Abstract
Background
Biallelic mutations in the TBX19 gene cause severe early-onset adrenal failure due to isolated ACTH deficiency (IAD). This rare disease is characterized by low plasma ACTH and cortisol levels, with normal secretion of other pituitary hormones. Herein, we report a patient with IAD due to a novel TBX19 gene mutation, who is also of tall stature.
Case report
A 48/12-year-old girl was presented with loss of consciousness due to hypoglycemia. The patient was born at term with a birth weight of 3800 g. Her parents were first-degree cousins. She had a history of several hospitalizations for recurrent seizures, abdominal pain, and vomiting. At presentation, her weight and height were + 1.8 and + 2.2 SDS, respectively. Serum glucose was 25 mg/dl (1.4 mmol/L), with normal sodium, potassium, and insulin concentrations. The child was hypocortisolemic (0.1 μg/dl), and ACTH levels were extremely low (< 5.0 pg/ml). A diagnosis of IAD was made and hydrocortisone treatment was started. Hypoglycemic episodes, seizures, and recurrent gastrointestinal complaints disappeared after hydrocortisone replacement. Magnetic resonance imaging of the pituitary was normal. Whole exome sequencing revealed a novel homozygous c.302G > A (W101*) mutation in the TBX19 gene.
Conclusion
We report a new mutation in the TBX19 gene in a patient with isolated ACTH deficiency. While overgrowth is a known feature of some types of adrenal insufficiencies, including MC2R gene defects and POMC deficiency, it may be a novel feature for TPIT deficiency, as in our patient.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adrenocorticotropic hormone (ACTH) is required for the synthesis of glucocorticoids and adrenal sex steroids. Impaired synthesis of ACTH from any genetic or acquired cause may result in secondary adrenal insufficiency. Congenital isolated ACTH deficiency (IAD) (MIM #201400) is characterized by low plasma ACTH and cortisol levels without deficiency of other pituitary hormones [1]. Recessively inherited mutations in the TBX19 gene are reported to cause IAD. T-box factor 19 (TBX19) is the first T-box gene identified in the pituitary, and encodes T-box pituitary restricted transcription factor (TPIT). TPIT is important for both proopiomelanocortin (POMC) gene expression and differentiation of cells expressing POMC [2]. In the absence of TPIT, intermediate lobe melanotropes and corticotropes (POMC lineages) fail to differentiate. Furthermore, inactivation of this transcription factor results in the change of cell fate and in melanotropes differentiating into gonadotropes in mice [2,3,4].
The TBX19 gene (MIM*604614) is located in chromosome 1q24.2, consisting of 8 exons, and mutations of this gene are associated with early-onset IAD in humans [4, 5]. IAD associated with TBX19 gene mutations is very rare, with 25 different mutations having been described so far [6]. Patients with TBX19 gene defects usually present with severe ACTH deficiency and adrenal insufficiency in the neonatal period or early childhood. Hypoglycemia and prolonged jaundice are frequently observed, and death may result if not diagnosed early. The pituitary gland is structurally normal [2, 7]. However, pituitary function other than ACTH deficiency has not been reported in detail in previous studies.
Herein, we report a patient with congenital IAD due to a novel TBX19 gene mutation. In addition to early-onset IAD, she also had accelerated growth, which is discussed in light of the current literature on TPIT deficiency.
Case report
A 48/12-year-old girl was admitted to the pediatric emergency unit with loss of consciousness. She was found to be hypoglycemic, with a capillary glucose level of 25 mg/dl (1.4 mmol/L). Past medical history revealed that she was born at term with a birth weight of 3800 g (+ 1.6 SDS) and was hospitalized for 10 days in a neonatal care unit for respiratory distress and hyperbilirubinemia. Her parents were first-degree cousins (Fig. 1a). Her brother died on the 23rd third postnatal day of unknown causes, and a preterm born sister died on the postnatal day after esophageal atresia surgery. Her mother had also lost a male fetus during the second trimester of unclear causes. Maternal prenatal evaluation and ultrasound imaging were unremarkable. The patient had not begun to walk until the age of 2, while first tooth eruption was also delayed (at 18 months). She was reported as having frequent viral respiratory tract infections and also a history of emergency department admissions due to vomiting, abdominal pain, and diarrhea. At about 3 years of age, she had recurrent seizures, especially in the morning. She was hospitalized in a pediatric intensive care unit with loss of consciousness and hypoglycemia at the age of 4. Hypoglycemic episodes were diagnosed as ketotic hypoglycemia after prolonged fasting.
