Abstract
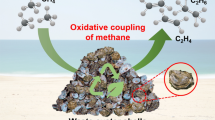
Agricultural wastes are equipped with unique physicochemical traits that may be harnessed to obtain valuable end products. Therefore, the present research focused on using waste eggshells as precursors for the synthesis of catalysts for partial oxidation of methane. Catalysts were synthesized via impregnation of copper on calcined eggshell surfaces. The catalysts were tested for selective oxidation of methane at 650° C and atmospheric pressure. Eggshells loaded with 2% copper activated partial oxidation pathway to enhance yield for syngas, while 5% and 10% loading led to C2–C6 hydrocarbons as a result of oxidative coupling of methane. Oxygen concentration in the feed along with feed flow rate and temperature influenced both fractional conversion and product selectivities. Limited-oxygen supply and adequate flow rate of 0.8 L min−1 at 650° C provided better conversion with 10% Cu-loaded catalyst. While the fresh catalyst exhibited a uniform distribution of copper and a smooth catalyst surface, little surface deformation was observed in the spent catalyst. Prolonged catalytic activity was also investigated via catalyst reactivation. Our results suggest that waste eggshells may be used as catalyst supports for metals to be used in oxidation reactions.
Graphic Abstract

Article highlights
-
Seven billion kilograms of chicken eggshells are disposed of every year.
-
Eggshell-supported copper catalysts for partial oxidation of methane for the first time.
-
CuO along with CaO activates methane and converts methane into value-added chemicals including C2–C6 hydrocarbons.
-
Excellent recyclabilities were obtained.
-
Value addition of eggshells can provide additional revenue to poultry producers and minimize waste disposal problems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There is a significant interest in utilizing agricultural wastes as precursors for the synthesis of value-added products (Santana-Méridas et al. 2012). Agricultural wastes are abundant and have unique physical and chemical properties that may be harnessed to obtain valuable end products (Demirbaş 2001). For example, the production of eggs for food is a common agricultural practice worldwide and results in an enormous quantity of eggshells (Laca and Laca 2017). To put things in perspective, the world egg production, based on 2017 report by the Food and Agricultural Organization is about 81 × 109 kg (Food and Agricultural Organization 2019). Consequently, considering that the shell accounts for about 11% of the total mass of the egg, about 9 × 109 kg of egg shells are available for value addition (Stadelman 2000).
Eggshells are predominantly composed of calcium carbonate along with other minerals and semi-permeable membrane structures (Wei et al. 2009; Boro et al. 2014; Tsai et al. 2006). In one of their articles, Oliveira et al. (2013) proposed several opportunities to sustainably utilize eggshells including adsorbents for mitigation of metals, heterogeneous catalysts for transesterification, edible calcium and protein supplements for human health, and others. As a result, several researchers have employed eggshells as adsorbents for mitigation of heavy metals (Mittal et al. 2016; Guru and Dash 2014). Eggshells are found to adsorb heavy metals such as chromium (III), iron, gold, and copper and hence find numerous applications in wastewater treatment (Mittal et al. 2016; Ahmad et al. 2012; Daraei et al. 2015; Zheng et al. 2007; Chojnacka 2005; Vijayaraghavan et al. 2005). Similarly, the calcium oxide obtained from calcined eggshell was exploited as a heterogeneous base catalyst in transesterification reactions (Buasri et al. 2013; Niju et al. 2014; Tan et al. 2015; Pandit and Fulekar 2017; Shan et al. 2018). In addition to acting as a heterogeneous base catalyst in transesterification reactions, calcium oxide allows for additional catalytic applications, including oxidation of methane (Carreiro and Baerns 1989; Lunsford 1995).
