Abstract
The semiconductive cadmium sulfide (CdS) nanoparticles were coated on the surface of Methanosarcina barkeri (M. barkeri) by self-assembly method to form the M. barkeri-CdS biohybrid in this work. It proved to be an effective and selective catalyst for the solar-driven conversion of CO2 to CH4, enabling the upgrading of biogas from anaerobic digestion. The physicochemical properties of the synthesized biohybrid were characterized, and the effect of various conditions on the CH4 production of the biohybrid was also investigated. It was revealed that the CdS dosage, pH, cysteine, and concentration of sodium bicarbonate were key factors influencing the performance of the biohybrid. Additionally, it was observed that CH4 was produced under both light and dark conditions. Finally, the mechanisms involved in the CH4 production by the biohybrid under light and dark conditions were discussed.
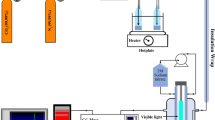
Graphical abstract
Methanosarcina barkeri–cadmium sulfide biohybrid can effectively convert carbon dioxide to methane, achieving biogas upgrading.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Transforming organic pollutants into biogas by anaerobic digestion is an effective method for recovering renewable energy from wastewater [1, 2]. CH4 with a high calorific value is the main content in the biogas [3, 4]. However, more than 30% of CO2 is typically present in biogas, reducing its energy density and confining its further applications [5, 6]. The conversion of CO2 into valuable products such as biodiesel, bioethanol, or additional biogas has aroused wide concern with a view to diminish carbon footprint [7,8,9,10]. Among the various approaches, a feasible strategy to comply with the specifications of natural gas pipelines is the promotion of CO2 biomethanation and biogas upgrading [11,12,13,14,15].
Various methods have been applied to purify and upgrade biogas from anaerobic digestion, including membrane separation [16], cryogenic separation [17], pressure swing adsorption [18], water scrubbing [19], physical scrubbing, chemical adsorption [20], and biological conversion [6]. Bio-electrochemical systems (BES) are recognized as an effective biological conversion method, gaining attention for their capability to increase the CH4 content in biogas up to 98% [21]. The semi-artificial photosynthetic biohybrid, composed of semiconductors and archaea, is considered as another environmentally friendly biological conversion method [22]. The biohybrid integrates the light-harvesting capability of semiconductors with the replicative abilities of microorganisms to fulfill photocatalytic objectives, thereby enhancing solar power conversion efficiency in comparison to conventional photosynthesis in plants [23,24,25]. Carbonate solutions were employed to selectively absorb CO2 components in biogas, and the biohybrid subsequently converted the separated CO2 into CH4. Under illuminated environments, semiconductors capture photons directly on their surface, generating electron–hole pairs [26, 27]. These pairs undergo spatial separation facilitated by sacrificial reagents, releasing electrons and forming reduction equivalents [28]. By establishing appropriate connections between membrane-bound cytochrome proteins and semiconductors, archaea can specifically utilize the electrons to reduce CO2 to CH4 [29].
Recently, Methanosarcina Barkeri–cadmium sulfide (M. barkeri-CdS) biohybrid has been successfully synthesized and demonstrated to be effective in converting CO2 to CH4 with a yield of 0.19 μmol/h [30]. In order to improve the CH4 production efficiency, Ni was further doped on the CdS, forming the M. barkeri-Ni: CdS biohybrid and the CH4 yield was increased by ~ 2.5 times compared to the M. barkeri-CdS biohybrid [31]. To minimize the production of H2 as a common by-product, Ni and Cu were integrated at the interface between the CdS and M. barkeri, and the obtained M. barkeri-NiCu@CdS biohybrid demonstrated a CH4 selectivity of 100% and a quantum yield of 12.41 ± 0.16% [32]. To address the efficiency mismatch between electron production and utilization in M. barkeri-CdS biohybrid, a metal-free polymer carbonitride (CNx) modified with a unique capacitor cyanamide (NCN) group was used as the semiconductor to form the M. barkeri -NCNCNx biohybrid and the CH4 selectivity achieved 92.30% in this biohybrid [33]. An M. barkeri–carbon dot-functionalized polymeric carbon nitrides (CDPCN) photocatalytic system was developed by assembling M. barkeri and CDPCN, achieving a CH4 selectivity of nearly 100% with the assistance of CO2 [34]. Besides, an R. palustris/CdS biohybrid was also found to effectively convert CO2 to CH4 [35].
For upgrading biogas from the anaerobic wastewater treatment, M. barkeri has been identified as a suitable microorganism for biohybrid due to its obligate production of CH4, high tolerance of adverse environments, and extracellular electron transfer ability [36, 37]. CdS, owing to the advantages of modifiable band gaps (Eg ~ 2.4 eV), multiple binding sites, good biocompatibility, and effective light capture, is an appropriate semiconductor for the biohybrid [38,39,40,41,42]. M. barkeri-CdS biohybrid has been demonstrated to effectively convert CO2 to CH4; thus, it is supposed to be a proper biohybrid for biogas upgrading. However, the effect of the key factors such as CdS dosage, pH, cystine, and sodium bicarbonate concentration on the conversion efficiency of the biohybrid still needs to be further investigated to obtain the optimal conditions for biogas upgrading.
In this work, a biohybrid was constructed using the CdS and M. barkeri as semiconductors and electroactive methanogens, respectively. The M. barkeri-CdS biohybrid was characterized, and the effect of CdS dosage in the biohybrid, pH, cystine, and sodium bicarbonate concentration on the transformation efficiency was investigated. Finally, the elemental composition and photochemical properties of the biohybrid with and without light were investigated to understand the mechanism of converting CO2 to CH4 by the M. barkeri-CdS biohybrid.