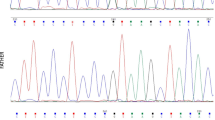
At presentation to our clinic at the age of 48/12 years, her weight and height were 23.1 kg (+ 1.8 SDS) and 116.5 cm (+2.2 SDS), respectively, despite having a midparental target height of − 0.8 SDS. She had no facial dysmorphism, hypo/hyperpigmentation, or red hair. Cardiac and abdominal examination was normal. Her pubic and breast stage were Tanner I. At initial evaluation, her developmental milestones were delayed compared to chronological age, while fine motor skills were appropriate for her age. Laboratory investigations in the emergency unit confirmed hypoglycemia and serum glucose 25 mg/dl (1.4 mmol/L), with normal sodium and potassium levels, mildly elevated AST (66 U/L), and normal ALT, creatinine, and bilirubin. Capillary ketone level was 2.2 mmol/L, while serum insulin and C-peptide were undetectable; hence, hyperinsulinemia was excluded. Blood lactate (0.7 mmol/L) and ammonia (20.1 μmol/L) were normal and she had no metabolic acidosis. The child was hypocortisolemic (0.1 μg/dl) with an extremely low ACTH level (< 5.0 pg/ml (0–46)). Other anterior pituitary hormones were normal (growth hormone 14.8 μg/L) at the time of hypoglycemia. Serum amino acids and urinary organic acid profile were normal. Insufficient cortisol response (0.4 μg/dl) was detected in the low-dose (1 μg intravenous) ACTH stimulation test. Diagnosis of central adrenal insufficiency due to ACTH deficiency was made, and hydrocortisone treatment (10 mg/m2/day in three divided doses) was started. Her hypoglycemic episodes and recurrent infections disappeared after hydrocortisone replacement. Magnetic resonance imaging of the pituitary was normal (Fig. 1b). Whole exome sequencing revealed a novel homozygous c.302G > A (W101*) mutation in the TBX19 gene, producing a truncated protein.
In the first year of follow-up, her height was + 1.6 SDS. Her pubic and breast stages were Tanner I. At the age of 5 4/12 years, she had Tanner 2 breast development with advanced bone age (7.8 years) (Fig. 1c) and high basal serum LH value (0.63 mIU/ml). Pelvic USG revealed enlarged ovaries (right: 33 × 17 × 12 mm, left: 25 × 16 × 15 mm) and uterus volume was 33 × 10 × 6 mm. However, an LHRH stimulation test result was in the prepubertal range (peak LH: 2.35 mIU/ml and peak FSH: 11.4 mIU/ml). The patient was observed for the tempo of puberty, and her breast stage was regressed during follow-up.
She was also investigated because of her being in the upper centiles of the growth chart and tall for target height, despite having had several vomiting/diarrhea and hypoglycemia episodes in the past. Her IGF-1 and IGFBP3 values were < 25.0 ng/ml (50–303) and 1.02 μg/ml (1.1–6.1), respectively, not suggestive of the etiology of overgrowth. Reevaluation of whole exome sequencing data revealed no pathogenic variant in the MC2R gene.
She was normoglycemic during 2.5-years of follow-up with hydrocortisone replacement (10 mg/m2/day), during which period the patient had no record of infections and no need for hospitalizations. At her last visit at the age of 71/12 years (Fig. 1c), her weight and height were 28.7 kg (+ 1.3 SDS) and 126.1 cm (+ 0.9 SDS), respectively. Systemic evaluation was normal, apart from breast stage Tanner 2. Her bone age was still 7.8 years, and basal serum LH value was 0.37 mIU/ml. The ovaries were enlarged (right: 28 × 25 × 11mm, left: 22 × 17 × 13 mm) for her age, and uterus volume was 33 × 23 × 10 mm. Peak LH and FSH levels in repeated LHRH stimulation testing were 5.78 mIU/ml and 11.5 mIU/ml, respectively. However, height velocity was 3.2 cm/year and bone age was not progressive. Other anterior pituitary hormone levels were also normal during follow-up. GH, IGF-1, and IGFBP3 values were 0.47 ng/ml (0–10), 76.8 ng/ml (80–233), and 3.0 μg/ml (1.4–6.1), respectively.
