Abstract
Oily wastewater pollution is an urgent problem to be solved. Complicated preparation process, toxic hydrophobic modifiers and poor mechanical properties limit the application of polysaccharide-based aerogel in oil–water separation. Inspired by the Strider’s Leg structure in nature, an eco-friendly and reusable polysaccharide-based composite aerogel was prepared by hydrophobic modification with zein for efficient oil–water separation. The introduction of hydrophobic zein into aerogel by simple immersion method without the use of toxic modifiers can build micro/nanostructures similar to the villi on a water strider’s leg to increase the surface roughness and the hydrophobicity. And three degradable, non-toxic and economical polysaccharides including chitosan, carboxylated cellulose nanofibers and starch were used to construct aerogel skeleton, endowing aerogel with porous structures and good mechanical properties. The resulting composite aerogel (ZOMA) showed low density (0.11 g/cm3), good oil absorption capacity (9 g/g), high flux oil–water separation (5595 L m−2 h−1) and excellent oil–water separation performance (99.8%). And ZOMA still had good tensile strength and elasticity after 50 compression cycles. After 10 cycles of absorption and desorption, ZOMA aerogel remained still more than 90% of its initial absorption capacity. This study provides new insight for the design of environmentally friendly and efficient adsorbents for oil–water separation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The seawater pollution caused by oil spills and the growing discharge of industrial oily wastewater impact on water quality, aquatic ecosystems and human health, which is an urgent problem to be solved [1,2,3,4]. Aerogel as nanoporous material has great application prospects in oil–water separation due to their unique advantages of high specific surface areas, low densities and high pore volumes [5,6,7,8]. Polysaccharides such as chitosan (CS), cellulose and starch are suitable for the fabrication of environmentally friendly aerogel, due to eco-friendly, readily available, renewability and economical features [9, 10]. Although cellulose-based aerogel and chitosan-based aerogel have good ability and degradability, but the hydrophilic of cellulose, the skeleton structure of aerogel is easy to collapse and dissolve when exposed to water, which limits the application of oil–water separation [11]. Thus hydrophobic modification of aerogel is very important to improve the oil/solvent from water. The commonly used hydrophobic modification method for aerogel is chemical vapor deposition [12] and chemical grafting methods, such as cross-linking chitosan /cellulose nanofibers using glutaraldehyde as crosslinker [13] or modifying chitosan with methyltrimethoxysilane [14]. However, toxic and expensive substances such as fluorine chemicals or glutaraldehyde are always used during hydrophobic modification process, and the preparation process is too cumbersome [15]. Therefore, it is necessary to develop a simple, economical and safe hydrophobic modification methods to prepare composite aerogel with high capacity and environmentally friendly property for efficient oily wastewater treatment.
Zein has received increasing attention because of its biodegradability, cheapness and renewability [16]. It mainly comes from corn gluten meal (CGM), which is the waste of corn deep processing. Importantly, it has a variety of non-polar amino acid residues such as proline, leucine, alanine and glutamine, making it a hydrophobic vegetable protein [17]. Thus it has the potential for hydrophobic modification of composite aerogel.
In nature, the water strider can move freely on the water. This is because that a lot of villus exist on their legs to form a stable air film, which prevents water infiltration [18]. Inspired by this, hydrophobic zein was introduced into polysaccharide-based aerogel by simple immersion method without the use of toxic modifiers, which can build micro/nanostructures similar to the villi on a water strider's leg to increase the surface roughness and the hydrophobicity. And polysaccharide aerogel was prepared from chitosan (CS), Carboxylated Cellulose Nanofibers (CCNF) and starch. The cross-linking between the carboxyl group of CS and the amine group of CCNF can form a network structure, increasing the mechanical properties. The addition of starch was able to prevent the collapse of aerogel during freeze-drying [19]. And the existence of potential ionic bonds between starch and nanocellulose can effectively increase the flexible tensile strength and elasticity of aerogel. The mechanical properties of aerogels were evaluated by compared with traditional polysaccharide-based aerogels constructed by two polysaccharides. And the effect of zein on hydrophobicity, oil–water separation capacity and recyclability of the resulting composite aerogel was studied.
2 Experiment Section
2.1 Materials
Chitosan (CS, degree of deacetylation is 95%, viscosity is 100-200mpa.s, MW = 300,000), Carboxylated Cellulose Nanofibers (CCNF, diameter 4–10 nm, length 200 nm) and octadecanal ((C18H36O), ≥ 95.0%, 268.49) were purchased from Aladdin. Anhydrous ethanol, starch ((C6H10O5)n, AR) and glacial acetic acid (C2H4O2, AR) were acquired from the Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Zein (purity N = 14.12%, average molecular weight 25,000–45,000) was purchased from Bide Pharmaceuticals.