Partial oxidation of methane into chemicals is one of the most interesting and challenging areas of research (Hammond et al. 2012; Zhao et al. 2016). Methane is abundantly available from several sources, including exploration of natural gas, landfills, and anaerobic digestion of solid and liquid wastes (Chynoweth et al. 2001; Themelis and Ulloa 2007). Current estimates indicate that the United States alone produced about a record 90 billion cubic feet per day of methane (U.S. Energy Information Administration 2019). As a result, there has been a significant interest in converting methane into chemicals and other oxygenates via catalysis (Ravi et al. 2017). However, partial oxidation of methane is difficult to achieve because of high bond dissociation energy (440 kJ mol−1) associated with its balanced tetrahedral structure coupled with the absence of dipole movement (Schwarz 2011; Schwach et al. 2017; Zhao et al. 2016). In that context, calcium oxide-based catalysts have been found to be effective in oxidation of methane into C2 hydrocarbons (Karoshi et al. 2015). It was proposed that O2− and Ca2+ in CaO were responsible for the activation of methane (Maiti 1998).
Previous research also suggested that copper can effectively catalyze selective oxidation of methane into oxygenates or higher hydrocarbons (Schwarz 2011; Shiota and Yoshizawa 2000). Based on the two-state reactivity, Schwarz (2011) predicted that cationic CuO+ would be one of the most powerful oxidants although its generation in the gas phase has not been very successful due to a significantly lower bond dissociation energy (37.4 kcal mol−1). Nonetheless, CuO is reported to be effective for oxidative coupling of methane when promoted with LiCl or alkali metal nitrates (Hatano and Otsuka 1988). Cu-based catalysts (CuO/Al2O3 and Mo–CuO/Al2O3) have been studied in a plasma reactor to facilitate methane oxidation to methanol between 50 and 300° C (Huang et al. 2011). Similarly, in one of their recent reports, Narsimhan et al. (2016) tested copper-exchanged zeolite catalysts for partial oxidation of methane into methanol at temperatures between 210 and 225° C. Copper-exchanged mordenite zeolite synthesized via ion exchange was also investigated as a catalyst for conversion of methane into methanol using water-derived oxygen as an oxidant (Sushkevich et al. 2017).
Considering the catalytic properties of calcium oxide and copper towards methane activation and oxidation, it may be hypothesized that copper combined with CaO in eggshells can facilitate partial oxidation of methane into products. By synthesizing a Cu/CaO catalyst, whose CaO is derived from eggshells, waste disposal problems associated with eggshells could be solved along with valorization of eggshells simultaneously. To our knowledge, eggshell-supported copper catalysts were never investigated for partial oxidation of methane. Therefore, this research investigated the possibility of eggshell-doped copper as a partial oxidation catalyst for oxidation of methane. Specifically, the objectives were to study the effects of (1) copper loading, (2) CH4:O2 ratio, (3) flow rate, and (4) temperature on the methane conversion and products.
Experimental
Catalyst Synthesis
Copper-impregnated eggshell catalysts were synthesized in three different concentrations, 2%, 5% and 10% by weight of copper to represent low, medium, and high loading, respectively, via the wet impregnation method, using copper chloride dihydrate (CuCl2·2H2O) solution. Briefly, eggshell particles of 2–5 mm size were mixed with aqueous copper chloride solution (1 mL g−1 of eggshells) of deionized water, for 5 h (300 rpm) at room temperature. The preparation further involved heating the mixtures at 105° C for 8 h and activation at 1000° C for 4 h in sequence. Large batches of catalysts were synthesized to maintain uniformity in the analysis.
Catalyst Characterization
The catalysts were characterized via variable pressure scanning electron microscopy (VPSEM) and time-of-flight secondary ion mass spectrometer (TOF–SIMS). For VPSEM, the samples were prepared by sputter coating the catalyst surfaces with ~ 35 nm of gold (60%) and palladium (40%) as described in Karoshi et al. (2015). The morphological data were acquired via a Hitachi S 3200 equipped with a 20 keV beam @ 10−6 Torr. Further, the sample surfaces were studied using a TOF SIMS V (ION TOF, Inc. Chestnut Ridge, NY) instrument equipped with a Bim+n (n = 1–5, m = 1, 2) liquid metal ion gun, Cs+ sputtering gun which were angled at 45° to the sample surface normal while maintaining the pressure inside the chamber below 5.0 × 10−9 mbar.
