Abstract
Glass-ceramics are commonly used as sealing materials for planar solid oxide fuel cells (SOFCs). The major requirements of stack and module builders for these materials are the stability of the coefficient of thermal expansion (CTE), the excellent bonding (sticking) behaviour and the absence of volatile ingredients, which can lead to changes of the material properties and the sealing ability. In this study, the thermal stability of glasses in the BaO-Al2O3-La2O3-B2O3-SiO2 system was investigated, to develop a suitable sealing glass for planar solid oxide fuel cell operating at 800–850°C. The developed glasses were characterised through measurement of different properties, coefficient of thermal expansion (CTE), glass transition temperature (T g), dilatometric softening temperature (T d), crystallisation behaviour during prolonged heat-treatment, density etc. At a target operating temperature of 750°C, the long-term coefficient of thermal expansion (CTE) of one particular composition (G4) was found to be particularly stable, due to devitrification to a mixture of glasses and ceramic phases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
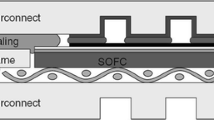
Solid oxide fuel cell (SOFC) is an energy conversion device that converts chemical energy of a fuel (such as hydrogen and methane) into electricity through a series of electrochemical reactions; no combustion process is involved. As a result, fuel cell efficiencies are not limited by Carnot efficiencies. This system produces electricity by the electrochemical reaction between a fuel and an oxidant [1–3]. Solid oxide fuel cells are being developed for a broad range of applications including portable electronic devices, automobiles, power generation, aeronautics etc. The salient features of SOFC are all solid construction and high temperature electrochemical reaction-based operation, resulting in clean and efficient power generation from a variety of fuels. There are two different designs of SOFCs, tubular and planar, and are currently under development. The planar SOFCs (pSOFC) are mostly preferred due to several advantages such as simple manufacturing and relatively short current path resulting in higher power density and efficiency. In the main structure of a fuel cell, the device consists of an anode electrode (exposed to fuel), an electrolyte and a cathode electrode (exposed to oxidant). The repeating unit of a planar solid oxide fuel cell is formed by anode-electrolyte-cathode and interconnects [2, 4]. However, planar SOFCs require hermetic seals to separate and contain fuel and oxidant within the cell and to bond cell components together [4–6]. The sealants for planar SOFCs must meet some important requirements: they have to be hermetic in order to prevent mixing of the fuel and oxidant and should have a thermal expansion coefficient close to those of the interconnect and the electrolyte. Moreover, the sealant must be mechanically and thermochemically stable in both oxidising and wet-reducing environments at 800 °C and must not undergo any reaction with the other cell components [6]. There are generally two types of seals for pSOFC stacks: compressive seals and rigid seals [7, 8]. Compressive seals suffer from problems of oxide scaling and chemical stability in addition to the drawback of an externally applied force [2, 3, 9]. Rigid seals are therefore more popular energy than compressive seals. Among the rigid seals developed, glasses and glass-ceramics are the most promising ones to be used in pSOFC systems. Glass or glass-ceramic may offer best perspectives as seal for planar system SOFCs because of the inertness to oxidising and reducing conditions, as well as thermal and mechanical stability [6, 10]. By carefully choosing the glass composition, glasses and glass-ceramics meet, in principle, most of the requirements of an ideal sealant. Glass-ceramics, which can be prepared by controlled sintering and crystallisation of glasses, possess superior mechanical properties and higher viscosity at the SOFC operating temperature than those of glasses. Sohn et al. [7] investigated the thermal stability of SiO2-B2O3-BaO-Al2O3 glass and chemical compatibility with electrolyte (8YSZ). They found that the TEC (thermal expansion coefficient) values of their glasses decreased with the formation of celsian.
Yang et al. [11] investigated the chemical interactions of SiO2-CaO-B2O3-BaO-Al2O3-based sealing glasses with oxidation-resistant alloys. They found that the formation of BaCrO4 led to the physical separation of the sealing glass from the interconnect alloys due to high thermal expansion mismatch. There are still some problems in improving the high temperature resistance of glass-ceramic materials. This area is still open for research for new investigations for fuel cell sealant applications. In order to improve the high temperature properties and solve the problem of mismatch thermal expansion coefficient, this study is worked out. The purpose of this study is to investigate properties of different compositions of BaO/RO-Al2O3-R2O3-B2O3-SiO2 glass-ceramic in comparison to those of the literature.
Methods and procedures
Different chemical compositions of the glasses studied during the present work are given in Table 1. To prepare a suitable glass-sealing composition, B2O3 and SiO2 were chosen as glass formers wherein B2O3/SiO2 ratio was kept at around 0.69. Other ingredients such as BaO were added to increase the CTE, La2O3, Nd2O3 and Y2O3 to control the viscosity and Al2O3 to prevent rapid crystallisation during heat-treatment as well as to control the surface tension of the glass. Anode-supported bilayers, consisting of NiO/5YSZ (zirconia stabilised with 5 mol% yttria) as the anode and 8YSZ as the electrolyte, and thin gage stainless steel (FeCrAlY ferritic alloys) (Crofer 22 APU) were chosen as model substrates in this study.