2.2 Preparation of Original Aerogel
Original aerogel was prepared by a one-pot method in aqueous solution according to literature [13, 20, 21]. Briefly, 5 g of chitosan powder was put into 100 ml of deionized water. Subsequently, 3 g of carboxylated cellulose nanofibers was added to ensure that the ratio of chitosan and cellulose was close to 1.6:1. Then 1.5 g of starch was added to the mixture to make the ratio of cellulose content to starch content 2:1. Glacial acetic acid was used to adjust the pH of the aqueous solution (pH = 4). Then 0.048 g EDC and 0.029 g NHS were added to the system and stirred slowly for 24 h at 40℃ to form a hydrogel. The hydrogel was placed in an oven at 50℃ for 6 h, then set in a freeze dryer at − 20℃ for 24 h, and was freeze-dried for 48 h to obtain the original aerogel.
2.3 Preparation of ZOMA Aerogel
Zein solution with a concentration of 5%, 10% and 15% was obtained in 75% ethanol solution. The original aerogel was soaked into the zein solution until saturated with the solution. Removing the excess solution by squeezing, the aerogel was put into an oven (50℃) for drying. The above operations were repeated three times to prepare Zein Modified Aerogel (ZMA). ZMA aerogel was further immersed in octadecanal solution and dried at 50℃ to obtain Zein/octadecanal modified plysaccharide-based composite aerogel (ZOMA).
2.4 Characterization and Materials
Field Emission Scanning Electron Microscope (SEM, SIGMA500, China) was used to characterize the surface morphology of as-prepared aerogels. Fourier Transform Infrared Spectrometer (FTIR, IS50, China) was carried out to analyze the chemical structure with all the spectra recorded from 4000 to 500 cm−1 in transmittance mode. X-ray photoelectron spectroscopy (XPS, Thermo Scientific ESCAlab 250xi) was used to analyze elemental composition with Al Kα radiation source. X-ray diffraction (XRD, Bruker D8 Advance, Germany) was implemented to research the phase structure of aerogel equipped with an unmonochromated aluminum Kα X-ray source (1486.6 eV) and conducted at 15 kV under current of 8 mA. The water contact angle (WCA) was measured by a contact angle analyzer (Dataphysics OCA15, Germany). The water concentration of the purified filtrates was analyzed using Karl Fischer Titrator (Mettler Toledo DL31, Switzerland).
3 Results and Discussion
3.1 Preparation and Characterization of Composite Aerogels
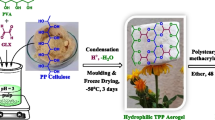
The preparation of plysaccharide-based composite aerogel (ZOMA) consisted of the following two steps: (i) synthesizing the original aerogel, and (ii) sequential hydrophobic modification of aerogels by hydrophobic zein and octadecanal, (Fig. 1). First, plysaccharide gel was prepared by mixing CS, CCNF and starch in an acidic aqueous solution. And the carboxyl group of CS is crosslinked with the amine group of CCNF under the catalysis of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide (EDC) and N-hydroxysuccinimide (NHS). Thus, chitosan and cellulose are cross-linked through covalent bond scheme1 under the action of cross-linker, and then the unreacted amino group and hydroxyl group form hydrogen bond cross-linking with hydroxyl group in starch, forming an aerogel-like structure network [22]. After the freeze-drying process, hydrogel was transformed into original aerogel (OA) with a three-dimensional porous aerogel framework network. The hydrophobic zein was added to OA for hydrophobic modification to prepare ZMA by simple soaking-drying method, Since zein is dissolved in 75% ethanol, ethanol evaporates faster than water when the solvent evaporates. As the evaporation continues, the solvent becomes more hydrophilic, and the resulting change in solvent polarity drives zein to spiral into pieces, accumulate into bands, and self-assemble onto the material skeleton [23]. Then ZOMA was obtained by grafting octadecanal onto aerogels through the reaction of the aldehyde groups of octadecanal with the amine groups of CS.
The ZOMA is an aerogel, which could be easily placed on fog pine without bending (Fig. 2a). And after hydrophobic modification in various ways, such as only Zein Modification Aerogel (ZMA), only Octadecanal Modification Aerogel (OMA), Zein and Octadecanal co-modification Aerogel (ZOMA), the densities of the aerogels did not change significantly (Fig. 2b). They were between 0.101 and 0.114 g/cm3, which still fell within the definition of aerogels and calculation the porosity of these aerogels were about 90%.