Catalyst Testing
A temperature resistant, 304-metric type stainless steel tubular reactor (2.5 cm diameter and 15 cm height) was loaded with 10 g of fresh catalyst for every experiment. High purity methane (99%) and oxygen (99.9%) were co-fed by adjusting the flow rates to obtain the required CH4:O2 molar ratio. Prior to each experiment, catalyst activation was carried out, in situ, at an operating temperature of 650° C for 1 h. After equilibration, gas samples were collected from the reactor at 10 min and 15 min intervals. Each sampling was followed by measurement of total inlet and outlet flow rates. Gas samples were then analyzed on gas chromatography with mass spectrometry (HP-5MS) and thermal conductivity detector (Carbosieve SII). Methane fractional conversion and selectivity of products were determined as described previously (Karoshi et al. 2015). In this research, we studied the effects of metal loading (2, 5, and 10%) and methane: oxygen ratio (1.0, 1.67, and 3.0) on the fractional conversion of methane and product selectivity. In addition, we also tested the effect of flow rate (0.4, 0.8, 1.2 L min−1) to accommodate residence times between 3 and 12 s and temperature (650, 700, and 750° C) by maintaining a molar ratio of CH4:O2 of 1.67 using a catalyst with 10%-Cu loading. The reaction temperatures (650–750° C) were chosen to initiate oxidation of methane but at the same time minimize the overoxidation of the products. Further, the longevity of catalyst was also investigated using the same batch of catalyst over several cycles where each cycle represented co-feeding of CH4 and O2 for 15 min at a CH4:O2 molar ratio of 3.0 and flow rate of 0.8 L min−1, followed by flow of pure O2 (0.8 L min−1) for 15 min and helium (1.0 L min−1) for 5 min to remove excess oxygen from the system. Inlet and outlet gas samples were obtained at the end of each cycle to determine methane conversion rates and selectivity of end products.
Results and Discussion
Catalyst Characterization
Analysis of the catalyst via SIMS-TOF revealed that the copper was dispersed all over the surface with few concentrated clusters in association with CaO active phase as shown in Fig. 1a, b. Overlaid mass spectral image of copper-coated eggshell catalyst indicates emission of Ca+, Cu+ and 65Cu+ secondary ions from all over the surface (Fig. 1c). However, since the catalyst surface was not planar, secondary ions were observed to emit from most of the surface but not all during SIMS-TOF analysis. Interestingly, chlorine was observed to migrate and interact with calcium upon high-temperature activation at 1000° C. This further suggests that copper loading might have masked CaO active sites significantly via interaction with the eggshell support upon calcination.
The effect of calcination and the interaction between copper and eggshell surface resulting in the formation of active clusters were confirmed by energy-dispersive spectroscopy (EDS) (Fig. 2 ). Similar interaction of nanosized copper oxide with the cordierite support upon calcination at 350–700° C was also observed by El-Shobaky and Fahmy (2006). VPSEM data (Fig. 2a), suggested that copper and chlorine were distributed all over the catalyst surface with few concentrated clusters of Cu. Copper and chloride-rich zones were observed both in fresh and spent catalyst suggesting strong interaction with eggshell support (Fig. 2b). Fresh catalyst surface appeared to be smooth with relatively less porosity as shown in Fig. 2c. However, slight surface deformation and change in porosity were observed in the spent catalyst. The appearance of ridges on the spent catalyst suggested that the flow of feed gas mixture at high temperature physically impacted the catalyst surface.
VPSEM analysis for eggshell catalyst with 10%-Cu loading. (a 10% Cu-loaded fresh catalyst representing heterogeneous surface with multiple active sites; b Cu clusters observed both on fresh and spent catalyst and c 10% Cu-loaded fresh and spent catalyst representing the change in overall surface morphology)
Effect of Metal Loading
The effect of metal loading on the fractional conversion of methane is shown in Fig. 3a. The fractional conversion seems to increase at first then decrease with an increase in metal loading (from 2 to 10% loading). Analysis of the products from the outlet indicated the presence of 2-propenal, 1,3 butadiene, benzene, ethane and ethylene, carbon monoxide and carbon dioxide, along with traces of chloromethane, perhaps derived from the copper precursor (CuCl2.2H2O). Interestingly 2%-Cu loading enhanced methane conversion substantially when compared to the control catalyst (eggshell without metal loading) along with higher selectivity for syngas (H2 + CO) as shown in Fig. 3b. A subsequent increase in metal loading to 5% and 10% decreased fractional conversion as well as selectivity towards syngas significantly. However, increased metal loading enhanced the production of C2–C6 hydrocarbons along with CO2 as depicted in Fig. 3b, c. Increase in production of CO2 and the target products of C2–C6 hydrocarbons with increasing metal loading suggested oxidation of methane by copper oxide clusters. The catalytic activity of such copper clusters was also reported by Tomkins et al. (2016) in their recent research on low-temperature catalytic conversion of methane into methanol.
