Abstract
A simple poly(arginine) film-modified carbon nanotube paste electrode (PAMCNTPE) was prepared using cyclic voltammetry (CV). The devised sensor was subjected to field-emission scanning electron microscope (FESEM) and CV characterization. The sensing of 0.1 mM pyridoxine (PY) was upgraded at PAMCNTPE as compared to the bare carbon nanotube paste electrode (BCNTPE). The PAMCNTPE detects the 0.1 mM PY at a specific potential 0.727 V with a current response of 10.68 µA. In the case of BCNTPE, the PY appeared at 0.798 V with a current 2.90 µA. The proposed analytical method was optimized by prime parameters such as the impact scan rate, pH and PY concentration. Under optimal conditions, the concentration of PY is directly proportional to oxidation current (Ipa) in linear range 2–10 µM, and 10–80 µM with a detection limit (LOD) of 9.6 × 10−7 M and limit of quantification (LOQ) of 3.21 × 10−6 M. The simultaneous determination, concentration variation analysis of PY is performed with riboflavin (RF) and interference analysis in detecting PY also examined. The proposed sensor was effectively applied for the determination of PY in natural food supplement with excellent recovery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
PY (vitamin B6) is an aqueous soluble B-vitamin, and it is involved in human metabolism and biochemical reactions. PY is required for the human body to maintain physical and psychological health. It is having a significant role in the synthesis of noradrenaline, dopamine, serotonin, and histamine in the human body. It is involved in the production of red blood cells (RBC) and reduces the risk of colorectal cancer. It acts as a coenzyme for many enzymatic reactions. Its inadequacy causes neurological and skin disorders [1,2,3,4,5]. RF (vitamin B2) belongs to B-group of vitamins which are found in an egg, meat, liver, and milk as a micronutrient. It is essential for the human body. Lack of RF in the human body causes skin and eye problems. These B-vitamins play a substantial role in human biochemistry; thus, it is necessary to develop a simple and quick analytical method for the determination of PY and RF [6,7,8]. There were previously reported a variety of analytical techniques such as chromatography, spectrophotometry, fluorescence spectroscopy [9,10,11], etc. However, these methods are costly and time-consuming analytical methods. The best substitute for the above methods are electroanalytical approaches like mesoporous TiO2 and SnO2 nanocatalyst-modified glassy carbon electrode, cobalt hexacyanoferrate-modified carbon paste electrode, carbon paste electrode modified with crown ethers, multi-wall carbon nanotube-modified carbon-ceramic electrode, copper nanoparticle-modified polycrystalline gold electrode, platinum electrode, electro-deposition of gold nanostructure-modified carbon paste electrode, etc. [12,13,14,15,16,17,18].
In sensor fabrication, the carbon nanotubes (CNTs) were frequently used due to their characteristic properties such as high electrical conduction, extreme stability, low noise, long voltage domains, and easy renewability [19, 20]. The polymeric films such as poly(glycine), poly(nigrosine), poly(methyl Orange), poly(rosaniline), solid R, etc. were used for the voltammetric analysis of biomolecules, because the polymerization films on the surface of electrode provide conducting path, swift electron transfer, and larger effective surface area. The surface modification by a polymer is a simple and effective approach in dealing with electroanalysis of bioactive molecules [21,22,23,24]. To the best of our knowledge, there is no electrochemical sensor based on poly(arginine)-modified carbon nanotube paste electrode for the voltammetry of pyridoxine in the presence of RF.
2 Experimental Part
2.1 Chemicals and Preparations
Pyridoxine, Riboflavin, and Arginine procured from Molychem, India. Carbon nanotubes are purchased from Sisco laboratories Maharashtra. Silicone oil is brought from Nice Chemicals India. All other chemicals are of A.R grade. The 0.1 M phosphate buffer solution (PBS) of various pH is prepared by intermixing of suitable amount Na2HPO4 and NaH2PO4. The 0.1 mM RF, PY, and 1 mM arginine solutions were freshly prepared using double distilled water.
2.2 Apparatus Used
All CV measurements were performed through CHI-6038E (Electrochemical Analyser, USA) in connection with a standard three-electrode arrangement and a computer for information storage and handling. The BCNTPE and PAMCNTPE were used as a working electrode, and a saturated calomel electrode (SCE) and a platinum wire were utilized as reference electrode and auxiliary electrode, respectively. All current measurements are taken with background current. All electrochemical experiments were done at lab temperature.