2 Materials and methods
2.1 Synthesis of M. barkeri-CdS biohybrid
Firstly, M. barkeri was incubated in the medium (Supplementary Table S1) in the serum bottle at 37 °C in a constant temperature incubator (FCE-3000, Kuntian, Shanghai, China) until it reached the late exponential phase. The culture was subsequently transferred into 50 mL of fresh substrate medium at a ratio of 1:5 for the secondary cultivation for 1–2 days. Once the OD600 of the culture reached 0.2 [43], 10 mL of the solution was added to a 20-mL anaerobic tube with CdCl2 solution and then incubated for an additional 2–3 days. The culture was conducted in a constant temperature shaker (SHZ-82, LICHEN, Shanghai, China) in darkness. The speed was maintained at 120 rpm/min, and the temperature was controlled at 37 ℃. When the color of the solution changed to bright yellow, the solid was separated from the culture, obtaining the M. barkeri-CdS biohybrid.
2.2 Characterizations of the biohybrid
The morphology of the biohybrid was characterized using a scanning electron microscope (SEM 500, Zeiss, Germany) [44], whereas the elemental composition was determined through energy dispersive spectroscopy (EDS; AMETEK, Octane elect super, USA). The biohybrid was immobilized with glutaraldehyde and underwent gradient elution with ethanol before the detection. Further microstructural insights were obtained using field emission transmission electron microscopy (FETEM, Tecnai G2 F30, FEI, America) following the previous method [45]. The functional groups of the biohybrid were analyzed by Fourier transform infrared spectrometry (FTIR; Vertex 70, Bruker, Germany), and sample preparation followed the established protocols in the literature [46]. The valence of elements in the biohybrids was determined by using X-ray photoelectron spectroscopy (XPS, 250XI, Escalab, Britain) [47]. The optical properties of the biohybrid were evaluated through ultraviolet–visible (UV–vis) spectroscopy to determine the absorbance at different wavelengths via the coefficient spectrum test. The band gap (Eg) of the biohybrid was calculated following the method in the literature [48]. Photocurrent (I-t) and electrochemical impedance spectroscopy (EIS) were conducted using a CHI620E electrochemical workstation (Chenhua, Shanghai, China). A glass carbon, platinum sheet, and silver chloride were used as anode, cathode, and reference electrode, respectively. A polarization potential of − 0.4 V was applied using a constant potential polarization mode.
2.3 CH4 production by the biohybrid
The medium without organic substrates was aerated and sterilized for further use in the experiments (Supplementary Table S1). For each trial, 10 mL of the synthesized biohybrid was added to a 50-mL medium in an anaerobic serum bottle. The bottle was sealed and injected with 40 mL of mixed gas consisting of 20% CO2 and 80% N2 [49]. Then, the mixture was incubated in an incubator maintained at 37 ℃. A 25-W LED light functioned as a solar energy simulator. The biogas generated was collected by extracting 1 mL of biogas from the serum bottles using a syringe. To maintain internal pressure, 1 mL of mixed gas (20% CO2 and 80% N2) was then injected into the bottle. The gas chromatograph (GC7900, Yunneng International Scientific Instruments, Beijing, China) was used to analyze the CH4 content in the biogas [50], with nitrogen serving as the carrier gas at a pressure of 0.4 MPa. The temperatures of the column oven, injector, and thermal conductivity detector were maintained at 120 °C, 140 °C, and 150 °C, respectively.
3 Results and discussion
3.1 Characterization of the biohybrid
SEM images showed the spherical morphology of the M. barkeri-CdS biohybrid with a rough surface, and the particles were attached to the surface of the M. barkeri (Fig. 1a). TEM images further illustrated that the CdS particles were uniformly distributed on the surface of the M. barkeri (Fig. 1b). EDS mapping showed a similar distribution of sulfur (S) and cadmium (Cd) elements (Fig. 1c,d). The uniformly distributed S and Cd elements as well as the well-dispersed carbon (C) and oxygen (O) elements confirmed the successful synthesis of CdS particles on the surface of M. barkeri (Supplementary Fig. S1), indicating the formation of M. barkeri-CdS biohybrid.
XPS analysis confirmed the presence of the S, C, Cd, and O elements in the biohybrid (Supplementary Fig. S2). The Cd 3d XPS spectrum showed the peaks located at 404.9 eV and 411.5 eV, which corresponded to Cd 3d5/2 and 3d3/2 (Fig. 1e). This indicated Cd in the biohybrid existed in the form of Cd2+ state [51, 52]. The S 2p3/2 peaks at 161 eV and 161.3 eV were attributed to S2− (Fig. 1f), whereas the peak at 164 eV indicated elemental sulfur (S0) [53], with the predominance of S2− peaks in the spectra. These results verified the adhesion of CdS on the surface of M. barkeri, confirming the successful formation of the M. barkeri-CdS biohybrid. The stability of intrinsic functional groups within the biohybrid was evaluated by FT-IR (Fig. 1g). Characteristic peaks at 550 cm−1 and 1399 cm−1 for Cd-S tensile vibrations, along with high-intensity peaks at 1028 cm−1 and 3389 cm−1 for hydroxyl group vibrations were observed [45]. Notably, the hydroxyl peak at 1028 cm−1 showed a red shift in the biohybrid, indicating the interaction between the CdS and M. barkeri in the biohybrid. The groups play a pivotal role in inhibiting hole-electron recombination and enhancing active site exposure [54], thereby facilitating electron transfer in the CH4 production process.