Clinical and laboratory findings of the patient at diagnosis and last evaluation are illustrated in Table 1.
Hormonal assays
Serum cortisol concentrations were analyzed by commercial kits based on electrochemiluminescence immunoassay (Beckman Coulter, USA). Plasma ACTH concentrations were analyzed by two-site sequential chemiluminescent immunoassay (Immulite 2000, Siemens). IGF-1 and IGFBP3 were analyzed by immunoassay (Immulite 2000, Siemens). LH and estradiol (E2) were analyzed by electrochemiluminescence immunoassay method (ECLIA) (Cobas e601, Roche). FSH concentrations were analyzed by electrochemiluminescence immuassay (Beckman Coulter, USA).
Molecular analyses
Written informed consent was obtained from the parents. DNA was extracted from peripheral blood using DNeasy blood extraction kits based on the manufacturer’s protocol (QIAGEN, http://www.qiagen.com/). Genomic DNA from the patient and family was sequenced and analyzed at Bezmialem Medical Faculty Department of Medical Genetics Molecular Laboratory. Amplified PCR samples were used for generating gene libraries by Nextera Rapid Capture Enrichment (Illumina, USA) kit protocol. Exome sequencing was performed on the NextSeq500 platform. Critical variants were analyzed using Illumina Variant Studio, and pathogenicity of the candidate variants was evaluated using Alamut Visual and HGMD Professional databases. As the parents were consanguineous, we initially filtered homozygous rare variants with a minor allele frequency of < 0.01 in the 1000 Genomes Project data (http://www.1000genomes.org/), searching for autosomal recessive inheritance. Whole exome sequencing revealed a novel homozygous c.302 G > A change predicting a termination codon in the 101st aminoacid position in the TBX19 gene. This variant was not detected in our in-house database, nor was it found in public databases, such as ExAC, dbSNP, ESP, and 1000 Genomes Project data. The variant was predicted to be disease-causing according to in silico software, including Polyphen-2, SIFT, and Mutationtaster [8,9,10]. Pathogenic or probable pathogenic variants in other adrenal insufficiency-related genes (PITX, MC2R, and POMC), and also overgrowth-related genes, were not detected.
Discussion
Congenital IAD is a rare and potentially life-threatening disease and may therefore be underestimated due to the low level of suspicion in clinical practice. As in our case, patients usually have several hospitalizations for hypoglycemia and episodes of abdominal pain and vomiting before the diagnosis is established. Thus, clinicians evaluating a hypoglycemic patient must consider the possibility of adrenal insufficiency and it is important to take critical samples of cortisol at the time of hypoglycemia. Congenital IAD is classified as early-onset (neonatal) and late-onset (juvenile) type, according to the onset of hypoglycemia. Metherell et al. [11] have defined onset within 1 year of age as early-onset IAD. Vallette Kasic et al. [2] have reported that the first symptoms appear in the neonatal period in all early-onset cases and the diagnosis is generally made between birth and 2 years of age. TBX19 gene mutation was detected in most of the early-onset (neonatal) IAD cases, but not in juvenile onset cases. Thus, TBX19 gene analysis is recommended, especially in IAD presenting in the neonatal period or early childhood [2, 11].
Hypoglycemia, seizures, and jaundice are the most common symptoms of IAD during the neonatal period [11,12,13]. Our patient had a history of neonatal hyperbilirubinemia but she had no cholestatic jaundice. A patient with compound heterozygous TBX19 gene mutation, presenting with recurrent lower respiratory tract infections without hypoglycemia, was reported recently [14]. Our patient had frequent respiratory tract infections and was hypoglycemic at admission, as are many reported cases.
Clinical presentations of previously reported patients with congenital IAD due to TBX19 gene mutations are summarized in Table 2. To date, 25 different TBX19 gene mutations have been identified [6]. TBX19 mutations are found throughout the gene, especially in the T-box region, resulting in DNA-binding defects [1]. In our patient, whole exome sequencing revealed a novel homozygous nonsense c.302 G > A (W101*) mutation localized in the T-box region of the TBX19 gene. TBX19 gene mutations identified in congenital IAD are illustrated in Fig. 2.
