Abstract
Tissue engineering is nowadays an emerging approach that aims to replace or regenerate diseased or damaged organs with engineered constructs. Considering the key role of growth factors (GFs) in the tissue regeneration process, these biomolecules are considered an important part of the tissue engineering process, so the presence of growth factors in engineered scaffolds can accelerate tissue regeneration by influencing the behavior of cells. Platelet-rich plasma (PRP), as an autologous source of a variety of growth factors, is considered a therapeutic agent for the treatment of degenerative diseases. Regarding its ability to promote the healing process and tissue regeneration, PRP therapy has attracted great attention in bone and cartilage tissue engineering. Incorporating PRP and its derivatives into engineered scaffolds not only bioactivates the scaffold, but the scaffold matrix also acts as a sustained and localized growth factor release system. In addition, the presence of a scaffold can promote the bioactivity of GFs by providing an environment that facilitates their interaction, leading to enhanced effects compared to their free form. This review presents a brief overview of PRP's role in bone and cartilage tissue regeneration with the main focus on scaffold-mediated PRP delivery. In addition, the classification of platelet-rich products, current extraction techniques, terminology, and scaffold bioactivation methods are presented to provide a better understanding of the basics and the key aspects that may affect the effectiveness of therapy in bone and cartilage tissue engineering.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Bone and cartilage tissue, the major load-bearing connective tissues of the human body, are usually injured for various reasons, including trauma, surgeries, and degenerative diseases. Nowadays, millions of patients are suffering from bone and cartilage defects globally, and their quality of life is significantly compromised. Although bone and cartilage are both classified in the orthopedic category, they are pretty different tissues [1]. Bone is the primary constituent of the musculoskeletal system that has a vital role in preserving the form of the body, transmitting the muscular forces, and protecting the bone marrow as well as soft tissues within the cranial, pelvic, and thoracic cavities because it can provide rigidity and hardness due to the high mineralization of its Extracellular Matrix (ECM) [2]. The highly complex structure of bone ECM is comprised of organic and inorganic components. The collagen fibers, mostly type-I collagen, make the organic phase responsible for tissue flexibility. In contrast, the inorganic phase is composed of calcium phosphate, especially Nanocrystalline Hydroxy Apatite (nHAp), and provides mechanical strength and toughness [2, 3]. Furthermore, four cell types are supported by bone ECM to contribute to osteogenesis, including osteoblasts, osteocytes, bone lining cells, and osteoclasts [3]. Bone tissue is also highly vascularized, which is necessary for the distribution of nutrients and oxygen, as well as the removal of waste products [4]. On the other hand, cartilage is an elastic and strong avascular connective tissue found in several organs such as the nose, ear, and rib cage that performs critical biomechanical functions, including wear resistance, load bearing, and shock absorption [5]. The ECM of human cartilage is mainly composed of proteoglycans, which include a core protein with covalently linked Glycosaminoglycans (GAGs) and a collagenous network. Collagen fibers are responsible for tensile strength and shear stress tolerance, whereas GAGs contribute to the cartilage's capacity to resist compressive loads [2]. According to the collagen/GAG compositions in the ECM, different cartilage types with various mechanical properties exist. Fibrous cartilage, for example, is rich in collagenous fibers (both type I and II collagens), but hyaline cartilage has a higher content of GAGs and a lower content of collagen fibers (mainly type II collagen) [2, 3]The interface between articular cartilage and bone, known as osteochondral tissue, exhibits gradual gradients in multiple properties including structure, biochemistry, mechanics, electrical conductivity, and metabolism, ranging from the stiff and dense characteristics of bone to the more flexible and gel-like properties of cartilage [6, 7]. Articular cartilage, calcified cartilage, and subchondral bone are the three major layers of this gradient tissue [8, 9]. For example, the porosity, pore size, collagen, water concentration, and compressive modulus vary over the full thickness spanning the osseous tissue to the cartilage region [2, 8, 10].
In general, considering the vital functions of bone and cartilage, efficient treatment of their defects is still a challenging issue clinically [2]. Human bones have exceptional regenerative properties because of their outstanding dynamic structure, so most bone injuries, such as fractures, defects, and local necrosis, can be treated routinely. However, this ability is ineffective in repairing significant bone defects (with a critical size limit of 2.5 cm) caused by fractures, traffic accidents, and bone tumor resection. In these cases, it is essential to induce and support osteogenesis to repair the defect. Current bone therapies (autografts and allografts) have drawbacks such as limited bone mass, difficulties at the donor site, infection, and immunological rejection, restricting their clinical applications [11,12,13]. In cartilage tissue, wound healing is inhibited because it is avascular, and nutrients and cell infiltration required for tissue repair cannot reach the lesion. Autografting, microfractures, and Autologous Chondrocyte Implantation (ACI) are all current treatments for facilitating cartilage regeneration; nevertheless, they all have disadvantages and are unable to repair functional hyaline cartilage, making long-term prognosis unclear. If healing occurs, it frequently results in the development of fibrous cartilage, which causes stiffer tissue at the injury site and long-term performance problems. On the other hand, osteochondral defects are extremely difficult to treat due to the highly different characteristics of subchondral bone, calcified cartilage, and articular cartilage [13, 14]. In the face of the staggering growth in demand for regenerative solutions for bone and cartilage, scientists have developed new areas to meet the needs of patients.
Nowadays, the tissue engineering approach has attracted extensive attention in the field of tissue regeneration [11]. Its goal is to develop 3D scaffolds that mimic the natural tissue, working as a porous structure for the growth, migration, and adhesion of cells to replace the injured tissue [15, 16]. Several requirements must be met by an ideal scaffold, including well-mimicking the natural environment of ECM, biocompatibility, especially after degradation, good mechanical characteristics in loadbearing conditions, and optimal structure for nutrients and gas diffusion [17]. A thorough understanding of the composition and structure of the tissue leads to the fabrication of a superior scaffold to solve the clinical challenges of bone and cartilage defects [18]. Developing scaffolds with high mechanical support, biocompatibility, biodegradability, and osteoinductive characteristics is the basis for bone and cartilage tissue engineering [1, 2]. Ceramics, metals, and polymers have been used to substitute natural tissue at injury sites [15]. All of these biomaterials have advantages and disadvantages, allowing researchers to choose one or a combination of them based on their application to fabricate a suitable scaffold for injured tissue healing [11, 19, 20].
In the field of tissue engineering, both biological factors and physical characteristics of scaffolds can contribute to improved tissue regeneration. While the natural healing process is complicated and differs between bone and cartilage, understanding this basic biology is essential for biomaterial design. To improve the bioactivity of scaffolds, bioactive materials, functional groups, proteins, and peptides must be included on the surface or bulk of the scaffolds. The tissue engineering scaffold could function as a delivery agent and a cell-anchoring entity or contribute to cell colonization, adhesion, differentiation, and proliferation by incorporating biological agents such as cells, growth factors (GFs), and drugs [1, 15, 21].
The use of GFs has received considerable clinical interest among biochemical approaches. GFs are important polypeptide molecules that facilitate cell-ECM interaction and send particular messages to a subset of cells. By binding to cell surface receptors and activating signaling pathways, GFs, which are secreted by a fraction of cells, can up-regulate or down-regulate cellular activities (adhesion, proliferation, and differentiation) [22]. Because GFs have been demonstrated to be present during tissue development and regeneration, their controlled release from scaffolds is a common approach for improving tissue repair. The direct use of GFs on their own or in scaffolds has a variety of challenges, including the rate of release, appropriate dosage, half-life, stability, cost, and long-term side effects. Therefore, despite successful in-vitro results, only a few studies have proceeded to clinical trials. Different GFs are involved in the regeneration of diverse tissues. Several studies have shown the perspective of GFs utilization in bone and cartilage tissue engineering. For example, bone growth can be stimulated by the interaction of Bone Morphogenetic Protein-2 (BMP-2) or Bone Morphogenetic Protein-7 (BMP-7) with osteoblast precursors. Furthermore, Transforming GF (TGF) or Connective Tissue GF (CTGF) can stimulate cartilage development, while Vascular Endothelial GF (VEGF) promotes vascular or neural formation [21, 23].