3.2 Chemical Structure and Surface Analysis
The chemical structures of aerogels were analyzed by FTIR spectra. As shown in Fig. 3a, there is a co-existence of characteristic peaks of chitosan and cellulose in the spectrum of all aerogels, such as the N–H peaks at 1500–1600 cm−1, 1410 cm−1, O–H peaks at 3340 cm−1, 2924 cm−1 and 1030 cm−1 and the C-H asymmetric stretching at 2850 cm−1, indicating the good preservation of the basic structure of chitosan and cellulose. The strong peaks of ZMA and ZOMA at 1649 cm−1 and 1653 cm−1, respectively, were due to the introduction of more C = O by depositional zein.
The X-ray diffraction (XRD) patterns of the aerogels before and after hydrophobic modification was measured to study the change in crystal form. As shown in Fig. 3b, four aerogels (OA, ZMA, OMA, and ZOMA) all showed the convex peaks at 2θ = 21.0°, indicating that the prepared aerogels in this work were pure natural oil–water separation materials. And the increase of diffraction peak area around 20° was related to the increase of phase after modification. Compared with OA, the N1S peak in the full XPS spectrum of ZOMA was obviously prominent, indicating the successful loading of zein (Fig. 3c).
Compared with white original aerogel (OA), zein/octadecanal composition aerogel (ZOMA) exhibited darker yellow and more compact surface, indicating successful loading of zein (Fig. 4a and e). The morphology of OA and ZOMA was observed by SEM. As seen in Fig. 4b–d, OA showed a layered structure with macropores, which may be caused by the sublimation of ice crystals during freeze-drying [24, 25]. And the three-dimensional bridged structure can be clearly observed on the surface of OA. Moreover, there is no obvious small particle precipitation on the surface of the chitosan framework. It can be speculated that the soluble starch had good compatibility with the chitosan framework, which was conducive to enhancing the toughness of the framework. And powder structures were not obviously observed in the whole aerogel microstructure, and no agglomeration appears in the optical photos, indicating that the ratio of CS to CCNF (2:1) was suitable for preparing aerogel with intact block structures [26]. This also revealed that the interaction between CS and CCNF effectively enhanced the supporting strength of the scaffold, which was consistent with previous reports [27]. As shown in Fig. 4f–h, compared to OA, the entire surface of ZOMA modified with 10% zein and 0.5% octadecanal showed more intricately interlaced structures. During evaporation, zein spirals into fragments driven by the change of solvent polarity, accumulates into bands and finally forms filamentous structures. Such filamentous “tentacle”s structures (similar to Strider’s Leg structure [18]) can be clearly observed in the enlarged images of ZOMA surface, which effectively increases the roughness. This unique structure was beneficial for oil–water separation, as water droplets can be supported by “tentacles” when they contacted the surface of ZOMA, resulting in a higher hydrophobic angle and thus improved hydrophobicity. As observed from EDS mapping images (Fig. 4i–l), the S and P atoms on the surface of ZOMA were brighter and more uniform than those on OA surface, which was caused by the successful loading of zein. And uniform distribution of zein on the surface of ZOMA was conducive to the formation of uniform surface roughness.
