Abstract
Consumers prefer fresh, mildly preserved fishes without added chemicals. Due to increasing awareness among consumers on health aspects, demand for fish is ever increasing. The conventional preservation methods do not offer fresh-like product for longer duration, which limits the availability of fish in distant markets. Hence, the present study was undertaken to assess the effect of mono- and multilayered packaging material on the quality of seer fish steaks during chilled storage. For this, seer fish (Scomberomorus commerson) steaks were packed in LDPE (monolayer) and polyester–polyethylene (multilayer) pouches and the packs were sealed and stored in ice. Sampling was carried out at regular intervals to assess the changes in headspace gas composition, sensory quality, biochemical, physical and microbial quality. Significant reduction in O2 and increase in CO2 content was observed for both the packs. Lower microbial counts were observed for seer fish packed in multilayered pouches as compared to LDPE. Significant (P < 0.05) lower TVB-N values were observed for seer fish packed in multilayered pouches. Reduction in TBA values and TVBN content was observed for samples packed in multilayer compared to monolayer film. No significant variation in flavor, firmness and taste was observed among the samples up to 7th day. Sensorily, seer fish steaks packed in LDPE were acceptable up to ~ 11–12 days compared to ~ 13–14 days in multilayered pouches indicating better quality in multilayered pouches.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fresh seer fish is highly preferred by the consumer due to its aroma, texture and other culinary attributes. In India, seer fish forms one of the most commercially important fisheries and fetches very high price in both domestic and export markets [1]. Among the five species of seer fishes, the species Scomberomorus commerson and Scomberomorus guttatus commonly occur in Indian waters and the production of seer fish has shown a steady increase in the last five decades. Of the potential 75,076 tons of seer fishes in Indian waters, S. commerson contributes to 66–67% of catch [2]. Seer fish in fresh form is commonly sold for immediate consumption at retail fish stores or at fish market mainly as steaks for preparing grilled or fried product or as curry form. In spite of availability of many advanced packaging technology, cold chain facilities, supply of fresh quality marine fishes is still a major problem in majority of tropical countries. The highly perishable nature of fish due to bacteriological activity and biochemical changes is attributed to the rapid spoilage. Various preservation methods have been in place to overcome the spoilage of fish [3]. Chilling and refrigeration is the most preferred preservation method as it helps in preserving fresh-like quality. Chilling or icing is reducing the temperature of fish so as to prolong the lag phase of bacteria and helps in reducing the spoilage rate. Fish being one of the most perishable foods, its freshness is rapidly lost even when stored under chilled conditions. Further, consumers demand to have fish in as fresh a state as possible so that the characteristic flavors are retained. Bulk transportation of fresh fish in ice has several limitations like limited extension of shelf life, unnecessary expenditure on freight due to ice, difficulty in handling and maintaining hygienic conditions due to leaching of ice melt water with leaching losses of soluble nutrients and flavoring compounds. Proper packaging of fish in retail packs will help in considerable extension of shelf life. Packaging assists the preservation of the world’s resources through the prevention of product spoilage and wastage, and by protecting products until they have performed their function. Every food product including fishery product needs some kind of packaging in their life cycle until they reach consumer. This accounts to nearly 99.8% of all food items undergoes some sort of packaging. A number of packaging materials ranging from monolayer to multilayer packaging materials are available in the market with varying properties. The selection of suitable packaging material is very critical for any preservation method as it influences the quality of stored product. The comparative studies on the mono- and multilayer packaging material on the quality of fishes is very scant. Hence, the present study was undertaken to assess the influence of mono (LDPE) and multilayer (polyester–polyethylene) packaging material on the shelf life of seer fish during chilled storage.
