Abstract
The aim of this in-vitro study is to compare the shear bond strengths of ceramic brackets bonded to zirconia surfaces by using different primers. Forty rectangular-shaped zirconia blocks were manufactured at 10 × 5 × 2.5 mm. Zirconia surfaces were sandblasted with 30-μm aluminum-oxide particles. Afterward, thermocycling for 2000 cycles and a shear bond strength test was applied. Universal primer (Monobond-Plus, Z Prime Plus, Clearfil) was applied to all specimen surfaces. The upper central ceramic brackets were bonded with light cure adhesive paste (3 M Transbond XT) to zirconia surfaces. The samples were equally divided into four groups according to the primer used in the bonding procedure: group I (control group), group II (Clearfil group), group III (Monobond-Plus group), group IV (Z Prime Plus group). The Z Prime Plus adhesive primer showed statistically higher shear bond strength values than that in the Monobond-Plus and control group. The results in the control group were statistically significantly different from the values obtained in all other groups. Monobond Plus, Z-Prime Plus, and Clearfil ceramic primer agents can be effective methods for bonding zirconia restorations with ceramic orthodontic brackets.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introductıon
Orthodontic treatment has attained popularity among adults [1]. Orthodontists face new challenges in managing more aged, restored, and periodontally vulnerable dentitions. They must be prepared to adequately bond to crowns, and fortunately, the materials exist to accomplish this feat, as alluded to earlier. The prevalence of porcelain-fused-to-metal (PFM) and full metal crowns is decreasing while the use of all-ceramic crowns is increasing. Zirconia-based ceramics have developed and risen to prominence in the dental field over the last 20 years. Because of its esthetic natural appearance, high mechanical strength, toughness, and chemical stability, it has a wide clinical application [2]. Besides its superior mechanical properties, it has made it a preference for patients and clinicians aesthetically with its natural tooth color appearance. All these features for dental applications create the ideal set of properties required from a material used. However, the non-reactive surfaces of zirconia exhibit low adhesion with other substrates [3].
Zirconia ceramics are very good aesthetically, mechanically, and biologically. But the adhesion ability of zirconia ceramics is still controversial. For this reason, surface modifications are applied to the zirconia surface to increase the adhesion ability of the surface, or different adhesives are applied to increase the adhesion [4].
Ceramic brackets are more preferred in adult patients because of their aesthetic properties. There are two different ceramic brackets, although aluminum oxide is composed of polycrystalline and monocrystalline aluminum. Polycrystalline brackets are currently easier to produce and available in large quantities [5,6,7].
The most significant difference between polycrystalline and monocrystalline brackets is in their optic clarity. Monocrystalline brackets are clearer up to translucent than polycrystalline brackets. Both monocrystalline and polycrystalline brackets resist staining and discoloration [7].
There is a higher failure rate in bonding orthodontic brackets to porcelain restorations in adult orthodontic patients with different porcelain restorations [8]. Bond strength is mostly affected by the bracket material type and retention mode, material properties of the bonding adhesive, the porcelain material type, surface conditioning, the light-curing source, as well as the skill of the clinician [9]. The strength of the bond between the attachment-adhesive and the surface area of the attachment are factors in withstanding tensile or shearing forces [10].
Different surface conditioning methods have been proposed to achieve high adhesion of zirconia strength, such as sandblasting or tribochemical silica coating [11]. While using acids to achieve the effect of micro etching, air abrasion (AA), also called sandblasting, causes macro etching [12]. With aluminum oxide, sandblasting may be provided to increase surface area, surface energy, and wettability for the proper bonding procedure [13].
The aim of this study was to eliminate failed bonding of orthodontic brackets to porcelain restorations in adult orthodontic patients with zirconia ceramic restorations and to determine the primers with high bond strength with zirconia. For these reasons, the aim of this study is to compare the shear bond strength (SBS) of ceramic orthodontic brackets bonded to zirconia surfaces by using different primers. The null hypothesis of this study is that there was no difference in the shear bond strength of the orthodontic ceramic bracket bonded to zirconia surfaces using different primers and universal adhesive.
