, C.Z. Cao, Ironmaking 40 (2021) No. 5, 1–8.
Z.Y. Chen, Y.X. Qu, C. Zeilstra, J. Stel, J. Sietsma, Y.X. Yang, J. Iron Steel Res. Int. 26 (2019) 1285–1294.
H.T. Wang, W. Zhao, M.S. Chu, C. Feng, Z.G. Liu, J. Tang, J. Iron Steel Res. Int. 24 (2017) 751–769.
M. Abdul Quader, S. Ahmed, S.Z. Dawal, Y. Nukman, Renew. Sustain. Energy Rev. 55 (2016) 537–549.
J.X. Fu, G.H. Tang, R.J. Zhao, W.S. Hwang, J. Iron Steel Res. Int. 21 (2014) 275–281.
X.Y. Zhang, K.X. Jiao, J.L. Zhang, Z.Y. Guo, J. Clean. Prod. 306 (2021) 127259.
Y.R. Liu, Y.S. Shen, Prog. Energy Combust. Sci. 87 (2021) 100952.
C.C. Xu, D.Q. Cang, J. Iron Steel Res. Int. 17 (2010) No. 3, 1–7.
E. Mousa, C. Wang, J. Riesbeck, M. Larsson, Renew. Sustain. Energy Rev. 65 (2016) 1247–1266.
T. Okosun, S. Nielson, C. Zhou, JOM 74 (2022) 1521–1532.
X. Peng, J. Wang, H. Zuo, G. Wang, X. She, Q. Xue, JOM 74 (2022) 860–868.
C. Yilmaz, J. Wendelstorf, T. Turek, J. Clean. Prod. 154 (2017) 488–501.
Z. Fan, S.J. Friedmann, Joule 5 (2021) 829–862.
A. Hasanbeigi, M. Arens, L. Price, Renew. Sustain. Energy Rev. 33 (2014) 645–658.
D. Khasraw, T. Theint Htet, X. Yang, V. Degirmenci, H. Hage, K. Meijer, Z. Li, Fuel 309 (2022) 122210.
B. Li, H.J. Guo, J. Guo, G.Y. Sun, Chinese Journal of Engineering 39 (2017) 1653–1660.
G. Sun, B. Li, H. Guo, W. Yang, S. Li, J. Guo, Energies 14 (2021) 1999.
H. Guo, Metallurgical Equipment 223 (2015) No. 6, 1–9+59.
V. Shatokha, Int. J. Miner. Metall. Mater. 29 (2022) 1851–1861.
J. Tang, M.S. Chu, F. Li, C. Feng, Z.G. Liu, Y.S. Zhou, Int. J. Miner. Metall. Mater. 27 (2020) 713–723.
B.S. Tian, Xinjiang Iron and Steel (2021) No. 4, 1–3.
Acknowledgements
The author are thankful for the support from the National Natural Science Foundation of China (Nos. U1560203, 51704021, and 51274031) and Beijing Key Laboratory of Special Melting and Preparation of High-End Metal Materials.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no conflict of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, Hj. Physicochemical principles of hydrogen metallurgy in blast furnace. J. Iron Steel Res. Int. 31, 46–63 (2024). https://doi.org/10.1007/s42243-023-01057-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42243-023-01057-6