Abstract
The water quality of Owan river, a very important river in Ovia North-East Local Government Area of Edo State, Nigeria was assessed. American Public Health Association (APHA) standard analytical procedures for water and sediment samples were used. Elemental composition of the sediment samples were determined by Energy Dispersive X-ray Fluorescence Spectrometer (EDXRF). Results obtained were statistically analysed (basic descriptive). In water, concentrations (mg/L) of nitrate—(2.65–21.55), sulphate—(58.6–80.0), calcium —(3.21–8.60), dissolved oxygen (5.5–6.53), total hardness (12.60–42.0), alkalinity (32.0–50.5), Cd (0.01), Cr (0.1–0.15), As (0.01–0.05), and Zn (1.4–2.04), electrical conductivity (32.0–38.0 µS/cm) and pH (6.10–6.85) were within environmental standards. However, Fe and Pb were slightly above the standards. In the sediment samples, Al, Si, Fe, and K are the most abundant elements. The highest values were detected as follows: Al—8.09 (SW7), Si—25.08 (SW5), Fe—16.72 (SW6), and K—2.1 (SW7). Urgent checks and monitoring by relevant agencies, government representatives, public health practitioners, and community leaders are required.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Springs, streams, hand-dug wells, and boreholes are some of the most common drinking water sources in underdeveloped and developing countries; in most cases, they are untreated (Owamah et al., 2013; Abulude et al., 2019). These water sources are sometimes polluted from human activities rendering them not potable. Furthermore, since the industrialization, environmental damage is now a worldwide critical issue affecting both developed and developing countries, with the resultant creation of inorganic pollutants such as heavy metals and organic pollutants such as cleaning products, fertilisers, pesticides, pharmaceuticals, and biomaterials. Pathogens, aromatic substances, and toxicants are the most common sources of water pollution (Akinnusotu & Arawande, 2016; Verma et al., 2018), because many are materials of natural accumulation, such as weathering reactions, bioactivity, volcanic eruptions, and rock solubility (Nnorom et al., 2019). When water is contaminated with toxic substances such as potentially toxic elements (PTEs), it becomes unfit for drinking and other domestic uses (Nazeer et al., 2014; Popoola et al., 2019).
More crucially, the possible dangers to human health and aquatic ecosystems end up making trace element contamination of water systems a continual environmental issue (Farahat & Linderholm, 2015). Groundwater and surface water are inherently connected, hence it is frequently difficult to distinguish between them because they recharge one another thus can pollute each other (Trcek & Zojer, 2010; Akinnusotu et al., 2015).
The organic fraction of the sedimentary column of aquatic environments trades nutrients with the overlying water column. Sediments, in general, are more than just a storage facility for products found in the water column; (i) they also serve as a recycling compartment for some biological pathways involving compounds, physicochemical, chemical, and transport processes (ii) the bioavailability and toxicity of heavy metals in sediments are influenced by sediment properties. (iii) it affects mobility, substrate quality, and other (Burton, 2017; Ivanić et al., 2020; Rose et al., 2018).
Owan river is in Ovia North-East Local Government Area, Edo State. The river flows through Okpokhumi and Sabongidda communities before emptying into the Ose river. This area is known for its extensive agricultural practices such as cocoa farming, plantain plantations and cultivation of food crops. Many of these farms are located along the edge of the river. Agrochemicals are constantly being used on these farms to safeguard crops from pest infestation and achieve food security (Omoigberale et al., 2014). The Owan river is the community's primary source of water for domestic uses—drinking, preparing food, washing, bathing, agro product processing, swimming and for irrigation purposes during the dry season. Other human activities include canoe and boat repair and these activities can easily resuspend contaminants in the sediments.
The purpose of this study was to determine the suitability of Owan river for drinking purposes. Therefore the study was aimed to (i) assess the physicochemical properties of the river water samples, (ii) characterise the metal contents of the water samples, and (iii) characterise the occurrence and distribution of metals and non-metals (Na, Ca, K, Mg, Cd, As, Pb, Zn, Ni, and Cr) in the sediment samples.