Effect of metal loading on a fractional conversion of methane, b production of C2 hydrocarbons and carbon oxides, c production of C4–C6 hydrocarbons, and d production of C2 hydrocarbons using copper-coated eggshell catalyst (n = 2). Reaction conditions: 650° C, 800 mL min−1, 10 g catalyst and CH4:O2 = 1:1 (control = 0% loading, i.e., calcined eggshell without metal loading)
The initial drop in carbon dioxide production and concomitant increase in CO production with 2% Cu when compared to control eggshell catalyst can be attributed to the shift in the pathway of partial oxidation (Fig. 3b). Increased conversion of methane (~ 50%) with 2%-Cu loading vs. other concentrations, may be attributed to the dominant pathway that leads to syngas production. It may be possible that CO once formed, would either be oxidized completely into CO2 or may remain unreacted and elute out of the reactor. This would increase the number of available active sites on the catalyst unlike in a coupling reaction, where, the larger intermediate molecules could block adsorption of methane molecules resulting in unreacted methane molecules eluting reactor. Further, an increase in metal loading resulted in reduced methane conversion (Fig. 3a) suggesting that methane activation occurred dominantly from the CaO phase of eggshell than the CuO phase.
Increased metal loading (5% and 10% Cu) would provide several active sites in close proximity that participate actively in oxidation which in turn would have resulted in overoxidation with high CO2 production. This mechanism is further evident from Fig. 3d which depicted increased oxidation with increased metal loading via relatively enhanced selectivity for ethylene when compared to ethane concentration. However, 2%-Cu loading might have the optimum arrangement with appropriate sequestration between the active clusters. It is possible that the CaO active sites inherent to eggshell catalyst may have activated methane by the abstraction of the hydrogen atom and causing coupling reaction to form higher hydrocarbons. On the other hand, CuO clusters may have oxidized the carbon atoms to different oxygenates including 2-propenal and carbon oxides. However, production of 2-propenal was observed only with 10% metal loading. The distribution of chloride species was observed all over the catalyst surface, which believed to have produced methyl chloride after interaction with methyl radical, generated on the catalyst surface. Lower catalyst loading of 2%-Cu did not yield any methyl chloride because of the dominant oxidation reaction to form syngas when compared to the interaction of radicals. Observance of methyl chloride further supports the possibility of surface bound-reactions than in the gas phase.
Effect of CH4:O2 on Catalytic Activity
As discussed in Karoshi et al. (2015), methane oxidation and coupling are known to be oxygen-dependent. As expected, the decrease in oxygen concentration in the feed resulted in a drop in methane conversion (Fig. 4a). At CH4:O2 ratio of 3:1 in the feed, the degree of drop in fractional conversion was substantial and may be attributed to the inability of oxygen to reactivate the catalyst quickly, due to the relatively higher concentration of methane which may compete with O2 for adsorption sites on the catalyst surface. Thus, diffusional constraints along with an adequate supply of oxygen play a critical role in fractional conversion. It is also possible that similar to studies focused on nickel, palladium, rhenium, and other metal-catalyzed methane oxidations (Torniainen et al. 1994; Freni et al. 2000), methane may have contributed partially in deactivating the catalyst via carbon deposition leading to overall reduced conversion as shown in Fig. 4a.
Decreased oxygen concentration resulted in a significant drop in selectivity for carbon dioxide indicating selective partial oxidation as shown in Fig. 4b. However, the drop in selectivity for carbon dioxide was not compensated by a corresponding increase in the production of target products identified in our analysis. Repeated experiments yielded similar results and the carbon balance was observed to have a significant gap suggesting the existence of a different set of oxygenated products in the product stream which was not detected by the gas chromatographic column.