3.2 Methane production by the biohybrid
CdS particles act as the semiconductor for the electron generation in the biohybrid, which plays a vital role in CH4 production. CdCl2 was used as the percussor for CdS; thus, its concentration affects CdS formation in the biohybrid, and excessive Cd2+ inhibits methanogenesis [55]. The effect of CdCl2 addition for the biohybrid on the conversion of CO2 to CH4 under light exposure was investigated (Fig. 2a). Initially, 0.094-mL CH4 was generated from the system added with pure M. barkeri, which was mainly attributed to residual organic matters in the system. When the CdCl2 dosage was 0.5 mM (maintaining a 1:1 CdCl2:Na2S molar ratio), the maximum CH4 production rate reached 65.42 μL/h on the first day, and the cumulative CH4 yield was 1.76 mL. Then, the CH4 production decreased with the increase in CdCl2 dosage. The addition of 0.75-mM CdCl2 initially slowed down CH4 production, but on the fourth day, the CH4 yield reached 37.72 μL/h. When CdCl2 dosage was increased to 1 mM (CdCl2:Na2S ratio of 2:1), CH4 production was significantly reduced to 0.043 mL. This is consistent with the results reported in the previous literature [30, 56]. A high concentration of Cd2+ stimulated the release of reactive oxygen species and suppressed the activity of M. barkeri, inhibiting CO2 reduction to CH4. Thus, the optimal CdCl2 dosage for the biohybrid was recommended to be 0.5 ~ 0.75 mM.
Bicarbonate solution was used as a carbon source for CO2 reduction by the M. barkeri-CdS biohybrid [57]. pH value of the solution determines the forms of the carbon, which significantly influences the metabolic processes of M. barkeri [58]. The effect of the pH of the bicarbonate solution on CH4 production by the M. barkeri-CdS biohybrid was investigated. As shown in Fig. 2b, CH4 generation was minimal at a pH below 3.5 due to the highly acidic conditions inhibiting the growth, metabolism, and acid–base balance of M. barkeri in the biohybrid. Moreover, 3.44 mL, 4.00 mL, 2.18 mL, and 3.96 mL CH4 were produced from the systems at pH levels of 6.52, 8.36, 10.08, and 12.03, respectively. The highest CH4 production rate of 0.5 mL/h was detected at pH 8.36, and no obvious decrease was observed when the pH of the system changed. As was known, H2CO3 and HCO3−, the main forms of carbon existing in the solution under the acidic condition, are challenging to use by the M. barkeri in the biohybrid, which leads to a low CH4 production rate. CO2 and CO32− are the main forms of carbon when the pH of the reaction system is neutral and alkaline. The CO2 is readily available for utilization by the M. barkeri, and the M. barkeri is more active under neutral conditions [59], which resulted in a high CH4 production rate. However, the CH4 production rate remained high when the pH was increased to 12.03, which indicated that the M. barkeri in the biohybrid displayed strong resistance to alkaline solution. This is important for the utilization of biohybrid in biogas upgrading.
Bicarbonate acts as a CO2 source for CH4 production and buffer solution in the system [33, 60]. The effect of bicarbonate concentration on the CH4 production by M. barkeri-CdS biohybrid was investigated (Fig. 2c); 2.81 mL of CH4 was detected in the system with 10 mM of bicarbonate, and the CH4 production decreased obviously with increasing bicarbonate concentration in the solution. This indicates that increased bicarbonate levels can negatively affect CH4 generation, possibly due to the toxicity of Na+ on the activity of M. barkeri in the biohybrid [61, 62]. Cysteine (Cys) has a dual role as a sulfur source for CdS synthesis and a reducing agent for capturing the generated holes in the biohybrid [63]. The effect of Cys dosage on CH4 production by the biohybrid was evaluated (Fig. 2d). High CH4 production was detected when the Cys dosage was 0.05 wt%, and it had a slight decrease when the Cys dosage increased; 4.15 mL of CH4 was produced when 0.06 wt% of Cys was added into the reaction system. The added Cys captured the holes generated from the CdS under illumination, which avoids the damage of M. barkeri by the oxidative intermediates. It can also promote electron–hole separation, facilitating photoelectron utilization and CO2 reduction by M. barkeri [64, 65].
3.3 Electron transfer in the biohybrid
CH4 production by the biohybrid was detected through the light–dark cycle, each consisting of 24 h of light followed by 24 h of darkness. There was a significant increase in CH4 production, with a total yield of 4.30 mL after four cycles of incubation (Fig. 3a). It was revealed that CH4 can be produced during both the light and dark periods. It can be inferred that the photoelectrons generated by CdS acted as the electron donors for the reduction of CO2 to CH4 by the biohybrid. Theoretically, no electrons can be generated under dark conditions, while CH4 was still generated at these stages. Moreover, the CH4 generation rate during the light period was higher than during the dark period counterpart. It was deduced that a part of the generated electrons was stored in the biohybrid during the light period, and these stored electrons can be released and used by the M. barkeri to reduce CO2 to CH4 under dark conditions. Moreover, the stored reductive intermediates (e.g., NADH, NADPH, ferredoxin, acetyl-CoA) during the light period might also be used by the biohybrid for CO2 reduction into CH4 [66].
(a) CH4 yields of the synthesized biohybrid; (b) the I-t curve of pure M. barkeri, CdS, and biohybrid during light on/off cycles; (c) Nyquist plots of pure M. barkeri, CdS, and biohybrid under illumination; (d) Nyquist plots of the biohybrid under illumination and dark; (e) UV–Vis absorption spectra of pure M. barkeri, CdS, and biohybrid with different concentrations of Cys; (f) FT-IR of the pure M. barkeri and biohybrid before and after illumination
The efficiency of photoelectron-hole separation in the M. barkeri-CdS biohybrid was determined by the photocurrent transient analysis (Fig. 3b). The M. barkeri exhibited a minimal and consistent linear photocurrent signal (14.9 μA/cm2), reflecting bioelectric feedback from irradiation [67]. In contrast, CdS displayed a marginal increase in current density over time, indicating limited photocatalytic activity due to poor charge carrier separation [68]. Conversely, the M. barkeri-CdS biohybrid showed pronounced current fluctuations during light–dark cycles, achieving a significantly high current density (230 μA/cm2) under light conditions. This enhancement is attributed to the superior photon absorption of CdS and a lower Fermi level, facilitating charge transfer across the interface. This stable current density suggests a more effective separation of electron–hole pairs, credited to the utilization of outer membrane-bound electron acceptors of the biohybrid [69]. Overall, the biohybrid has an outstanding performance on photoelectron generation as well as hole separation, which facilitates the CH4 production by the M. barkeri-CdS biohybrid.