Materials and methods
Study area
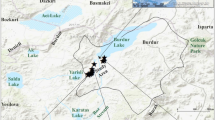
The Owan river is one of many freshwater bodies that exist in South Western of Nigeria. Owan river is located in between equator's interception points of 6° 45′ 23.6″ N 5° 43′ 53.8″ E (Fig. 1). It is a tropical metropolitan river that provides water supply to the majority of the riparian neighbourhoods (Edegbene & Arimoro, 2014). The river runs southward from Otuo in Owan East Local Government Area via Aken, Uokha, Ovbiowun, Afuze, Ogute, Evbiamen, Okpokhumi, Ojavun, Sabon-gidda Ora, Uhonmora in Owan West to Uzebba and into River Ose in Edo State. Agricultural activities take place within the watershed of the river. Cassava, plantain, maize are the most commonly grown food crops while cocoa, palm fruits etc. are some of the cash crops (Talabi et al., 2016).
The samples were collected in seven different locations. The locations were 1 km apart. The samples collected were coded WS1, WS2, WS3, WS4, WS5, WS6 and WS7. Generally, all the sample locations experienced fishing and farming activities which is part of the livelihood of the community. Agrochemicals and fertilisers were commonly used to boost agricultural productions. WS3 was located near the market while WS4 was located by a trunk A where activities like cars and cloth washings, bathing, waste dumping and vehicular activities were observed.
Water and sediment samples collection
Water samples were collected and transported to laboratories in accordance with standard procedures (APHA, 1998). 5 Litres polyethylene bottles were used for the collection of the water samples. The date and station number were written on the sample bottles. The samples were collected after rinsing with surface water from the sampling points and were immediately transported to the laboratory in coolers containing ice to avoid sample degradation before analysis. A day before use, all glassware was washed with 1–2 percent HCl solutions, rinsed with distilled water, and dried in the drying oven. Sediment samples were collected using a stainless steel grab sampler at the same point as the water samples. Sediment samples were placed in zipper bags with a polyethylene spoon to avoid contamination and securely sealed. The sediment samples were taken to the laboratory in ice chests and stored at 4 °C prior to elemental analysis (Ustaoglu & Tepe, 2019). All sampling stations were geo-referenced Garmin GPSMAP, model 76CSx.
Physico-chemical analysis
In-situ measurements of pH, dissolved oxygen (DO), electrical conductivity (EC), and total dissolved solids (TDS) were made using YSI pro1030 and Hach Lange HQ40D multi-parameter instruments. Total alkalinity (TA), total hardness (TH), and biochemical oxygen demand (BOD5) were determined using standard methods (APHA, 1998).
A Bulk scientific atomic absorption spectrometer (AAS) model 210 VGP was used to determine the concentrations of the elemental composition (Cd, Cu, Pb, As, Pb etc.) in the water samples. An air-acetylene flame was used in all of the analyses, and their respective wavelengths set in line with standard laboratory procedure. Sodium (Na) and potassium (K) was analysed using Bulk Scientific flame photometer model PF 710 after calibration with standards. These analyses were carried out in the Chemistry laboratory, Department of Science Laboratory Technology, Rufus Giwa Polytechnic, Owo, Nigeria.
Sediment analysis
Sediment samples were air dried and sieved with a 2 mm mesh sieve in the Chemistry laboratory, Department of Science Laboratory Technology, Rufus Giwa Polytechnic, while the elemental analysis of the sediment samples was carried out at Engineering and Material Development Institute, Akure, Nigeria using Energy Dispersive X-ray Fluorescence Spectrometer EDXRF (Skyray Instrument Inc. USA) equipment model EDX 3600B.
Quality control and assurance
Laboratory chemicals and reagents used were of analytical grade (99% purity). Distilled water was used for the preparation of reagents. De-ionised water was used for elemental analyses. Laboratory apparatuses were washed with detergent, rinsed with distilled water and oven-dry before use. Equipment was calibrated in line with manufacturers’ specifications and instructions.
Statistical analysis
Mini Tab (version 16) statistical package was used to perform basic descriptive statistical analyses of the data generated.
Results and discussion
Physico-chemical parameters of water samples
The pH of the water samples ranged between 6.10 and 6.85. According to WHO standards, the pH of two of the samples was within the acceptable range for drinking purposes (Table 1). SW1 and 6 are within the acceptable range of 6.5–8.5. These are within the acceptable range for freshwaters, which is typically between pH 6.5 and 8.5, the acceptable range prescribed by WHO (2007) and SON (2007). A low pH indicates that the water is acidic, whereas, a high pH indicates that the water is alkaline. When the pH of water exceeds 8.5, the taste of the water becomes more bitter. While this higher pH poses no health risks, it can cause skin to become dry, itchy, and irritated (Sila, 2019).