Platelet-rich plasma (PRP) serves as a biological material that is harvested from autologous Whole Blood (WB) and enriched in GFs such as Platelet-derived GF (PDGF), Transforming GF β1 (TGF-β1) and Transforming GF β2 (TGF-β2). The high content of GFs and their autologous source make them a therapeutic agent for the treatment of degenerative diseases [24]. Nowadays, regarding its ability to promote wound healing and tissue regeneration, PRP therapy has attracted great attention in various biomedical fields, including orthopedic and plastic surgery, and dermatology [25, 26]. Incorporation of PRP as a source of GFs into the tissue engineering scaffolds may be advantageous. In addition, PRP-activated scaffolds may serve as a localized system for GF sustained delivery. The publication record (Fig. 1) indicates that the volume of research carried out on the use of PRP in cartilage and bone tissue engineering has increased since 2012. For instance, the quantity of scientific studies published in 2021 pertaining to cartilage tissue engineering and bone tissue engineering has exhibited an approximately twofold increase in comparison to the number of publications recorded in 2012.
This review presents a brief overview of PRP role in bone and cartilage tissue regeneration with the main focus on scaffold-mediated PRP therapies.
2 Introduction to PRP
PRP refers to the term used for platelet concentrates in hematology that attempts to take advantage of PRP’s functional properties for the treatment of severe thrombopenia [27]. This term was first used by Kingsley et al., who worked on blood coagulation disorders in patients who suffer from congenital factor V deficiency [27, 28]. Some attempts were reported by Owren et al. to recognize the plasma-containing factors affecting blood coagulation (factors V and VI) [28]. The first PRP investigations were conducted on the components of the blood coagulation process.
The idea of topical use of platelet concentrate was announced by Matras in 1970 [29]. Schulz et al. studied the effect of various fibrin preparations on reimplantation in rat skin, which indicated that PRP acts as an excellent adhesive and hemostatic agent [30]. After the first publications by Matras in the early 1970s, the application of autologous platelet-fibrinogen-thrombin mixtures was evaluated in different fields of medicine, such as ophthalmology [27]. Rosenthal et al. used this biological mixture as a corneal adhesive for sutureless lamellar keratoplasty in the rabbit [31], and then they utilized the same mixture as a sealant for experimental penetrating corneal wounds [32]. In the field of surgery and neurosurgery, this mixture was used as a sealant-adhesive for microvascular anastomosis [33] and a physiological sealant for cerebrospinal fluid leakages, respectively [34]. Two years later, Fischer et al. investigated the application of gelatin platelets (gel foam) to be a part of a suture-free method of anastomosis for nerve transplantation. In a way, the gelatin platelet is formed like a tube and wrapped around the ends of the anastomosis to prevent suture-induced reactions in the vicinity of the anastomosis [35].
The first clinical report about the wound-healing potential of PRP was reported by Knighton et al. in 1986 [27]. They studied the treatments of nonhealing chronic wounds with autologous Platelet-derived Wound Healing Factors (PDWHF) and achieved acceptable results, indicating PDWHF potential in the acceleration of the healing process [36].
Platelet concentrate-based technologies were introduced in oral and maxillofacial surgery by Marx et al. in 1998 for bone grafts [27]. This idea was inspired by Tayapongsak et al.’s 1994 study, where they incorporated Autologous Fibrin Adhesive (AFA) with cancellous bone for mandibular continuity reconstructions [37]. The polymerization of blood fibrinogen into a fibrin gel serves as the foundation for both platelet concentrates and fibrin glues [27]. Since the early 1990s, Marx and colleagues have conducted research on the biological effects of GFs within platelets, specifically PDGFs, on enhancing bone graft continuity in the mandible. They have also evaluated the impact of PRP on increasing bone formation rate and improving bone graft integrity and density for up to six months. Gradient density centrifugation was used in their study to isolate and concentrate platelets to obtain PRP as an autologous source of PDGFs. Their results demonstrated that PRP, as a source of GFs, could lead to greater bone density [37].
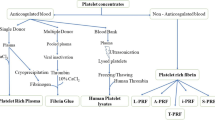
2.1 Classification of Platelet-rich Products
Platelet concentrate products, generally known as PRP or Platelet-rich Fibrin (PRF), are extensively utilized in regenerative medicine and as surgical adjuvants. Due to the lack of a specific manufacturing technique or even an appropriate standard for characterization and classification, there were ambiguities in developing concentrated platelet-based products about 40 years ago [38].
The current classification system of concentrated platelet-based products contains four main categories according to their fibrin architecture and cell content (mostly leukocytes). They are mainly classified as Pure PRP (P-PRP) or Leukocyte-Poor PRP, Leukocyte-and Platelet-Rich Plasma (L-PRP), Pure PRF (P-PRF), or Leukocyte-Poor PRF, and Leukocyte-and PRF (L-PRF) (Fig. 2). P-PRP and L-PRP products are used as an injectable liquid solution for sports lesions or as wound dressing and sutures in an activated gel form. P-PRF and L-PRF products are only employed in a strongly activated gel form and behave like a real solid material [38].
2.2 Extraction Methods
In 1954, when Kingsley, for the first time, used PRP, there was no particular protocol for PRP isolation [28]. German authors released the first report on the method of PRP preparation in the 1970s, focusing on the number of platelets and assessing their function [39]. Knighton et al. [36]employed a procedure that required double centrifugation to prepare PDWHF in 1986. Then, in 1997, Withman et al. prepared PRP with a gradient cell separator, a typical method in hematology labs. The obtained product was referred to as PRP, but it was known that the eventual product would be fibrin gel; hence, platelet gel was the first product of this protocol [40]. RP was also made by Marx et al. using a cell separator [37]. Anitua introduced one of the first PRP preparation procedures in 1999, which involves a single centrifugation stage and many pipetting steps. PRGF contained no leucocytes and had lower platelet and GF content than other products. PRGF gel was produced by activating PRGF using materials such as calcium chloride. Biotechnology Institute (BTI), one of the pioneer companies in platelet concentrate products, developed this cost-effective manual protocol [27, 41].
Nowadays, PRP is obtained from autologous blood by differential centrifugation. In this process, blood constituents are sedimented according to differences in specific gravity, so each layer includes the components with the same density; the lower density is at the top of the centrifugation tube, and the higher density is at the bottom. In general, the PRP preparation process can be done manually using either the “PRP method” or the “buffy-coat approach” [42]. These procedures need two-step centrifugation, but their primary differences are the centrifugation rate and the WB storage temperature prior to the procedure.
In the “PRP method,” blood is collected into a tube containing anti-coagulants such as Acid Citrate Dextrose (ACD). It is important to highlight that blood should not be cooled before or during the procedure. The blood tube is then centrifuged with low forces and constant acceleration to separate erythrocytes from the remaining components of WB, which are sorted into three layers based on their densities (Fig. 3a). The top and middle layers are transferred to an anticoagulant-free tube to prepare PRP. The platelet concentrate is obtained by centrifuging the new tube at a higher speed. After the second centrifugation, PRP is extracted by discarding the top two-thirds of the volume (known as poor-platelet plasma, or PPP) and then resuspending the remaining amount to be homogenized [42].
In the “buffy-coat approach”, a blood sample is taken from the patient and kept between 20 and 24 degrees Celsius. For the first stage, it is centrifuged at high speed. Then, three layers are created (Fig. 3b), similar to the “PRP approach”. To separate leucocytes, the middle layer, also known as the buffy-coat layer, is transferred to another tube and centrifuged at a lower speed. However, leukocyte filters can be employed to separate leukocytes instead of the second centrifuge [42]. The PRP prepared using these procedures (or similar ones) is known as “homemade PRP” [43].
Today, some companies commercialize PRP kits based on centrifugation principles. These commercial kits allow us to make PRP from a small amount of blood (50ml) in a reproducible manner [42]. The kits vary greatly in terms of centrifuge type, time from isolation to consumption, cost of equipment and reagents, and method ergonomy and complexity [41]. Although there are differences between the various kits and methods, it is impossible to determine which one is better because different applications need different concentrations of PRP [42].
2.3 GFs Content
PRP has been known as a rich source of GFs since the 1990s [44]. The existence of PDGF, TGF-b1, and TGF-b2 was proved by Marx in 1998 that have beneficial effects on the enhancement of bone grafts used in oral and maxillofacial surgery [37].
When platelets are activated, they release a large number of different factors and participate in the formation of fibrin scaffolds and, subsequently, fibrin clots [40]. Fibrin clot formation is essential for hemostasis and is one of the key steps in the wound-healing process. At first, fibrin tissue adhesives were the only usage of platelet-rich products (not as healing stimulators). The platelets were only responsible for supporting a stronger fibrin polymerization and, therefore, a more efficient tissue sealing than basic fibrin glues [27].
