Abstract
Cement production causes serious environmental problems and requires large amounts of energy. Studies have proven that the use of geopolymers can significantly reduce carbon dioxide emission and energy consumption arisen from cement production. Although there are many studies on the geopolymers, the number of studies dealing with geopolymers containing natural pozzolans other than metakaolin is very limited. In this study, the compressive strength and pore structure of geopolymers produced with trass (a type of natural pozzolan) were investigated. The mineralogical composition of trass, morphology of trass, and microstructure of geopolymer paste as well as the weight loss of the paste were determined using XRD, SEM, and TGA examinations, respectively. Moreover, the pore size distribution of the paste mixtures was obtained with Micro-CT analysis. The trass was used both in the natural form and after calcining at 550 °C for 6 h. The strength of geopolymer mortars produced with calcined trass, cured at 90 °C for 24 and 96 h, was found to be 86.7% and 81.6% higher than that of their counterparts containing natural trass. In addition, calcination of the trass resulted in 12.8–22.9% reduction in the porosity of geopolymer paste, compared to that of the paste containing natural trass. In XRD and TGA investigations, it was determined that the trass calcination performed at 550 °C did not make a significant change in the paste crystal structure and the weight loss-temperature graphs. SEM images proved that the reactivity of trass in geopolymerization increased with calcination and the matrix structure became denser and more homogeneous.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Concrete is the most used building material in the world and the need for concrete, and resultantly for cement, is still increasing day by day [1]. During the production of portland cement, high amount of CO2 is emitted due to the utilization of fossil fuels and calcination of limestone accounts for approximately 1 ton per ton of cement [2]. Cement production also causes depletion of natural resources, soil, and land pollution [3]. International Energy Agency [4] reported that approximately 4.3 billion tons of cement was produced in the world in 2020. This amount gives an idea about the extent of the damages arisen from cement production.
In order to reduce the environmental problems and high-energy consumption caused by cement production, more environmentally friendly building materials are needed. Geopolymers have emerged as an alternative to traditional concrete, both by reducing CO2 emissions and energy consumption and by protecting natural resources [5]. It was pointed out that using geopolymers instead of cement will reduce CO2 emissions effectively. While Duxson et al. (2007) stated that the amount of reduction would be at least 80% [6], Davidovits (2013) reported that the use of by-product slag in geopolymer production provided 80% less CO2 emission as well as 59% less energy consumption compared to that of the cement production [7]. Geopolymers are generally produced by using aluminosilicates such as fly ash, blast furnace slag, and metakaolin with alkali activators such as sodium hydroxide and sodium silicate [8]. The strength and durability of geopolymer concretes produced by appropriate materials, curing conditions, activator concentration, and mix proportions are comparable to those of the conventional concretes [9]. Moreover, geopolymers have a lot of advantages such as high strength and durability as well as ensuring the disposal of waste materials such as fly ash, slag, and construction demolition waste [10]. In addition to the industrial wastes and metakaolin [11], other aluminosilicates such as kaolin, rice husk ash, and red mud [12] can also be used in the production of geopolymers. Considering their chemical composition, natural pozzolans are also potential aluminosilicates to be used in the geopolymer mixtures. There are many different factors that affect the reactivity of natural pozzolans in geopolymerization. In addition to the chemical and mineralogical composition, the variability in the formation process of natural pozzolan is also effective in its reactivity. Some researchers have stated that in addition to amorphous phases, crystalline aluminosilicate phases can also contribute to the geopolymerization. All these reasons limit the formulations of general rules for geopolymerization of natural pozzolans and more studies are needed on this kind of geopolymers [13]. Although natural pozzolans have a high potential for the production of geopolymers, limited research has been done on natural pozzolan–based geopolymers. Robayo-Salazar and Gutierrez (2018) reported that there were around 60 studies on the natural volcanic-based geopolymer/alkali-activated materials during 2000–2018 years [14].