3.3 Wetting Ability of Aerogels
The wetting ability of the adsorbent material is very important for the separation of oil/water mixtures. The wetting properties of aerogel before and after hydrophobic modification were studied by water contact angle (WCA) determination. As shown in Fig. 5a and f, when water droplets (dyed with methyl blue) landed on the surface of OA aerogel, they spread rapidly within 1 s and were absorbed by the aerogel, indicating that OA has a strong super-hydrophilic surface and was not suitable for oil–water separation engineering. And water contact angle of OA was nearly zero, which also proved this. These results were caused by the fact that OA had many hydrophilic groups such as hydroxyl groups. After modifying with zein (10%), the contact angle of aerogel (ZMA) increased to 118° (Fig. 5b and f), which was related to the roughness produced by zein on the surface of the aerogel. Zein built up uneven “tentacles” on the surface of the aerogel to trap the air in the gap to form an air cushion, reducing the contact area between the water droplet and the surface and improving the hydrophobicity of the aerogel [28]. With the addition of high concentration of zein (15%), the contact angle of aerogel decreased slightly (Fig. S1). To further decrease surface energy of the aerogel, octadecanal with long-chain alkane groups was introduced into ZMA for hydrophobic modification. After the addition of octadecanal at same concentration, the composite aerogel modified with zein and octadecanal (ZOMA) showed higher contact angle than the aerogel modified with octadecanal (OMA) (Table S1), confirming the synergistic effect of zein and octadecanal for hydrophobic modification. And the contact angle of OMA with the addition of 1% octadecanal was 128°, while the contact angle of ZOMA reached 148°, requiring only 0.5% octadecanal (Fig. 5d and e). Such a small amount of modifier added to the aerogel was advantageous to avoid affecting the structure and function of other components. Importantly, compared with OA, ZMA and OMA, the ZOMA exhibited the highest contact angle (148°) close to superhydrophobicity (Fig. 5f), which proved that hydrophobic modification by using zein and octadecanal was effective. Thus the ZOMA aerogel showed rapidly absorb oil (dyed with sudan IV) while repelling water, as shown in Fig. 5c. Because the ZOMA aerogel modified with 10% zein and 0.5% octadecanal showed the best wetting property, it was selected for the following studies. In addition, the immersion-type biomass hydrophobization method in this study did not require any specific equipment, or additional energy, and was more cost-effective and simpler than previously reported hydrophobic modification of polysaccharide-based aerogel [29,30,31].
Wetting properties of aerogels. Digital images of water droplet (dyed with methyl blue) deposited on the surface of OA (a); ZMA (b) and OMA (d); ZOMA (e). c Images of the absorbed oil droplet (dyed with Sudan IV) and standing water droplet (dyed with methyl blue) on ZOMA. f Water contact angles on the surface of different aerogels
3.4 Oil–Water Separation Capacity of Aerogels
Encouraged by the good hydrophobicity and oil-absorbing property of the zein/octadecanal modified composite aerogel (ZOMA), we further evaluated its oil–water separation capacity. As shown in Fig. 6a–b, the ZOMA aerogel rapidly and completely adsorbed the n-hexane (light oil) floating on the surface within a few seconds, when being placed in a beaker of n-hexane/water mixture. After picking the aerogel up with tweezers, there was no obvious n-hexane in the water, and the n-hexane was well retained in the aerogel (Fig. 6c). Besides, the ability of ZOMA aerogel to adsorb trichloromethane (heavy oil) in the wastewater was also studied (Fig. 6e–g). The aerogel selectively absorbed trichloromethane in a few seconds, not the water. And there is no obvious heavy oil residue in the water.
The capacity of ZOMA aerogel for various oils was investigated, including 6 kinds of light oils (n-hexane, n-hexadecane, cyclohexane, toluene, isooctane and rapeseed oil) and 4 kinds of heavy oils (trichloromethane, dichloromethane, 1,2-dichloroethane and carbon tetrachloride). The capacity of heavy/light oil by aerogel was 3 times to 9 times the weight of the aerogel (Fig. 6d and h). The oil absorption capacity by aerogel increased with increasing oil density, which is consistent with previously reported work [30]. Among them, aerogel showed the highest absorption capacity of trichloromethane and the lowest absorption capacity of cyclohexane. As shown in Fig. 5d and h, the oil retention capacity by aerogel was above 96%. Such good oil capacity and retention capacity may be attributed to the numerous three-dimensional pore structures of the aerogel, and rough surface and low surface energy produced by hydrophobic modification of the aerogel by zein and octadecanal. The rough surface and low surface energy facilitate the diffusion of oil or solvent through the pores of the aerogel surface and the rapid entry into the interior of the three-dimensional structure. From the surface morphology, the pores formed by aerogels are consistent and regular in orientation, which makes aerogel have better oil capacity and easy recovery, while the capacity of different oils is slightly different, which may be due to the influence of pore volume on oil of different densities during forming. The three-dimensional pore structure provides a great deal of space for storing absorbed oil. Capillary force causes the adsorbed oil to gradually accumulate in the porous structure [32]. We further discussed the selective of oil in water by aerogel interface. Since the material is obtained by hydrophilic skeleton modified by zein, such weak water still exists in the process of of oil in oil–water mixture. The pores contain very little water, mostly oil, it may be that the oil and water in contact with the material interface, external surface and inner hole in direct competition with the point, and the larger molecular weight in the oil in the whole process of occupy advantage, in line with previous studies [33], this shows that our material has good selective ability. Thus, obtaining ZOMA may be an ideal absorbent for treating oil spills and remediating oily wastewater.