Platelets could release some GFs, including PDGF (a-b), TGF (α-β), VEGF, Epidermal GF (EGF), Fibroblast GF (FGF), CTGF, and Insulin-like GF - 1 (IGF -1). Each GF plays a special role in the stimulation of chemotaxis, mitogenesis, differentiation of stem cells, angiogenesis, protein secretion/synthesis, and wound healing (Fig. 4) [42].
Effect of PRP on stem cells in bone and cartilage formation [56]
3 Scaffold Bioactivation by PRP
Scaffolds are porous 3D-constructed structures that facilitate cell-biomaterial interactions while also supporting cell survival, proliferation, and differentiation temporarily. While scaffolds degrade and are gradually replaced by native ECM, it is necessary to provide adequate transport of gases, nutrients, and regulatory factors within the structure [45, 46].
A scaffold that promotes cell ingrowth, adhesion, and differentiation can be considered ideal. Utilizing bioactive biomaterials that can facilitate the interaction/adhesion of cells to scaffolds has attracted significant interest in this area [47]. Moreover, physical, and external stimuli (e.g., mechanical or electrical stimulation) and biochemical signaling can be utilized to create a bioactive scaffold. Increasing the bioactivity of the scaffolds enhances cell growth and the scaffold's adhesion to the host tissue and increases the differentiation of immature cells in combination with an improvement in angiogenesis. As a result, bioactivated scaffolds may accelerate tissue regeneration, leading to more functional tissues.
Bioactive compounds are biochemical signals and biological cues that improve cell-scaffold communication and thereby promote scaffold biocompatibility. Bioactive agents, such as binding proteins or peptides, genes, and GFs, have extensive therapeutic applications in a variety of fields, including cancer therapy [48], vascularization, and regenerative medicine [49, 50]. GFs are commonly employed in regenerative medicine as a supplement to the tissue culture media or scaffold, among other biological substances. Additionally, GFs can be injected directly into the lesion site or put within the scaffold; the scaffold complex containing the GFs can subsequently be injected or implanted into the lesion site. The most significant issue with direct injection of GFs is the potential for a short initial burst release, necessitating re-injection. Furthermore, because GFs have a limited half-life, they may lose their activity before interacting with a target cell. In recent years, GF delivery has emerged as one of the most significant Drug Delivery System (DDS) applications in tissue engineering. Providing a sustained pattern of GF release while maintaining their bioactivity in the therapeutic window appears to be challenging. The incorporation of PRP into a tissue engineering scaffold is one of the bioactivation strategies based on GF delivery. Autologous PRP avoids the need for expensive recombinant GFs or animal-derived products that involve a risk of an immune reaction. In contrast to scaffold-mediated PRP delivery, the rapid release and short half-life of the PRP-containing GFs in direct injection of PRP at the lesion site restricts their adequate accessibility for the longer time periods required for tissue repair. Due to the short platelet lifespan of 7 to 10 days, several injections of PRP are required to generate an effective response. In general, PRP products are mechanically unstable, which restricts their use in mechanically injured tissue such as the joints [51]. For instance, to repair cartilage, it is important to release GFs gradually over 30 days at the site of the lesion. Therefore, finding a strategy to improve the retention duration of GFs produced by PRP at the lesion site is necessary. In this approach, the combination of designed scaffolds and PRP may induce a superposition effect in which PRP improves the bioactivity of the scaffold, and the scaffold supports the bioactivity of GF and enables its sustained release through immobilization.
Therefore, pre-encapsulation of PRP into an appropriate carrier under mild conditions may be useful to provide further protection against scaffold manufacturing. Compared to pre-formed scaffolds, injectable hydrogels that are formed in situ could be exploited with less interference or harm for the localized distribution of PRP and its derivatives. The biomaterial's charge is one of the characteristics that affect the encapsulation rate of GFs, which is crucial when selecting a scaffold material. In physiological conditions, GFs have a positive charge; therefore, selecting biomaterials with a negative charge, such as Hyaluronic Acid (HA), is an effective technique for forming ion interactions and controlling the release behavior and GF loading capacity [11].
4 Bone Regeneration via PRP-Delivering Scaffold
Bone loss or injury can arise from diverse factors, including surgical procedures, advanced age, accidents, resection of bone tumors, and other causative agents. Even though bones are capable of self-repair, this ability can fail in cases of extensive damage [52,53,54]. BTE, as a potential alternative to the conventional use of bone grafts, is an innovative technique for regenerating and repairing bone defects that combines material science, engineering principles, and cell biology. BTE consists of a combination of cells, GFs, and an adequate substrate that exhibits osteoinductive and osteoconductive properties. Due to the increasing interest and development of BTE over the years, it focuses on novel therapies that address the limitations of existing grafting materials (i.e., immune rejection, secondary injury, pathogen transfer, and restricted availability) and achieve great regeneration. In this field, which aims to mimic the structure and function of the natural bone ECM, cells, bioactive compounds, and biomaterial scaffolds offer a three-dimensional environment for bone regeneration [55,56,57,58].
PRP and its derivatives have been recently used for many therapeutic purposes and tissue engineering applications [59, 60]. According to the crucial biological function of PRP-containing GFs in cell-ECM interactions during regenerative activity, they are being employed extensively in BTE [61]. GFs such as PDGF, TGF-, and IGF are associated with bone development and regeneration because they contain proteins known to be present during wound healing and to mimic bone healing conditions. These factors are abundant in PRP and can be applied to cells to promote bone repair. In the presence of these stimuli, stem cells are more likely to differentiate into an osteogenic lineage. In addition, PRP minimizes inflammation, promotes superior tissue healing, and minimizes the risk of disease transmission and immunogenic reactions [56].
In addition to delivering important signals as a natural reservoir of GFs in hard and soft tissue regeneration, PRP has been used with natural or synthetic biomaterials for the fabrication of engineered scaffolds to address its inadequacies. It has been claimed that the purpose of employing PRP in such combinations is to promote wound healing, angiogenesis, cell proliferation, migration, and differentiation. Combinations of natural hydrogels, such as HA/PRP for the treatment of osteoarthritis and chitosan/PRP for osteoblast development, have been developed previously [59].
Since the first use of PRP in bone formation and growth, it has been utilized in many forms, such as hydrogels, intra-articular injections, and artificial bone grafts [55]. PRP-derived GFs’ actions are summarized in Table 1 [56].
PRP has been widely used in the repair and regeneration of cartilage and bone as a scaffold and/or as part of a composite [62]. Although several research have been conducted to evaluate the effect of PRP on bone defect regeneration, the results are controversial.
Marx et al. employed PRP for the repair of maxillofacial defects and observed that it accelerated the development of autogenous bone transplants and increased bone density. Further clinical studies indicate an osteogenic potential of PRP but did not include control groups [63].
PRP has been shown to improve the aggregation and cohesiveness of bone substitutes since the PRP’s GFs could provide a nutritive environment to the Mesenchymal Stem Cells (MSCs). In-vitro studies indicate that a concentration of 2–5% PRP is ideal for the osteogenic differentiation of the MSCs, and any concentration below or above can inhibit the osteogenic potential of MSCs. Likewise, an increase in the concentration of MSCs causes a significant enhancement in cell proliferative activity. However, conditions need to be optimized for clinical studies [56].
According to the results of clinical research, even though PRP contains an abundance of GFs that promote bone repair, it is inefficient. The reason for this can be attributed to the fact that these GFs are released almost immediately upon exposure to body fluid, with complete exhaustion of the factors occurring within the first day. Since bone repair occurs over a period of 4 to 6 weeks, the effect of the PRP is insignificant. This necessitates that the PRP be delivered in such a way that the GFs can be delivered sustainably throughout the period of bone regeneration. Consequently, it is essential to distribute PRP via a biodegradable carrier with a controllable degradation rate [56]. In this regard, PRP has been combined with various biomaterials, such as gelatin hydrogels, chitosan, Poly Lactic-co-Glycolic Acid (PLGA) mesh, and tricalcium phosphate scaffolds, to prolong the release of GFs. Different in-vitro and in-vivo studies have revealed that the combination of PRP with these various biomaterials is substantially more effective than pure PRP [64].