Glass preparation
The thoroughly mixed batches were taken in an alumina crucible and melted in an electric furnace (Protherm, PLF 160/30) at 1400 °C for 2 h. To obtain the glass, the melts were quenched by pouring into cooled water. It was rinsed with acetone to remove impurity and then dried at 80 °C for 2 h. The dried glass frit was milled in a ball mill below 100-μm particle size. These amorphous glasses were called as GX (X is changed from 1 to 4). Starting composition and code of samples were given in Table 1. G1 is a standard composition which is selected from literature [12].
Glass-ceramic preparation
The prepared glass powders were mixed with 3 wt% polyethylene glycol (PEG) solution as a binder and then uniaxially pressed into discs with 40 mm in diameter under 30 MPa pressure. Five tablets were prepared for each composition.
Characterisation
Thermal analysis
Crystallisation capacity of glass-ceramics, glass transition (T g), crystallisation (T c) and melting (Tm) temperatures was determined by differential thermal analyser (DTA, Netzsch STA 409 DTA PC). The milled frit with particle size under 63 μm was used for thermal analysis.
Coefficient of thermal expansion (CTE) of glass powder was measured by a dilatometer (Netzsch DIL 402 PC) characterisation. Sample was uniaxially pressed into a bar with dimensions of 25 × 10 × 4 mm. They were analysed with 10°/min heating rate by subtracting to 1400 °C. In order to show the changes in CTEs (coefficients of thermal expansion), values of the glass-ceramics dilatometric measurements were also performed on the rectangular (25 × 10 × 4 mm) bulk samples sintered heating to 850 °C with a heating rate 10 °C/min.
Phase characterisation
In order to explore the evolution process of crystalline phases, the green discs were heattreated at 800 °C for different durations of 12 and 24 h with heating rate of 10 °C/min. The heat-treated pellets were analysed using an X-ray diffractometer (Rigaku Rint 2000) with CuKα1 (λ = 1.5056 Å) radiation at 40 kV and 40 mA, with 2Φ = 10–60° and 2°/minute to confirm the amorphous nature (as-prepared glass) and to identify the crystalline phases (heat-treated glass).
Results and discussion
Physical properties of developed glasses
The physical properties of each glass are almost identical for all the developed composition. G1 and G3 samples have similar densities (∼3.37 g/cm3), whereas G2 has the lowest (Table 2). These differences are directly related with the starting composition of glasses. The G2 has CaO, which is not in other composition, and due to low decomposition temperature of this material, the density of G2 is the lowest. On the other hand, G1 and G3 have a similar amount starting raw and main difference is the amount of rare earths (La2O3 and/or Nd2O3) in the composition. Melting temperature and molecular weight of these rare earths are similar (2315–2233 °C and 6.51–7.24 g/cm3, respectively); therefore, these results are expected for these compositions. However, G4 glass-ceramic has the highest density (∼3.43 g/cm3) due to the different rare earths used in the composition. Y2O3 has more refractory properties compared with those of other rare earths (T melting is 2425 °C), whereas its molecular weight is lower (225 g/mol) than that of others (La2O3 and Nd2O3 have 325.805 and 336.48 g/mol, respectively). Therefore, in the same ratio, the amount of Y2O3 is higher than that of La2O3 and/or Nd2O3 in the composition. And the amount of liquid phase will be higher than that of other composition to increasing sintering behaviour of system. These reasons explain why the G4 has the highest T g, T c and density. Due to the similar physical and thermal properties to G1, G4 was found to be very promising. Hence, this particular glass composition has been referred extensively in this study.
The T g values for the developed glasses lie within the temperature range 620–645 °C, which is below the SOFC operating temperature (800 °C). The CTE values of all these glasses (∼(7.88 to 8.35) × 10−6/K) from room temperature to T g are in the same range as those of other cell components, zirconia electrolyte in particular [13]. According to results of G1 and G2, which indicate that T g and T sintering are almost independent of the change in BaO content under constant B2O3/SiO2 ratio (0.69), this results are in agreement with literature which are carried out in borosilicate glasses [6]. The results show that introduction of rare earth oxides increases the coefficient of thermal expansion and Nd2O3 is an exception in viscosity. The glass transition temperature of BaO/RO-Al2O3-R2O3-B2O3-SiO2 glass doped with rare earth oxides increases with increasing cationic field strength (r cation/values of cation) of corresponding rare earth ions [14]. G4 glass exhibited the highest CTE value of ∼8.336 × 10−6/K. In this system, all the rare earths have the same charge (3+), whereas their cationic radius (r cation) is changing. Y+3 has the lowest cationic radius in these three; therefore, its cationic field strength is highest in these cations. These results explain why G4 exhibited the highest CTE value.