The geopolymerization process is affected by several parameters such as mix design, temperature, duration and type of curing, alkali type, and concentration as well as activity of the aluminosilicate associated with its type, glassy phase content, and fineness [15]. Although it is possible to use different methods such as grinding or applying chemical processes to increase the pozzolanic activity of natural pozzolans, it has been stated that the most effective method is calcination. Upon calcination, the structural water in the clay evaporates and the clay structure becomes distorted. At temperatures of 500–800 °C, the structural water in the octahedral layer of clay is removed. With the disappearance of the OH groups that bond the octahedral and tetrahedral layers, the structure becomes either semi-crystalline or amorphous and the activity of clay increases [16]. It was shown that calcination of the pozzolans can improve the geopolymer strength substantially. Ghani et al. (2021) examined some properties of the geopolymer pastes produced with lateric clays which were not calcined and calcined at different temperatures for 1 h. The geopolymer produced with uncalcined clay could not attain any strength. However, the pastes produced with calcined clay at 500, 700, and 900 °C gained compressive strengths of 5.3, 11.5, and 24.8 MPa, respectively [17]. Nikolov et al. (2017) conducted a study on natural zeolite and calcined zeolite obtained with the calcination of natural zeolite at 900 °C for 4 h. It was stated that the activity of zeolite increased with calcination so that compressive strengths of 25.5 MPa and 43.1 MPa were obtained in the natural zeolite and calcined zeolite-based geopolymers containing potassium hydroxide and potassium silicate solution as activator [18]. Antoni et al. (2013) investigated the calcination of mud erupted from Sidoarjo volcano in Indonesia at different temperatures on mud-based geopolymer mortars. In the research, mud was calcined at 700, 800, and 900 °C for 5 h and a mixture of sodium silicate-sodium hydroxide was used as activator. Researchers stated that 800 °C was the most suitable calcination temperature and reported that the compressive strengths of the mortars produced with mud calcined at 700, 800, and 900 °C were 26.6, 36.7, and 18.3 MPa, respectively [19].
In recent years, many researchers have been working on geopolymer/alkali-activated materials. However, the number of studies on natural pozzolans other than clay is limited. There is a lack of studies on the effect of calcination of natural pozzolan on the geopolymers microstructure and porosity. In this study, effect of the calcination of a natural type of pozzolan (trass) obtained from İzmir Cumaovasi region on the properties of geopolymer mixtures was investigated. For this purpose, geopolymer mortars and pastes were produced by using natural trass and calcined trass obtained by calcining this natural trass in a muffle furnace at 550 °C for 6 h. Compressive strength and pore size distributions were determined on mortar specimens; SEM, XRD, and TGA examinations were carried out on paste samples. It is aimed to examine the changes in the pore ratio, pore distribution, compressive strength, and microstructure of trass-based geopolymers by calcination. For this purpose, SEM, XRD, TGA, and Micro-CT examinations were carried out. 3-D pore size distribution images were obtained at different curing times. The beneficial effects of calcination of trass on the compressive strength of geopolymer mortar were found to be more pronounced than its effect on the porosity of the mix.
Materials and method
Materials
In the study, a granular trass with the 64 mm maximum particle size obtained from Izmir Cumaovasi was used. The trass was ground in a planetary type laboratory ball mill and sieved through 125-µm sieve. Geopolymer mortar mixtures were produced with both natural trass and trass calcined at 550 °C for 6 h. In order to determine the most suitable calcination condition, 15 different calcined trasses were prepared by applying 5 different calcination temperatures (450, 550, 650, 750, 850 °C) and 3 different calcination times (2, 4, 6 h) by Bascik (2019). The most appropriate calcination condition was found to be 550 °C-6 h in terms of geopolymer compressive strength [20]. It was determined that the 28-day strength activity indices of natural and calcined trass (at 550 °C for 6 h) according to ASTM C311 [21] were 76% and 91%, respectively.
SEM images of the natural and calcined trass are presented in Fig. 1 which denotes no considerable change in the morphology of trass particles upon calcination. Besides, particle size distribution and chemical composition/physical properties of the trass are shown in Fig. 2 and Table 1, respectively. The Blaine specific surface and d50 value of the trass which was used in the geopolymer mixtures were 4620 cm2/g and 21.2 µm, respectively.
A sodium silicate solution consisting of 27% SiO2, 8% Na2O, 65% H2O, and sodium hydroxide pellets with 97% purity was used as alkali activator in the geopolymer mixtures. An activator solution with an Ms ratio (SiO2/Na2O ratio by weight) of 1.6 was produced by dissolving the sodium hydroxide pellets in the sodium silicate solution. The activator solution was used after resting in the laboratory for 24 h. Tap water was used to improve the workability of the mixtures. Standard CEN sand in accordance with TS EN 196–1 [22] was used as aggregate in the geopolymer mortars.