In addition to PRP, bone-derived biomaterials such as HAp have been utilized in biomedical applications. HAp, which is the primary mineral component of bone tissue, has been extensively applied with positive results to clinical bone tissue engineering efforts. One clinical study on the repair of induced periapical lesions using HAp scaffolds utilized HAp/PRP, HAp alone, PRP alone, and no intervention as the control group. In this 20-patient trial, full bone regeneration was observed in all treated groups, with the most rapid regeneration occurring in the HAp/PRP group at six months, followed by the PRP and HAp groups at nine months and one year, respectively. Thus, a combinatorial approach accelerates healing, whereas HAp scaffolds and PRP alone result in complete tissue regeneration [65].
Nowadays, the fabrication of PRP-activated scaffolds has attracted increasing attention due to their outstanding potential in bone repair. In a study, a novel therapeutic platform for the treatment of Rheumatoid Arthritis (RA) was obtained by combining Black Phosphorus Nanosheets (BPNs) with PRP/chitosan thermoresponsive hydrogel (Fig. 5). PRP can effectively enhance the adhesion and capability of MSCs on hydrogels. The BPNs/Chitosan/PRP hydrogel greatly reduced the degree of edema in arthritic mice, as shown by the study's findings. Consequently, this multi-component hydrogel based on the unique photothermal properties of BPNs and consequently phosphorus-driven osteogenesis, as well as cell compatibility provided by PRP, opens a new door for treating RA [66].
Effect of PRP on stem cells in bone and cartilage formation [56]
In another study, Wu et al. reported fabricating a scaffold encapsulated with living human Adipose-derived Stem Cells (hASCs) and PRP using a vapor-phase sublimation and deposition procedure. Sterile water was used to produce ice templates for accommodating the cells and PRP during the process. Under regulated processing conditions, water molecules were evaporated by vapor sublimation of ice templates while poly-p-xylylene replaced the templates through the vapor-phase deposition. Parylene served as the matrix of the final scaffold, which was encapsulated with living hASCs and PRP [67].
In addition, Alkaline Phosphatase (ALP) expression at an early stage and calcium mineralization at a later stage demonstrated a considerable increase in osteogenic differentiation of hASCs directed by the PRP (Fig. 6). In contrast, investigations of adipogenic activity by lipid droplet formation revealed inhibition of adipogenesis with decreased intracellular lipid accumulation, and significant downregulation of adipogenic differentiation was observed in comparison to osteogenic results. This fabrication method for scaffolds combines delicate living hASCs with PRP within the structure, which is a straightforward and clean process [67].
Evaluation of the osteogenesis activities scaffolds with and without PRP based on their a early-stage osteogenesis marker ALP expression at day 10 and b mature-stage marker calcium mineralization formation (with Alizarin red staining) at day 21. c Fluorescence images of osteocalcin expression (green channel) at day 21 [67]
There are several BTE studies that employ PRP as a bioactive factor to promote bone regeneration. Table 2 contains their fabrication strategy and their key findings. The utilization of PRP alongside novel carriers and biomaterials provides controlled release of GFs, while new methodologies underscore its adaptability. With its versatile attributes, PRP emerges as a potent tool for advancing bone repair strategies and reshaping the approach of regenerative medicine.
5 Cartilage Regeneration via PRP-Delivering Scaffold
The articular cartilage diseases have long-lasting harmful effects because of the tissue's low potential for spontaneous healing. The current techniques for articular cartilage regeneration are non-surgical, microfracturing, intra-articular injections, and engineered scaffolds with or without cell inclusion. These approaches have not yet produced hyaline cartilage with an organized zonal structure and sufficient mechanical properties. Additionally, these methods for entering the clinic must be accessible and cost-effective [81].
Recently, there has been a growing emphasis on the fabrication of biomaterials and scaffolds from autologous tissues for use in tissue engineering and regenerative medicine. It could prevent the risk of animal disease transmission and foreign body rejection. Despite the limited availability of autologous human tissues, blood-derived sources remain one of the most intriguing. As previously stated, PRP's GFs contribute to tissue repair and wound healing. These GFs bind to certain membrane receptors (such as Tyrosine Kinases (RTKs)) and activate the passive messenger proteins in the cell cytoplasm. The activated proteins then activate the genes for cell division. After mRNA transcription is upregulated, the initiation of cascades promotes tissue repair and regeneration [82].
Exogenous materials, such as autologous PRP can facilitate the self-repairing stimulation of endogenous tissue [81]. PRP is utilized as an autologous source of GFs, cytokines, and proteins to stimulate cell proliferation and induce chondrogenic differentiation, which may repair cartilage defects. Moreover, numerous studies have indicated that PRP has a high potential for cartilage regeneration due to its biocompatibility and efficacy in stimulating chondrogenesis [83].
In-vitro, in-vivo, and preclinical research on articular cartilage tissue engineering have shown that PRP and its derivatives have beneficial and unique features. The presence of PDGF can drive proliferation and collagen synthesis; TGF-β can decrease the catabolic activity of Interleukin-1(IL-1) and boost the proliferation, ECM synthesis, and synthetic activity of chondrocytes, and FGF assists in the activation of several anabolic pathways. Migration of MSCs and human subchondral progenitor cells may be triggered by the co-action of FGF and TGF-β signaling molecules. GFs can enhance the proliferation rate of MSCs regardless of the donor's age and stimulate chondrogenic differentiation of stem cells. In addition, PRP exhibits anti-inflammatory properties because its GFs recruit resident stem cells to the site of injury, where they are induced to release additional GFs and anti-inflammatory cytokines (IL-4, IL-10, and IL-13), resulting in a greater increase in collagen and matrix synthesis, and consequently cartilage regeneration. The inhibitory effect of PRP-containing GFs (e.g., IGF-1) on apoptotic factors may suppress the expression of programmed cell death proteins [82, 84].
Drengk et al. [85] examined the effects of PRP on chondrocyte and MSC proliferation and chondrogenic differentiation. PRP was employed as a potent source of autologous GFs and an autologous scaffold on freshly isolated chondrocytes and MSCs. First, chondrocytes were isolated from pieces of cartilage from the femoral condyle of an adult female sheep, and MSCs were isolated from the sheep's bone marrow. Next, PRP was prepared from the blood that was collected from the ear vein of the anesthetized sheep. Finally, the isolated cells were subjected to a two- or three-dimensional growth system, with or without PRP. They found that PRP had a proliferative effect on chondrocytes in a 3-Dimensional (3D) environment and on MSCs in a monolayer environment. They also noticed that as chondrocyte proliferative activity increased, the chondrogenic phenotype decreased. Although their results were promising, they did not discuss how PRP functions.
Elder et al. [86] used a double centrifugation process to prepare allogenic PRP to investigate the chondrogenesis differentiation capability of three-dimensional PRP hydrogel constructs. Stromal cells from canine bone marrow were encapsulated in the form of beads made from 2% sodium alginate or an alginate/PRP mixture (3:1 mixture of freshly prepared PRP and 2% alginate). The prepared beads were then cultured in a chemically defined chondrogenic medium with and without TGF-β3. The results indicated that the proliferative effect of PRP in the absence of TGF-β3 was minimal. However, compared to alginate beads, alginate/PRP beads exhibited greater chondrogenesis potential. The addition of TGF-β3 substantially increased the chondrogenic potential of alginate/PRP beads.
Liou et al. [24] conducted a study on the chondroinductive activities of PRP on adult human MSCs derived from Infrapatellar Fat Pad Adipose Stem Cells (IFP-ASCs) and Bone Marrow (BM-MSCs). Their findings indicated that increased PRP concentration and exposure time could hinder chondrogenesis. Although PRP is helpful as a joint pain reliever, its mechanism of action is not believed to directly involve the stimulation of MSC-mediated hyaline cartilage formation; instead, its effect on other proliferative and differentiating factors may aid in cartilage regeneration.
In recent years, lab and clinical interest have increased in PRP-activated scaffolds. Scaffolds developed for cartilage tissue engineering could be combined with PRP to improve chondrogenesis potential and localized sustained release of GFs at the defect site, which may result in accelerated healing. A study explored the cartilage regenerative potential of a complex containing autologous PRP and injectable HA hydrogel. On the medial femoral condyle of the porcine model, focal cartilage lesions of different sizes were created. After six months of observation, the models were sacrificed for additional analysis. In the HA/PRP-treated group, there was more integration with native tissue and ECM synthesis without hypertrophic cartilage, indicating hyaline-like cartilage regeneration (Fig. 7). These results demonstrate the efficacy of HA/PRP in regenerating cartilage defects measuring 8.5 mm in diameter and osteochondral defects measuring 6.5 mm in diameter and 5 mm in depth [81].