Known TBX19 mutations identified in isolated ACTH deficiency patients. Transcribed sequences are boxed. Small boxes include 5′ and 3′ untranslated regions. The coding sequence of TBX19 gene is represented by the bigger boxes. The dark gray boxes indicate the T-Box domain. The nonsense and deletional mutations are indicated at the upper part of the panel, missense and splice site mutations are shown in the lower panel
There are a limited number of reports about prenatal diagnosis of IAD by low estriol levels in maternal serum triple marker screening [16,17,18]. Low estriol levels with normal fetal ultrasonography and exclusion of placental sulfatase deficiency and Smith-Lemli-Opitz (SLO) syndrome may suggest a defect in fetal steroidogenesis. Weintrob et al. [18] reported a patient with low estriol in maternal serum triple screening with normal fetal sonography, who was diagnosed with ACTH deficiency postnatally. Subsequently, the patient was found to have a novel TBX19 gene mutation and was treated with hydrocortisone. Our case also had a history of intrauterine death of a sibling and postnatal death of two siblings of unknown causes. Her mother had been followed by an obstetrician and she had amniocentesis for the history of the deaths of unknown etiology of her three children. Results were reportedly “normal.” Awareness about the low estriol observed in relation to adrenal insufficiency may increase the number of patients with prenatal and early diagnosis of IAD.
Despite severe hypoglycemic episodes and recurrent infections and hospitalizations, our patient had accelerated growth. Furthermore, she had low IGF1 and insulin levels. On follow-up, she was observed to have premature thelarche, but did not develop progressive precocious puberty which would have accounted for the significant advancement of linear growth. Therefore, premature thelarche may only partially have contributed to growth phenotype in our case. Reports to date on the pubertal development of patients with TBX19 mutations are inadequate. Animal studies report that TPIT is a negative regulator of gonadotroph differentiation. Cells would switch to gonadotroph cell line in the case of TBX19 gene inactivation [4, 5]. TPIT-deficient mice had mildly increased levels of gonadotroph cells in the intermediate and anterior lobe of the pituitary. Nevertheless, an increased number of gonadotrophs is not sufficient to affect gonadotroph function. The TPIT mutant proteins may additionally repress steroidogenic factor-1 transcriptional activity and, hence, prevent the cell fate change to gonadotrophs [2, 4]. Therefore, the effect of these mutations on the pituitary-gonadal axis is not obvious.
Tall stature was reported in two other cases with adrenal insufficiency, MC2R gene defects (which cause primary adrenal insufficiency), and POMC deficiency (which causes central adrenal insufficiency). MC2R is a G protein-coupled receptor and ACTH exerts its role through this receptor [19, 20]. Familial glucocorticoid deficiency (FGD) type 1 caused by MC2R mutations presents with primary adrenal insufficiency [21]. Additionally, FGD-1 cases have been reported with tall stature, the underlying mechanisms of which remain unclear. Bone growth is stimulated by IGF-binding protein 5 (IGFBP5). Glucocorticoids inhibit IGFBP5, and cortisol deficiency results in lack of negative inhibition. Tall stature in these patients may be related to this lack of inhibition of IGFBP5 [22,23,24]. Based upon previous reports, cortisol deficiency and lack of inhibition of IGFBP5 could, hypothetically, also be a mechanism underlying our case. A second hypothesis to explain tall stature in FGD-1 cases is the ACTH effect on the chondrogenic phenotype. In vitro, ACTH at high concentrations could activate melanocortin receptors in bone and the cartilaginous growth plate and stimulate growth. On the other hand, low ACTH in congenital IAD is incompatible with the mechanism of ACTH effect of chondrocytes. Any coexisting pathogenic variant of the MC2R gene was excluded in our patient in the WES analysis. It is suggested that glucocorticoid replacement normalizes advanced growth in FGD; however, the possibility of overtreatment is an issue [24]. In our case, height was in the upper centiles, despite a declining trend from the 97th to 75th centile during follow-up.
Accelerated growth together with central adrenal insufficiency has also been observed in a few cases with POMC deficiency [25,26,27]. Although growth was not adequately described in these previous cases with POMC deficiency, obesity and hyperinsulinism were suspected to be causing growth acceleration [28]. However, Ozsu et al. [29] reported two siblings with overgrowth and POMC deficiency. One of them had insulinopenic diabetes and, hence, had low-normal serum IGF1 and low insulin concentrations. Therefore, regulation of accelerated linear growth with POMC deficiency, at least in this case, cannot be explained by hyperinsulinism.