Materials and Methods
Fish Preparation and Packaging
Freshly landed seer fish (S. commerson) with average body length of 85–87 cm with weight of 8–9 kg were procured from Matsyafed unit at Thoppumpady, Kochi, India. The fishes were washed in chilled potable water, gutted and washed in chilled water. Steaks of 2 cm thickness weighing 300 ± 10 g was prepared and used for packing. The steaks prepared were brought to the laboratory in iced condition and washed again with potable chilled water. Commercially available packaging material, mono layered LDPE with thickness of 45 ± 3 µm and 12 µm polyester laminated with 75 µm low density polyethylene (Pradeep Laminates, Pune, India) was used in the study as multilayered polybag. Oxygen transmission rate of LDPE and multilayered pouch was 860 ± 4.8 and 236.38 ± 3.89 cc m−2 24 h−1 at 1 atm pressure, respectively. The seer fish steaks were divided into two lots and one steak from each lot was packed in LDPE and polyester polyethylene poly bags (b × l, 20 × 25 cm size) and stored on ice in alternative layers of ice and fish sample. Three pouches from each batch were drawn at regular intervals to monitor head space gas, sensory, biochemical, physical and microbial quality changes of seer fish. Ice melt water was drained every day and ice was replenished every day.
Head Space Gas Analysis
Head space gas composition comprising O2 and CO2 was measured using a gas analyzer (PBI Dansensor, Checkmate 9900, Ronnedevej, Denmark), which functions based on a solid-state O2 ion conductive material (zirconium oxide). Gas analysis was performed by drawing headspace gas sample by piercing the syringe needle through a rubber septum glued on the film.
Sensory Analysis
Sensory quality of seer fish steaks packed in monolayer and multilayer packaging material was carried out by seven trained researchers. On the day of samples, the sample was removed from the polybag and washed with potable water and served in a coded plate after cooking for 10 min in boiling water with 2% salt (NaCl, w/v) and cooling for 1–2 min. The panellists were asked to assign a score of 1–9 as prescribed by Meilgaard et al. [4] for the sensory attributes viz., appearance, color, odor, flavor, firmness and taste. Overall acceptability was estimated by adding the scores for all the attributes and dividing by the total number of attributes. An overall acceptability score of less than 5 was considered as the limit of acceptability.
Microbiological Analysis
Ten gram of fish sample was aseptically weighed and homogenized with 90 ml of normal saline (0.85%) for 1 min in a stomacher at 230 rpm (Seward Stomacher 400 Circular, London, UK). The homogenized sample was serially diluted using sterile 9 ml saline for bacteriological analysis. Total Aerobic plate count (APC) and Psychrophilic Counts were estimated using 3 M Petrifilm™ Aerobic Plate Count Plates as per AOAC [5]. Petri films were incubated at 35 ± 1 °C for 48 ± 3 h for aerobic plate counts and 7 °C for 7 days for psychrophilic counts. The average counts were calculated and expressed as colony forming units per gram (cfu g−1) of the sample.
Biochemical Analysis
Proximate composition and energy value was monitored for fresh seer fish. Moisture content of fish was determined by drying a known quantity of fish meat in an oven at 105 ± 1 °C for 16 h [6] and expressed as % on wet weight basis. Crude fat content was extracted using Soxhlet apparatus with petroleum ether following AOAC method [6]. The total nitrogen present in the sample was determined according to AOAC [6] and crude protein content was calculated by multiplying with a factor 6.25. Ash content was determined by heating the sample at 550 ± 2 °C for 4–5 h in a muffle furnace [6]. The energy value of fish was determined using the factors 4.27 and 9.02 kcal g−1 for protein and fat, respectively, and expressed as kcal g−1 edible part [7]. Total volatile base nitrogen (TVB-N) content was determined using Conway diffusion method [8]. pH value was measured by homogenizing the fish muscle with distilled water (1:2 w/v) by using a glass electrode digital pH meter (Cyberscan 510, Eutech Instruments, Singapore) [9]. Oxidation stability of the fish as thiobarbituric acid (TBA) value was measured spectrophotometrically [10].