The photoelectrochemical characteristics of the M. barkeri-CdS biohybrid were further analyzed by EIS. In EIS Nyquist plots, a smaller semicircle radius indicates improved charge carrier transport [70]. In both dark and light conditions, the M. barkeri-CdS biohybrid exhibited a significantly small semicircle diameter compared to pure CdS and M. barkeri alone (Fig. 3c, Supplementary Fig. S3), suggesting a reduced electron transfer resistance and increased photocurrent density within the biohybrid. Furthermore, a decrease in impedance under light compared to dark conditions (Fig. 3d) indicated enhanced photoelectron production and conductance. UV–vis spectroscopy (Fig. 3e) showed the bandgap (Eg) of CdS reached 2.70 eV, likely due to quantum size effects [71]. The biohybrid displayed a wavelength of absorption edge (λg) at 470 nm and an Eg of 2.63 eV, confirming successful CdS doping on M. barkeri and extending the Eg of M. barkeri and λg of CdS. Importantly, the λg of M. barkeri-CdS (0.05 wt% Cys) exhibited a significant red-shift compared to M. barkeri-CdS (0.06 wt% Cys), indicating increased photocatalytic activity. The optimal Cys concentration led to a narrowed bandgap, optimizing visible light absorption without introducing localized states between the valence bands and conduction bands [72], which led to a higher CH4 production.
The impact of light exposure on the chemical properties of the biohybrid was assessed by FT-IR (Fig. 3f). No obvious change in the peak positions of the biohybrid was observed, indicating no significant alterations for functional groups of the biohybrid before and after light exposure. This stability showed the robust structural integrity of this biohybrid under light exposure. Notably, a peak corresponding to the hydroxyl group (1028 cm−1) was present in the biohybrid under the light condition. It was inferred that the photogenerated electrons interacted with the hydroxyl group, which facilitated electron transfer and inhibited electron–hole recombination in the biohybrid. Besides, M. barkeri is hydrophilic due to abundant electron-rich hydroxyl groups, contributing to the interaction with the CdS via hydrogen bond and electrostatic interactions. Under light conditions, CdS was capable of generating electrons and holes, with the hydroxyl anion/radical redox couple efficiently transferring holes from the CdS to the scavenger. The hydroxyl group transfers from CdS to hydroxyl oxygen by accepting electrons, potentially enhancing the photocatalytic efficiency of the photocatalyst during the reaction.
XPS analysis was used to examine the valence changes of elements within the biohybrid before and after light exposure, providing insights into the electron transfer mechanism involved in CO2 reduction to CH4 by the biohybrid. The XPS spectrum identified C, O, S, and Cd as the main elements (Supplementary Fig. S2), with C, O, and Cd in the biohybrid maintaining stable valence states throughout the reaction (Fig. 4a–c). Notable changes were observed in the S 2p spectra before and after light exposure (Fig. 4d). It was proposed that the S can act as an effective electron transfer hub in biohybrid catalysts through valence state adjustments [73]. Initially, the S 2p spectrum peaked at 161 eV and 161.7 eV for S2− and 164 eV for S0. After light exposure, the peak location shifted, revealing predominant peaks for S2− at 161.1 eV and 162.3 eV, while the peak for S0 decreased. This indicates the dynamic electron interactions under light conditions. Therefore, the generated photoelectrons under light conditions were mainly used to convert CO2 to CH4 by the M. barkeri-CdS hybrid.
The valence state of the elements was also determined to disclose the electron transfer during CH4 production by the biohybrid under dark conditions. The biohybrid was predominantly composed of C, O, Cd, and S (Fig. 5a–c). The changes in S valence were detected in biohybrid (Fig. 5d). The peaks at 164 eV corresponded to S0, whereas those at 161.7 eV and 160.9 eV corresponded to S2− [69]. Both S2− and S0 were detected in the biohybrid, with an increased intensity of S0 after the reaction. It was inferred that S2− was oxidized to S0, providing electrons for the reduction of CO2 to CH4 by M. barkeri under dark conditions.
4 Conclusion
The M. barkeri-CdS biohybrid was successfully developed to enhance CO2 reduction for CH4 production under illuminated conditions. Optimal performance was achieved with a CdCl2 dosage of 0.75 mM, pH of 8.36, NaHCO3 concentration of 10 mM, and Cys concentration of 0.05 wt%. The biohybrid was demonstrated to be efficient in photoelectron generation, leading to a current with reduced impedance under light conditions. Photoelectrons generated under light conditions primarily drive the conversion of CO2 to CH4 by the M. barkeri-CdS biohybrid, whereas S2− serves as the potential electron donor under dark conditions.
Data availability
The datasets generated from the current study will be provided upon reasonable request.