A typical linear expansion curve for the same G1 glass is shown in Fig. 1 and compared with that of the CTE of YSZ and Crofer 22 APU [12]. The slope of the curve between T g and T d shows a dramatic increase in expansion just before the glass structure deforms by viscous flow. When fuel cell stacks are cooled to room temperature, stresses begin to develop as the temperature drops below T g and with further decrease in temperature, the stress enhances significantly due to the thermal expansion mismatch. Therefore, to minimise the total stress produced, the T g at which the viscosity equals to 1013 to 1013.6 dPas should be as low as practicable with adequate rigidity at the cell operating temperature of around 800 °C. Figure 1 also reveals that the difference in CTE values between the optimised glass G1 and YSZ electrolyte is small and exhibiting a thermal expansion mismatch.
Comparison of thermal expansion for G1 glass (−) and glass-ceramics (sintered at 800 °C for 50 and 100 h) with YSZ and Crofer 22 APU (metallic interconnect) [12]
The thermal expansion results of glasses are given in Fig. 2. According to these results, it is possible to conclude that G4, the best compositions in four, shows a similar behaviour to G1 and YSZ and Crofer 22 APU and is a good candidate for using in this application.
Phase characterisation of glass-ceramics
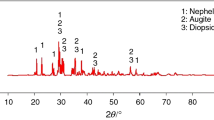
The X-ray diffraction analysis results of glass-ceramics, heat-treated at 800 °C for 12 and 24 h, are given in Figs. 3, 4, 5, and 6. The XRD analysis results show that the glass compacts sintered at 800 °C for 12 and 24 h have a crystalline structure. Figure 3 shows the XRD pattern of G1 glass-ceramics heat-treated at 800 °C for 12 and 24 h. The results show that two different morphologies of BAS glass phases are investigated as hexacelsian and celcian. As investigated by Ghosh et al., the high temperature phase, hexacelsian (BaAl2Si2O8-JCPDS: 01-072-7502), forms metastable after heat-treatment at 800 °C for 12 h and always crystallises out first in these BAS glasses. When the glasses were heat-treated for longer period of time (24 h), the hexacelsian phase begins to transform to the monoclinic celsian phase (BaAl2SiO8-JCPDS: 01-077-0185) as the latter is thermodynamically stable below ∼1590 °C. The formation of celsian phase is, however, undesirable for SOFC sealing application as the same has a very low CTE of ∼2.29 × 10−6 K−1 in between 30 and 1000 °C. The hexacelsian phase, on the contrary, has a relatively higher CTE value of ∼8 × 10−6 K−1 within 30–1000 °C and is more compatible with the parent glasses compared to that of celsian in terms of CTE mismatch. Therefore, crystallisation of hexacelsian is always desirable in the preparation of glass sealant for SOFCs, whereas, rapid and progressive transformation from hexacelsian to celsian is considered undesirable [12]. Figure 3 shows that main phase in the structure is BaAl2Si2O8 (hexacelsian) (JCPDS: 00-028-0124). Additionally, SiO2 is observed as the secondary phase in this glass composition. This standard composition, chosen from literature, is in agreement with literature [12]. G2 glass composition, heat-treated for 12 h, analysis results show that it has BaAl2Si2O8 as main phase in its structure, whereas this phase transforms to celsian phase (JCPDS: 00-038-1450) after 24 h heat-treatment (Fig. 4). Additionally, it was observed that some amount of anorthite (CaAl2Si2O8) is present due to CaCO3 in its starting composition. Fergus also investigated similar results in his study [9]. G3 glass analysis result is different from that of the others. This composition has BaAl2SiO4 phase as main phase in the structure of heat-treated for 12 h. Additionally, it has a celsian morphology as the secondary phase. The transformation of hexacelsian to celsian is also observed in this sample after 24 h heat-treatment. Low amount of unreacted Nd2O3 has also seen in the result (Fig. 5). The results of G4 sample are different from those of the others. It has BaAl2Si8O8 (hexacelsian) phase as the main phase in all conditions (12 and 24 h heat-treatment). Therefore, this composition is more suitable for SOFC sealing applications according to literature.
The polishing surface scanning electron microscopy (SEM) of samples is given in Figs. 7 and 8. Al matrix represents the hexacelsian phase as an elongated form in Fig. 6a for G1 sample. G2 sample has both phases as hexaselcian and celsian in its morphology (Fig. 7b). G3 sample has two different phases. The celsian phase is observed as grey, whereas hexacelsian phase as elongated form (white) in its structure (Fig. 8a). As it is seen in Fig. 8b, G4 sample has a hexacelsian phase in its structure. All the microstuctural results are in agreement with phases analysed of samples.