Physical Quality Assessment
Instrumental Color Measurement
The color of the homogenized sample was measured with a Hunter’s colorimeter (Hunter Lab colorimeter, MiniScan® XE Plus Hunter Associates Lab inc., Reston, Virginia, USA). The sample was filled up to half of the circular 2.5 inch glass cell, fitted into the grove provided for the measurement. An opaque sample cup cover was covered and sample was measured at three different locations. CIELAB L* (lightness), a* [redness/greenness (±)] and b* [yellowness/blueness (±)] were measured using D65 illuminant, 10° standard observer. L* is the luminance or lightness component, which ranges from 0 to 100, and parameters a* [redness/greenness (±) and b* (yellowness/blueness (±)] are the two chromatic components, which range from − 120 to + 120 were measured [12]. Other color attributes like chroma (C), hue angle (h), whiteness index and ΔE were calculated using the equations given below.
Texture Profile Analysis
Changes in instrumental texture of seer fish were measured using Universal Testing Machine (Lloyd Instruments LRX plus, Lloyd Instruments Ltd, Hampshire, UK). Cylindrical probe of 50 mm dia with a load cell of 50 N was used for the measurement. Uniform sample size of 2 cm2 was maintained throughout the storage period for texture analysis. The sample was placed exactly below the centre of cylindrical probe and the two compression cycles of 40% was used with a crosshead speed of 12 mm min−1 with a trigger of 0.05 kg f. Force by time data from each test were used to calculate the texture attributes like hardness 1 and 2, chewiness, cohesiveness, springiness, gumminess and fracture force as described by Bourne [11].
Statistical Analysis
The results are expressed as mean ± standard deviation (sd). Significant differences for different attributes over the study period were evaluated at P < 0.01 for microbiological counts and at P < 0.05 for all other attributes by analysis of variance (ANOVA) using the software SPSS version 10.00. Mean separations were determined by Duncan’s multiple range test.
Results and Discussion
Proximate Composition and Energy Value of Seer Fish
Seer fish is one of the highly priced commercially important fish. It is preferred both in domestic as well as export markets due to its culinary attributes. The chemical composition of fish varies greatly from one species and one individual to another depending on nutrition, fish size, sex, age, environment and season. Therefore, a considerable variation is observed for the constituents of fish muscle [13]. The seer fish used in the present study had moisture content of 75.25% (Table 1) which is similar to the reported values [14] and higher than the values reported [15]. The variation in moisture content of same fish species by different authors could be due to the temporal changes and other species related factors. The crude protein and crude fat content was 18.3 and 4.98%, respectively. Similar results were reported by Yesudhason [14] for seer fish. Variation in lipid values was reported for cultured and temperate fishes [16, 17]. The fat content is influenced by season and geographical location, with lower lipid content in fish from tropical waters. Crude protein observed in the present study is less than the reported value for seer fish [15], yellowfin tuna [18] and more than Indian oil sardine [19]. Ash content of the fish was 1.34% which is similar to values reported for Indian oil sardine [19]. Energy value of seer fish was 123.1 kcal 100 g−1, which is higher than yellowfin tuna [18].
Changes in Headspace Gas Composition
The gas composition in the packaging material packed with food products varies with the storage days due to the growth of microorganisms and other biochemical changes. This can be used indirectly as a means of assessing the freshness condition of the packed food. In the present study, the headspace gas composition varied with the storage days in all the packs (Table 2). For both LDPE and multilayered pouches, the initial O2 content was 21.8%, which decreased with the storage period. On the day of sensory rejection O2 level of 1.5 and 0% was observed for LDPE and multilayered pouches, respectively. The initial level of CO2 in both the packs was < 0.1%, which increased with the storage period, reaching level of 5.3 and 9.4% for LDPE and multilayered pouches, respectively. The variations in the headspace gas composition, particularly CO2 and O2 could be used as an effective and easy index of microbial growth. The large variation of the gas composition of the control air packed samples could be due to microbial growth as supported by the increasing microbial counts with the storage period. Similar results were reported for seer fish packed in EVOH pouches [15] but the values for CO2 was higher compared to the present study.