Materıal and methods
Forty rectangular-shaped sintered zirconia samples (ZirkonZahn Bruneck, Italy) measuring 10 × 5 × 2.5 mm were used for this in vitro study. Each zirconia sample was embedded individually in a mold with auto-polymerizing acrylic resin. To get the roughness on the surface of zirconia samples, water cooling and a hand under pressure using a silicon carbide abrasive paper polishing machine (Phoenix Beta, Buehler, Germany) for 1 min are polished.
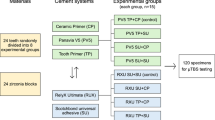
Samples obtained from the application of the surface treatment were ultrasonically cleaned in distilled water for 15 min and then air-dried and randomly distributed to one of the following surface treatment protocols. Air abrasion on the zirconia surfaces was sandblasted with 30-μm aluminum-oxide (A2O3) particles (Cojet Sand, 3 M-ESPE, Germany). A universal primer (Monobond-Plus, Z Prime Plus, Clearfil) and an orthodontic adhesive composite (3 M Transbond XT Light cure adhesive paste) were applied to all specimen surfaces. Experimental materials and their characteristics were given in Table 1. The orthodontic adhesive composite was applied to the upper central ceramic brackets for bonding to zirconia surfaces. Then, it was photopolymerized. A flow chart of experimental groups and their respective treatment processes is given in Fig. 1.
The four experimental groups were composed according to primer applied (N: 40 and n: 10 in each group): group I (control group) no primer was applied, group II (Clearfil ceramic primer group), group III (Monobond-Plus group), group IV (Z Prime Plus group).
The orthodontic bracket bonding procedure
The upper central incisor ceramic brackets were used by Orthoclassic (Evrclear, Orthoclassic, McMinnville, OR, USA). Evrclear brackets are monocrystalline brackets made of a fully transparent monocrystalline material. The four different primers were applied on zirconia surfaces and cured for 15 s before applying composite resin cement. Then, a little amount of adhesive composite cement (3 M Transbond XT Light cure adhesive paste) was applied to the bracket base, and the bracket was placed tightly on the zirconia surfaces. Abundance bonding resin was removed with a small instrument and the bonding composite resin under the bracket base was cured with a light-emitting diode for the 40 s.
Thermal cycling and shear bond strength test (SBS)
Initially, all the specimens were held in distilled water at 37 °C for 24 h. Afterwards, thermocycling for 2000 cycles between 5 ± 2 °C and 55 ± 2 °C in 20 s of standby time and 10 s of transfer time. For the shear bond strength test, the adhesion properties of each surface of the zirconia group were evaluated at a crosshead speed of 1 mm/min until an adhesion fracture occurred. The knife-edge was positioned between the bracket’s wing and the base, which is perpendicular to the slot of the bracket and parallel to the base (Fig. 2). A computer recorded the first bond strength force values of breaking and converted them into megapascals (MPa).
Field emission scanning elektron microscope (FE-SEM) examination
The morphologies of bracket bases were imaged using an FE-SEM (field emission scanning electron microscope, HITACHI Regulus 8230, 0.7 nm/1 kV, Tokyo, Japan). The specimens were covered with 4 nm Au–Pd (LEICA EM ACE600 sputter coater) and imaged.
Statistical analysis
All analyses were performed by the Statistical Package for Social Sciences (SPSS) (version 19.0, Chicago, USA). The power analysis of this study was performed using reference information from previous similar studies [8]. As a result of the analysis made by taking alpha (α) = 0.05, effect size (f) 2.672566, and power (1-β) = 0.95, it was calculated that there should be at least 10 specimens in each group.
The Shapiro–Wilk test was used to evaluate normality and indicated that the data for shear bond strength values was normally distributed. Parametric statistical one-way ANOVA tests were used to compare the SBS of the group. Further comparisons with Tukey multiple comparison tests in between groups were conducted. The significance for the statistical test was set at p < 0.05.
Results
The mean values and standard deviations of SBS for all groups were summarized in Table 2. The highest mean SBS was recorded in group IV (Z Prime Plus group) (13,64 MPa), followed by group II (Clearfil group) (11,80 MPa). In addition, Tukey’s test showed that a significant difference was found between group III and group IV and no significant difference was found between groups II–group III and group II–group IV. The results in the control group were statistically significantly different from the values obtained in all other groups (Table 2, Fig. 3).