2.3 Development of Carbon Nanotube Paste Electrode
The carbon nanotube paste electrode (CNTPE) is fabricated by hand mixing of carbon nanotubes and silicone oil (60:40%, w/w) in an agate mortar to attain uniform paste, and the obtained paste is packed into the tip of Teflon tube and leveled out by tissue paper.
3 Results and Discussion
3.1 Electrochemical Deposition of Poly(arginine) on Electrode Surface
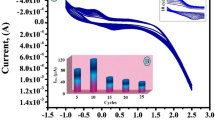
Amino acid functionalization and polymerization were extensively used for surface modification of electrode due to their high biocompatibility, mechanical strength, easy modification, little cost, and outstanding film-forming capability. The –NH2 and –COOH groups of amino acids play a key role in the electro-polymerization process. The polymer modified electrode surface shows excellent electrocatalytic properties in sensing the bioactive molecules. CV is a suitable approach to develop a polymer layer on an electrode surface. The electro-deposition poly(arginine) was performed in potential domain − 0.2 to 1.5 V with the presence of 1 mM arginine solution in 0.1 M PBS of pH 5.5, at potential sweep rate 0.1 V/s for 10 consecutive cycles as depicted in Fig. 1. It can be seen in figure that the cyclic voltammetric curves were gradually sloped downwards as cyclic period rises. This shows the formation of poly(arginine) thin layer on the surface of the carbon nanotube paste electrode. The poly(arginine)-coated carbon nanotube paste electrode is cleaned with little water to arise the unreacted arginine and used as a sensor for PY detection. The ten multiple cycles are optimized for electrochemical analysis PY.
3.2 Electroanalytical Characterization of BCNTPE and PAMCNTPE
The voltammetric behavior of BCNTPE and PAMCNTPE for standard 0.1 mM K4 [Fe(CN)6] was performed by CV in 0.1 M KCl at 0.1 V/s sweep rate as displayed in Fig. 2. The BCNTPE (curve a) characterizes the K4 [Fe(CN)6] oxidation at 0.327 V with a current 5.25 µA and reduction at 0.164 V with 3.22 µA current response. PAMCNTPE improves the electrochemical profile of K4[Fe(CN)6] by detecting anodic potential at 0.283 V with 10.85 µA current response and anodic potential at 0.187 V with 8 µA current response, so the constructed arginine polymer layer on carbon nanotube paste electrode improves the current–voltage characteristics of K4Fe(CN)6 with quasi-reversible behavior.
3.3 FESEM Characteristics of BCNTPE (curve a) and PAMCNTPE (curve b) Surfaces
The electroanalytical sensing is a surface-based phenomenon; hence, the surface topography of electrode is important in electroanalysis. This influences the sensing capacity of the electrode, effective surface area, electrode kinetics, etc. Therefore, it is so essential to know the surface morphology of the electrode. The surface characterization was performed using FESEM for BCNTPE and PAMCNTPE, as illustrated in Fig. 3a, b, respectively. The BCNTPE morphology was irregular arrangement whereas in case MCNTPE symmetric surface was observed, which confirms the modification BCNTPE with poly(arginine).
3.4 Electrocatalytic Effect in Sensing PY
The electrooxidation of 0.1 mM PY was analyzed by CV in the potential range 0.2–1.0 V at sweep rate 0.1 V/s in 0.1 M PBS of pH 6.5, as illustrated in Fig. 4. At BCNTPE PY was detected at potential 0.798 V with a small current response (2.9 µA), but at PAMCNTPE, the overpotential is reduced, and the current response was enriched due to the catalytic effect of poly(arginine) in sensing PY. The fine-tuned sensing of PY at PAMCNTPE appeared at specific potential 0.727 V with current response 10.71 µA with irreversible behavior. In absence of PY (curve b) the characterized CV portrays no peak at PAMCNTPE.