The filtration ability of ZOMA aerogel for heavy oil/water mixtures was also tested. The aerogel was placed in the middle layer of the assembled filtration device, in which the upper part is the filter cup, and the lower part is the triangular beaker for collecting the filtrate (Fig. 7a). After the addition of the heavy oil/water mixture into the upper part, the aerogel was filled with oil (Fig. 7b). only under the gravity of the water column above, the intrusion pressure breaks through the capillary force, resulting in the occurrence of oil–water separation [34]. After 18.6 s, the oil completely penetrated the aerogel and entered the collection device below (Fig. 7c). The Karl Fischer Titrator moisture measurement was performed on the filtered oil. The calculated separation efficiency was 99.8%, and the separation flux was 5595 L m−2 h−1 (Fig. 6d). The reusability of the aerogel was evaluated after up to ten oil/water separation cycles (Fig. 6d). The separation efficiency decreased slightly after ten cycles, and still remained above 98.5%. And the aerogel could still achieve the flux of 3980 L m−2 h−1 after 10 cycles.This may be because that intramolecular interaction and van der Waals forces made the oil adhere to the surface of the aerogel skeleton, resulting in the blockage of small pores [35]. Overall, ZOMA aerogel had good oil–water separation and reusability properties, which will be promising for industrial oil-polluted water treatment.
3.5 Recyclability, Thermal Stability and Mechanical Properties of Aerogels
The recyclability and mechanical properties of aerogel as oil or solvent absorbents are one of the key criteria for practical application in oil purification. The surface of aerogels was discussed above. The of oil/water mixture on aerogels is a spontaneous physical process, in which the oil will occupy the pore space of the material by discharging air and eventually fill the pore space of the whole material. In terms of the recovery of adsorbents and adsorbents, the recovery performance of physical is often better than that of chemical [33], so our elastic aerogels can be recycled and reused economically. The reusability of ZOMA aerogel was investigated by successive absorption-extrusion of rapeseed oil. As shown in Fig. 8a, the mechanical extrusion method was proven to collect the absorbed oil in ZOMA aerogel. And the aerogel recovered its original shape without mechanical extrusion. The amount of oil absorbed by the aerogel in absorption cycle and left in recovery cycle was calculated by the determination of aerogel weight before and after extrusion. About 10% of the oil remained in the aerogel after squeezing in each cycle (Fig. 8b). After 10 cycles of absorption and desorption, ZOMA aerogel remained still more than 90% of its initial absorption capacity, indicating that the aerogel structure remained good. This may be due to the good toughness and flexibility provided by the synergistic effect of chitosan, carboxylated cellulose and starch as the main components of the aerogel [29]. And the absorption capacity of the aerogel for light oil (n-hexane) or heavy oil (carbon tetrachloride) decreased slightly after 10 cycles (Fig. 8c and d), due to blocking the pore structure of the aerogel by the oil [36]. Compared with other reported methods, such as solvent flushing, heating and burning, mechanical extrusion method is competitive due to the advantages of the simplicity and sustainability [37]. The thermal stability of the material was studied by using a thermogravimetric analyzer, and it was shown from the test results (Supporting information, Fig. S2) that there was a significant thermal decomposition weight loss between 100 and 500 degree. The thermal decomposition of 100, 250, 350 and above 350 degree, respectively, corresponds to water decomposition, decomposition of small molecules, decomposition of molecular chains of biomass components, and decomposition of glycosylated rings of polysaccharides [38]. When the temperature reaches 600 degree, the equilibrium is reached, and the thermal decomposition weight loss is 76%. It is worth mentioning that the nitrogen content of zein is higher, and the weight loss is faster between 150 and 200 degree.
The recyclability property of ZOMA aerogel. a Oil collected process by absorption-extrusion method. b Oil recovery ability of the aerogel after absorption and desorption cycles by mechanical squeezing. c and d The capacity of the aerogel for light oil (n-hexane) or heavy oil (carbon tetrachloride) within 10 cycles
The mechanical properties of ZOMA aerogel (test sample length 6 cm, width 4 cm, height 1 cm) composed of three kinds of plysaccharide (chitosan, carboxylated cellulose and starch) were investigated by compression cycle test at the RH of 60% and at the temperature of 27 degree. And the two aerogels (chitosan/carboxylated cellulose aerogel and carboxylated cellulose/starch aerogel) composed of two kinds of plysaccharide were control groups. As shown in Fig. 9a–c, the ZOMA aerogel showed a good compress-recovery property in the radial direction. The Compressive strength of chitosan/carboxylated cellulose aerogel and carboxylated cellulose/starch aerogel is about 80 MPa and 100 MPa, respectively (Fig. 9d and e). And their curve areas were significantly different between the first and subsequent cycles, indicating poor resilience performance, which was consistent with previous reports [13], Energy dissipation and hysteresis are one of the key functions of porous nanostructures. Our aerogels also exhibit hysteresis during compression, which is caused by the densification of the structure and friction between adjacent nanofibers [39]. After 60% strain, there is some local damage, but it still restores to 78% of the original height of the material, which has an advantage over some previous works [40]. After 5 cycles, the energy loss coefficient is about 65%, which is mainly caused by plastic deformation, fracture and friction between adjacent nanofibers. And gradually decreased within 50 cycles, and tended to be stable, showing that the prepared material has good mechanical stability.