References
Chen X, Liu W, Zhao Y, He H, Ma J, Cui Z, Yuan X (2023) Optimization of semi-continuous dry anaerobic digestion process and biogas yield of dry yellow corn straw: based on “gradient anaerobic digestion reactor.” Biores Technol 389:129804. https://doi.org/10.1016/j.biortech.2023.129804
Wang Z, He H, Yan J, Xu Z, Yang G, Wang H, Zhao Y, Cui Z, Yuan X (2024) Influence of temperature fluctuations on anaerobic digestion: optimum performance is achieved at 45 °C. Chem Eng J 492:152331. https://doi.org/10.1016/j.cej.2024.152331
Lu J, Yang Y, Zhing Y, Hu Q, Qiu B (2022) The study on activated carbon, magnetite, polyaniline and polypyrrole development of methane production improvement from wastewater treatment. ES Food Agrofor 10:30–38. https://doi.org/10.30919/esfaf802
Noori MT, Vu MT, Ali RB, Min B (2020) Recent advances in cathode materials and configurations for upgrading methane in bioelectrochemical systems integrated with anaerobic digestion. Chem Eng J 392:18. https://doi.org/10.1016/j.cej.2019.123689
Aghel B, Behaein S, Alobaid F (2022) CO2 capture from biogas by biomass-based adsorbents: a review. Fuel 328:18. https://doi.org/10.1016/j.fuel.2022.125276
Wu L, Wei W, Song L, Wozniak-Karczewska M, Chrzanowski L, Ni BJ (2021) Upgrading biogas produced in anaerobic digestion: biological removal and bioconversion of CO2 in biogas. Renew Sustain Energy Rev 150:22. https://doi.org/10.1016/j.rser.2021.111448
AbdulRasheed T, Afotey B, Ankudey EG, Anang DA (2023) Synthesis and characterization of novel calcium oxide/calcium ferrite, CaO/CaFe2O4 composite nanocatalyst for biodiesel production. ES Mater Manuf 22:922. https://doi.org/10.30919/esmm922
Das S, Goswami T, Ghosh A, Bhat M, Hait M, Jalgham RTT, Vidya L, Das R, Goswami J, Roymahapatra G (2024) Biodiesel from algal biomass: renewable, and environment-friendly solutions to global energy needs and its current status. ES Gen https://doi.org/10.30919/esg1133
Jamradloedluk J, Trisupakitti S (2023) Two-step biodiesel production from black acid oil waste using calcium oxide from charcoal ash as a catalyst. Eng Sci 28:1053. https://doi.org/10.30919/es1053
Roymahapatra G, Pradhan S, Sato S, Nakane D, Hait M, Bhattacharyya S, Saha R, Akitsu T (2023) Computational study on docking of laccase and cyanide-bridged Ag-Cu complex for designing the improved biofuel cell cathode. ES Energy Environ 21:957. https://doi.org/10.30919/esee957
Batlle-Vilanova P, Rovira-Alsina L, Puig S, Balaguer MD, Icaran P, Monsalvo VM, Rogalla F, Colprim J (2019) Biogas upgrading, CO2 valorisation and economic revaluation of bioelectrochemical systems through anodic chlorine production in the framework of wastewater treatment plants. Sci Total Environ 690:352–360. https://doi.org/10.1016/j.scitotenv.2019.06.361
Fu S, Angelidaki I, Zhang Y (2021) In situ biogas upgrading by CO2-to-CH4 bioconversion. Trends Biotechnol 39:336–347. https://doi.org/10.1016/j.tibtech.2020.08.006
Iniyan S, Ren J, Deshmukh S, Rajeswaran K, Jegan G, Hou H, Suryanarayanan V, Murugadoss V, Kathiresan M, Xu B, Guo Z (2023) An overview of metal-organic framework based electrocatalysts: design and synthesis for electrochemical hydrogen evolution, oxygen evolution, and carbon dioxide reduction reactions. Chem Rec 23:e2023003. https://doi.org/10.1002/tcr.202300317
Shi J, Wang A, An Y, Chen S, Bi C, Qu L, Shi C, Kang F, Sun C, Huang Z, Qi H, Hu J (2024) Core@shell-structured catalysts based on Mg-O-Cu bond for highly selective photoreduction of carbon dioxide to methane. Adv Compos Hybrid Mater 7:2. https://doi.org/10.1007/s42114-023-00801-6
Hamukwaya SL, Zhao Z, Hao H, Abo-Dief HM, Abualnaja KM, Alanazi AK, Mashingaidze MM, El-Bahy SM, Huang M, Guo Z (2022) Enhanced photocatalytic performance for hydrogen production and carbon dioxide reduction by a mesoporous single-crystal-like TiO2 composite catalyst. Adv Compos Hybrid Mater 5:2620–2630. https://doi.org/10.1007/s42114-022-00545-9
Brunetti A, Barbieri G (2022) Multi-step membrane process for biogas upgrading. J Membr Sci 652:10. https://doi.org/10.1016/j.memsci.2022.120454
Pellegrini LA, De Guido G, Langé S (2018) Biogas to liquefied biomethane via cryogenic upgrading technologies. Renew Energy 124:75–83. https://doi.org/10.1016/j.renene.2017.08.007
Vilardi G, Bassano C, Deiana P, Verdone N (2020) Exergy and energy analysis of biogas upgrading by pressure swing adsorption: dynamic analysis of the process. Energy Convers Manag 226:14. https://doi.org/10.1016/j.enconman.2020.113482
Wang H, Ma C, Yang Z, Lu X, Ji X (2020) Improving high-pressure water scrubbing through process integration and solvent selection for biogas upgrading. Appl Energy 276:11. https://doi.org/10.1016/j.apenergy.2020.115462