3.5 Potential Sweep Rate Effect
The potential scan rate study is a key parameter in electroanalysis; it is carried out to analyze the electrode kinetics. The impact of sweep rate from 0.1 to 0.250 V/s on the electrocatalytic oxidation of PY is analyzed by CV in 0.1 M PBS pH 6.5, as shown in Fig. 5a. The scan rate affects the current sensitivity of 0.1 mM PY linearly. The plot v1/2 vs. Ipa (Fig. 5b) shows the linear dependence of the oxidation peak current of PY by validating linear equation Ipa (A) = 1.08 × 10−5 + 6.485 × 10−5v1/2 (V/s) with a coefficient of correlation of 0.99. This elucidates that the electrode process is under diffusion controlled. The number of electrons transferred in PY oxidation can be calculated using the standard equation [25]:
where n is the number of electrons transferred, R = 8.314 J.K/mol (real gas constant), T is the temperature, F is the Faraday’s constant (96,485 C/mol), and α (charge transfer coefficient) is assumed to be 0.5 in a completely irreversible electrode phenomena [26] and electron transfer evaluated as 1.72 ~ = 2. Electron transfer mechanism of PY is predicted as shown in Scheme 1. The effective surface area of PAMCNTPE can be determined using a standard formula [27]:
where Ip is the peak current (A), n is the number of electrons, A is the electroactive area (cm2), D is the coefficient of diffusion (cm2/s), C is the concentration of the electroactive analyte (mol/cm3), and v1/2 is the square root of sweep rate (V/s). The diffusion coefficient value was obtained from the slope of Ipa vs. v1/2 plot. The active surface area of the PAMCNTPE electrode is calculated using the Randles equation and found to be 0.082 cm2.
3.6 Impact of pH of Supporting Electrolyte
The pH of supporting electrolyte has marked effects on the electroanalysis of bioactive molecules, and the pH optimization can improve the analytical performance of the electrode. Therefore, the influence of pH from 5.5 to 7.5 for the electroanalytical determination of PY was studied by using CV at sweep rate 0.1 V/s as displayed in Fig. 6a. As the pH rises, peak potential of PY is lifted towards the negative direction. The graphical plot of pH vs. Ipa (Fig. 6b) gives the high current signal at pH 6.5 at which rapid electron transfer takes place; for further increase or decrease in the pH, the peak current decreases. Therefore, pH 6.5 was the fine-tuned pH for whole electrochemical experiments. PY oxidation current decreases at acidic pH which may be due to protonation of pyridine group, and also after pH 6.5, the anodic current declines due to degradation of PY molecule forming the aldehyde-free radical.
3.7 Analytical Performance of PAMCNTPE
CV responses were used to quantify PY at PAMCNTPE in 0.1 M PBS solution of pH 6.5 with a 0.1 V/s sweep rate. Under calibrated conditions, the anodic peak current of PY as a function of concentration was investigated, as shown in Fig. 7a. The electrooxidation peak current is linearly proportional to the concentration over a range 10-80 µM (Fig. 7b); actually, the two linear curves are obtained between 2–10 and 10–80 µM, but we considered the fine linear 10–80 µM with linear equation Ipa (A) = 5.2 × 10−6 + 0.043 C (M) with correlation coefficient (R) of 0.99. The LOD is estimated to be 9.6 × 10−7 M and LOQ is 3.21 × 10−6 M with a sensitivity 0.04361 A/M. The LOD and LOQ was evaluated as LOD = 3SD (blank)/SC and LOQ = 10SD (blank)/SC [28], the SD (blank) is the standard deviation of the blank (five measurements), and SC is the slope of standard calibration graph.
Table 1 [29,30,31,32,33,34,35] compares the PAMCNTPE with other modified electrodes which are previously reported in the literature for sensing PY. The detection limit of 9.6 × 10−7 M of the proposed sensor is lower than that of disposable screen-printed electrode/CNTs [29], silver-doped poly(arginine)/GCE [30], boron-doped diamond electrode [31], vanadyl (IV)–salen complex-modified carbon paste [32] and close to screen-printed electrode/Ru nanoparticle [34], and graphene/ZnO nanocomposite [35], higher than the complicated preparation such as GCE/Au-CuO/MWCNTs [33]. This method provides a simple, disposable, and effective sensor for the PY determination in the presence of RF.
3.8 Analytical Applications
The devised sensor PAMCNTPE was applied to analyze PY in natural food supplement under optimized conditions using CV. The PY containing sample is dissolved in 0.1 M PBS (pH 6.5) to a known concentration of PY. The PY estimation was performed by standard addition strategy. The PAMCNTPE detects and quantifies the PY in natural food supplement with excellent recovery from 97.0% to 100.1% with RSD 1.27%. These results shows the analytical applicability of the proposed sensor (Table 2).
3.9 Estimation of Repeatability, Reproducibility, and Stability
The repeatability, reproducibility, and stability in sensing the PY were estimated by CV under optimal conditions. Good repeatability was obtained for five consecutive measurements with RSD 1.2% and excellent reproducibility for five separate measurements was attained by the renewal of electrode with RSD 2.9%. Stability was studied by running 20 cycles continuously with 90% of primary current and potential signals were retained even after 20 cycles.