Total alkalinity ranged between 32.0 and 50.5 mg/L. They are relatively low. The total alkalinity values are characteristic of freshwater bodies (Cambers et al., 2008). Even though total alkalinity is an assessment of a water's ability to resist changes in pH when acid is added to it, it implies that such water sources have stable pH (Cambers et al., 2008). In addition, because alkalinity is caused primarily by CO3, HCO3, and OH ions, it indicates that such ions were less abundant in the water samples. Most values reported by Irenosen et al. (2019) were.
The dissolved oxygen (DO) concentration ranged from 5.5 to 6.53 mg/L. There is no DO limit for drinking water though well aerated drinking water tastes better. WS3 had the highest concentration of DO (6.53 mg/L). The DO concentration ranges for the samples are nearly identical. As a result, the nutrient load and rate of oxygen consumption by oxidisable matter in these locations may be the same. The DO concentrations measured in the water sources may be good for high-quality potable water for domestic use and recreational purposes (e.g., bathing, swimming, boating, and fishing).
The high concentration of dissolved oxygen in the Owan river can be attributed to wind action mixing, boat movements, as well as photosynthetic activities of aquatic plants and surrounding plant species (Asante et al., 2005). The recorded DO concentrations (5.5–6.53 mg/L) were within the ranges recorded in other rivers in Nigeria—5.2–0.8 mg/L (Edegbene & Arimoro, 2014); 3.79–4.84 mg/L (Ghali et al., 2020); 5.97–8.47 mg/L (Irenosen et al., 2019).
The electrical conductivities (EC) of the river ranged between 32.0 and 38.0 µS/cm. The overall conductivity trend showed that higher conductivity values in WS2 and WS6, while lower conductivity values are obtained in WS4. The high values in WS2 and WS6 could be due to the natural (wastes, animal and vegetation, dust and wildfires, and agriculture and animal husbandry) and man-made activities (plastics, burning of fossil fuels, agricultural activities, and may be illegal mining) within the locations. All the conductivity values were lower than the acceptable limit of 1000 μS/cm set by WHO (2018). The electrical conductivity values reported in this study are lower than the values reported by Irenosen et al. (2019) and Ghali et al., (2020), which are 118.60–194.80 and 115.9–149.0 µS/cm, respectively. Electrical conductivity is the ability of water to conduct electricity. Because electric current is conducted by the movement of ions in solution, EC can also provide information about ion concentrations or total dissolved salts (TDS) in the water being tested for EC (Rahmanian et al., 2015).
Table 1 showed a nitrate content with lower and highest concentrations of 2.65 and 21.55 mg/L, respectively. Only the WS1 sample is less than the WHO's limit is 50 mg/L to prevent blue baby syndrome (methaemoglobinaemia) in infants. The nitrate content of the water samples could have resulted leaching of agrochemicals and fertilisers from the farms along the banks of the river. Excess nitrate in water has been linked to hypertension in adults (Mkadmi et al., 2018) and methaemoglobinaemia in infants (Bruning-Fann & Kanaeme, 1993).
The sulphate concentrations ranged from 58.67 to 80.0 mg/L. The lowest concentration of 58.67 mg/L was recorded in WS2 while the highest concentration of 80 mg/L was recorded in WS4. All values were lower than the WHO recommended limit of 250 mg/L. Sulphate accumulation in water can cause low pH, resulting in acidosis (Asamoah & Amorin, 2011). Acidosis is caused by prolonged consumption of acidic water. Sulphate values in excess of the acceptable limit result in taste impairment (WHO, 2017).