Changes in Sensory Quality of Seer Fish
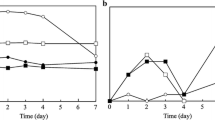
Sensory changes in seer fish packed under different atmosphere are given in Fig. 1. Initially, the fish used in the study had very good appearance, shining skin characteristic to the species. The steaks had characteristic bright color and up on exposure to light, the steaks appeared light pink color. No discoloration was observed in any of the concentric rings. The fish had very firm texture and sweet seaweedy odor and sweet taste indicating the very fresh nature of fish used in the study. The sensory score for all the attributes including flavor, firmness and taste for fresh fish was rated 9.0 by the panelists, which showed a decreasing trend for all the samples with storage period (Fig. 1). Among mono- and multilayered pouches, samples packed in multilayered pouches rated better for all the attributes throughout the storage period. On 5th day, no marked variations were observed in any of the sensory attributes in both the samples. Appearance was very fresh with no discoloration and no change in odor for the sample packed in multilayered pouches. Discoloration of up to 3 concentric ring was observed for samples stored in monolayered pouches and no discoloration was observed for multilayered pouch samples. On 7th day, slight change in appearance, odor, color and taste was observed. Shrunken skin was observed for both the mono and multilayered pouches. Sweet characteristic odor of seer fish was lost in both the samples. Characteristic pink coloration of the meat was not observed in both the samples. Visible drip loss was observed more in monolayered pouches compared to multilayered pouches. Discoloration of up to 5 concentric rings was observed in monolayer pouches and 3 concentric rings for multilayered pouch. Brown discoloration of red meat was observed in both the samples. Up to 7th day, prime quality of seer fish was maintained in both the packaging materials, which showed slight variation during the subsequent storage period. There were no significant differences (P < 0.05) in the sensory quality among the two samples during the initial storage period. Overall acceptability score decreased with storage period (Table 3) and reached less than 5 on 13 and 15th day for samples packed in mono- and multilayered pouches, indicating a shelf life of ~ 11–12 and ~ 13–14 days, respectively. The extension of shelf life could be attributed to the good barrier property of multilayered film as indicated by its oxygen transmission rate, which is 3.6 times less than monolayer, LDPE. The shelf life obtained in the present study is comparable with the results reported for seer fish [20, 21] and salmon [22].
Changes in Microbial Quality
Changes in total aerobic plate counts and psychrophilic counts are given in Fig. 2. Initially, the aerobic plate counts were 6.08 and 5.96 log cfu g−1 for mono and multilayered samples, respectively, which showed a progressive increase for the samples packed in monolayer pouch, after an initial lag of 5 days. On the day of sensory rejection, aerobic plate count of 6.72 log cfu g−1 was observed for seer fish packed in monolayer pouch. For the samples packed with multilayered packaging material, after initial lag of 5 days, aerobic plate counts showed a decrease in their counts to 5.69 log cfu g−1 on 7th day. There after an increasing trend was observed. However, the increase was significantly lower (P < 0.01) compared to samples packed in monolayer pouches. The variation in the aerobic plate counts among the packaging material could be attributed to the barrier property, which indicated significant low oxygen content in the multilayered packs during storage period compared to monolayer pouch (Table 2). However, the aerobic plate counts of seer fish packed in monolayer pouch did not exceeded the maximum recommended limit of 107 log cfu g−1 in fresh fish [23] throughout the storage period. For seer fish packed in multilayered film, this limit was exceeded on 15th day corresponding to sensory rejection. Microbial shelf life of 8 days [24] and 12 days were reported for seer fish [25] which is comparable with the present observation. The initial psychrophilic counts in the seer fish was 4.79 and 4.56 log cfu g−1 for samples packed in mono and multilayered pouches, respectively. The psychrophilic counts did not show lag phase for both the packaging material and showed a progressive increase with the storage period. However, significant differences (P < 0.01) were not observed among the packaging materials. Psychrophilic counts exceeded the maximum recommended limit of 107 log cfu g−1 on the day of sensory rejection in both the samples. The study indicates that psychrophilic counts are better indicator of microbial spoilage for ice stored fish compared to aerobic plate counts.