Yang H, Wang X, Liu J, Liu W, Gong Y, Sun Y (2022) Amine-impregnated polymeric resin with high CO2 adsorption capacity for biogas upgrading. Chem Eng J 430:8. https://doi.org/10.1016/j.cej.2021.132899
Fu X, Li J, Pan X, Huang L, Li C, Cui S, Liu H, Tan Z, Li W (2020) A single microbial electrochemical system for CO2 reduction and simultaneous biogas purification, upgrading and sulfur recovery. Biores Technol 297:4. https://doi.org/10.1016/j.biortech.2019.122448
Wang S, Han X, Zhang Y, Tian N, Ma T, Huang H (2020) Inside-and-out semiconductor engineering for CO2 photoreduction: from recent advances to new trends. Small Structs 2:49. https://doi.org/10.1002/sstr.202000061
Lee C-Y, Zou J, Bullock J, Wallace GG (2019) Emerging approach in semiconductor photocatalysis: towards 3D architectures for efficient solar fuels generation in semi-artificial photosynthetic systems. J Photochem Photobiol C 39:142–160. https://doi.org/10.1016/j.jphotochemrev.2019.04.002
Sahoo PC, Pant D, Kumar M, Puri SK, Ramakumar SSV (2020) Material-microbe interfaces for solar-driven CO2 bioelectrosynthesis. Trends Biotechnol 38:1245–1261. https://doi.org/10.1016/j.tibtech.2020.03.008
Cestellos-Blanco S, Zhang H, Kim JM, Shen Y, Yang P (2020) Photosynthetic semiconductor biohybrids for solar-driven biocatalysis. Nat Catal 3:245–255. https://doi.org/10.1038/s41929-020-0428-y
Wang R, Wang Y, Mao S, Hao X, Duan X, Wen Y (2021) Different morphology MoS2 over the g-C3N4 as a boosted photo-catalyst for pollutant removal under visible-light. J Inorg Organomet Polym Mater 31:32–42. https://doi.org/10.1007/s10904-020-01626-2
Chamanehpour E, Sayadi MH, Hajiani M (2022) A hierarchical graphitic carbon nitride supported by metal–organic framework and copper nanocomposite as a novel bifunctional catalyst with long-term stability for enhanced carbon dioxide photoreduction under solar light irradiation. Adv Compos Hybrid Mater 5:2461–2477. https://doi.org/10.1007/s42114-022-00459-6
Hu L, Yang J, Xia Q, Zhang J, Zhao H, Lu Y (2024) Chemico-biological conversion of carbon dioxide. J Energy Chem 89:371–387. https://doi.org/10.1016/j.jechem.2023.10.058
Li L, Xu Z, Huang X (2021) Whole-cell-based photosynthetic biohybrid systems for energy and environmental applications. ChemPlusChem 86:1021–1036. https://doi.org/10.1002/cplu.202100171
Ye J, Yu J, Zhang Y, Chen M, Liu X, Zhou S, He Z (2019) Light-driven carbon dioxide reduction to methane by Methanosarcina barkeri-CdS biohybrid. Appl Catal B-Environ 257:8. https://doi.org/10.1016/j.apcatb.2019.117916
Ye J, Ren G, Kang L, Zhang Y, Liu X, Zhou S, He Z (2020) Efficient photoelectron capture by Ni decoration in Methanosarcina barkeri-CdS biohybrids for enhanced photocatalytic CO2-to-CH4 conversion. iScience 23:101287. https://doi.org/10.1016/j.isci.2020.101287
Ye J, Wang C, Gao C, Fu T, Yang C, Ren G, Lu J, Zhou S, Xiong Y (2022) Solar-driven methanogenesis with ultrahigh selectivity by turning down H2 production at biotic-abiotic interface. Nat Commun 13:6612. https://doi.org/10.1038/s41467-022-34423-1
Hu A, Ye J, Ren G, Qi Y, Chen Y, Zhou S (2022) Metal-free semiconductor-based bio-nano hybrids for sustainable CO2-to-CH4 conversion with high quantum yield. Angew Chem-Int Ed 61:6. https://doi.org/10.1002/anie.202206508
Ye J, Chen Y, Gao C, Wang C, Hu A, Dong G, Chen Z, Zhou S, Xiong Y (2022) Sustainable conversion of microplastics to methane with ultrahigh selectivity by a biotic-abiotic hybrid photocatalytic system. Angew Chem-Int Ed 61:e202213244. https://doi.org/10.1002/anie.202213244
Chen M, Fang Z, Xu L, Zhou D, Yang X, Zhu H, Yong Y (2021) Enhancement of photo-driven biomethanation under visible light by nano-engineering of Rhodopseudomonas palustris. Bioresources Bioprocess 8:9. https://doi.org/10.1186/s40643-021-00383-5
Zhang W, Chen B, Li A, Zhang L, Li R, Yang T, Xing W (2019) Mechanism of process imbalance of long-term anaerobic digestion of food waste and role of trace elements in maintaining anaerobic process stability. Biores Technol 275:172–182. https://doi.org/10.1016/j.biortech.2018.12.052
Zhao Z, Wang J, Li Y, Zhu T, Yu Q, Wang T, Liang S, Zhang Y (2020) Why do DIETers like drinking: metagenomic analysis for methane and energy metabolism during anaerobic digestion with ethanol. Water Res 171:14. https://doi.org/10.1016/j.watres.2019.115425
Cheng L, Xiang Q, Liao Y, Zhang H (2018) CdS-based photocatalysts. Energy Environ Sci 11:1362–1391. https://doi.org/10.1039/c7ee03640j
Sun Q, Wang N, Yu J, Yu J (2018) A hollow porous CdS photocatalyst. Adv Mater 30:1804368. https://doi.org/10.1002/adma.201804368
Li J, Li Y, Qi M, Lin Q, Tang Z, Xu Y (2020) Selective organic transformations over cadmium sulfide-based photocatalysts. ACS Catal 10:6262–6280. https://doi.org/10.1021/acscatal.0c01567