Method
Preparation of mortar mixes
A total of 1350 g of standard sand, 450 g of trass (natural or calcined), 297.5 g of activator (Na2O content of the activator solution was adjusted to be 10% by the weight of trass), and 10 g of water were put into the mixer bowl, respectively, and the mixer was operated at low speed for 90 s. After scraping off the mortar adhered to the bowl, the mixer was operated for another 90 s. The fresh mortar mixtures were placed in 50-mm cubic steel molds in two layers. Each layer was dropped 25 times on the jolting table after tamped 25 times by a 15-mm diameter ceramic rod. Samples were placed in a laboratory type oven with their molds immediately after preparation. The temperature was increased to 90 °C at a rate of ~ 10 °C/min. The specimens were cured at 90 °C for 24 or 96 h. Afterwards, the specimens were removed from the oven and demolded after being cooled to the room temperature. The compressive strengths of specimens were determined with a 2000 kN hydraulic testing machine at a loading rate of 0.9 kN/s. Three 50-mm cubes were used in the experiment and the average of the three specimens was recorded as compressive strength.
SEM, XRD, Micro-CT, and TGA investigations
For SEM, XRD, and TGA investigations, paste samples containing either natural or calcined trass and cured at 90 °C for 24 or 96 h were used. The production methods of the pastes were the same as that of the mortars. For the XRD and TGA analyses, the pastes were crushed, ground, and tested in powder form after being screened through 125-µm sieve. For morphology determination, SEM examinations were carried out on the broken sample surfaces. XRD analysis was performed in the range of 5–80° 2θ Cu, Kα at 0.0130° step sizes. Thermogravimetric analysis was performed at a temperature range of 25–1000 °C with a temperature increase rate of 10 °C/min and the sample was tested in nitrogen environment.
The pore structure of the samples and the effect of calcination on the pore size distribution as well as the total porosity were investigated with Micro-CT on 25-mm cube mortar specimens (regarding the sample size capacity of the device). The sample was examined with 20 × 20 × 20 µm-sized voxels. The pore size calculations were carried out by using “µCT Evaluation Program V6.5–3” software.
Results and discussion
Compressive strength
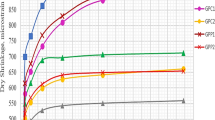
The compressive strength values of geopolymer mortars produced with natural and calcined trass cured for 24 or 96 h at 90 °C are shown in Fig. 3. As can be seen, the compressive strength of mortars produced with calcined trass and cured for 24 h is 86.7% greater than that of its counterpart mortar containing natural trass. The corresponding value for the mortars cured for 96 h is 81.6%. Antoni et al. (2013) and Nikolov et al. (2017) reported that with the calcination, the activity of natural pozzolans increases so the geopolymer produced with calcined pozzolans achieves higher compressive strength [18, 19]. In addition, it is seen that the strength of the natural trass-based mortars, cured for 96 h, is approximately equal to the strength of calcined trass mortars cured for 24 h. Hardjito et al. (2003) stated that the progress in polymerization with the increase of curing time improved the mechanical properties of the geopolymer [23]. Chithambaram et al. (2019) and Bing-hui et al. (2014) also reported improvement in the strength of geopolymer mixtures with increasing the curing time [24, 25].
Porosity and pore size distributions
The porosity of geopolymer mortars produced with natural and calcined trass cured for 24 or 96 h at 90 °C and the images of pore structure of geopolymer mortars obtained from Micro CT analysis are shown in Figs. 3 and 4, respectively. It is seen that, regardless of the duration of curing, maximum pore size in the samples produced with calcined trass is greater than that of those produced with natural trass. The fact may be arisen from the loss of water from calcined trass during its calcination resulting in absorbing of some of the mixing water and agglomeration of trass particles in some parts of the mortar sample (especially in the central zone). Considering the distribution of the pores, it is evident that the large pores are mostly concentrated in the inner parts of the samples produced with calcined trass. However, in spite of the greater maximum pore size, the total porosity of the calcined trass-based mortars is lower than that of the natural trass-based counterparts. The increase in curing time from 24 to 96 h did not affect the amount of pores in the mortar produced with natural trass. However, the porosity decreased by 10.2% and the compressive strength increased from 21.1 to 40.5 MPa with increasing of the curing time in the sample produced with calcined trass. These facts were attributed to the progress of geopolymerization resulting in a denser structure. The SEM images shown in Fig. 7 also indicate that by increasing the curing time from 24 to 96 h, the microstructure of paste produced with calcined trass became more homogeneous and denser. Thus, the total pore ratio decreased from 14.3 to 12.8%.