Images illustrating the macroscopic appearance of the repaired area. FT stands for full thickness; HA for hyaluronic acid hydrogel [81]
In another study, Seker et al. [82] developed a 3D macroporous cryogel scaffold for tissue engineering applications using oxidized dextran (OD; 0.5, 1, 2, and 4%) and Platelet Lysate (PL). After implantation, the PL/OD cryogels with a macroporous network and pore diameters ranging from 10 to 200 μm degraded entirely in 90 to 240 days (depending on the OD concentration). Histochemical studies revealed a high level of cell and tissue penetration through the porous structure of PL/OD. Among four distinct OD concentrations, only PL/OD4 had quite toxic effects on cells, whereas the other three samples were non-toxic. After a week of seeding hASCs on PL/OD2, the results indicated that 3D cryogels were able to maintain cell viability and revealed significant cell spreading and filopodia formation. PL/OD2 also increased the chondrogenic potential of hASCs by chondroinductive factors.
In 2020, Bolandi et al. [87] designed a localized sustained release system for articular cartilage regeneration by fabricating a composite hydrogel based on alginate/polyvinyl alcohol incorporating PRP-encapsulated Alginate sulfate microbeads. They showed that by using alginate sulfate microbeads, which serve as a sustained growth factor delivery system, PRP could preserve its bioactivity significantly and have a continuous presence during the healing process. The sulfated groups in the alginate sulfate microbeads played the role of binding sites, so GFs attached to them were released gradually by cell requirement and departed from the system. In addition, MSCs encapsulated into this composite hydrogel illustrated a higher proliferation rate and upregulated expression of collagen type ΙΙ, Aggrecan, and SOX9, as cartilage-critical genes, compared to the direct treatment by PRP.
Li and his colleagues [88] have designed a bioink composed of Silk Fibroin (SF) and PRP (v/v) concentrations of 12.5%, 25%, and 50%. According to the release profiles, SF-PRP bioinks provide a controlled release of GFs. Then, scaffolds of SF/PRP bioinks were created using the bioprinting technique. Characterizations of scaffolds revealed an excellent porous structure, good mechanical characteristics, and a suitable degradation rate. Furthermore, the live-dead analysis showed that 3D-printed scaffolds were biocompatible. In addition, the histological and immunohistochemical results of in-vitro tests were superior to those of the control group (pure SF). The higher collagen and GAG contents of SF/PRP scaffolds (more than 50% PRP, v/v) compared to the control group confirmed the potential use of these 3D-printed scaffolds in cartilage regeneration.
Table 3 lists the recent studies on PRP-activated scaffolds for cartilage tissue engineering. These investigations underscore the central role of PRP in conducting cellular responses and chondrogenesis. Additionally, studies exploring PRP-activated scaffolds provide a promising frontier, offering a potent strategy to enhance chondrogenesis and facilitate targeted GF release for accelerated healing. This confluence of research underscores the multifaceted potential of PRP in cartilage tissue engineering, suggesting an exciting approach for future advancements in regenerative medicine.
6 Conclusion and Future Perspective
The regeneration of bone and cartilage tissue is a physiological process that is intricate and well-coordinated. This process involves several molecular, cellular, and biochemical processes. Multiple compounds, including GFs, are involved in tissue repair. These GFs are well-known for their impact on cell adhesion, proliferation, and differentiation. PRP delivers a natural combination of autologous GFs and is a promising therapy for accelerating tissue healing, allowing for a quick recovery after bone or cartilage defects. Even though various papers have elaborated on the benefits of blood derivatives, especially PRP, in tissue engineering applications, there are still several obstacles to be addressed. Depending on the preparation method, the composition of PRP may be altered. This necessitates additional study for the establishment of standard protocols. Moreover, the autologous nature of PRP is a crucial consideration because its composition varies between patients based on age, sex, and patient comorbidities, which might decrease the comparability and reproducibility of different PRP studies. A comprehensive analysis, such as a high-throughput assay, should resolve these challenges to get more robust and predictable clinical outcomes. In addition to the composition of PRP, how it is delivered has a significant impact on its regenerative properties. Combining biomaterials with PRP is an intriguing method for PRP delivery. Currently, PRP therapy requires multiple treatments, such as multiple injections. This leads to highly variable GF concentrations, which reduces therapeutic predictability. Biomaterials can function as controlled-release devices, enabling sustained delivery of certain GF combinations. Therefore, biomaterial properties such as stiffness, degradability, porosity, and bioactivity are expected to affect the release profile of PRP and bone/cartilage repair. Although several studies have started to investigate the concept of combining PRP with biomaterials, comprehensive approaches are required not only to affect PRP half-life during scaffold fabrication positively but also to prolong its release depending on the application.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Cheng, A., Schwartz, Z., Kahn, A., Li, X., Shao, Z., Sun, M., Ao, Y., Boyan, B. D., & Chen, H. (2019). Advances in porous scaffold design for bone and cartilage tissue engineering and regeneration. Tissue Engineering Part B: Reviews, 25(1), 14–29.
De Mori, A., Peña Fernández, M., Blunn, G., Tozzi, G., & Roldo, M. (2018). 3D printing and electrospinning of composite hydrogels for cartilage and bone tissue engineering. Polymers, 10(3), 285.
Kim, Y. S., Majid, M., Melchiorri, A. J., & Mikos, A. G. (2019). Applications of decellularized extracellular matrix in bone and cartilage tissue engineering. Bioengineering & Translational Medicine, 4(1), 83–95.
Han, X., Sun, M., Chen, B., Saiding, Q., Zhang, J., Song, H., Deng, L., Wang, P., Gong, W., & Cui, W. (2021). Lotus seedpod-inspired internal vascularized 3D printed scaffold for bone tissue repair. Bioactive Materials, 6(6), 1639–1652.
Liu, J., Yang, B., Li, M., Li, J., & Wan, Y. (2020). Enhanced dual network hydrogels consisting of thiolated chitosan and silk fibroin for cartilage tissue engineering. Carbohydrate Polymers, 227, 115335.
Zhang, N., Wang, Y., Zhang, J., Guo, J., & He, J. (2021). Controlled domain gels with a biomimetic gradient environment for osteochondral tissue regeneration. Acta Biomaterialia, 135, 304–317.
Ansari, S., Khorshidi, S., & Karkhaneh, A. (2019). Engineering of gradient osteochondral tissue: From nature to lab. Acta Biomaterialia, 87, 41–54.
Gonçalves, A. M., Moreira, A., Weber, A., Williams, G. R., & Costa, P. F. (2021). Osteochondral tissue engineering: The potential of electrospinning and additive manufacturing. Pharmaceutics, 13(7), 983.
Wei, W., & Dai, H. (2021). Articular cartilage and osteochondral tissue engineering techniques: Recent advances and challenges. Bioactive Materials, 6(12), 4830–4855.
Nowicki, M., Zhu, W., Sarkar, K., Rao, R., & Zhang, L. G. (2020). 3D printing multiphasic osteochondral tissue constructs with nano to micro features via PCL based bioink. Bioprinting, 17, e00066.
Zhang, Y., Liu, X., Zeng, L., Zhang, J., Zuo, J., Zou, J., Ding, J., & Chen, X. (2019). Polymer fiber scaffolds for bone and cartilage tissue engineering. Advanced Functional Materials, 29(36), 1903279.
Chiesa, I., De Maria, C., Lapomarda, A., Fortunato, G. M., Montemurro, F., Di Gesù, R., Tuan, R. S., Vozzi, G., & Gottardi, R. (2020). Endothelial cells support osteogenesis in an in vitro vascularized bone model developed by 3D bioprinting. Biofabrication, 12(2), 025013.
Ding, H., Cheng, Y., Niu, X., & Hu, Y. (2020). Application of electrospun nanofibers in bone, cartilage and osteochondral tissue engineering. Journal of Biomaterials Science, Polymer Edition, 32(4), 536–561.
Tran, H. D., Park, K. D., Ching, Y. C., Huynh, C., & Nguyen, D. H. (2020). A comprehensive review on polymeric hydrogel and its composite: Matrices of choice for bone and cartilage tissue engineering. Journal of Industrial and Engineering Chemistry, 89, 58–82.
Qasim, M., Chae, D. S., & Lee, N. Y. (2019). Advancements and frontiers in nano-based 3D and 4D scaffolds for bone and cartilage tissue engineering. International Journal of Nanomedicine, 14, 4333.