Lin C, Zhang Y, Zhang S, Wang XX, Yang J, Li J, Lu X, Liu B, School Of Chemistry And Chemical Engineering X S Y U, School Of Materials Science Engineering Shan Dong University S J C (2023) Facile fabrication of a novel g-C3N4/CdS composites catalysts with enhanced photocatalytic performances. ES Energy Environ 20:860. https://doi.org/10.30919/esee8c
Jasmine J, Ponvel KM (2023) Synthesis of Ag2CdS2/carbon nanocomposites for effective solar-driven dye photodegradation and electrochemical application. ES Energy Environ 20:898. https://doi.org/10.30919/esee898
Liu G, Gao F, Zhang H, Wang L, Gao C, Xiong Y (2021) Biosynthetic CdS-Thiobacillus thioparus hybrid for solar-driven carbon dioxide fixation. Nano Res 16:4531–4538. https://doi.org/10.1007/s12274-021-3883-0
Ouyang L, Huang W, Huang M, Qiu B (2022) Polyaniline improves granulation and stability of aerobic granular sludge. Adv Compos Hybrid Mater 5:1126–1136. https://doi.org/10.1007/s42114-022-00450-1
Lu H, Zhao B, Pan R, Yao J, Qiu J, Luo L, Liu Y (2014) Safe and facile hydrogenation of commercial degussa P25 at room temperature with enhanced photocatalytic activity. RSC Adv 4:1128–1132. https://doi.org/10.1039/c3ra44493g
Zhang K, Liu X, Bi J, BaQais A, Xu BB, Amin MA, Hou Y, Liu X, Li H, Algadi H, Xu J, Guo Z (2023) Bimetallic NiCe/Lay catalysts facilitated co-pyrolysis of oleic acid and methanol for efficiently preparing anaerobic hydrocarbon fuels. New J Chem 47:18272–18284. https://doi.org/10.1039/d3nj01359f
Wang Y, Lu H, Wang Y, Qiu J, Wen J, Zhou K, Chen L, Song G, Yao J (2016) Facile synthesis of TaOxNy photocatalysts with enhanced visible photocatalytic activity. RSC Adv 6:1860–1864. https://doi.org/10.1039/c5ra23087j
Ka F, Saidi I, Ben Jannet H, Khairy M, Abdulkhair BY, Al-Ghamdi YO, Abdelhamid HN (2022) Chitosan-CdS quantum dots biohybrid for highly selective interaction with copper(II) ions. ACS Omega 7:21014–21024. https://doi.org/10.1021/acsomega.2c01793
Kim J, Cestellos-Blanco S, Shen YX, Cai R, Yang P (2022) Enhancing biohybrid CO2 to multicarbon reduction via adapted whole-cell catalysts. Nano Lett 22:5503–5509. https://doi.org/10.1021/acs.nanolett.2c01576
Huang Y, Cai B, Dong H, Li H, Yuan J, Xu H, Wu H, Xu Z, Sun D, Dang Y, Holmes DE (2022) Enhancing anaerobic digestion of food waste with granular activated carbon immobilized with riboflavin. Sci Total Environ 851:158172. https://doi.org/10.1016/j.scitotenv.2022.158172
Kumar V, Singh N, Jana S, Rout SK, Dey RK, Singh GP (2021) Surface polar charge induced Ni loaded CdS heterostructure nanorod for efficient photo-catalytic hydrogen evolution. Int J Hydrogen Energy 46:16373–16386. https://doi.org/10.1016/j.ijhydene.2020.06.176
Bai L, Li S, Ding Z, Wang X (2020) Wet chemical synthesis of CdS/ZnO nanoparticle/nanorod hetero-structure for enhanced visible light disposal of Cr(VI) and methylene blue. Colloid Surface A. https://doi.org/10.1016/j.colsurfa.2020.125489
Jiang Z, Wang B, Yu J, Wang J, An T, Zhao H, Li H, Yuan S, Wong PK (2018) AglnS2/In2S3 heterostructure sensitization of Escherichia coli for sustainable hydrogen production. Nano Energy 46:234–240. https://doi.org/10.1016/j.nanoen.2018.02.001
Yuan G, Zhao X, Liang Y, Peng L, Dong H, Xiao Y, Hu C, Hu H, Liu Y, Zheng M (2019) Small nitrogen-doped carbon dots as efficient nanoenhancer for boosting the electrochemical performance of three-dimensional graphene. J Colloid Interface Sci 536:628–637. https://doi.org/10.1016/j.jcis.2018.10.096
Wang J, Li X, Yan J, Yang Y (2022) Effects of heavy metal ions on microbial reductive dechlorination of 1, 2-dichloroethane and tetrachloroethene. Front Mar Sci 9:11. https://doi.org/10.3389/fmars.2022.881950
Hu A, Fu T, Ren G, Zhuang M, Yuan W, Zhong S, Zhou S (2022) Sustained biotic-abiotic hybrids methanogenesis enabled using metal-free black phosphorus/carbon nitride. Front Microbiol 13:10. https://doi.org/10.3389/fmicb.2022.957066
Wang J, Fu R, Wen S, Ning P, Helal MH, Salem MA, Xu BB, El-Bahy ZM, Huang M, Guo Z, Huang L, Wang Q (2022) Progress and current challenges for CO2 capture materials from ambient air. Adv Compos Hybrid Mater 5:2721–2759. https://doi.org/10.1007/s42114-022-00567-3
He P, Duan H, Han W, Liu Y, Shao L, Lü F (2019) Responses of Methanosarcina barkeri to acetate stress. Biotechnol Biofuels 12:14. https://doi.org/10.1186/s13068-019-1630-5
Duan H, He P, Zhang H, Shao L, Lü F (2022) Metabolic regulation of mesophilic Methanosarcina barkeri to ammonium inhibition. Environ Sci Technol 56:8897–8907. https://doi.org/10.1021/acs.est.2c01212
Lee E, Bittencourt P, Casimir L, Jimenez E, Wang M, Zhang Q, Ergas SJ (2019) Biogas production from high solids anaerobic co-digestion of food waste, yard waste and waste activated sludge. Waste Manag 95:432–439. https://doi.org/10.1016/j.wasman.2019.06.033