Chloride concentrations ranged from 10.93 to 25.60 mg/L (Table 1), with WS4 and WS7 having the lowest and highest concentrations, respectively. All concentrations measured fell below the WHO’s maximum permissible limit of 250 mg/L. The presence of chlorides could be attributed to chloride-containing soils and rocks leaching into the river (Aremu et al., 2011). Chlorides is a necessary component for activities that involve human body metabolism. Too much chloride concentrations in water, on the other hand, may cause (1) a laxative effect, (2) corrosion of metallic pipes, and (3) unsuitability of water for agricultural irrigation (Raviprakash & Krishna, 1989). The concentration of chloride varies depending on the type of water and has been discovered to exist naturally in the form of sodium and potassium salts (WHO, 2017). It is a stable water component whose concentration is unaffected by both bio- and physico-chemical processes (Gorde & Jadhav, 2013).
COD and BOD levels were 28.5–48.5 mg/L and 2.0–3.2 mg/L, respectively (Table 1). All values were low making them suitable for domestic use. The values reported could be due to natural phenomenon in the river, the surroundings, and agrochemicals (Sumant et al., 2015). A BOD level of 1–2 ppm is regarded as good which implies that all the water samples in this study are good in terms of BOD indicating little or low organic wastes in the water. A BOD level of 3–5 ppm in a water supply is considered moderately clean. COD measures the amount of oxygen required for chemical oxidation of organic matters to occur with the help of a strong chemical oxidant, whereas BOD measures the amount of oxygen required for carbonaceous matter biodegradation in a sample.
Magnesium is the eighth most abundant element on the earth crust and a naturally occurring constituent of water (Aviprakash & Krishna 1989). It is found in minerals such as dolomite and magnetite and is necessary for the proper functioning of living organisms. Magnesium is found in the human body in approximately 25 g. (60 percent in bones and 40 percent in muscles and tissues). Magnesium levels in water samples ranged from 0.94 to 3.20 mg/L in the study areas. Sample WS2 had the lowest magnesium concentration. Meride and Ayenew (2016) report a similar value for Ethiopian drinking water. The result showed that magnesium concentrations in the Owan river were low.
Maximum and minimum calcium levels in the study areas are 6.60 mg/L (SW6) and 3.21 mg/L (SW2), respectively (Table 1). Water containing calcium carbonate at concentrations 0 and 60 mg/L is considered soft; water containing calcium carbonate at concentrations between 60 and 120 mg/L is considered moderately hard; water containing calcium carbonate at concentrations between 120 and 180 mg/L is considered hard; and water containing calcium carbonate at concentrations greater than 180 mg/L is considered extremely hard. Calcium is the fifth most abundant element in the earth’s crust and is crucial to human cell physiology and bone formation. The bones and teeth store approximately 95 percent of the calcium in the human body. A high calcium deficiency in humans can result in rickets, poor blood clotting, bone fractures, and other health problems, while exceeding the calcium limit can result in cardiovascular disease. Its allowable range in drinking water is 75 mg/L, according to standards.
Elemental contents of water samples
The concentrations of all elements (macro and micro) are presented in Table 2. From the table it is observed that Mn, Cd, Ni, Cr, Pb, and As have values of less than 0.6 mg/L, while Fe and Zn concentrations are found to be less than 7 mg/L. This finding contrasts with the findings of Ayotunde et al., (2012), Omoigberale et al., (2014), Ochelebe et al., (2020) and Edori and Iyama (2020) for Ikom Beach (Fe 0.043 ± 0.03 and Zn 0.004 ± 0.00), Owan river (Fe 0.210–1.250 and 0.010–0.320 mg/L), Ikpaya stream (Fe ND and Zn ND), and Edagberi Creek (Fe 0.028–0.075 and Zn 0.016–0.076 mg/L), respectively.
The Zn concentrations were low (1.40–2.04 mg/L) and lower than the acceptable limits of 3.00 mg/L (Table 2). All the Mg concentrations (4.3–8.60 mg/L) exceeded the allowable limit of 0.20 mg/L; WS1 having the highest concentrations. Magnesium ion, along with Ca, are well-known cause of water hardness. Diarrhoea may be caused by an increase in Mg salt consumption. Studies on the other hand, have noted an inverse relationship between the risk of cardiovascular disease and high magnesium intake, particularly in men (Daramola et al., 2019; Lacey & Shaper, 1984; Sengupta, 2013). Although the WHO (2018) drinking water guideline shows that excessive magnesium intake has no significant adverse effects, it has been proposed that consumption of magnesium above the body requirement is especially dangerous for people who have cancerous cells (Castiglioni & Maier, 2011).