Baghersad, S., Bahrami, S. H., Mohammadi, M. R., Mojtahedi, M. R. M., & Milan, P. B. (2018). Development of biodegradable electrospun gelatin/aloe-vera/poly (ε-caprolactone) hybrid nanofibrous scaffold for application as skin substitutes. Materials Science and Engineering: C, 93, 367–379.
Baghersad, S., Hivechi, A., Bahrami, S. H., Milan, P. B., Siegel, R. A., & Amoupour, M. (2022). Optimal Aloe vera encapsulated PCL/Gel nanofiber design for skin substitute application and the evaluation of its in vivo implantation. Journal of Drug Delivery Science and Technology, 74, 103536.
Vyas, C., Mishbak, H., Cooper, G., Peach, C., Pereira, R. F., & Bartolo, P. (2020). Biological perspectives and current biofabrication strategies in osteochondral tissue engineering. Biomanufacturing Reviews, 5(1), 1–24.
Xing, F., Xiang, Z., Rommens, P. M., & Ritz, U. (2020). 3D bioprinting for vascularized tissue-engineered bone fabrication. Materials, 13(10), 2278.
Leucht, A., Volz, A.-C., Rogal, J., Borchers, K., & Kluger, P. J. (2020). Advanced gelatin-based vascularization bioinks for extrusion-based bioprinting of vascularized bone equivalents. Scientific Reports, 10(1), 1–15.
Oliveira, É. R., Nie, L., Podstawczyk, D., Allahbakhsh, A., Ratnayake, J., Brasil, D. L., & Shavandi, A. (2021). Advances in growth factor delivery for bone tissue engineering. International Journal of Molecular Sciences, 22(2), 903.
Koons, G. L., & Mikos, A. G. (2019). Progress in three-dimensional printing with growth factors. Journal of Controlled Release, 295, 50–59.
Goonoo, N., & Bhaw-Luximon, A. (2019). Mimicking growth factors: Role of small molecule scaffold additives in promoting tissue regeneration and repair. RSC Advances, 9(32), 18124–18146.
Liou, J.-J., Rothrauff, B. B., Alexander, P. G., & Tuan, R. S. (2018). Effect of platelet-rich plasma on chondrogenic differentiation of adipose-and bone marrow-derived mesenchymal stem cells. Tissue Engineering Part A, 24(19–20), 1432–1443.
Parmaksiz, M. (2022). Decellularized tendon-based heparinized nanocomposite scaffolds for prospective regenerative applications: Chemical, physical, thermal, mechanical and in vitro biological evaluations. Journal of the Mechanical Behavior of Biomedical Materials, 134, 105387.
Bhatnagar, P., Law, J. X., & Ng, S.-F. (2022). Delivery systems for platelet derived growth factors in wound healing: A review of recent developments and global patent landscape. Journal of Drug Delivery Science and Technology., 71, 103270. https://doi.org/10.1016/j.jddst.2022.103270
Dohan Ehrenfest, D. M., Bielecki, T., Mishra, A., Borzini, P., Inchingolo, F., Sammartino, G., Rasmusson, L., & Evert, P. A. (2012). In search of a consensus terminology in the field of platelet concentrates for surgical use: platelet-rich plasma (PRP), platelet-rich fibrin (PRF), fibrin gel polymerization and leukocytes. Current Pharmaceutical Biotechnology, 13(7), 1131–1137.
Kingsley, C. S. (1954). Blood coagulation: Evidence of an antagonist to factor VI in platelet-rich human plasma. Nature, 173(4407), 723–724.
Matras, H. (1970). Die Wirkungen vershiedener fibrinpraparate auf kontinuitat-strennungen der rattenhaut. Österreichische Zeitschrift für Stomatologie, 67(9), 338–359.
Schulz, V., Kochsiek, K., Köstering, H., & Walther, C. H. (1971). Zur Gewinnung „plättchenreicher Plasmen” für Thrombocyten-Zählungen und -Funktionsprüfungen. Clinical Chemistry and Laboratory Medicine., 9(4), 324–328. https://doi.org/10.1515/cclm.1971.9.4.324
Rosenthal, A. R., Harbury, C., Egbert, P. R., & Rubenstein, E. (1975). Use of a platelet-fibrinogen-thrombin mixture as a corneal adhesive: Experiments with sutureless lamellar keratoplasty in the rabbit. Investigative Ophthalmology & Visual Science, 14(11), 872–875.
Rosenthal, A. R., Egbert, P. R., Harbury, C., Hopkins, J. L., & Rubenstein, E. (1978). Use of platelet-fibrinogen-thrombin mixture to seal experimental penetrating corneal wounds. Albrecht von Graefes Archiv Für Klinische Und Experimentelle Ophthalmologie, 207(2), 111–115.
Pearl, R. M., Wustrack, K. O., Harbury, C., Rubenstein, E., & Kaplan, E. N. (1977). Microvascular anastomosis using a blood product sealant-adhesive. Surgery, Gynecology & Obstetrics, 144(2), 227–231.
Silverberg, G. D., Harbury, C. B., & Rubenstein, E. (1977). A physiological sealant for cerebrospinal fluid leaks. Journal of Neurosurgery, 46(2), 215–219.
Fischer, H. (1979). A method of suture-free anastomosis of nerve transplantation is being reported, using facial nerve as the example (author’s transl). Laryngologie, Rhinologie, Otologie, 58(2), 154–156.
Knighton, D. R., Ciresi, K. F., Fiegel, V. D., Austin, L. L., & Butler, E. L. (1986). Classification and treatment of chronic nonhealing wounds. Successful treatment with autologous platelet-derived wound healing factors (PDWHF). Annals of Surgery, 204(3), 322.
Marx, R. E., Carlson, E. R., Eichstaedt, R. M., Schimmele, S. R., Strauss, J. E., & Georgeff, K. R. (1998). Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology, 85(6), 638–646.
Ehrenfest, D. M. D., Andia, I., Zumstein, M. A., Zhang, C.-Q., Pinto, N. R., & Bielecki, T. (2014). Classification of platelet concentrates (Platelet-Rich Plasma-PRP, Platelet-Rich Fibrin-PRF) for topical and infiltrative use in orthopedic and sports medicine: Current consensus, clinical implications and perspectives. Muscles, Ligaments and Tendons Journal, 4(1), 3.
Zhu, Y., Yuan, M., Meng, H. Y., Wang, A. Y., Guo, Q. Y., Wang, Y., & Peng, J. (2013). Basic science and clinical application of platelet-rich plasma for cartilage defects and osteoarthritis: A review. Osteoarthritis and Cartilage, 21(11), 1627–1637.
Whitman, D. H., Berry, R. L., & Green, D. M. (1997). Platelet gel: An autologous alternative to fibrin glue with applications in oral and maxillofacial surgery. Journal of Oral and Maxillofacial Surgery, 55(11), 1294–1299.
Ehrenfest, D. M. D., Rasmusson, L., & Albrektsson, T. (2009). Classification of platelet concentrates: From pure platelet-rich plasma (P-PRP) to leucocyte-and platelet-rich fibrin (L-PRF). Trends in Biotechnology, 27(3), 158–167.
Dhurat, R., & Sukesh, M. (2014). Principles and methods of preparation of platelet-rich plasma: A review and author’s perspective. Journal of Cutaneous and Aesthetic Surgery, 7(4), 189.
Bausset, O., Giraudo, L., Veran, J., Magalon, J., Coudreuse, J.-M., Magalon, G., Dubois, C., Serratrice, N., Dignat-George, F., & Sabatier, F. (2012). Formulation and storage of platelet-rich plasma homemade product. BioResearch Open Access, 1(3), 115–123.
Castillo, T. N., Pouliot, M. A., Kim, H. J., & Dragoo, J. L. (2011). Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. The American Journal of Sports Medicine, 39(2), 266–271.
Ma, P. X. (2008). Biomimetic materials for tissue engineering. Advanced Drug Delivery Reviews, 60(2), 184–198.
Furth, M. E., Atala, A., & van Dyke, M. E. (2007). Smart biomaterials design for tissue engineering and regenerative medicine. Biomaterials, 28(34), 5068–5073.
Nour, S., Baheiraei, N., Imani, R., Rabiee, N., Khodaei, M., Alizadeh, A., & Moazzeni, S. M. (2019). Bioactive materials: A comprehensive review on interactions with biological microenvironment based on the immune response. Journal of Bionic Engineering, 16(4), 563–581.