Lee J, Kim E, Hwang S (2021) Effects of inhibitions by sodium ion and ammonia and different inocula on acetate-utilizing methanogenesis: methanogenic activity and succession of methanogens. Biores Technol 334:125202. https://doi.org/10.1016/j.biortech.2021.125202
Valença RB, dos Santos LA, Firmo AL, da Silva LC, de Lucena TV, Santos AF, Jucá JF (2021) Influence of sodium bicarbonate (NaHCO3) on the methane generation potential of organic food waste. J Clean Prod 317:8. https://doi.org/10.1016/j.jclepro.2021.128390
Chen M, Zhou X, Yu Y, Liu X, Zeng R, Zhou S, He Z (2019) Light-driven nitrous oxide production via autotrophic denitrification by self-photosensitized Thiobacillus denitrificans. Environ Int 127:353–360. https://doi.org/10.1016/j.envint.2019.03.045
Wang B, Xiao K, Jiang Z, Wang J, Yu J, Wong P (2019) Biohybrid photoheterotrophic metabolism for significant enhancement of biological nitrogen fixation in pure microbial cultures. Energy Environ Sci 12:2185–2191. https://doi.org/10.1039/c9ee00705a
Zhang K, Li R, Chen J, Chai L, Lin Z, Zou L, Shi Y (2024) Biohybrids of twinning Cd0.8Zn0.2S nanoparticles and Sporomusa ovata for efficient solar-driven reduction of CO2 to acetate. Appl Catal B: Environ 342:11. https://doi.org/10.1016/j.apcatb.2023.123375
Ye J, Chen Y, Gao C, Wang C, Hu A, Dong G, Chen Z, Zhou S, Xiong Y (2022) Sustainable conversion of microplastics to methane with ultrahigh selectivity by a biotic-abiotic hybrid photocatalytic system. Angew Chem-Int Ed 61:8. https://doi.org/10.1002/anie.202213244
Wu H, Feng X, Wang L, Chen C, Wu P, Li L, Xu J, Qi F, Zhang S, Huo F, Zhang W (2023) Solar energy for value-added chemical production by light- powered microbial factories. CCS Chem 10.31635. https://doi.org/10.31635/ccschem.023.202303011
Guo C, Tian K, Wang L, Liang F, Wang F, Chen D, Ning J, Zhong Y, Hu Y (2021) Approach of fermi level and electron-trap level in cadmium sulfide nanorods via molybdenum doping with enhanced carrier separation for boosted photocatalytic hydrogen production. J Colloid Interface Sci 583:661–671. https://doi.org/10.1016/j.jcis.2020.09.093
Yi X, Liu S, Luo M, Li Q, Wang Y (2022) An outer membrane photosensitized Geobacter Sulfurreducens-CdS biohybrid for redox transformation of Cr(VI) and tetracycline. J Hazard Mater 431:11. https://doi.org/10.1016/j.jhazmat.2022.128633
Kang F, Jiang X, Wang Y, Ren J, Xu B, Gao G, Huang Z, Guo Z (2023) Electron-rich biochar enhanced Z-scheme heterojunctioned bismuth tungstate/bismuth oxyiodide removing tetracycline. Inorg Chem Front 10:6045–6057. https://doi.org/10.1039/d3qi01283b
Zhang S, Li C, Ke C, Liu S, Yao Q, Huang W, Dang Z, Guo C (2023) Extracellular polymeric substances sustain photoreduction of Cr(VI) by Shewanella oneidensis-CdS biohybrid system. Water Res 243:120339. https://doi.org/10.1016/j.watres.2023.120339
Wang Y, Zhang Y, Lu H, Chen Y, Liu Z, Su S, Xue Y, Yao J, Zeng H (2018) Novel N-doped ZrO2 with enhanced visible-light photocatalytic activity for hydrogen production and degradation of organic dyes. RSC Adv 8:6752–6758. https://doi.org/10.1039/c7ra12938f
Wang C, Yu J, Ren G, Hu A, Liu X, Chen Y, Ye J, Zhou S, He Z (2022) Self-replicating biophotoelectrochemistry system for sustainable CO methanation. Environ Sci Technol 56:4587–4596. https://doi.org/10.1021/acs.est.1c08340
Funding
This work is sponsored by the Beijing Nova Program (20220484080). The authors extend their appreciation to the Deanship of Scientific Research at Northern Border University, Arar, KSA, for funding this research (NBU-FPEJ-2024–249-04).
Author information
Authors and Affiliations
Contributions
Ziyu Wang: data curation, formal analysis, methodology, writing-original draft, writing-review & editing.
Mingyu Gou: data curation, visualization, methodology, formal analysis, writing-original draft, writing-review & editing.
Qiyuan Zheng: data curation, formal analysis.
Haiyu Xu: Methodology, writing-review & editing.
Saad Melhi: formal analysis, methodology, review & editing,
Zeinhom M. El-Bahy: formal analysis, methodology, review & editing.
Eman Ramadan Elsharkawy: formal analysis, methodology, review & editing.
Yan Dang: Conceptualization, methodology, writing-review & editing.
Bin Qiu: Conceptualization, project administration, investigation, methodology, writing-original draft, writing-review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Z., Gou, M., Zheng, Q. et al. Enhanced biogas upgrading by photocatalytic conversion of carbon dioxide to methane by Methanosarcina barkeri–cadmium sulfide biohybrid. Adv Compos Hybrid Mater 7, 111 (2024). https://doi.org/10.1007/s42114-024-00926-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42114-024-00926-2