Conclusion
BaO/RO-Al2O3-R2O3-B2O3-SiO2 glass-ceramic system was investigated for application as potential SOFC sealants. In this study, as different from literature, dual and single rare earth addition was studied. The results showed that addition of La2O3 and La2O3-Nd2O3 to composition affects the CTEs and transformation from hexacelsian to celsian phase. However, using of Nd2O3 in composition (G4) helps to improve the CTE and prevent phase transformation during the heat-treatment process. According to results of reference [12], CTE of G4 was found to be well matched with the zirconia electrolyte, although there was less than 10% mismatch with the Crofer 22 APU (interconnect metal), which was within the tolerable limit for application as a sealing material. As a result of heat-treatment process, these glasses (G1, G2 and G3) were found to generate mainly two different polymorphs, celsian and hexacelsian, and also little amount of niobium, anorthite and boron oxide. Celsian phase is undesirable due to the high kinetic barrier associated with the transformation from hexacelsian to celsian. Therefore, G4 glass composition was observed as a good candidate due to prevention of this transformation and similar CTE value to that of SOFC.
References
Singhal, S.C.: Solid oxide fuel cells: facts and figures. In: Irvine, J.T.S., Corner, P. (eds.) Past, present and future, perspectives for SOFC technologies, pp. 1–2. Springer London Heildelberg, New York Dordrecht
Smeacetto, F., Salvo, M., Cho, J., Boccaccini, A.R.: Glass-ceramic seal to join Crofer 22 APU alloy to YSZ ceramic in planar SOFCs. J European Ceramic Soc. 28(1), 61–68 (2008)
Singh, R.N.: Sealing technology for solid oxide fuel cells (SOFC). Int JAppl Ceram Technol. 4, 134–144 (2007)
Smeacetto, F., Salvo, M., Ferraris, M., Casalegno, V., Asinari, P.: Glass and composite seal for the joining of YSZ to metallic interconnect in solid oxide fuel cells. J. European Ceramic Soc. 28(1), 611–616 (2008)
Menzler, N.H., Sebold, D., Zahid, M., Gross, S.M., Koppitz, T.: Interaction of metallic SOFC interconnect materials with glass-ceramic sealant in various atmosphere. J Power Sources. 145, 46–57 (2005)
Namwong, P., Laorodphan, N., Thiemson, W., Jaimasith, M., Wannakon, A., Chairuangsri, T.: A barium-calcium silicate glass for use as seals in planar SOFCs. Chiang Mai J Sci. 37(2), 231–242 (2010)
Sohn, S.B., Choi, S.Y., Kim, G.H., Song, H.S., Kim, G.D.: Stable sealing glass for planar solid oxide fuel cell. J Non-Cryst Solids. 297, 103–112 (2002)
Basu, R.M.: Materials for solid oxide fuel cells in recent trends in fuel cell science and technology, jointly published by Anamaya Publisher, New Delhi (India) and springer. New York, Chapter. 12, 284–329 (2006)
J.W. Fergus, Sealants for solid oxide fuel cells, Vol. [147], (2005), 46–57.
Smeacetto, F., Salvo, M., Ferraris, M., Casalegno, V., Asinari, P., Chrysanthou, A.: Characterization and performance of glass-ceramic sealant to join metallic interconnects to YSZ and anode-supported-electrolyte in planar SOFCs. J. European Ceramic Soc. 28, 2521–2527 (2008)
Lahl, N., Sing, K., Singheiser, L., Hilpert, K., Bahadur, D.: Crystallisation kinetics in AO-Al2O3-SiO2-B2O3 glasses (A=Ba, Ca, Mg). J Mater Sci. 35, 3089–3096 (2000)
Ghosh, S., Kundu, P., Sharma, A.D., Basu, R.N., Maiti, H.S.: Microstructure and property evaluation of barium aluminosilicate glass-ceramic sealant for anode-supported solid oxide fuel cell. J. European Ceramic Soc. 28, 69–76 (2008)
Reis, S.T., Brow, R.K.: Designing sealing glasses for solid oxide fuel cell. JMEPEG. 15, 410–413 (2006)
Wang, M.T., Cheng, J.S.: Viscosity and thermal expansion of rare earth containing soda–lime–silicate glass. J Alloys Compd. 504(1), 273–276 (2010)
Acknowledgements
The authors wish to thank the Scientific & Technological Research Council of Turkey (Project: 1919B011303387) for its generous financial support of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kurama, S., Saydam, G. Investigation properties of BaO/RO-Al2O3-R2O3-B2O3-SiO2 glass-ceramic sealants for solid oxide fuel cell. J Aust Ceram Soc 53, 293–298 (2017). https://doi.org/10.1007/s41779-017-0036-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41779-017-0036-8