In contrast, the cyclic curve area of ZOMA aerogel after multiple compression cycles changed slightly (Fig. 9f), proving that the aerogel showed excellent toughness. This may be due to the uniform distribution of starch on the chitosan framework to enhance the toughness of the framework. And the carboxylated cellulose is connected between the chitosan frameworks to enhance the compressibility (Supporting information, Fig.S3). And the aerogel remained undamaged after 50 compression cycles, the contact Angle only decreased from 148° to 130° (Supporting information, Fig.S4), which is a great improvement over previous work (Supporting information,Table S2).
4 Conclusion
In summary, we prepared an economical and environmentally friendly zein/octadecanal modified polysaccharide-based composite aerogel with good properties and recyclability for efficient oil–water separation. The mixing and cross-linking of chitosan, carboxylated cellulose nanofibers and starch to construct aerogel skeleton enhanced the toughness and flexibility of the aerogel. And the hydrophobic modification of the aerogel by zein and octadecanal through a simple soaking method improves the surface roughness and hydrophobicity of the aerogel. ZOMA aerogel can absorb oil and organic solvent from water. And the oil retention capacity by aerogel was above 96%. The oil–water separation efficiency of the aerogel as a thick filter membrane was 99.8%. After 10 cycles of absorption and desorption, the aerogel remained still more than 90% of its initial absorption capacity. The aerogel prepared by using environmentally friendly and cheap raw materials and simple hydrophobic modification method, has great application prospects in oily wastewater treatment.
Data Availability
The data that support the findings of this article are avaible in Journal of Bionic Engineering webstite (Springer) with the DOI of the article.
References
Yang, Y., Li, X. J., Zheng, X., Chen, Z. Y., Zhou, Q. F., & Chen, Y. (2018). 3D-printed biomimetic super-hydrophobic structure for microdroplet manipulation and oil/water separation. Advanced Materials, 30, 1704912.
Fu, Y., & Guo, Z. G. (2022). Natural polysaccharide-based aerogels and their applications in oil–water separations: A review. Journal of Materials Chemistry A, 10, 8129–8158.
Xu, C. L., Luo, Y. T., Zhou, L., Bi, Y. W., & Sun, H. (2022). Fabrication of durable superhydrophobic stainless steel mesh with nano/micro flower-like morphologies for self-cleaning and efficient oil/water separation. Journal of Bionic Engineering, 19, 1615–1624.
Tian, Y. L., Feng, J. K., Cai, Z. X., Chao, J. Q., Zhang, D. W., Cui, Y. X., & Chen, F. (2021). Dodecyl mercaptan functionalized copper mesh for water repellence and oil-water separation. Journal of Bionic Engineering, 18, 887–899.
Wang, Y. X., Su, Y. H., Wang, W. L., Fang, Y., Riffat, S. B., & Jiang, F. T. (2019). The advances of polysaccharide-based aerogels: Preparation and potential application. Carbohydrate Polymers, 226, 115242.
Ziegler, C., Wolf, A., Liu, W., Herrmann, A. K., Gaponik, N., & Eychmuller, A. (2017). Modern inorganic aerogels. Angewandte Chemie International Edition, 56, 13200–13221.
Wang, W., Dong, C. X., Liu, S. R., Zhang, Y. H., Kong, X. Q., Wang, M., Ding, C., Liu, T. Y., Shen, H. L., & Bi, H. C. (2023). Super-hydrophobic cotton aerogel with ultra-high flux and high oil retention capability for efficient oil/water separation. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 657, 130572.