3.10 Cyclic Voltammetric Response of RF
The redox nature of RF (0.1 mM) at BCNTPE (curve a) and PAMCNTPE (curve b) was deliberated by CV in 0.1 M PBS of pH 6.5 at a potential scan rate of 0.1 V/s as demonstrated in Fig. 8. The BCNTPE detects the RF (0.1 mM) oxidation at − 0.383 V and reduction at − 0.486 V with minor current responses. The PAMCNTPE has a marked effect in sensing 0.1 mM RF the anodic potential appeared at − 0.407 V and cathodic potential at − 0.524 V with enhanced current signals. The sensing of RF was accelerated at PAMCNTPE with ΔEP as 0.117 V, so the RF electroreduction was quasi-reversible.
3.11 Influence of Sweep Rate
The sweep rate analysis from 0.1 to 0.3 V/s for RF detection was performed using cyclic voltammetry in 0.1 M PBS of pH 6.5 at PAMCNTPE, as shown in Fig. 9a. As the sweep rate increases, the anodic and cathodic current signals also increase, and the cathodic and anodic potentials shift opposite to each other with an increase in ∆Ep values, which shows that the RF reduction is quasi-reversible. The plot v1/2 vs oxidation and reduction peak currents of RF (Fig. 9b) shows a linear relationship by agreeing linear equations Ipa (A) = 6.57 × 10−5 + 2.70 × 10−5v1/2 (V/s) and Ipc (A) = 1.38 × 10−5 + 1.72 × 10−4v1/2 (V/s) with R = 0.99. This illustrates the electrochemical probe in detecting RF was under diffusion controlled. The generally accepted reduction mechanism of RF is shown in the Scheme. 2.
3.12 Concurrent Separation of RF and PY Using CV
The electrochemical separation of RF and PY was performed in the potential window − 0.8 to 1.2 V at PAMCNTPE (curve b) and BCNTPE (curve a) using CV in 0.1 M PBS of pH 6.5 at sweep rate 0.1 V/s, as shown in Fig. 10. The RF oxidation and reduction were sensed at − 0.413 V and − 0.525 V, respectively, with excellent current responses and PY detected at 0.771 V with enhanced current responses with peak separation 0.308 V. In the case of BCNTPE, the RF anodic and cathodic peaks appeared at − 0.376 V and − 0.501 V, and PY was detected at 0.772 V with a low current response and peak separation 0.396 V. In the concurrent analysis, the RF reduction was quite sensitive than RF oxidation, because during the forward scan, the RF interacts less strongly may be due to interference of PY, but during a reverse scan, RF interacts more strongly with electrode yields more sensitivity for RF.
3.13 PY Variation in Presence of RF
The concentration variation of PY from 0.1 to 0.16 mM under optimal conditions was performed with the presence of 0.1 mM RF at PAMCNTPE using CV as revealed in Fig. 11a. At each successive increment in PY concentration is linearly proportional to the PY oxidation current without affecting much to RF peak characteristics. Moreover, the graphical plot of concentration variation PY vs. Ipa (Fig. 11b) gives excellent linearity with a coefficient of correlation 0.99.
3.14 RF Variation in Presence of PY
To check the detection ability of PAMCNTPE towards the detection of RF (0.1–0.15 mM) in the presence of 0.1 mM PY solution under optimal conditions using CV as demonstrated in Fig. 12a. The peak currents of RF vary linearly with each addition without affecting PY peak currents and potentials. This shows the possibility of determination RF in the presence of PY. The plot of peak currents of RF as a function of RF addition gives a straight line with a correlation coefficient of 0.99 (Fig. 12b).
3.15 Interference
The common species that affect for PY sensing such as ascorbic acid, biotin, folic acid, riboflavin, tyrosine, estriol, uric acid, and glycine (0.1 mM) were examined under optimal conditions using CV and observed that there is no significant effect in detection of PY with these interfering molecules and limit of interference was below ± 5. Therefore, the projected sensor exhibits excellent selectivity.
4 Conclusion
The simple and active electroanalytical sensor method is established for the determination of PY. The proposed sensor delivers excellent analytical performance after modification with poly(arginine) as compared to the unmodified electrode. The devised electrochemical sensor provides large effective area, and high electrical conductance through the polymer layer. Moreover, the fabricated sensor exhibits good repeatability, stability, simultaneous analyzing ability with less interference effect, effective analytical applications in pharmaceutical formulations. These excellent features make the developed sensor another alternative for the detection and quantification of PY.