As suggested earlier, the data obtained from our experiments also suggested that copper-impregnated eggshell catalyst may have promoted multiple pathways of methane conversion due to the existence of different active sites located in the close proximity to each other. Potential of CuO cluster to catalyze oxidation is evident from observed increase in CO2 production with an increase in metal loading on the eggshell support as shown in Fig. 3b. Such CuO clusters located in close proximity along with CaO active sites of the eggshell support may have been involved in partial oxidation of higher hydrocarbons. Propene would have undergone partial oxidation into 2-propenal due to the abstraction and insertion of hydrogen and lattice oxygen, respectively, as suggested by Reitz and Solomon (1998). In addition, the formation of methyl chloride suggests that the methyl radicals so formed due to activation of methane, interact on the catalyst surface to cleave away the chloride ions from the catalyst surface. Enhanced selectivity towards syngas by the catalyst with 2% Cu can be attributed to an appropriate arrangement of active sites where CaO would have involved in methane activation and CuO in enhanced partial oxidation. Further, partial dissolution of CuO into support lattice of the eggshell is possible (El-Shobaky and Fahmy 2006). Such dissolution can be significant with increasing metal loading which either may have exhibited synergistic effect or would have acted as an independent site to give rise to an alternative pathway. However, the CuO cluster may have induced selective oxidation of methane to syngas as suggested by Schwarz (2011). Further, with a bond energy of 157 kJ mol−1 in Cu–O, the oxygen remains more mobile when compared to the bond energy of 400 kJ mol−1 in Ca–O (Schwarz 2011; Maiti 1998; deB Darwent 1970).
Effect of Flow Rate
Methane conversion increased significantly with an increase in flow rate. Among three flow rates tested, a flow rate of 0.8 L min−1 appeared to provide highest fractional conversion of methane (Fig. 5a).
As shown in Fig. 5b, high selectivity for CO2 was observed for both cases of the lower flow rate of 0.4 L min−1 and a maximum tested the flow rate of 1.2 L min−1. However, lower selectivity for CO2 at 0.8 L min−1 with simultaneous reduction of selectivity for target products suggests the possibility of the existence of an alternative pathway for methane conversion leading to a different set of products. Conversions and selectivities for this condition were also confirmed by an additional replicated experiment. While the observed high selectivity for CO2 with the lowest flow rate (0.4 L min−1) can be attributed to an enhanced residence time of reactants and overoxidation, increased tortuosity resulting in exposure of activated radicals to oxygen-rich vicinity with the highest flow rate of 1.2 L min−1 also increase CO2 selectivity. On the other hand, products including C3–C6 hydrocarbons were found in traces with variations in flow rates. Thus, it is evident that the production of higher hydrocarbons via coupling reaction involves multiple adsorption and desorption steps of the reactants on to the catalyst surface.
Effect of Temperature
The data suggested that temperature did not influence the methane conversion rate substantially (Fig. 6a). This may be explained by the fact that the CuO and chloride species distributed on the surface may have masked the CaO sites capable of activating methane. The higher concentration of CuCl2 (10%) along with its relatively low melting point (~ 620° C) might have led to a greater extent of masking of CaO sites. Such sintering of Cu-based catalysts has been reported to occur above 300° C in catalytic partial oxidation (Rabe and Vogel 2008). Further, partial dissolution of Cu into the eggshell support along with its interaction with the support could have also reduced its methane activation ability. Nonetheless, temperature affected product selectivity. As shown in Fig. 6b, CO2 production increased with temperature suggesting overoxidation of target products. On the other hand, ethylene production was favored with an initial increase in temperature as shown in Fig. 6c. With further increase in temperature, the observed drop in ethylene production could be because of its overoxidation into CO2 (Farrell et al. 2016).
The data suggested that the temperature can enhance selective oxidation of ethane to ethylene but beyond the threshold increase of temperature, overoxidation will be favored to produce CO2 and H2O. Similarly, it is evident from Fig. 6d that production of higher hydrocarbons (C3–C6) was preferred with an initial increase in temperature from 650 to 700° C possibly because of the excess energy from the additional heat supplied. However, with further increase in temperature to 750° C, a significant drop in selectivity for C3–C6 hydrocarbons was observed, which can be attributed to overoxidation.