The FE-SEM images (× 70 and × 1.00 k magnification) of the zirconia surfaces were applied with the four different methods shown in Figs. 4 and 5. The control group specimen (a) shows a smooth surface while the other group specimens (b, c, d) show different surface morphologies (Fig. 4). Since no primer was used in the control group, the composite resin did not adhere to the zirconia surface. While the control group (a) does not show composite resin remaining on the zirconia surface, there seems to be a greater amount of remaining composite resin in other groups. Some surface roughness with a granulated texture was observed on the group II, III and IV (Fig. 5).
Discussion
In this study, the effect of different primer agents on the shear bond strength of ceramic orthodontic brackets bonded to zirconia ceramic surfaces that were used in prosthetic restorations was evaluated. Incompatible with the results, the null-hypothesis was rejected since there was a difference in shear bond strength of the orthodontic ceramic bracket bonded to zirconia surfaces using different primers and universal adhesive. While Z Prime Plus is the most effective agent in bonding ceramic brackets to the zirconia surface, Monobond Plus, and Clearfil ceramic primers have been found effective.
The light transmittance at the adhesive-bracket interface, which allows for better photo-polymerization and reduced stress, is the reason for the higher bond strength of ceramic brackets [14,15,16]. In most studies on SBS values, the mean shear bond strength of the ceramic brackets is significantly greater than metallic brackets [17, 18]. Therefore, monocrystalline ceramic brackets are preferred in this study.
In some other studies, the ceramic brackets might be expected to have greater bond failure rates because ceramic bracket mesh base designs have considerably fewer mechanical undercuts [7]. This problem may be overcome by using different applications on the bracket base. The ceramic brackets used in our study have a coated base system. The coated base system forms a base that gives very good bonding strength.
Besides the bracket base design affecting the bond strength, there are various other factors, including etching time, type of bonding resin, surface conditioning methods, and preparation of teeth [19, 20]. The air abrasion from surface conditioning methods on the zirconia surfaces sandblasted with 30-μm aluminum-oxide (A2O3) particles has been widely used. Kwak et al. found that the use of 30-μm aluminum oxide combined with silane resulted in a higher bond strength than hydrofluoric acid with silane [21]. In our study, zirconia surfaces were sandblasted with 30-μm silica-coated aluminum oxide particles to increase the surface energy, surface area and wettability. The surface conditioning methods alone are not capable of producing reliable bond strength between zirconia and adhesive resin agents. At the same time, it is also necessary to apply primers to zirconia surfaces. But if so, a combination of both pretreatment methods is reachable to achieve higher bond strength values. Because the SBS test is simple to apply and fast to produce results, it was preferred. Thermally, 2000 cycles were applied in each bath. All the samples were held in distilled water at 37 °C for 24 h before testing. Shear bond testing and thermocycling may be advised as a standard method of testing the bond strength of brackets to different surfaces [14, 15]. Other studies were stored in distilled water for 30 days or thermal cycles [22,23,24].
During mastication, the average force transmitted to a bracket has been reported to be between 40 and 120 N. In some studies about the optimal adhesive force that is required to ensure sufficient adhesion of the brackets without the danger of surface damage during bracket removal was found between 6 and 8 MPa (Reynold 60–80 kg/cm2, Whitlock 6–8 MPa) [7, 10, 25,26,27]. According to these findings, group II, group III, and group IV had acceptable shear bond strength values in this study. Monobond shear bond strength values above the optimal range required for orthodontic treatment were detected. When compared to Monobond and Monobond S, Monobond S had higher shear bond strength values [28]. Additionally, Monobond-Plus occurred at high enough SBS levels for all restorative materials tested [22]. In this study, in the Monobond-Plus group (group III), we found high SBS levels (10.85 MPa).
In a similar study by Lee et al. with ceramic brackets, the control group shear bond strength value was 1.07 ± 0.81 MPa, while the Z Prime Plus primer shear bond strength value was found to be 10.47 ± 2.69 MPa [8]. In this study, it was found to be control group 2.48 ± 0.46 MPa, while the Z Prime Plus primer shear bond strength value was 13.64 ± 0.83 MPa.