Wang, N. N., Wang, H., Wang, Y. Y., Wei, Y. H., Si, J. Y., Yuen, A. C. Y., Xie, J. S., Yu, B., Zhu, S. E., Lu, H. D., Yang, W., Chan, Q. N., & Yeoh, G. H. (2019). Robust, lightweight, hydrophobic, and fire-retarded polyimide/MXene aerogels for effective oil/water separation. ACS Applied Materials and Interfaces, 11, 40512–40523.
Delbianco, M., & Seeberger, P. H. (2020). Materials science based on synthetic polysaccharides. Materials Horizons, 7, 963–969.
Yang, W.-J., Yuen, A. C. Y., Li, A., Lin, B., Chen, T. B. Y., Yang, W., Lu, H.-D., & Yeoh, G. H. (2019). Recent progress in bio-based aerogel absorbents for oil/water separation. Cellulose, 26, 6449–6476.
Zhang, Y., Yin, M. L., Li, L., Fan, B. J., Liu, Y., Li, R., Ren, X. H., Huang, T. S., & Kim, I. S. (2020). Construction of aerogels based on nanocrystalline cellulose and chitosan for high efficient oil/water separation and water disinfection. Carbohydrate Polymers, 243, 116461.
Qin, H. F., Zhang, Y. F., Jiang, J. G., Wang, L. L., Song, M. Y., Bi, R., Zhu, P. H., & Jiang, F. (2021). Multifunctional superelastic cellulose nanofibrils aerogel by dual ice-templating assembly. Advanced Functional Materials, 31, 2106269.
Zhang, M. L., Jiang, S., Han, F. Y., Li, M. M., Wang, N., & Liu, L. F. (2021). Anisotropic cellulose nanofiber/chitosan aerogel with thermal management and oil absorption properties. Carbohydrate Polymers, 264, 118033.
Dong, T., Tian, N., Xu, B., Huang, X. H., Chi, S., Liu, Y. M., Lou, C.-W., & Lin, J.-H. (2022). Biomass poplar catkin fiber-based superhydrophobic aerogel with tubular-lamellar interweaved neurons-like structure. Journal of Hazardous Materials, 429, 128290.
Mulyadi, A., Zhang, Z., & Deng, Y. (2016). Fluorine-free oil absorbents made from cellulose nanofibril aerogels. ACS Applied Materials and Interfaces, 8, 2732–2740.
Kasaai, M. R. (2018). Zein and zein-based nano-materials for food and nutrition applications: A review. Trends in Food Science & Technology, 79, 184–197.
Yu, X., Li, C. M., Tian, H. F., Yuan, L., Xiang, A. M., Li, J. L., Wang, C. Y., & Rajulu, A. V. (2020). Hydrophobic cross-linked zein-based nanofibers with efficient air filtration and improved moisture stability. Chemical Engineering Journal, 396, 125373.
Gao, X. F., & Jiang, L. (2004). Water-repellent legs of water striders. Nature, 432(7013), 36–36.
Zhu, F. (2019). Starch based aerogels: Production, properties and applications. Trends in Food Science & Technology, 89, 1–10.
Zhao, J., Xi, X. T., Ouyang, H., Yang, J. Y., Wang, Y., Yi, L. F., Song, D. Y., Song, Y. J., & Zhao, L. J. (2021). Acidic and alkaline gas sensitive and self-healing chitosan aerogel based on electrostatic interaction. Carbohydrate Polymers, 272, 118445.
Wang, H., Kong, L. Y., & Ziegler, G. R. (2019). Fabrication of starch-nanocellulose composite fibers by electrospinning. Food Hydrocolloids, 90, 90–98.
Zhang, F., Chi, H., Wang, C., Wang, X. Y., Wang, Y. C., Zhang, H., Xu, K., Bai, Y. G., & Wang, P. X. (2021). Multifunctional starch-based material for contaminated emulsions separation and purification. Carbohydrate Polymers, 269, 118354.
Wang, Y., & Padua, G. W. (2012). Nanoscale characterization of zein self-assembly. Langmuir, 28, 2429–2435.
Ma, X. T., Lou, Y., Chen, X.-B., Shi, Z., & Xu, Y. (2019). Multifunctional flexible composite aerogels constructed through in-situ growth of metal-organic framework nanoparticles on bacterial cellulose. Chemical Engineering Journal, 356, 227–235.
Jimenez-Saelices, C., Seantier, B., Cathala, B., & Grohens, Y. (2017). Spray freeze-dried nanofibrillated cellulose aerogels with thermal superinsulating properties. Carbohydrate Polymers, 157, 105–113.