Changes in Biochemical Quality
Changes in Total Volatile Base Nitrogen Content
The changes in total volatile base nitrogen content of seer fish packed in mono and multilayered polybag is shown in Fig. 3a. The fish used in the study had good quality as indicated by low initial TVBN content (4.2 mg N2 100 g−1). The concentration of TVB-N in freshly caught fish ranges between 5 and 20 mg N2 100 g−1 [26, 27]. The level of TVBN increased with the storage period in both the samples. No significant variation (P < 0.05) was observed in both the samples throughout the storage period. However, the sample packed in multilayer polybag exhibited slightly lower TVB-N values from 7th day onwards. No significant variations (P < 0.05) were observed for TVB-N values between the packaging material in the beginning, but showed a slightly higher values for the sample stored with monolayered pouch. On the day of sensory rejection, TVB-N value of 21.4 and 22.2 mg N2 100 g−1 was observed for samples in LDPE and multilayered polybags, respectively. The increase in TVB-N value during storage period is mainly attributed to the increase in bacterial counts (Fig. 2) and other endogenous enzymes [28,29,30,31]. TVB-N value may be considered as a quality index for fish and levels of 30–35 mg N 100 g−1 flesh are generally regarded as the limit of acceptability [26, 27], which was not observed in both the samples.
Changes in pH Values
Changes in the mean pH of seer fish steaks packed in different packaging material over the storage period is shown in Fig. 3b. The initial pH of seer fish steak was 6.22, indicating the freshness of the sample. Low pH observed in the present study could be due to the depletion of energy reserves, mainly glycogen with the production of lactate which indirectly indicates the stress at the time of catching [32,33,34]. During storage, pH value of seer fish increased with the storage period in the LDPE with slight variation. In multilayered film, a slight decrease in pH initially on 5th day and later increased with the storage period reaching a value of 6.68 on the day of sensory rejection compared to 6.66 for samples packed in LDPE. The increased pH during storage could be attributed to the production of alkaline compounds such as total volatile bases, ammonia by spoilage bacteria [35, 36]. This increased pH during storage indicates bacterial growth and possible spoilage [36].
Changes in TBA
Degree of lipid oxidation is commonly assessed using thiobarbiutiric acid value which measures malonaldehyde content [37]. Fatty acid, particularly polyunsaturated fatty acids reacts with oxygen leads to the formation of hydroperoxides and further oxidation leading to the formation of malonaldehyde. Being highly stable compound, malonaldehyde provides useful information on the oxidation of food products [38]. TBA values for seer fish packed in different packaging material are given in Fig. 3c. The initial TBA value of seer fish was 0.315 mg malonaldehyde kg−1 of fish which increased with the storage period. TBA value of seer fish packed in multilayered polybag were significantly lower (P < 0.05) than in the monolayer pouch. This could be attributed to the lower oxygen content in the multilayered pouch (Table 2). On the day of sensory rejection, TBA value of 5.9 and 2.8 mg malonaldehyde kg−1 was observed for fish packed in monolayer and multilayer pouches, respectively. Results of the present study are in agreement with the results reported by Yesudhason [14] for seer fish.