The pore size distributions of 96-h cured natural- and calcined trass-based geopolymer mortars are shown in Figs. 5 and 6. The average pore sizes of natural and calcined trass mortars are 0.19 and 0.25 mm, respectively. Although the average and maximum pore sizes of the calcined trass mortar are higher than those of the natural trass mortar, the total porosity of the former mixture is lower. As it was mentioned earlier, it seems that the reason for the greater pore size in the calcined trass mortar is the higher water requirement of the mix as compared to that of the natural trass mortar. However, the water content of the mortar mixtures was kept constant. Thus, the workability of calcined trass mortar was somewhat lower than that of the natural trass mortar. This resulted in a lower uniformity and flocculation of trass particles as well as difficulty in the proper compaction of the specimens, particularly in their central part.
SEM investigation
SEM images of geopolymer pastes produced with calcined trass, cured for 24 and 96 h, are presented in the Fig. 7. Cracks and pores are indicated by arrows, meanwhile unreacted, and partially reacted trass particles are shown by yellow and orange circles, respectively. As can be seen from the figure, there are large pores, large wide and long cracks, and an inhomogeneous structure in the paste cured for 24 h. However, with the increase of the curing time, the microstructure became more homogeneous and denser, providing greater strength. With the increase of the curing time, more trass particles participated in the geopolymerization; thus, the structure became more homogeneous and the low porosity coupled with a more homogeneous microstructure increased the compressive strength of the matrix. Micro-CT examination also supports these results and shows reduction in total porosity either by elongation of curing or by the calcination of trass.
TGA and XRD investigation
The temperature-weight loss graphs, obtained from thermogravimetric analysis, of geopolymer pastes produced with natural and calcined trass are shown in Fig. 8. The number of studies on the properties of geopolymers containing natural pozzolans other than metakaolin using TGA is limited. Firdous and Stephan (2019) stated that it is possible to examine the weight losses in TGA analysis of natural pozzolan-based geopolymers in 3 different temperature ranges, i.e., 50–150, 150–600, and 600–850 °C. It was reported that the reason for the weight loss in the first range is the loss of physically bound water in the geopolymer pores. According to these researchers, the weight loss in the second region is due to the loss of chemically bound water molecules and OH groups, while the reason for the weight loss at temperatures above 600 °C is the decomposition of carbonate groups [26]. When the TGA graphics obtained within the scope of this study are examined, there is a sudden weight loss up to 100 °C in geopolymer pastes. It is thought that this weight loss is due to the evaporation of free water and physically bound water from the paste. This was more evident in the pastes cured for 24 h. This is an indication of the presence of considerable amount of free water in the 24-h cured paste. It seems that the water that does not enter the structure as chemical water evaporates as physical water at this temperature zone. A considerable and continuous weight loss occurred at temperatures between 100 and 500 °C but at a relatively lower rate. This was due to both the loss of chemically combined water, OH groups, and the loss of some physical water. The effect of free (uncombined) water on the paste weight loss decreases as the temperature increases. The weight loss at temperatures beyond 500 °C was attributed to the loss of small amount of water still remaining in the specimen.
XRD spectra of natural and calcined trass-based geopolymer pastes cured at 90 °C for 96 h are shown in Fig. 9. Quartz, albite, and orthoclase peaks were detected in the samples. Both samples showed similar spectrum, although their peak intensities varied somewhat.
Conclusions
Considering the materials and methods used in this study, the following conclusions were drawn:
-
Compressive strength of the natural trass-based geopolymer mortar cured at 90 °C for 96 h was 22.3 MPa; upon increasing the activity of trass by calcination, the compressive strength of geopolymer increased to 40.5 MPa.
-
Total porosity of the geopolymer mortars produced with calcined trass was 12.8% and 22.9% less than those of natural trass-based mortars cured for 24 and 96 h, respectively. Moreover, increasing the curing duration from 24 to 96 h had no effect on the porosity of natural trass geopolymer mortar. However, the porosity of calcined trass mortar decreased by 10.5% upon prolonged curing.