Zhang, Z., Tan, J. W., Gu, W. H., Zhao, H. Q., Zheng, J., Zhang, B. S., & Ji, G. (2020). Cellulose-chitosan framework/polyailine hybrid aerogel toward thermal insulation and microwave absorbing application. Chemical Engineering Journal, 395, 125190.
Huang, J. Y., Li, D. W., Zhao, M., Ke, H. Z., Mensah, A., Lv, P., Tian, X. J., & Wei, Q. F. (2019). Flexible electrically conductive biomass-based aerogels for piezoresistive pressure/strain sensors. Chemical Engineering Journal, 373, 1357–1366.
Zhang, G. H., Liu, Y., Chen, C., Long, L., He, J. S., Tian, D., Luo, L., Yang, G., Zhang, X. H., & Zhang, Y. Z. (2022). MOF-based cotton fabrics with switchable superwettability for oil–water separation. Chemical Engineering Science, 256, 117695.
Liu, Q., Yu, H. H., Zeng, F. M., Li, X., Sun, J., Li, C., Lin, H., & Su, Z. M. (2021). HKUST-1 modified ultrastability cellulose/chitosan composite aerogel for highly efficient removal of methylene blue. Carbohydrate Polymers, 255, 117402.
Li, Z. D., Zhong, L., Zhang, T., Qiu, F. X., Yue, X. J., & Yang, D. Y. (2019). Sustainable, flexible, and superhydrophobic functionalized cellulose aerogel for selective and versatile oil/water separation. ACS Sustainable Chemistry & Engineering, 7, 9984–9994.
He, J., Zhao, H. Y., Li, X. L., Su, D., Zhang, F. R., Ji, H. M., & Liu, R. (2018). Superelastic and superhydrophobic bacterial cellulose/silica aerogels with hierarchical cellular structure for oil absorption and recovery. Journal of Hazardous Materials, 346, 199–207.
Zanini, M., Lavoratti, A., Lazzari, L. K., Galiotto, D., Pagnocelli, M., Baldasso, C., & Zattera, A. J. (2016). Producing aerogels from silanized cellulose nanofiber suspension. Cellulose, 24, 769–779.
Huang, J. K., & Yan, Z. F. (2018). Adsorption mechanism of oil by resilient graphene aerogels from oil-water emulsion. Langmuir, 34, 1890–1898.
Wang, R., Zhu, L., Zhu, X., Yan, Z. C., Xia, F. J., Zhang, J. Q., Liu, X. L., Yu, J. P., & Xue, Q. Z. (2023). A super-hydrophilic and underwater super-oleophobic membrane with robust anti-fouling performance of high viscous crude oil for efficient oil/water separation. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 658, 130662.
Dilamian, M., & Noroozi, B. (2021). Rice straw agri-waste for water pollutant adsorption: Relevant mesoporous super hydrophobic cellulose aerogel. Carbohydrate Polymers, 251, 117016.
Chen, X. M., Weibel, J. A., & Garimella, S. V. (2016). Continuous oil–water separation using polydimethylsiloxane-functionalized melamine sponge. Industrial & Engineering Chemistry Research, 55, 3596–3602.
Saini, H., Otyepková, E., Schneemann, A., Zbořil, R., Otyepka, M., Fischer, R. A., & Jayaramulu, K. (2022). Hierarchical porous metal–organic framework materials for efficient oil–water separation. Journal of Materials Chemistry A, 10, 2751–2785.
Horvat, G., Fajfar, T., Perva Uzunalić, A., Knez, Ž, & Novak, Z. (2016). Thermal properties of polysaccharide aerogels. Journal of Thermal Analysis and Calorimetry, 127, 363–370.
Jiang, S. H., Uch, B., Agarwal, S., & Greiner, A. (2017). Ultralight, thermally insulating, compressible polyimide fiber assembled sponges. ACS Applied Materials and Interfaces, 9, 32308–32315.
Wang, H. L., Zhang, X., Wang, N., Li, Y., Feng, X., Huang, Y., Zhao, C. S., Liu, Z. L., Fang, M. H., Ou, G., Gao, H. J., Li, X. Y., & Wu, H. (2017). Ultralight, scalable, and high-temperature–resilientceramic nanofiber sponges. Science advances, 3(6), e1603170.
Acknowledgements
This work is supported by the National Nature Science Foundation of China (no. 51735013).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest/competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fu, Y., Ai, S., Guo, Z. et al. Nature-inspired Polysaccharide-based Aerogel for Oil–water Separation. J Bionic Eng 20, 1956–1966 (2023). https://doi.org/10.1007/s42235-023-00370-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42235-023-00370-w