Changes in Physical Quality of Seer Fish
Changes in Instrumental Colour
The color of the food products is very important as it decides the final acceptability by the consumers. Instrumental color analysis provides unbiased color value of the product. Variation in color parameters of seer fish packed in monolayer pouch and multilayer pouch is given in Table 4, respectively. The initial lightness (L*) value of seer fish was 64.84, which is higher than the values reported for fresh tuna [18, 39]. Among the different packs, the L* value did not show any variation with the storage period. However, it increased in both the packs up to 7th day and decreased during subsequent storage period. On the day of sensory rejection, L* value of 63.76 and 64.18 was observed for seer fish packed in monolayer and multilayered pouches. a* value of fresh seer fish was 0.29 indicating red colour of the meat, which changed to − 1.37 on 5th day for monolayer and to and − 0.3 on 7th day for multilayered pouches, indicating change of color from red to green. Significant differences (P < 0.05) was observed for a* values among different sampling days for both the samples. b* value which indicates yellowness when values are positive and blueness when values are negative and chroma values did not show significant differences (P < 0.05) among the different pouches. During the storage period, b* and chroma values increased from an initial value of 14.5 to ~ 16.1, on the day of sensory rejection for both the samples. b* values observed in the present study are comparable with the values reported for tuna [18, 39]. The denaturation of myoglobin and oxidation of carotenoid pigments could be attributed to the increased b* values. Hue angle ranged between 84.43–89.69 and 85.37–88.87 for seer fish packed in monolayer and multilayer pouches, respectively. The whiteness index and ΔE showed slight fluctuation during the storage period in both the samples and ranged between 60.38–66.99 and 26.34–33.25, respectively. In both the samples, a slight increase in the whiteness was observed with the storage days, which could be attributed to the oxidation of pigments leading to bleached appearance of the meat.
Changes in Instrumental Texture
Instrumental texture analysis provides useful information on the state of fish with the storage period. The initial hardness 1 and 2 of seer fish ranged between 10.8–12.4 and 9.1–10.3 N which showed a decreasing trend up to 7th day in both the pouches (Table 5). The decrease was faster for samples packed in monolayer pouch compared to multilayered pouch. The decrease in hardness could be attributed to the loss of binding property of connective tissue with the storage period which increased the visible drip content in the pouches. The increase in hardness 1 and 2 after 7th day could be attributed to the reduced water content of the fish muscle due to increased drip loss. Values of hardness 1 and 2 of seer fish packed in both the mono- and multilayer were significantly different (P < 0.05) during different sampling days. Cohesiveness, gumminess and fracture force exhibited a decreasing trend with the storage period for both the samples. Cohesiveness of seer fish did not show any significant differences (P < 0.05) among different sample intervals in both the packaging materials. Both springiness and chewiness were higher for samples packed in monolayer pouches initially, which decreased faster compared to multilayered pouches with the storage period. On the day of sensory rejection, springiness and chewiness decreased to 29.2 and 58.2% in monolayer pouch from an initial value compared to 21 and 34.6% for multilayered pouches, respectively. Decreasing trend of springiness and chewiness was observed in control as well modified atmosphere packaged seer fish during storage [14, 15]. The present study indicates that use of multilayered pouches with good barrier property has advantage in preserving the fish quality better compared to monolayer pouches.
Conclusion
Selection of suitable packaging material is very vital for preserving the quality of perishable food like fish. Any method which provides extension of shelf life will be easily adopted by the seafood industry. The study indicated that use of multilayered pouch had advantage over monolayered LDPE in preserving the quality and extending the shelf life. Better barrier property of multilayered film reduced the lipid oxidation and other quality changes. A shelf life of ~ 11–12 days was observed for seer fish steaks packed in LDPE in iced condition compared to ~ 13–14 days for multilayered pouch.