Kitayama et al. stated that the primer containing a silane coupling agent, phosphonic acid monomer, a phosphate ester monomer, including 6-MHPA and MDP were effective in improving the bonding of resin cement to zirconia ceramic [29]. In particular, the primers containing MDP (10-methacryloyloxydecyl dihydrogen phosphate) can react with the zirconium oxide and this increases the bond strength [8, 30, 31]. The Z-Prime Plus and Clearfil primers that were used in this study contain MDP (10-methacryloyloxydecyl dihydrogen phosphate) and showed higher bond strength values.
In the study of Magne et al., the SEM micrograph of the abraded zirconia with Al2O3 airborne particle was characterized by a uniform presence of irregularities and shallow pits. In contrast with, the primed surfaces are uniformly smooth. Additionally, on the non-primed surface groups, the adhesive failure mode is clearly seen [24]. These findings are in accordance with this study that found in the non-primed control groups, the adhesive composite resin had not adhered to the zirconia surface.
Performing this study in vitro is one of its limitations. There are differences between the clinical and the experimental conditions. In the mouth, brackets are influenced by different tensile stretch forces with chewing force. In addition, humidity, acidity, temperature, and microbial plaque exist are different from experimental conditions. Because of these reasons, in vivo studies are needed to validate the present finding.
Conclusion
Using the zirconia primers may increase the bond strength between the ceramic bracket and zirconia restoration. The Z Prime Plus group showed the highest shear bond strength. Ceramic orthodontic brackets bonded to zirconia restorations with Monobond Plus and Clearfil ceramic primer agents can also be an effective method for bonding zirconia restorations with ceramic orthodontic brackets.
References
Proffit, W.R., Fields, H.W., Sarver, D.M.: Contemporary Orthodontics, 5th edn. Mosby, St. Louis (2013)
Bielen, V., Inokoshi, M., Munck, J.D., et al.: Bonding effectiveness to differently sandblasted dental zirconia. J. Adhes. Dent. 17(3), 235–242 (2015)
Akay, C., Tanış, M., Mumcu, E., et al.: Influence of nano alumina coating on the flexural bond strength between zirconia and resin cement. J. Adv. Prosthodont. 10(1), 43–49 (2018)
Tanış, M., Akay, C., Karakış, D.: Resin cementation of zirconia ceramics with different bonding agents. Biotechnol. Biotechnol. Equip. 29(2), 363–367 (2015)
Birnie, D.: Ceramic brackets. Br. J. Orthod. 17(1), 71–74 (1990)
Karamouzos, A., Athanasiou, A.E., Papadopoulos, M.A.: Clinical characteristics and properties of ceramic brackets: a comprehensive review. Am. J. Orthod. Dentofac. Orthop. 112(1), 34–40 (1997)
Swartz, M.L.: Ceramic brackets. J. Clin. Orthod. 22(2), 82–88 (1988)
Lee, J.Y., Kim, J.S., Hwang, C.J.: Comparison of shear bond strength of orthodontic brackets using various zirconia primers. Korean J. Orthod. 45(4), 164–170 (2015)
Grewal Bach, G.K., Torrealba, Y., Lagravère, M.O.: Orthodontic bonding to porcelain: a systematic review. Angle. Orthod. 84(3), 555–560 (2014)
Reynolds, I.R., von Fraunhofer, J.A.: Direct bonding of orthodontic brackets–a comparative study of adhesives. Br. J. Orthod. 3(3), 143–146 (1976)
Kern, M., Wegner, S.M.: Bonding to zirconia ceramic: adhesion methods and their durability. Dent. Mater. 14(1), 64–71 (1988)
Reisner, K.R., Levitt, H.L., Mante, F.: Enamel preparation for orthodontic bonding: a comparison between the use of a sandblaster and current techniques. Am. J. Orthod. Dentofacial. Orthop. 111(4), 366–373 (1997)