Catalytic Life
Data obtained from catalyst reusability studies (four cycles) are presented in Fig. 7a. It appeared that methane conversion rapidly increased from the third cycle of operation and remains consistent thereafter. As depicted in the micrograph in Fig. 2, the constant flow of gases on to catalyst bed could have disrupted the catalyst surface, increasing porosity and hence, increased the number of active sites for methane conversion. Production of carbon oxides as well as C2–C6 hydrocarbons except for ethane was observed to increase with each cycle of operation as shown in Fig. 7b–d. Further, it is evident from Fig. 7c that production of ethylene increased significantly from the second cycle with a drop in production of ethane with each cycle of operation. It is suggested that selective oxidation of ethane into ethylene increases with every cycle perhaps due to availability of active sites and combined with the availability of heat energy released from methane oxidation retained by the catalyst (Enger et al. 2008).
Effect on catalyst reusability on a fractional conversion of methane, b selectivity for C2-hydrocarbons and carbon oxides, c effect on selectivity for C2-hydrocarbons, and d selectivity for C3–C6 hydrocarbons. Experimental conditions: CH4:O2 = 3.0 with flow rate of 0.8 L min−1, pure O2 (0.8 L min−1 for 15 min) and helium (1.0 L min−1 for 5 min)
Handling and Valorization of Spent Catalyst
From a waste management perspective, the copper-loaded eggshell catalysts, even after complete deactivation, can still be used as a precursor for the synthesis of novel materials because copper is chemically bonded to calcium and is not prone to leaching. For example, Souza et al. (Souza et al. 2017) employed egg shells as foaming agents for synthesis of glass foams that could be used as insulation materials. Similarly, there is a significant interest in sustainable and light-weight construction materials. In that context, eggshells are being increasingly explored as alternative building materials (Raji and Samuel 2015) and the spent copper-loaded catalysts may be recycled as environment-friendly foaming agents or binders in building construction-related applications.
Conclusion
Chicken eggshells were valorized and used as a precursor for synthesis and testing of copper-supported calcium oxide catalysts for partial oxidation of methane. The results suggested that while low concentration of copper on eggshells favored syngas production, further increase in copper loading resulted in the synthesis of higher order hydrocarbons. However, increasing copper loading resulted in reduced methane conversion suggesting that catalyst properties may be manipulated by varying metal loading on eggshell surfaces. The data indicated that the oxygenated products were generated mainly from the CaO–CuO active sites and the higher order hydrocarbons were derived mainly from the CaO phase. While flowrate and CH4:O2 impacted both conversion and product selectivity, the temperature did not influence conversion rate except leading to higher production of CO2 and H2O. The catalyst can be reactivated and be used for at least four cycles and the conversion was observed to rapidly increase with multiple cycles of operation possibly because of the exposure of several active sites over time. These results clearly suggest that eggshells can serve as a catalyst supports for metal impregnated catalysts. However, future research focused on minimizing overoxdiation of methane and maximizing the product selectivity for C3–C6 hydrocarbons is suggested.