Data in the literature about the growth and height potential of cases with TBX19 gene mutations is limited. A case reported by Akcan et al. [14] concerned a prepubertal male with compound heterozygous TBX19 mutation, who was admitted at 3 years old with IAD. His height was + 0.5 SDS at admission, and + 0.3 SDS at last evaluation at the age of 7.2 years. In a recent study reporting two siblings with IAD due to TBX19 gene mutation, their height and weight SDS were also in normal ranges, despite a history of hypoglycemic episodes and hospitalizations [13]. The anthropometric data of the patients with TBX19 gene mutations reported so far are presented in Table 3.
In conclusion, we have reported a novel nonsense TBX19 gene mutation. In addition to early-onset IAD, the patient had coexisting tall stature. While overgrowth is a known feature of various types of adrenal insufficiencies, including MC2R gene defects and POMC deficiency, it may be a novel feature of TPIT deficiency, as in our patient.
References
Couture C, Saveanu A, Barlier A et al (2012) Phenotypic homogeneity and genotypic variability in a large series of congenital isolated ACTH-deficiency patients with TPIT gene mutations. J Clin Endocrinol Metab 97:486–495
Vallette- Kasic S, Brue T, Pulichino AM et al (2005) Congenital isolated adrenocorticotropin deficiency: an underestimated cause of neonatal death explained by TPIT gene mutations. J Clin Endocrinol Metab 90:1323–1331
Pulichino AM, Vallette-Kasic S, Couture C, Brue T, Drouin J (2004) Tpit mutations reveal a new model of pituitary differentiation and account for isolated ACTH deficiency. Med Sci (Paris) 20:1009–1013
Pulichino AM, Vallette-Kasic S, Tsai JP, Couture C, Gauthier Y, Drouin J (2003b) Tpit determines alternate fates during pituitary cell differentiation. Genes Dev 17:738–747
Lamolet B, Pulichino AM, Lamonerie T et al (2001) A pituitary cell-restricted T box factor Tpit, activates POMC transcription in cooperation with Pitx homeoproteins. Cell 104:849–859
Stenson PD, Mort M, Ball EV et al (2017) The human mutation database: towards a comphrehensive repository of inherited mutation data for medical research, genetic diagnosis and next generation sequencing studies. Hum Genet 136:665–677
Déchelotte P, Darcha C, Labbé A, Vanlieféringhen P, Beaufrère AM, Malpuech G (1994) Congenital adrenal hypoplasia due to isolated familial ACTH deficiency. Pediatr Pathol 14:377–380
Adzhubei I, Jordan DM, Sunyaev SR (2013) Predicting functional effect of human missense mutations using PolyPhen-2. Curr Protoc Hum Genet 7:Unit7.20
Sim NL, Kumar P, Hu J, Henikoff S, Schneider G, Ng PC (2012) SIFT web server: predicting effects of amino acid substitutions on proteins. Nucleic Acids Res 40:452–457
Schwarz JM, Cooper DN, Schuelke M, Seelow D (2014) MutationTaster2: mutation prediction for the deep-sequencing age. Nat Methods 11:361–362
Metherell LA, Savage MO, Dattani M et al (2004) TPIT mutations are associated with early onset, but not late-onset isolated ACTH deficieny. Eur J Endocrinol 151:463–465
Alsaleem M, Saadeh L, Misra A, Madani S 2016 Neonatal isolated ACTH deficiency (IAD): a potentialy life-threatening but treatable cause of neonatal cholestasis. BMC Case Rep. https://doi.org/10.1136/bcr-2016-215032
Unal E, Yıldırım R, Taş FF, Tekin S, Sen A, Haspolat YK (2018) A rare cause of neonatal hypoglycemia in two siblings: TBX19 gene mutation. Hormones (Athens) 17:269–273
Akcan N, Serakıncı N, Turkgenc B, Bundak R, Bahceciler N, Temel SG (2017) A novel TBX19 gene mutation in a case of congenital isolated adrenocorticotropic hormone deficiency presenting with recurrent respiratory tract infections. Front Endocrinol (Lausanne) 8:64
Pulichino AM, Vallette-Kasic S, Couture C et al (2003a) Human and mouse TPIT gene mutations cause early-onset pituitary ACTH deficiency. Genes Dev 17:711–716