References
Jyoti TB, Nandibewoor ST. Modification of glassy carbon electrode by polybromocresol using cyclic voltammetry as a sensor and its analytical applications in determination of pyridoxine hydrochloride in commercial drinks. Anal. Bioanal. Electrochem. 2019;10(9):1144–62.
Liang T, Qingji X, Shouzhuo Y. Electrochemical and spectroelectrochemical studies on pyridoxine hydrochloride using a poly(methylene blue) modified electrode. Electroanalysis. 2004;16(19):1592–7.
Nada FA, Ahmed G, Ekram HE, Asmaa RME. Effective and facile determination of vitamin B6 in human serum with CuO nanoparticles/ionic liquid crystal carbon based sensor. J Electrochem Soc. 2017;164(13):B730–8.
Hernandez SR, Ribero GG, Goicoechea HC. Enhanced application of square wave voltammetry with glassy carbon electrode coupled to multivariate calibration tools for the determination of B6 and B12 vitamins in pharmaceutical preparations. Talanta. 2003;61(5):743–53.
Qu W, Wu K, Hu S. Voltammetric determination of pyridoxine (vitamin B6) by use of a chemically-modified glassy carbon electrode. J Pharm Biomed Anal. 2004;36(3):631–5.
Tigari G, Manjunatha JG, Raril C, Hareesha N. Determination of riboflavin at carbon nanotube paste electrodes modified with an anionic surfactant. ChemistrySelect. 2019;4(7):2168–73.
Krystian W, Boguslaw B. Voltammetric characteristics and determination of riboflavin at the different metallic bulk annular band electrodes. J Electrochem Soc. 2018;165(7):H393–8.
Selvarajan S, Suganthi A, Rajarajan M. A facile synthesis of ZnO/manganese hexacyanoferrate nanocomposite modified electrode for the electrocatalytic sensing of riboflavin. J Phys Chem Solids. 2018;121:350–9.
Vinas P, Balsalobre N, Lopez-Erroz C, Hernandez-Cordoba M. Determination of vitamin B6 compounds in foods using liquid chromatography with post-column derivatization fluorescence detection. Chromatographia. 2004;59(5–6):381–6.
Jinghe Y, Rongjiang H, Benyu SU, Cunguo L, Naixing W, Jingtian HU. Simultaneous determination of four components in composite vitamin B tablets using a square root Kalman-filter. Anal Sci. 1998;14(5):965–9.
Marti-Andres P, Escuder-Gilabert L, Martin-Biosca Y, Sagrado S, Medina-Hernandez MJ. Simultaneous determination of pyridoxine and riboflavin in energy drinks by high-performance liquid chromatography with fluorescence detection. J Chem Educ. 2015;92(5):903–6.
Devaraj M, Saravanan R, Jiaqian Q, Elumalai S, Mehmet LY, Necip A, Gracia F, Rabah B, Gracia-Pinilla MA, Gupta VK. Heterostructures of mesoporous TiO2 and SnO2 nanocatalyst for improved electrochemical oxidation ability of vitamin B6 in pharmaceutical tablets. J Colloid Interface Sci. 2019;542:45–53.
Alemu M, Saini RC, Tadese A, Rishi P. Square wave voltammetric determination of pyridoxine in pharmaceutical preparations using cobalt hexacyanoferrate modified carbon paste electrode. J. Chem. Pharm. Res. 2014;6(1):544–51.
Purvi BD, Rahul MK, Ashwini KS. Electrochemical behaviour of pyridoxine hydrochloride (vitamin B6) at carbon paste electrode modified with crown ethers. J Solid State Electrochem. 2008;12(9):1067–75.
Habibia B, Phezhhana H, Pournaghi-Azarb MH. Voltammetric determination of vitamin B6 (pyridoxine) using multi wall carbon nanotube modified carbon-ceramic electrode. J Iran Chem Soc. 2010;7(2):S103–12.
Mohammed AK, Omar AH, Ohsaka T, Awad MI. Electroanalysis of pyridoxine at copper nanoparticles modified polycrystalline gold electrode. Electroanalysis. 2016;28(3):539–45.
Jazreen HQL, Yanni Y, Rakesh G, Richard DW. Electrochemical study of pyridoxine (vitamin B6) in acetonitrile. ChemElectroChem. 2014;2(3):412–20.