The Pb concentration is greater than 0.01 mg/L, which is the acceptable limit. This is a matter of public health concern. This high content could be attributed to the constant washing of vehicles in parts of the flowing river. In drinking water, Pb is a known potentially toxic metal (PTM) (Igwe et al., 2014). The potential effects of Pb on human health when ingested in any form are known to be accumulative (Igwe et al., 2014). Based on the WHO (2018) thresholds for drinking water, the possible health risks associated with high Pb intake (i.e. > 0.01 mg/L) includes cancerous growth, interference with Vitamin D metabolism, affect mental development in infants, as well as the toxicity of the central and peripheral nervous systems. Lead poisoning can be either acute or chronic (Jaishankar et al., 2014). The former causes appetite loss, headaches, renal dysfunction, insomnia, hypertension, abdominal pain, and fatigue (Martin & Griswold, 2009). Similarly, chronic Pb exposure in children can cause mental retardation, birth defects, psychosis, autism, allergic reactions, dyslexia, loss of weight, hyperactivity, paralysis, muscular weakness, brain damage, kidney damage, and even death (Jaishankar et al., 2014; Martin & Griswold, 2009).
Sodium levels were generally low and well below the allowable limit of 200 mg/L. On the contrary, Fe content was found to be high, exceeding the recommended limit of 0.3 mg/L. Although the World Health Organisation believes that adverse health effects associated with high K intake from potable water are unlikely in a healthy human, individuals with renal failure may be at risk due to pre-existing kidney damage (Gosselin et al., 1984). Medical conditions such as heart failure, chest tightness, diarrhoea, nausea and vomiting, hyperkalaemia, and shortness of breath have been identified as potential health risks from excessive K consumption. After ingestion, a potassium concentration of 11 g (21 g of salt substitute) has been shown to result in fatality due to hyperkalaemia and the resulting asystole (Restuccio, 1992).
Elemental content of sediment samples
Figure 2a–g summarises the minimum and maximum concentrations (represented by the peak) of metals in sediment samples. All heavy metals were found in all sediment samples. The concentrations (mg/kg) of Fe, Cr, Co, Ni, Cu, Zn, Cd, and Pb were 5.07–16.72, 0.0–0.04, 0.04–0.54, 0.06–0.09, 0.03–0.06, 0.09–0.14, ND 0.001, and 0.01–0.04, respectively. Silicon, the second most abundant element on the planet, had (18.97–25.08). The concentrations of major elements are as follows: K (1.17–2.71) and Ca (0.22–2.39). The findings of this study contradict the findings of a previous study (Ayotunde et al., 2012). The heavy metals (Ni and Fe) are more abundant in the sediments than in the water.
Conclusion
The findings show that the majority of the physicochemical and elemental qualities of the river water are generally below the recommended guidelines for drinking water, indicating that the water quality is good. With the exception of As and Cd, all sediment samples contained heavy metals. Their average concentrations were as follows: Fe > Ni > Cu > Co > Zn > Cr > Pb. The PTM concentrations such as Zn, Cu, Cr, and Ni, and Na and Ca are too low to pose an environmental risk. However, constant checks and monitoring by relevant agencies, government representatives, public health practitioners, and community leaders are required.
Data availability statement
The data presented in this study are available on request from the corresponding author.
Change history
17 September 2021
A Correction to this paper has been published: https://doi.org/10.1007/s42108-021-00148-y
Abbreviations
- PTEs:
-
Potentially toxic elements
- TM:
-
Trace metals
- NSDWQ:
-
Nigerian Standard for Drinking Water Quality
- USEPA:
-
United States Environmental Protection Agency
- BOD:
-
Biochemical oxygen demand
- COD:
-
Chemical oxygen demand
- NTU:
-
Nephelometric turbidity unit
- DO:
-
Dissolved oxygen
- WHO:
-
World Health Organisation
- WS:
-
Water sample
- TDS:
-
Total dissolved salts/solids
- EC:
-
Electrical conductivities
References
Abulude, F. O., Adebiyi, A. B., & Elemide, O. A. (2019). Physico-Chemical Parameters of Rainwater samples obtained in a Suburban Town in Ondo State, Nigeria. Continental Journal Environmental Sciences, 12(1), 21–38. https://doi.org/10.5281/zenodo.3575244
Akinnusotu, A., Abulude, F. O., & Abdulrasheed, Y. (2015). Determination of Physico-chemical Properties and Metal concentrations of hand dug wells around a dumpsite in Akure, Ondo State, Nigeria. Continental Journal Environmental Sciences, 9(2), 22–36.