Kretlow, J. D., Klouda, L., & Mikos, A. G. (2007). Injectable matrices and scaffolds for drug delivery in tissue engineering. Advanced Drug Delivery Reviews, 59(4–5), 263–273.
van Tomme, S. R., Storm, G., & Hennink, W. E. (2008). In situ gelling hydrogels for pharmaceutical and biomedical applications. International Journal of Pharmaceutics, 355(1–2), 1–18.
Hatefi, A., & Amsden, B. (2002). Biodegradable injectable in situ forming drug delivery systems. Journal of Controlled Release, 80(1–3), 9–28.
Masoudi, E. A., Ribas, J., Kaushik, G., Leijten, J., & Khademhosseini, A. (2016). Platelet-rich blood derivatives for stem cell-based tissue engineering and regeneration. Current Stem Cell Reports, 2(1), 33–42.
Najafloo, R., Baheiraei, N., & Imani, R. (2021). Synthesis and characterization of collagen/calcium phosphate scaffolds incorporating antibacterial agent for bone tissue engineering application. Journal of Bioactive and Compatible Polymers, 36(1), 29–43.
Koons, G. L., Diba, M., & Mikos, A. G. (2020). Materials design for bone-tissue engineering. Nature Reviews Materials, 5(8), 584–603.
Dehghan, F., Gholipour-Kanani, A., Kamali Dolatabadi, M., & Bahrami, S. H. (2022). Nanofibrous composite from polycaprolactone-polyethylene glycol-aloe vera as a promising scaffold for bone repairing. Journal of Applied Polymer Science, 139(26), e52463.
Kashef-Saberi, M. S., Roodbari, N. H., Parivar, K., Vakilian, S., & Hanaee-Ahvaz, H. (2018). Enhanced osteogenic differentiation of mesenchymal stem cells on electrospun polyethersulfone/poly (vinyl) alcohol/platelet rich plasma nanofibrous scaffolds. ASAIO Journal, 64(5), e115–e122.
Fernandes, G., & Yang, S. (2016). Application of platelet-rich plasma with stem cells in bone and periodontal tissue engineering. Bone Research, 4(1), 1–21.
Oustadi, F., Imani, R., Haghbin Nazarpak, M., & Sharifi, A. M. (2020). Genipin-crosslinked gelatin hydrogel incorporated with PLLA-nanocylinders as a bone scaffold: Synthesis, characterization, and mechanical properties evaluation. Polymers for Advanced Technologies, 31(8), 1783–1792.
Khalili, M., Keshvari, H., Imani, R., Sohi, A. N., Esmaeili, E., & Tajabadi, M. (2022). Study of osteogenic potential of electrospun PCL incorporated by dendrimerized superparamagnetic nanoparticles as a bone tissue engineering scaffold. Polymers for Advanced Technologies, 33(3), 782–794.
Sani, F., Mehdipour, F., Talaei-Khozani, T., Sani, M., & Razban, V. (2017). Fabrication of platelet-rich plasma/silica scaffolds for bone tissue engineering. Bioinspired, Biomimetic and Nanobiomaterials, 7(2), 74–81.
Zou, J., Shi, Z., Xu, H., & Li, X. (2017). In vitro studies on the degradability, bioactivity, and cell differentiation of PRP/AZ31B Mg alloys composite scaffold. BioMed Research International. https://doi.org/10.1155/2017/5763173
Li, J., Chen, M., Wei, X., Hao, Y., & Wang, J. (2017). Evaluation of 3D-printed polycaprolactone scaffolds coated with freeze-dried platelet-rich plasma for bone regeneration. Materials, 10(7), 831.
Zhang, Y., Niu, J., Wang, Z., Liu, S., Wu, J., & Yu, B. (2017). Repair of osteochondral defects in a rabbit model using bilayer poly (lactide-co-glycolide) scaffolds loaded with autologous platelet-rich plasma. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research, 23, 5189.
Oryan, A., Meimandi Parizi, A., Shafiei-Sarvestani, Z., & Bigham, A. S. (2012). Effects of combined hydroxyapatite and human platelet rich plasma on bone healing in rabbit model: Radiological, macroscopical, hidtopathological and biomechanical evaluation. Cell and Tissue Banking, 13(4), 639–651.
Dhillon, M. S., Patel, S., & Bansal, T. (2019). Improvising PRP for use in osteoarthritis knee-upcoming trends and futuristic view. Journal of Clinical Orthopaedics and Trauma, 10(1), 32–35.
Liao, J. C. (2019). Positive effect on spinal fusion by the combination of platelet-rich plasma and collagen-mineral scaffold using lumbar posterolateral fusion model in rats. Journal of Orthopaedic Surgery and Research, 14, 39. https://doi.org/10.1186/s13018-019-1076-2
Pan, W., Dai, C., Li, Y., Yin, Y., Gong, L., Machuki, J. O., Achwa Yang, Y., Qiu, S., Guo, K., & Gao, F. (2020). PRP-chitosan thermoresponsive hydrogel combined with black phosphorus nanosheets as injectable biomaterial for biotherapy and phototherapy treatment of rheumatoid arthritis. Biomaterials, 239, 119851.
Wu, C.-Y., Guo, C.-L., Yang, Y.-C., Huang, C.-W., Zeng, J.-Y., Guan, Z.-Y., Chiang, Y.-C., Wang, P.-Y., & Chen, H.-Y. (2020). Parylene-based porous scaffold with functionalized encapsulation of platelet-rich plasma and living stem cells for tissue engineering applications. ACS Applied Bio Materials, 3(10), 7193–7201.
Chen, M., Liu, Q., Xu, Y., Wang, Y., Han, X., Wang, Z., Liang, J., Sun, Y., Fan, Y., & Zhang, X. (2021). The effect of LyPRP/collagen composite hydrogel on osteogenic differentiation of rBMSCs. Regenerative Biomaterials, 8(1), rbaa053. https://doi.org/10.1093/rb/rbaa053
Sarkar, M. R., Augat, P., Shefelbine, S. J., Schorlemmer, S., Huber-Lang, M., Claes, L., Kinzl, L., & Ignatius, A. (2006). Bone formation in a long bone defect model using a platelet-rich plasma-loaded collagen scaffold. Biomaterials, 27(9), 1817–1823. https://doi.org/10.1016/j.biomaterials.2005.10.039
Yu, T., Pan, H., Hu, Y., Tao, H., Wang, K., & Zhang, C. (2017). Autologous platelet-rich plasma induces bone formation of tissue-engineered bone with bone marrow mesenchymal stem cells on beta-tricalcium phosphate ceramics. Journal of Orthopaedic Surgery and Research, 12(1), 178. https://doi.org/10.1186/s13018-017-0665-1
Cheng, G., Ma, X., Li, J., Cheng, Y., Cao, Y., Wang, Z., Shi, X., Du, Y., Deng, H., & Li, Z. (2018). Incorporating platelet-rich plasma into coaxial electrospun nanofibers for bone tissue engineering. International Journal of Pharmaceutics., 547(1), 656–666. https://doi.org/10.1016/j.ijpharm.2018.06.020
Zhang, M., Zhen, J., Zhang, X., Yang, Z., Zhang, L., Hao, D., & Ren, B. (2019). Effect of autologous platelet-rich plasma and gelatin sponge for tendon-to-bone healing after rabbit anterior cruciate ligament reconstruction. Arthroscopy The Journal of Arthroscopic & Related Surgery., 35(5), 1486–1497.