References
Muthiah C, Mohamad Kasim H, Pillai NGK, Yohannan TM, Manoj Kumar B, Said koya KP, Uma S, Bhat, Balasubramaian TS, Elayathu MNK, Manimaran C, Dhokia HK, Somaraju MV (2002) Status of exploitation of seer fishes in the Indian seas. In: Management of scombroid fisheries. ICAR-CMFRI, Kochi, India
Sinha MK, Premchand, Tiburtius A (2015) Status of seer fish fishery including some biological characteristic of Scomberomerus commerson in Indian waters. FAO-Indian Ocean Tuna Commission, Revision 1 (IOCT-2015-WPNT05-14 Rev_1), p 22
Mohan CO, Ninan G, Bindu J, Zynudheen AA, Ravishankar CN (2018) Value addition and preservation of fishery products In: Mohan CO, Carvajal-Millan E, Ravishankar CN, Haghi AK (eds) Food process engineering and quality assurance. Apple Academic Press, Waretown, NJ, USA, pp 439–458
Meilgaard M, Civille GV, Carr BT (1999) Sensory evaluation techniques, 3rd edn. CRC Press, Boca Raton, p 387
AOAC (2012) Aerobic plate count in foods (Petrifilm TM Method). In: Latimer GW (ed) AOAC official method 990.12, 19th edition. Official methods of Analysis of AOAC International, Rockville
AOAC (2000) Official methods of analysis, 17th edition, vol 2. Association of Official Analytical Chemists International, Rockville, pp 1–27
FAO (1989) Yield and nutritional value of the commercially more important fish species. FAO fisheries technical paper 309 Part I. FAO, Rome, pp 1–187
Conway EJ (1962) Micro-diffusion analysis and volumetric error, 5th edn. Crosby Lockwood and Son Ltd., London
IS: 2168 (1971) Specification for Pomfret canned in oil. Indian Standard Institute, New Delhi
Tarladgis GB, Watts MB, Younathan TM (1960) A distillation method for the quantitative determination of malonaldehyde in rancid foods. J AOCS 37:44–50
Bourne MC (1978) Texture profile analysis. Food Technol 33:62–66
Papdakis SE, Abdul Malek S, Kamdem RE, Yam KL (2000) A versatile and inexpensive technique for measuring color of foods. Food Technol 54:48–51
Pacheco-Aguilar R, Lugo-Sanchez ME, Robles-Burgueno M (2000) Postmortem biochemical and functional characteristic of Monterey sardine muscle stored at 0 °C. J Food Sci 65(1):40–47
Yesudhason P (2007) Effect of modified atmosphere packaging on the shelf life of commercially important fish. Ph.D Thesis, Central Institute of Fisheries Education, Mumbai, India, p 316
Mohan CO (2008) Shelf life extension of seer fish (Scomberomorus commerson) steaks using O2 scavenger and CO2 emitters in chilled condition. Ph.D. Thesis, Central Institute of Fisheries Education, Mumbai, India, p 248
Tejada M, Huidobro A (2002) Quality of farmed gilthead sea bream (Sparus aurata) during ice storage related to the slaughter method and gutting. Eur Food Res Technol 215:1–7
Alasalvar C, Taylor KDA, Oksuz A, Gartwaite T, Alexis MN, Grigoakis K (2001) Freshness assessment of cultured sea bream (Sparus aurata) by chemical, physical and sensory methods. Food Chem 72:33–40
Mohan CO, Remya S, Murthy LN, Ravishankar CN, Kumar KA (2015) Effect of filling medium on histamine content and quality of canned yellowfin tuna (Thunnus albacares). Food Control 50:320–327
Mohan CO, Ravishankar CN, Gopal TKS, Lalitha KV (2012) Effect of chitosan edible coating on the quality of double filleted Indian oil sardine (Sardinella longiceps) during chilled storage. Food Hydrocolloids 26(1):167–174
Mohan CO, Ravishankar CN, Gopal TKS, Ashok Kumar K (2009) Nucleotide breakdown products of seer fish (Scomberomorus commerson) steaks stored in O2 scavenger packs during chilled storage. Innov Food Sci Emerg Technol 10:272–278
Mohan CO, Ravishankar CN, Gopal TKS, Kumar KA, Lalitha KV (2009) Biogenic amines formation in seer fish (Scomberomorus commerson) steaks packed with O2-scavenger during chilled storage. Food Res Int 42:411–416
Sallam KI (2007) Chemical, sensory and shelf life evaluation of sliced salmon treated with salts of organic acids. Food Chem 101(2):592–600