Kim, B.K., Bae, H.E., Shim, J.S., Lee, K.W.: The influence of ceramic surface treatments on the tensile bond strength of composite resin to all-ceramic coping materials. J. Prosthet. Dent. 94(4), 357–362 (2005)
Zachrisson, Y.O., Zachrisson, B.U., Büyükyilmaz, T.: Surface preparation for orthodontic bonding to porcelain. Am. J. Orthod. Dentofacial. Orthop. 109(4), 420–430 (1996)
Bourke, B.M., Rock, W.P.: Factors affecting the shear bond strength of orthodontic brackets to porcelain. Br. J. Orthod. 26(4), 285–290 (1999)
Bishara, S.E., Olsen, M.E., VonWald, L., et al.: Comparison of the debonding characteristics of two innovative ceramic bracket designs. Am. J. Orthod. Dentofac. Orthop. 116(1), 86–92 (1999)
Viazis, A.D., Cavanaugh, G., Bevis, R.R.: Bond strength of ceramic brackets under shear stress: an in vitro report. Am. J. Orthod. Dentofac. Orthop. 98(3), 214–221 (1990)
Odegaard, J., Segner, D.: Shear bond strength of metal brackets compared with a new ceramic bracket. Am. J. Orthod. Dentofac. Orthop. 94(3), 201–206 (1988)
Ghafari, J., Skanchy, T.L., Mante, F.: Shear bond strengths of two ceramic brackets. J. Clin. Orthod. 26(8), 491–493 (1992)
Joseph, V.P., Rossouw, E.: The shear bond strengths of stainless steel and ceramic brackets used with chemically and light-activated composite resins. Am. J. Orthod. Dentofac. Orthop. 97(2), 121–125 (1990)
Kwak, J.Y., Jung, H.K., Choi, I.K., Kwon, T.Y.: Orthodontic bracket bonding to glazed full-contour zirconia. Restor. Dent. Endod. 41(2), 106–113 (2016)
Ebert, T., Elsner, L., et al.: Shear bond strength of brackets on restorative materials: comparison on various dental restorative materials using the universal primer Monobond® Plus. J Orofac. Orthop. 77(2), 73–84 (2016)
Cetik, S., Ha, T.H., Sitri, L., et al.: Comparison of shear strength of metal and ceramic orthodontic brackets cemented to zirconia depending on surface treatment: an ın vitro study. Eur. J. Dent. 13(2), 150–155 (2019)
Magne, P., Paranhos, M.P., Burnett, L.H.: New zirconia primer improves bond strength of resin-based cements. Dent. Mater. 26(4), 345–352 (2010)
Dumbryte, I., Vebriene, J., et al.: Enamel microcracks in the form of tooth damage during orthodontic debonding: a systematic review and meta-analysis of in vitro studies. Eur. J. Orthod. 40(6), 636–648 (2018)
Whitlock, B.O., Eik, J.D., Ackerman, R.J., et al.: Shear strength of ceramic brackets bonded to porcelain. Am. J. Orthod. Dentofac. Orthop. 106(4), 358–364 (1994)
Newman, G.V.: Epoxy adhesives for orthodontic attachments: progress report. Am. J. Orthod. 51(12), 901–912 (1965)
Franz, A., Raabe, M., Lilaj, B., et al.: Effect of two different primers on the shear bond strength of metallic brackets to zirconia ceramic. BMC Oral Health 19(1), 51 (2019)
Kitayama, S., Nikaido, T., Takahashi, R., et al.: Effect of primer treatment on bonding of resin cements to zirconia ceramic. Dent. Mater. 26(5), 426–432 (2010)
Milagres, F.D.S.A., Olivera, D.D., Silveira, G.S., et al.: Bond strength and failure pattern of orthodontic tubes adhered to a zirconia surface submitted to different modes of application of a ceramic primer. Materials (Basel) 12(23), 3922 (2019)
Byeon, S.M., Lee, M.H., Bae, T.S.: Shear bond strength of Al2O3 sandblasted Y-TZP ceramic to the orthodontic metal bracket. Materials (Basel) 10(2), 148 (2017)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kucukkaraca, E., Akay, C. Effect of different primer agents on shear bond strength of ceramic orthodontic brackets bonded to zirconia ceramics. J Aust Ceram Soc 58, 645–651 (2022). https://doi.org/10.1007/s41779-021-00692-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41779-021-00692-0