References
Ahmad R, Kumar R, Haseeb S (2012) Adsorption of Cu2 from aqueous solution onto iron oxide coated eggshell powder: evaluation of equilibrium, isotherms, kinetics, and regeneration capacity. Arab J Chem 5:353–359
Boro J, Konwar LJ, Deka D (2014) Transesterification of non edible feedstock with lithium incorporated egg shell derived CaO for biodiesel production. Fuel Process Technol 122:72–78
Buasri A, Chaiyut N, Loryuenyong V, Wongweang C, Khamsrisuk S (2013) Application of eggshell wastes as a heterogeneous catalyst for biodiesel production. Sustain Energy 1:7–13
Carreiro J, Baerns M (1989) Oxidative coupling of methane: II. Composite catalysts of basic materials. J Catal 2:396–403
Chojnacka K (2005) Biosorption of Cr(III) ions by eggshells. J Hazard Mater 121:167–173
Chynoweth DP, Owens JM, Legrand R (2001) Renewable methane from anaerobic digestion of biomass. Renew Energy 22:1–8
Daraei H, Mittal A, Noorisepehr M, Mittal J (2015) Separation of chromium from water samples using eggshell powder as a low-cost sorbent: Kinetic and thermodynamic studies. Desalin Water Treat 53:214–220
deB Darwent B (1970) Bond dissociation energies in simple molecules. http://www.nist.gov/data/nsrds/NSRDS-NBS31.pdf
Demirbaş A (2001) Biomass resource facilities and biomass conversion processing for fuels and chemicals. Energy Convers Manag 42:1357–1378
El-Shobaky HG, Fahmy YM (2006) Cordierite as catalyst support for nanocrystalline CuO/Fe2O3 system. Mater Res Bull 41:1701–1713
Enger BC, Lødeng R, Holmen A (2008) A review of catalytic partial oxidation of methane to synthesis gas with emphasis on reaction mechanisms over transition metal catalysts. Appl Catal A Gen 346:1–27
Farrell BL, Igenegbai VO, Linic S (2016) A viewpoint on direct methane conversion to ethane and ethylene using oxidative coupling on solid catalysts. ACS Catal 6:4340–4346
Food and Agricultural Organization (2019) Gateway to poultry production and products. http://www.fao.org/poultry-production-products/production/en/
Freni S, Calogero G, Cavallaro S (2000) Hydrogen production from methane through catalytic partial oxidation reactions. J Power Sour 87:28–38
Guru PS, Dash S (2014) Sorption on eggshell waste—a review on ultrastructure, biomineralization and other applications. Adv Colloid Interface Sci 209:49–67
Hammond C, Conrad S, Hermans I (2012) Oxidative methane upgrading. ChemSusChem 5:1668–1686
Hatano M, Otsuka K (1988) Alkali metal-doped transition metal oxides active for oxidative coupling of methane. Inorg Chim Acta 146:243–247
Huang L, Zhang X, Chen L, Lei L (2011) Direct oxidation of methane to methanol over cu-based catalyst in an ac dielectric barrier discharge. Plasma Chem Plasma Process 34:67–77
Karoshi G, Kolar P, Shah SB, Gilleskie G, Das L (2015) Calcined eggshell as an inexpensive catalyst for partial oxidation of methane. J Taiwan Inst Chem Eng 57:123–128
Laca, A., Laca, A., Diaz, M.: Eggshell waste as catalyst: A review. J. Environ. Manage. (2017)
Lunsford JH (1995) The catalytic oxidative coupling of methane. Angew Chem Int Edit Engl 34:970–980
Maiti GC (1998) Dehydrogenative coupling of CH4: nature of interaction over CaO surface. Studies in surface science and catalysis. Elsevier, New York, pp 487–495
Mittal A, Teotia M, Soni RK, Mittal J (2016) Applications of egg shell and egg shell membrane as adsorbents: a review. J Mol Liq 223:376–387
Narsimhan K, Iyoki K, Dinh K, Román-Leshkov Y (2016) Catalytic oxidation of methane into methanol over copper-exchanged zeolites with oxygen at low temperature. ACS Cent Sci 2:424–429
Niju S, Meera KM, Begum S, Anantharaman N (2014) Modification of egg shell and its application in biodiesel production. J Saudi Chem Soc 18:702–706
Oliveira DA, Benelli P, Amante ER (2013) A literature review on adding value to solid residues: egg shells. J Clean Prod 46:42–47
Pandit PR, Fulekar MH (2017) Egg shell waste as heterogeneous nanocatalyst for biodiesel production: optimized by response surface methodology. J Environ Manag 198:319–329