Akinnusotu, A., & Arawande, J. O. (2016). Qualities of Effluents from three Cocoa Processing factories in Ondo State, Nigeria. International Journal of Environment and Bioenergy, 11(1), 24–35.
APHA. (1998). Standard methods for the examination of water and wastewater (20th ed.). American Public Health Association.
Aremu, M. O., Olaofe, O., Ikokoh, P. P., & Yakubu, M. M. (2011). Physicochemi-cal characteristics of stream, well and borehole water sources in Eggon, Nasarawa State, Nigeria. Journal Chemical Society of Nigeria, 36(1), 131–136.
Asamoah, A. A., & Amorin, B. S. (2011). Assessment of the quality of bottled sachet water in the Tarkwa-Nsuaem municipality in Ghana. Research Journal of Applied Science, 3(5), 105–113.
Asante, K. A., Quarcoopome, T. F., & Amevenku, Y. K. (2005). Water quality of the Weija reservoir after 28 years of impoundment. West African Journal of Applied Ecology, 13, 112–114.
Ayotunde, E. O., Offem, B. O., & Ada, F. B. (2012). Heavy metal profile of water, sediment and freshwater cat fish, Chrysichthys nigrodigitatus (Siluriformes: Bagridae), of Cross River, Nigeria. Revista De Biología Tropical, 60(3), 1289–1301. https://doi.org/10.15517/rbt.v60i3.1807 PMID: 23025098.
Bruning-Fann, C. S., & Kanaeme, J. B. (1993). The effect of nitrate, nitrite, and N-nitro compound on human health. Veterinary and Human Toxicology, 35, 521–538.
Burton, G. A. (2017). Sediment toxicity assessment (1st ed.). CRC Press. https://doi.org/10.1201/9781351076555
Cambers, G., Muehlig-Hofmann, A., & Troost, D. (2008). Environment and development in coastal regions and in small Islands: a small Islands’ perspective. Accessed on 14th May, 2021. www.unesco.org/csi/wise/tenure.html.
Castiglioni, S., & Maier, J. A. M. (2011). Magnesium and cancer: A dangerous liason. Magnesium Research, 24(3), S92–S100. https://doi.org/10.1684/mrh.2011.0285
Daramola, J., Ekhwan, T. M., Adepehin, E. J., Mokhta, J., Lam, K. C., & Er, A. C. (2019). Seasonal quality variation and environmental risks associated with the consumption of surface water: Implication from the Landzun Stream, Bida Nigeria. Heliyon, 5, e02121. https://doi.org/10.1016/j.heliyon.2019.e02121
Edegbene, A. O., & Arimoro, F. O. (2014). Preponderance of Mollusca in Owan river, Niger Delta Area, Nigeria. Electronic Journal of Biosciences, 2(1), 67–76.
Edori and Iyama. (2020). Status of heavy metals contamination in water from Edagberi Creek, Engenni, Rivers State, South-South, Nigeria. Biomedical Journal of Science & Technological Research, 29(3), 22482–22488. https://doi.org/10.26717/BJSTR.2020.29.004810
EPA, (2018). Drinking Water Standards and Health Advisory Table. U.S. Environ-mental Protection Agency. https://www.epa.gov/sites/production/files/2018-03/documents/dwtable2018.pdf.
Farahat, E., & Linderholm, H. W. (2015). The effect of long-term wastewater irrigation on accumulation and transfer of heavy metals in Cupressus sempervirens leaves and adjacent soils. Science of the Total Environment., 512–513(9), 1–7. https://doi.org/10.1016/j.scitotenv.2015.01.032
Ghali, H., Osimen, E. C., Ogidiaka, E., Akamagwuna, F. C., Keke, U. N., & Edegbene, A. O. (2020). Preliminary assessment of the deteriorating state of a dam in north-westernNigeria using phytoplankton structural assemblage and environmental factors. Water Science, 34(1), 181–189. https://doi.org/10.1080/11104929.2020.1816152
Gorde, S. P., & Jadhav, M. V. (2013). Assessment of water quality parameters: A review. International Journal of Engineering Research and Applications., 3(6), 2029–2035.