-
In spite of having lower total porosity, the average pore size and maximum pore size of the calcined trass-based mortar were larger than those of the natural trass one. The fact was attributed to the higher water requirement of the calcined trass and the flocculation of trass particles in the central part of the mortar.
-
Irrespective of the trass type and curing duration, geopolymer pastes showed around 15 to 20% weight loss upon exposure to 600 °C. However, there was a negligible weight loss in the pastes at temperatures beyond 600 °C.
-
Weight losses in the thermogravimetric analysis and the spectra of XRD of mortars containing either calcined trass or natural trass were similar to each other, indicating that the calcination of trass had limited effect on the results of these analyses.
The data obtained within the scope of this study showed that calcination of trass before using in the geopolymer mixture has a significant positive effect on the compressive strength. Although with the calcination some energy consumption is made, the temperatures necessary for calcination are incomparably lower than that of the cement production. It is thought that calcined natural pozzolans will attract more attention in the future and more research will be done on this subject.
References
Sikandar, M.A., Jo, B.W., Baloch, Z., Khan, M.A.: Properties of chemically synthesized nano-geopolymer cement based self-compacting geopolymer concrete (SCGC). J. Wuhan. Univ. Technol. Mater. Sci. Ed. 34(1), 98–106 (2021). https://doi.org/10.1007/s11595-019-2021-2
Vikas, G., Rao, T.D.G.: Setting time, workability and strength properties of alkali activated fly ash and slag based geopolymer concrete activated with high silica modulus water glass. IJST-T CIV Eng. 45, 1483–1492 (2021). https://doi.org/10.1007/s40996-021-00598-8
Ganesh, A.C., Muthukannan, M.: Development of high performance sustainable optimized fiber reinforced geopolymer concrete and prediction of compressive strength. J. Clean. Prod. (2021). https://doi.org/10.1016/j.jclepro.2020.124543
International Enery Agency: Cement reports. https://www.iea.org/reports/cement. (2021) Accessed 1 January 2022
Gulsan, M.E., Alzeebaree, R., Rasheed, A.A., Nis, A., Kurtoglu, A.E.: Development of fly ash/slag based self-compacting geopolymer concrete using nano-silica and steel fiber. Constr. Build Mater. 211, 271–283 (2019). https://doi.org/10.1016/j.conbuildmat.2019.03.228
Duxson, P., Provis, J.L., Lukey, G.C., van Deventer, J.S.J.: The role of inorganic polymer technology in the development of ‘green concrete.’ Cem. Concr. Res. 37, 1590–1597 (2007). https://doi.org/10.1016/j.cemconres.2007.08.018
Davidovits, J.: Geopolymer cement: a review. Geopolymer Science and Technics, Technical Paper. 21, 1–11 (2013)
Prabha, V.C., Revathi, V., Reddy, S.S.: Ambient cured high calcium fly ash geopolymer concrete with dolomite powder. Eur. J. Environ. Civ (2021). https://doi.org/10.1080/19648189.2021.2012262
Irshidat, M.R., Abdel-Jawad, Y.A., Al-Sughayer, R.: Feasibility of producing sustainable geopolymer composites made of locally available natural pozzolans. J. Mater. Cycles Waste Manag. 20, 1751–1760 (2018). https://doi.org/10.1007/s10163-018-0742-5
Metekong, J.V.S., Kaze, C.R., Adesina, A., Nemaleu, J.G.D., Djobo, J.N.Y., Lemougna, P.N., Alomayri, T., Kamseu, E., Melo, U.C., Tatietse, T.T.: Influence of thermal activation and silica modulus on the properties of clayey-lateritic based geopolymer binders cured at room temperature. Silicon (2022). https://doi.org/10.1007/s12633-021-01566-7
Thokchom, S., Ghosh, P., Ghosh, S.: Effect of Na2O content on durability of geopolymer mortars in sulphuric acid. Int. Scholarly Sci. Res. Innov. 3(3), 193–198 (2009). https://doi.org/10.5281/zenodo.1334099
Salahuddin, M.B.M., Norkhairunnisa, M., Mustapha, F.: A review on thermophysical evaluation of alkali-activated geopolymers. Ceram. 41, 4273–4281 (2015). https://doi.org/10.1016/j.ceramint.2014.11.119
Firdous, R., Stephan, D.: Impact of the mineralogical composition of natural pozzolans on properties of resultant geopolymers. J. Sustain. Cem.-Based. Mater. 10(3), 149–164 (2021). https://doi.org/10.1080/21650373.2020.1809028
Robayo-Salazar, R.A., de Gutierrez, R.M.: Natural volcanic pozzolans as an available raw material for alkali-activated materials in the foreseeable future: a review. Constr Build Mater. 189, 109–118 (2018). https://doi.org/10.1016/j.conbuildmat.2018.08.174
Singh, N.B.: Fly ash-based geopolymer binder: a future construction material. Minerals (2018). https://doi.org/10.3390/min8070299
Hollanders, S.: Mineralogical study of the pozzolanic properties of calcined clays. Ph.D. Thesis. KU Leuven, Science, Engineering & Technology, Arenberg Doctoral School Faculty of Science (2017). Heverlee, Belgium.