ICMSF (International Commission on Microbiological Specifications for Foods) (1998) Microorganisms in foods. Microbial ecology of food commodities—fish and fish products, vol 6. Blackie Academic & Professional, Baltimore, pp 130–189
Yesudhason P, Lalitha KV, Gopal TKS, Ravishankar CN (2014) Retention of shelf life and microbial quality of seer fish stored in modified atmosphere packaging and sodium acetate pretreatment. Food Packag Shelf Life 1(2):123–130
Mohan CO, Ravishankar CN, Gopal TKS, Lalitha KV (2010) Effect of reduced oxygen atmosphere and sodium acetate treatment on the microbial quality changes of Seer fish (Scomberomorus commerson) steaks stored in ice. Food Microbiol 27:526–534
Huss HH (1988) Fresh fish: quality and quality changes. Fisheries Series No. 29, Food and Agriculture Organization (FAO) of the United Nations, Rome
Connell JJ (1995) Control of fish quality, 4th edn. Fishing News Books, Surrey
Kyrana VR, Lougovois VP, Valsamis DS (1997) Assessment of shelf-life of maricultured gilthead sea bream (Spratus aurata) stored in ice. Int J Food Sci Technol 32:339–347
Ozogul F, Polat A, Ozogul Y (2004) The effects of modified atmospheric packaging and vacuum packaging on chemical, sensory and microbiological changes of sardines (Sardina pilchardus). Food Chem 85:49–57
Ruiz-Capilllas C, Moral A (2005) Sensory and biochemical aspects of quality of whole bigeye tuna (Thunnus obesus) during bulk storage in controlled atmospheres. Food Chem 89:347–354
Vereltzis K, Koufidis D, Gavriilidou E, Papavergou E, Vasiliadou S (1997) Effectiveness of a natural rosemary (Rosmarinus officinalis) extract on the stability of filleted and minced fish during frozen storage. Z Lebensm Unters Forschung 205:93–96
Azam K, Mackie IM, Smith J (1989) The effect of slaughter method on the quality of rainbow trout (Salmo gairdneri) during storage on ice. Int J Food Sci Technol 24:69–79
Marx H, Brunner B, Weinzierl W, Hoffmann R, Stolle A (1997) Methods of stunning freshwater fish: impact on meat quality and aspects of animal welfare. Z Lebensm Unters Forsch A 204:282–286
Van de Vis JV, Oehlenschlager J, Kuhlmann H, Munkner W, Robb DHF, Schelvis-Smith AAM (2001) Effect of commercial and experimental slaughter of eels (Anguilla anguilla L.) on quality and welfare. In: Kestin SC, Warriss PD (eds) Farmed fish quality. Fishing News Books, Oxford, pp 234–248
Scott DN, Fletcher GC, Hogg MG (1986) Storage of snapper fillets in modified atmospheres at −1 °C. Food Technol Aust 38:234–239
Stammen K, Gerdes D, Caporaso F (1990) Modified atmosphere packaging of seafood. CRC Crit Rev Food Sci Nutr 29:301–308
Nishimoto J, Suwetja IK, Miki H (1985) Estimation of keeping freshness period and practical storage life of mackerel muscle during storage at low temperatures. Mem Fac Fish Kagoshima Univ 34:89–96
Mohan CO, Ravishankar CN, Srinivasa Gopal TK (2008) Effect of O2-scavenger on the shelf-life of catfish (Pangasius sutchi) steaks during chilled storage. J Sci Food Agric 88:442–448
Mohan CO, Remya S, Ravishankar CN, Vijayan PK, Gopal TKS (2014) Effect of filling ingredient on the quality of canned yellowfin tuna (Thunnus albacares). Int J Food Sci Technol 49:1557–1564
Acknowledgements
Authors would like to thank Director, ICAR-CIFT, Cochin for providing all the support to undertake this work and permitting to publish. The help and support rendered by the technical staffs of Fish Processing Division of the institute is highly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohan, C.O., Ashitha, V.A., Kishore, P. et al. Influence of Mono- and Multilayered Packaging Material on the Quality of Seer Fish (Scomberomorus commerson) During Chilled Storage. J Package Technol Res 2, 67–76 (2018). https://doi.org/10.1007/s41783-018-0025-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41783-018-0025-6