Gosselin, R. E., Smith, R. P., & Hodge, H. C. (1984). Clinical toxicology of commercial products (5th ed.). Williams & Wilkins.
Igwe, O., Adepehin, E. J., Iwuanyanwu, C., & Una, C. O. (2014). Risks associated with the mining of Pb–Zn minerals in some parts of the Southern Benue trough, Nigeria. Environmental Monitoring and Assessment, 186, 3755–3765. https://doi.org/10.1007/s10661-014-3655-3
Irenosen, G. O., Egbulefu, A. V. I., & Korede, A. O. (2019). The Microbial status and physico-chemical pollutants studies of rivers Owan and Evbiobe in Edo State, Nigeria. Science Journal of Analytical Chemistry, 7(2), 57–64. https://doi.org/10.11648/j.sjac.20190702.13
Ivanić, M., Durn, G., Škapin, S. D., & Sondi, I. (2020). Size-related mineralogical and surface physicochemical properties of the mineral particles from the recent sediments of the Eastern Adriatic Sea. Chemosphere, 249, 126531.
Jaishankar, M., Tseten, T., Anbalagan, N., Mathew, B., & Beeregowda, K. (2014). Toxicity, mechanism and health effects of some heavy metals. Interdisciplinary Toxicology, 7(2), 60–72. https://doi.org/10.2478/intox-2014-0009
Lacey, R. F., & Shaper, A. G. (1984). Changes in water hardness and cardiovascular death rates. International Journal of Epidemiology, 13(1), 18–24. https://doi.org/10.1093/ije/13.1.18
Martin, S., & Griswold, W. (2009). Human health effects of heavy metals. Environmental Science and Technology Brief Citizens 15, 1–6. www.engg.ksu.edu/CHSR/ Accessed 14th May, 2021.
Meride, Y., & Ayenew, B. (2016). Drinking water quality assessment and its effects on residents health in Wondo genet campus, Ethiopia. Environmental System Research, 5, 1. https://doi.org/10.1186/s40068-016-0053-6
Mkadmi, Y., Benabbi, O., Fekhaoui, M., Benakkam, R., Bjijou, W., Elaz-zouzi, M., Kadourri, M., & Chetouani, A. (2018). Study of the impact of heavy metals and physico-chemical parameters on the quality of the wells and waters of the Holcim area (Oriental region of Morocco). Journal of Materials and Environmental Science, 9(2), 672–679.
Nazeer, S., Hashmi, M. Z., & Malik, R. S. (2014). Heavy metals distribution, risk assessment and water quality characterization by water quality index of the River Soan, Pakistan. Ecological Indicators, 43, 262–270. https://doi.org/10.1016/j.ecolind.2014.03.010
Nnorom, I. C., Ewuzie, U., & Eze, S. O. (2019). Multivariate statistical approach and water quality assessment of natural springs and other drinking water sources in Southeastern Nigeria. Heliyon, 5(2019), e01123. https://doi.org/10.1016/j.heliyon.2019.e01123
Ochelebe, I., Kudamnya, E. A., & Nkebem, G. E. (2020). An assessment of heavy metals concentration in water around quarries and Barite mine sites in part of central cross River State, Southeastern Nigeria. Global Journal of Geological Sciences, 18, 89–95. https://doi.org/10.4314/gjgs.v18i1.8
Omoigberale, M. O., Oboh, I. P., Erhunmwunse, N. O., Ezenwa, I. M., & Omoruyi, S. O. (2014). An assessment of the trace metal contents of Owan river, Edo State, Nigeria. European International Journal of Science and Technology, 3(5), 88–98.