Mohammadhassan M, Mohsen BS, Mahdi G, Ebrahim H. Electro-deposition of gold nanostructures on carbon paste electrode: a platform with signal amplification for voltammetric study and determination of pyridoxine (vitamin B6). Russ J Electrochem. 2016;52(5):477–87.
Beitollahi H, Fahimeh M, Somayeh T, Shohreh J. A review on the effects of introducing CNTs in the modification process of electrochemical sensors. Electroanalysis. 2019;31(7):1195–203.
Punbusayakul N. Carbon nanotubes architectures in electroanalysis. Procedia Eng. 2012;32:683–9.
Manjunatha JG. Highly sensitive polymer based sensor for determination of the drug mitoxantrone. J. Surf Sci. Technol. 2018;34(1–2):74–80.
Manjunatha JG. Poly (nigrosine) modified electrochemical sensor for the determination of dopamine and uric acid: a cyclic voltammetric study. Int. J. Chem Tech Res. 2016;9(2):136–46.
Raril C, Manjunatha JG. Cyclic voltammetric investigation of caffeine at methyl orange modified carbon paste electrode. Biomed J Sci Tech Res. 2018;9(3):1–6.
Manjunatha JG, Kumara Swamy BE, Deraman M. Electrochemical studies of dopamine, ascorbic acid and their simultaneous determination at a poly (rosaniline) modified carbon paste electrode: a cyclic voltammetric study. Anal. Bioanal. Electrochem. 2013;5(4):426–38.
Raril C, Manjunatha JG. A sensitive and selective procedure for the voltammetric determination of brilliant indigo and acid yellow 23 at surfactant modified graphene paste electrode. J. Mater. Environ. Sci. 2019;10(6):510–9.
Sangili A, Veerakumar P, Chen SM, Rajkumar C, Lin KC. Voltammetric determination of vitamin B2 by using a highly porous carbon electrode modified with palladium-copper nanoparticles. Microchim Acta. 2019;186:299.
Raril C, Manjunatha JG. Sensitive electrochemical analysis of resorcinol using polymer modified carbon paste electrode: a cyclic voltammetric study. Anal. Bioanal. Electrochem. 2018;10(4):488–98.
Manjunatha JG. A novel poly (glycine) biosensor towards the detection of indigo carmine: a voltammetric study. J. Food Drug Anal. 2018;26(1):292–9.
Barbara B, Elio D. Voltammetric determination of vitamin B6 in food samples and dietary supplements. J Food Compos Anal. 2014;33(2):155–60.
Liu G, Wang YM, Sun DM. Simultaneous determination of vitamins B2, B6 and C using silver-doped poly(l-arginine)-modified glassy carbon electrode. J Anal Chem. 2016;71(1):102–9.
Darko K, Muslim K, Eda M, Ruqia N, Naser Ramdan RA, Dalibor MS. Determination of pyridoxine (vitamin B6) in pharmaceuticals and urine samples using unmodified boron-doped diamond electrode. Diam Relat Mater. 2016;64:184–9.
Marcos FST, Glimaldo M, Edward RD, Eder TGC. Voltammetric determination of pyridoxine (vitamin B6) at a carbon paste electrode modified with vanadyl(IV)–Salen complex. Anal Chim Acta. 2004;508(1):79–85.
Ranjith Kumar D, Manoj D, Santhanalakshmi J, Shim JJ. Au–CuO core-shell nanoparticles design and development for the selective determination of vitamin B6. Electrochim Acta. 2015;176:514–22.
Moreno V, Liorent-Martinez EJ, Zougagh M, Rios A. Decoration of multi-walled carbon nanotubes with metal nanoparticles in supercritical carbon dioxide medium as a novel approach for the modification of screen-printed electrodes. Talanta. 2016;161:775–9.
Hadiseh S, Beitollahi H. Graphene/ZnO nanocomposite for voltammetric sensing of vitamin B6 using modified glassy carbon electrode. Anal. Bioanal. Electrochem. 2016;8(6):732–40.
Acknowledgements
We gratefully acknowledge the financial support from the VGST, Bangalore, under Research Project No. KSTePS/VGST—KFIST (L1)2016–2017/GRD-559/2017-18/126/333, 21/11/2017.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
About this article
Cite this article
Tigari, G., Manjunatha, J.G. Electrochemical Preparation of Poly(arginine)-Modified Carbon Nanotube Paste Electrode and its Application for the Determination of Pyridoxine in the Presence of Riboflavin: An Electroanalytical Approach. J. Anal. Test. 3, 331–340 (2019). https://doi.org/10.1007/s41664-019-00116-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41664-019-00116-w