Rabe S, Vogel F (2008) A thermogravimetric study of the partial oxidation of methanol for hydrogen production over a Cu/ZnO/Al2O3 catalyst. Appl Catal B Environ 84:827–834
Raji SA, Samuel AT (2015) Egg shell as a fine aggregate in concrete for sustainable construction. Int J Sci Technol Res 4:8–13
Ravi M, Ranocchiari M, van Bokhoven JA (2017) The direct catalytic oxidation of methane to methanol—a critical assessment. Angew Chem Int Edn 56:16464–16483
Reitz JB, Solomon EI (1998) Propylene oxidation on copper oxide surfaces: electronic and geometric contributions to reactivity and selectivity. J Am Chem Soc 120:11467–11478
Santana-Méridas O, González-Coloma A, Sánchez-Vioque R (2012) Agricultural residues as a source of bioactive natural products. Phytochem Rev 11:447–466
Schwach P, Pan X, Bao X (2017) Direct conversion of methane to value-added chemicals over heterogeneous catalysts: challenges and prospects. Chem. Rev 117:8497–8520
Schwarz H (2011) Chemistry with methane: concepts rather than recipes. Angew Chem Int Edn 50:10096–10115
Shan R, Lu L, Shi Y, Yuan H, Shi J (2018) Catalysts from renewable resources for biodiesel production. Energy Convers Manag 178:277–289
Shiota Y, Yoshizawa K (2000) Methane-to-methanol conversion by first-row transition-metal oxide ions: ScO, TiO, VO, CrO, MnO, FeO, CoO, NiO, and CuO. J Am Chem Soc 122:1217–12326
Souza MT, Maia BG, Teixeira LB, de Oliveira KG, Teixeira AH, de Oliveira APN (2017) Glass foams produced from glass bottles and eggshell wastes. Process Saf Environ Prot 111:60–64
Stadelman WJ (2000) Eggs and egg products. In: Francis FJ (ed) Encyclopedia of food science and technology. Wiley, New York
Sushkevich VL, Palagin D, Ranocchiari M, van Bokhoven JA (2017) Selective anaerobic oxidation of methane enables direct synthesis of methanol. Science 356:523–527
Tan YH, Abdullah MO, Nolasco-Hipolito C, Taufiq-Yap YH (2015) Waste ostrich-and chicken-eggshells as heterogeneous base catalyst for biodiesel production from used cooking oil: catalyst characterization and biodiesel yield performance. Appl Energy 160:58–70
Themelis NJ, Ulloa PA (2007) Methane generation in landfills. Renew Energy 32:1243–1257
Tomkins P, Mansouri A, Bozbag SE, Krumeich F, Park MB, Alayon EMC, Ranocchiari M, van Bokhoven JA (2016) Isothermal cyclic conversion of methane into methanol over copper-exchanged zeolite at low temperature. Angew Chem Int Edn 55:5467–5471
Torniainen PM, Chu X, Schmidt LD (1994) Comparison of monolith-supported metals for the direct oxidation of methane to syngas. J Catal. https://doi.org/10.1016/0021-9517(94)90002-7
Tsai WT, Yang JM, Lai CW, Cheng YH, Lin CC, Yeh CW (2006) Characterization and adsorption properties of eggshells and eggshell membrane. Bioresour Technol 97:488–493
U.S. Energy Information Administration (2019) Today in energy. https://www.eia.gov/todayinenergy/detail.php?id=38692. Accessed 12 April 2019
Vijayaraghavan K, Jegan J, Palanivelu K, Velan M (2005) Removal and recovery of copper from aqueous solution by eggshell in a packed column. Miner Eng 18:545–547
Wei Z, Xu C, Li B (2009) Application of waste eggshell as low-cost solid catalyst for biodiesel production. Bioresour Technol 100:2883–2885
Zhao Z, Kulkarni A, Vilella L, Nørskov JK, Studt F (2016) Theoretical insights into the selective oxidation of methane to methanol in copper-exchanged mordenite. ACS Catal 6:3760–3766
Zheng W, Li X, Yang Q, Zeng G, Shen X, Zhang Y, Liu J (2007) Adsorption of Cd (II) and Cu (II) from aqueous solution by carbonate hydroxylapatite derived from eggshell waste. J Hazard Mater 147:534–539
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karoshi, G., Kolar, P., Shah, S.B. et al. Valorization of Eggshell Waste into Supported Copper Catalysts for Partial Oxidation of Methane. Int J Environ Res 14, 61–70 (2020). https://doi.org/10.1007/s41742-019-00238-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41742-019-00238-0