Owamah, H. I., Asiagwu, A. K., Egboh, S. H. O., & Phil-Usiayo, S. (2013). Drinking water quality at Isoko North communities of the Niger Delta Region, Nigeria. Toxicological & Environmental Chemistry, 95(7), 1116–1128. https://doi.org/10.1080/02772248.2013.847939
Popoola, L. T., Yusuff, A. S., & Aderibigbe, T. A. (2019). Assessment of natural groundwater physico-chemical properties in major industrial and residential locations of Lagos metropolis. Applied Water Science, 9, 191. https://doi.org/10.1007/s13201-019-1073-y
Rahmanian, N., Ali, S. H. B., Homayoonfard, M., Ali, N. J., Rehan, M., Sadef, Y., & Nizami, A. S. (2015). Analysis of physiochemical parameters to evaluate the drinking water quality in the State of Perak, Malaysia. Journal of Chemistry. https://doi.org/10.1155/2015/716125
Raviprakash, S. L., & Krishna, R. G. (1989). The chemistry of groundwater Paravada area with regard to their suitability for domestic and Irrigation purpose. India Journal of Geochemistry, 4(1), 39–54.
Restuccio, A. (1992). Fatal hyperkalemia from a salt substitute. American Journal of Emergency Medicine, 10(2), 171–173. https://doi.org/10.1016/0735-6757(92)90058-6
Rose, L. A., Karwan, D. L., & Aufdenkampe, A. K. (2018). Sediment fingerprinting suggests differential suspended particulate matter formation and transport processes across hydrologic regimes. Journal of Geophysical Research: Biogeosciences, 123(4), 1213–1229.
Sengupta, P. (2013). Potential health impacts of hard water. International Journal of Preventive Medicine, 4(8), 866.
Sila, O. N. (2019). Physico-chemical and bacteriological quality of water sources in rural settings, a case study of Kenya, Africa. Scientific African, 2, e00018. https://doi.org/10.1016/j.sciaf.2018.e00018
Standard Organization of Nigeria (SON). (2007). Nigerian standards for drinking water quality (NSDWQ), Industrial standards. SON, 554, 1–14.
Sumant, K. N. C., Ghosh, R. P., Singh, M. M., Sonkusare, S. S., & Sanjay, M. (2015). Assessment of water quality of lakes for drinking and irrigation purposes in Raipur City, Chhattisgarh, India. International Journal of Engineering Research and Applications, 5, 42–49.
Talabi, A. O., Ojo, O. F., Ajayi, C. A., Olaolorun, O. A., & Afolagboye, O. L. (2016). Hydrochemistry and environmental status of river Owan water, Edo State Nigeria. International Journal of Environment and Pollution Research, 4(5), 1–15.
Trcek, B., & Zojer, H. (2010). Recharge of springs. In N. Kresic & Z. Stevanovic (Eds.), Groundwater hydrology of springs: Engineering, theory, management, and sustainability (pp. 87–127). Butterworth-Heinemann.
Ustaoglu, F., & Tepe, Y. (2019). Water quality and sediment contamination assessment of Pazarsuyu Stream, Turkey using multivariate statistical methods and pollution indicators. International Soil and Water Conservation Research, 7(1), 47–56. https://doi.org/10.1016/j.iswcr.2018.09.001
Verma, S., Mohanty, B. P., Singh, K. P., Behera, B. R., & Kumar, A. (2018). Dependence of recipitation of trace elements on pH in standard water. Nuclear Instruments and Methods in Physics Research Section b: Beam Interactions with Materials and Atoms, 420, 18–22. https://doi.org/10.1016/j.nimb.2018.01.016
WHO. (2007). Guidelines of drinking water quality (3rd ed.). World Health Organization.
WHO (2017). Guidelines for drinking-water quality, 4th edition, incorporating the 1st addendum. Overview. https://www.who.int/publications/i/item/9789241549950. Accessed 12th Jun 2021
WHO. (2018). A global overview of National regulations and standards for drinking water quality (p. 104). WHO.
Acknowledgements
The authors wish to acknowledge the contributions of the people who assisted in the collection of the samples and the anonymous reviewers.
Funding
The authors wish to thank Tertiary Education Trust Fund (TETFund) for the research grant received (TETFUND/DRSS/POLY/OWO/2016/RP/VOL 1) through Rufus Giwa Polytechnic, Owo, Nigeria to carry out this research project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Institutional review board statement
Not applicable.
Informed consent statement
Not applicable.
Rights and permissions
About this article
Cite this article
Akinnusotu, A., Ukpebor, J.E., Okieimen, F.E. et al. Assessment of the physico-chemical characteristics and elemental properties of water and sediments from Owan river in Edo State, Nigeria. Int J Energ Water Res 6, 543–552 (2022). https://doi.org/10.1007/s42108-021-00144-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42108-021-00144-2