Qiao, S., Sheng, Q., Li, Z., Wu, D., Zhu, Y., Lai, H., & Gu, Y. (2020). 3D-printed Ti6Al4V scaffolds coated with freeze-dried platelet-rich plasma as bioactive interface for enhancing osseointegration in osteoporosis. Materials & Design, 194, 108825. https://doi.org/10.1016/j.matdes.2020.108825
Sadeghinia, A., Davaran, S., Salehi, R., & Jamalpoor, Z. (2019). Nano-hydroxy apatite/chitosan/gelatin scaffolds enriched by a combination of platelet-rich plasma and fibrin glue enhance proliferation and differentiation of seeded human dental pulp stem cells. Biomedicine & Pharmacotherapy., 109, 1924–1931. https://doi.org/10.1016/j.biopha.2018.11.072
Abazari, M. F., Nejati, F., Nasiri, N., Khazeni, Z. A. S., Nazari, B., Enderami, S. E., & Mohajerani, H. (2019). Platelet-rich plasma incorporated electrospun PVA-chitosan-HA nanofibers accelerates osteogenic differentiation and bone reconstruction. Gene., 720, 144096. https://doi.org/10.1016/j.gene.2019.144096
Wei, L., Wu, S., Kuss, M., Jiang, X., Sun, R., Reid, P., Qin, X., & Duan, B. (2019). 3D printing of silk fibroin-based hybrid scaffold treated with platelet rich plasma for bone tissue engineering. Bioactive Materials, 4, 256–260. https://doi.org/10.1016/j.bioactmat.2019.09.001
Liu, Z., Yuan, X., Fernandes, G., Dziak, R., Ionita, C. N., Li, C., Wang, C., & Yang, S. (2017). The combination of nano-calcium sulfate/platelet rich plasma gel scaffold with BMP2 gene-modified mesenchymal stem cells promotes bone regeneration in rat critical-sized calvarial defects. Stem Cell Research & Therapy, 8(1), 122. https://doi.org/10.1186/s13287-017-0574-6
Sajesh, K. M., Kiran, K., Nair, S. V., & Jayakumar, R. (2016). Sequential layer-by-layer electrospinning of nano SrCO3/PRP loaded PHBV fibrous scaffold for bone tissue engineering. Composites Part B: Engineering., 99, 445–452. https://doi.org/10.1016/j.compositesb.2016.06.026
Liu, C., Peng, Z., Xu, H., Gao, H., Li, J., Jin, Y., Wang, Y., Wang, C., Liu, Y., & Hu, Y. (2022). 3D printed platelet-rich plasma-loaded scaffold with sustained cytokine release for bone defect repair. Tissue Engineering Part A, 28(15–16), 700–711.
Li, J., Wang, K., Bai, X., Wang, Q., Lv, N., & Li, Z. (2021). Enhanced regeneration of bone defects using sintered porous Ti6Al4V scaffolds incorporated with mesenchymal stem cells and platelet-rich plasma. RSC Advances, 11(9), 5128–5138. https://doi.org/10.1039/d0ra10215f
Yan, W., Xu, X., Xu, Q., Sun, Z., Jiang, Q., & Shi, D. (2020). Platelet-rich plasma combined with injectable hyaluronic acid hydrogel for porcine cartilage regeneration: A 6-month follow-up. Regenerative Biomaterials, 7(1), 77–90. https://doi.org/10.1093/rb/rbz039
Şeker, Ş, Elçin, A. E., & Elçin, Y. M. (2020). Macroporous elastic cryogels based on platelet lysate and oxidized dextran as tissue engineering scaffold: In vitro and in vivo evaluations. Materials Science and Engineering: C., 110, 110703. https://doi.org/10.1016/j.msec.2020.110703
Jelodari, S., Ebrahimi Sadrabadi, A., Zarei, F., Jahangir, S., Azami, M., Sheykhhasan, M., & Hosseini, S. (2022). New insights into cartilage tissue engineering: Improvement of tissue-scaffold integration to enhance cartilage regeneration. BioMed Research International. https://doi.org/10.1155/2022/7638245
Moussa, M., Lajeunesse, D., Hilal, G., el Atat, O., Haykal, G., Serhal, R., Chalhoub, A., Khalil, C., & Alaaeddine, N. (2017). Platelet rich plasma (PRP) induces chondroprotection via increasing autophagy, anti-inflammatory markers, and decreasing apoptosis in human osteoarthritic cartilage. Experimental Cell Research., 352(1), 146–156. https://doi.org/10.1016/j.yexcr.2017.02.012
Drengk, A., Zapf, A., Stürmer, E. K., Stürmer, K. M., & Frosch, K.-H. (2009). Influence of platelet-rich plasma on chondrogenic differentiation and proliferation of chondrocytes and mesenchymal stem cells. Cells, Tissues, Organs, 189(5), 317–326.
Elder, S., & Thomason, J. (2014). Effect of platelet-rich plasma on chondrogenic differentiation in three-dimensional culture. The Open Orthopaedics Journal, 8, 78.
Bolandi, B., Imani, R., Bonakdar, S., & Fakhrzadeh, H. (2021). Chondrogenic stimulation in mesenchymal stem cells using scaffold-based sustained release of platelet-rich plasma. Journal of Applied Polymer Science., 138(12), 50075. https://doi.org/10.1002/app.50075
Li, Z., Zhang, X., Yuan, T., Zhang, Y., Luo, C., Zhang, J., Liu, Y., & Fan, W. (2020). Addition of platelet-rich plasma to silk fibroin hydrogel bioprinting for cartilage regeneration. Tissue Engineering Part A, 26(15–16), 886–895.
Rosadi, I., Karina, K., Rosliana, I., Sobariah, S., Afini, I., Widyastuti, T., & Barlian, A. (2019). In vitro study of cartilage tissue engineering using human adipose-derived stem cells induced by platelet-rich plasma and cultured on silk fibroin scaffold. Stem Cell Research & Therapy, 10(1), 369. https://doi.org/10.1186/s13287-019-1443-2
Şeker, Ş, Elçin, A. E., & Elçin, Y. M. (2019). Autologous protein-based scaffold composed of platelet lysate and aminated hyaluronic acid. Journal of Materials Science: Materials in Medicine, 30(12), 127. https://doi.org/10.1007/s10856-019-6334-7
Ruan, S., Deng, J., Yan, L., & Huang, W. (2018). Evaluation of the effects of the combination of bmp-2-modified bmscs and prp on cartilage defects. Experimental and Therapeutic Medicine, 16(6), 4569–4577. https://doi.org/10.3892/etm.2018.6776
Jooybar, E., Abdekhodaie, M. J., Alvi, M., Mousavi, A., Karperien, M., & Dijkstra, P. J. (2019). An injectable platelet lysate-hyaluronic acid hydrogel supports cellular activities and induces chondrogenesis of encapsulated mesenchymal stem cells. Acta Biomaterialia., 83, 233–244. https://doi.org/10.1016/j.actbio.2018.10.031
Sancho-Tello, M., Martorell, S., Roig, M. M., Milián, L., Gámiz-González, M. A., Ribelles, J. L. G., & Carda, C. (2017). Human platelet-rich plasma improves the nesting and differentiation of human chondrocytes cultured in stabilized porous chitosan scaffolds. Journal of Tissue Engineering. https://doi.org/10.1177/2041731417697545
Wang, Z., Qin, H., Feng, Z., & Zhao, Y. (2016). Platelet-rich plasma gel composited with nondegradable porous polyurethane scaffolds as a potential auricular cartilage alternative. Journal of Biomaterials Applications, 30(7), 889–899. https://doi.org/10.1177/0885328215604818
Zhang, X., Wang, J., Ren, M., Li, L., Wang, Q., & Hou, X. (2016). A novel collagen/platelet-rich plasma (COL/PRP) scaffold: Preparation and growth factor release analysis. Cell and Tissue Banking, 17(2), 327–334. https://doi.org/10.1007/s10561-016-9551-z
Tang, Y., Wang, H., Sun, Y., Jiang, Y., Fang, S., Kan, Z., Lu, Y., Liu, S., Zhou, X., & Li, Z. (2021). Using platelet-rich plasma hydrogel to deliver mesenchymal stem cells into three-dimensional PLGA scaffold for cartilage tissue engineering. ACS Applied Bio Materials, 4(12), 8607–8614.
Singh, B. N., Nallakumarasamy, A., Sinha, S., Rastogi, A., Mallick, S. P., Divakar, S., & Srivastava, P. (2022). Generation of hybrid tissue engineered construct through embedding autologous chondrocyte loaded platelet rich plasma/alginate based hydrogel in porous scaffold for cartilage regeneration. International Journal of Biological Macromolecules., 203, 389–405. https://doi.org/10.1016/j.ijbiomac.2022.01.054
KhaliliJafarabad, N., Behnamghader, A., Khorasani, M. T., & Mozafari, M. (2022). Platelet-rich plasma-hyaluronic acid/chondrotin sulfate/carboxymethyl chitosan hydrogel for cartilage regeneration. Biotechnology and Applied Biochemistry, 69(2), 534–547. https://doi.org/10.1002/bab.2130
Acknowledgements
The authors would like to appreciate support from Iran’s National Elites Foundation (INEF). Also, the authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Authors have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Baghersad, S., Bolandi, B., Imani, R. et al. An Overview of PRP-Delivering Scaffolds for Bone and Cartilage Tissue Engineering. J Bionic Eng 21, 674–693 (2024). https://doi.org/10.1007/s42235-023-00471-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42235-023-00471-6