Zachmann M, Girard J, Duc G, Illiq R, Prader A (1979) Low estriol during pregnancy caused by isolated ACTH- deficiency. Acta Paediatr Scand Suppl 277:26–31
Malpuech G, Vanlieferinghen P, Dechelotte P, Gaulme J, Labbe A, Guiot F (1988) Isolated familial adrenocorticotropin deficiency: prenatal diagnosis by maternal plasma estriol assay. Am J Med Genet 29:125–130
Weintrob N, Drouin J, Vallette- Kasic J et al (2006) Low estriol levels in the maternal triple-marker screen as a predictor of isolated adrenocorticotropic hormone deficiency caused by a new mutation in the TPIT gene. Pediatrics 117:322–327
Novoselova TV, Chan LF, Clark AJL (2018) Pathophysiology of melanocortin receptors and their accessory proteins. Best Pract Res Clin Endocrinol Metab 32:93–106
Fridmanis D, Roga A, Klovins J (2017) ACTH receptor (MC2R) specifity: what do we know about underlying molecular mechanisms? Front Endocrinol (Lausanne) 8:13
Naville D, Weber A, Genin E, Durand P, Clark AJ, Begeot M (1998) Exclusion of the adrenocorticotropin (ACTH) receptor (MC2R) locus in some families with ACTH resistance but no mutations of the MC2R coding sequence (familial glucocorticoid deficiency type 2). J Clin Endocrinol Metab 83:3592–3596
Yeh JK, Evans JF, Niu QT, Aloia JF (2006) A possible role for melanocortin peptides in longitudinal growth. J Endocrinol 191:677–686
Chida D, Nakagawa S, Nagai S et al (2007) Melanocortin 2 receptor is required for adrenal gland development, steroidogenesis, and neonatal gluconeogenesis. Proc Natl Acad Sci U S A 104:18205–18210
Chung TT, Chan LF, Metherell LA, Clark AJ (2010) Phenotypis characteristics of familial glucocorticoid deficiency (FGD) type 1 and 2. Clin Endocrinol 72:589–594
Anisimova AS, Rubtsov PM, Akulich KA, Dmitriev SE, Frolova E, Tiulpakov A (2017) Late diagnosis of POMC deficiency and in vitro evidence of residual translation from allele with c.-11C>a mutation. J Clin Endocrinol Metab 102:359–362
Darcan S, Can S, Goksen D, Asar G (2010) Transient salt wasting in POMC-deficiency due to infection induced stress. Exp Clin Endocrinol Diabetes 118:281–283
Özen S, Özcan N, Uçar SK, Gökşen D, Darcan Ş (2015) Unexpected clinical features in a female patient with proopiomelanocortin (POMC) deficiency. J Pediatr Endocrinol Metab 28:691–694
Aslan IR, Ranadive SA, Valle I, Kollipara S, Noble JA, Vaisse C (2014) The melanocortin system and insulin resistance in humans: insights from a patient with complete POMC deficiency and type 1 diabetes mellitus. Int J Obes 38:148–151
Ozsu E, Bahm A (2017) Delayed diagnosis of proopiomelanocortin (POMC) deficiency with type 1 diabetes in a 9-year-old girl and her infant sibling. J Pediatr Endocrinol Metab 30:1137–1140
Acknowledgements
The authors wish to express their gratitude to the parents and the patient who participated in this study.
Author contributions
Zehra Yavas Abali, Gozde Yesil, Tarik Kirkgoz, Sare Betul Kaygusuz, Mehmet Eltan, Serap Turan, Abdullah Bereket, and Tulay Guran conceptualized and designed the study. Zehra Yavas Abali and Tulay Guran drafted the initial manuscript. Serap Turan, Abdullah Bereket, and Tulay Guran critically reviewed the manuscript. Gozde Yesil and Tulay Guran performed the assays, analyzed data, and interpreted the results. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Patient confidentiality
The patient’s parents provided informed consent for publication of the submitted article and the results of the accompanying genetic analyses.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abali, Z.Y., Yesil, G., Kirkgoz, T. et al. Evaluation of growth and puberty in a child with a novel TBX19 gene mutation and review of the literature. Hormones 18, 229–236 (2019). https://doi.org/10.1007/s42000-019-00096-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42000-019-00096-7