Ghani, U., Hussain, S., Amin, N., Imtiaz, M., Khan, S.A.: Role of calcination on geopolymerization of lateritic clay by alkali treatment. J. Saudi Chem. Soc (2021). https://doi.org/10.1016/j.jscs.2021.101198
Nikolov, A., Rostovsky, I., Nugteren, H.: Natural and calcined zeolite (metazeolite) based geopolymers. Bulgarian Geological Society-Geosciences. 31–32 (2017)
Antoni, A., Geman, R., Tjondro, R.T., Anggono, J., Hardjito, D.: Effects of calcination temperature of Lusi mud on the compressive strength of geopolymer mortar. Adv. Mat. Res. 626, 224–228 (2013). https://doi.org/10.4028/www.scientific.net/AMR.626.224
Bascik, H.I.: Development of calcined natural pozzolan-based geopolymer mortar, Master Thesis, Ege University Graduate School of Natural and Applied Science Izmir. Turkey (2019)
ASTM C311/C311M-18: Standard test methods for sampling and testing fly ash or natural pozzolans for use in portland-cement concrete (2018). PA, USA.
TS EN 196–1: Methods of testing cement - part 1: determination of strength, Ankara, Turkey, 2016.
Hardjito, D., Wallah, S.E., Sumajouw, D.M.J., Rangan, B.V.: Geopolymer concrete: turn waste into environmentally friendly concrete. International Conference on Recent Trends in Concrete Technology and Structures, Incontest (2003). Coimbatore, India
Chithambaram, S.J., Kumar, S., Prased, M.M.: Thermo-mechanical characteristics of geopolymer mortar. Constr. Build Mater. 213, 100–108 (2019). https://doi.org/10.1016/j.conbuildmat.2019.04.051
Bing-hui, M., Zhu, H., Xue-min, C., Yan, H., Si-yu, G.: Effect of curing temperature on geopolymerization of metakaolin-based geopolymers. Appl. Clay Sci. 99, 144–148 (2014). https://doi.org/10.1016/j.clay.2014.06.024
Firdous, R., Stephan, D.: Effect of silica modulus on the geopolymerization activity of natural pozzolans. Constr. Build Mater. 219, 31–43 (2019). https://doi.org/10.1016/j.conbuildmat.2019.05.161
Acknowledgements
This work was supported by the Ege University Scientific Research Projects Coordination under grant number of 17-MUH-029. The authors also acknowledge the contribution of Ege University Central Research, Test and Analysis Laboratory for SEM, TG and Micro-CT analysis as well as Izmir Katip Celebi University Central Research Laboratory for XRD and particle size distribution determination.
Author information
Authors and Affiliations
Contributions
Conceptualization: Adil Gultekin, Semsi Yazici, Kambiz Ramyar; methodology: Adil Gultekin, Semsi Yazici, Kambiz Ramyar; investigation: Adil Gultekin, Kambiz Ramyar; writing—original draft preparation: Adil Gultekin; writing—review and editing: Kambiz Ramyar.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gultekin, A., Yazici, S. & Ramyar, K. Effect of trass calcination on properties of geopolymer mixtures. J Aust Ceram Soc 58, 1623–1631 (2022). https://doi.org/10.1007/s41779-022-00799-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41779-022-00799-y