Abstract
Aim
The aim of the study was to accurately assess the antibacterial effect of the combined Er,Cr:YSGG and InGaAsP 940 nm laser therapy on nine pathogenic bacteria in the treatment of periodontitis.
Materials and method
Fifty-six patients were selected for this pilot study. Five patients were excluded, whereas 51 of them completed the study. The patients were randomly allocated to either the combined 2780 nm Er,Cr:YSGG (Waterlase, Biolase) and 940 nm InGaAsP diode laser (EPIC, Biolase) therapy, adjunct to scaling and root planning (SRP) (experimental group), or to scaling and root planning alone (control group). The quantitative and qualitative analysis of the total number of bacteria and nine specific germs was performed using quantitative real-time polymerase chain reaction.
Results
The total bacterial load inside the periodontal pockets was reduced both for the laser plus SRP and for the SRP alone group at the 1-month and 6-month follow-ups (p < 0.05). The laser therapy group showed a more significant bacterial reduction than the control group at the 1-month and 6-month follow-ups. The germ number reduction was statistically strongly significant for the total number of germs and for eight out of nine analyzed bacteria.
Conclusions
The present study suggests that a combined Er,Cr:YSGG 2780 nm and diode InGaAsP 940 nm laser therapy added to the nonsurgical periodontal treatment brings an important benefit in bacterial reduction and stands as a reliable alternative to antibiotic prescriptions in periodontal treatment. The positive changes are also reflected in significant improvement of clinical periodontal parameters. The results suggest that newly formed bacterial microbiome inside the sulcus appears to be more beneficial, durable, and stable in the lased group.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The disruption of the harmonic relationship between the host and the commensal microbiota is considered to be an important factor for the development of oral diseases [1,2,3,4]. The periodontitis is initiated when the pathogenic bacteria species become predominant, overruling the host’s defense and triggering an enhanced immune response, which may lead to ineffective chronic inflammation [5,6,7,8]. The aim of the nonsurgical periodontal therapy is to reduce the loss of tooth supporting tissues induced by the presence of pathogens and their interaction with the human body but also to reduce the total number of periodontal pathogens, to prevent their recolonization, or to lessen the pathogenicity of a potential future biofilm. However, the periodontal pathogens cannot be completely eliminated by conventional scaling and root polishing, especially in deeper periodontal pockets [9].
Considering the polymicrobial immune-inflammatory nature of the periodontitis related to the loss of the gingival attachment, the research must be focused on any therapeutic technique or tool that can contribute to the decrease of the pathogenic periodontal bacteria inside the periodontal pockets. The use of lasers has been proposed for its bactericidal and detoxifying effect, but also for its capacity to stimulate local healing [10,11,12,13,14]. Despite the literature data showing controversial results and conclusions of various laser wavelengths used in periodontal therapy, only a few researches aimed to investigate the synergic effect promoted by the combination of different laser categories.
Aim of the study
The study aims to assess the role of the complementary therapy performed with InGaAsP 940 nm laser and Er,Cr:YSGG 2780 nm laser in restoring the microbiological balance during periodontal therapy. The comparative evaluation of the bacterial recolonization after treatment was performed 1 month after the baseline and 6 months after the baseline by comparison with the final results of the baseline evaluation made before the treatment.
Materials and method
Patient selection
For the purposes of this pilot study, 56 patients were selected who had at least one true 6 mm or deeper periodontal pocket per quadrant. All the subjects, patients of Krondent Dental Clinic, were selected through screening from a number of 300 new patients. The inclusion criteria were as follows: subjects without systemic diseases, subjects without antibiotherapy 3 months prior to the periodontal treatment, at least 12 natural teeth present in the oral cavity distributed in four quadrants. The exclusion criteria were the following: patients aged over 80 or under 18, the presence of diabetes mellitus types I and II, TBC, HIV, HBV, and HCV, patients undergoing immunosuppressive treatments or radiotherapy, patients with psychiatric diseases and/or antibiotherapy in the preceding 3 months, patients who had undergone any periodontal therapy in the preceding 12 months.
Approval of the Ethical Committee of Private Practice Krondent Dental Clinic Application (#101E/30.11.2015) was obtained. The research was conducted according to the Declaration of Helsinki (1964, revised in 2013). Fifty-six consent forms were randomly numbered by a qualified statistician (DP) using a randomization software (https://www.randomlists.com/, Google AdSense).
Patients were informed about the purpose and the protocol of the study. They received and signed a consent form. The patients were subjected to a medical and dental anamnesis. Four patients were excluded: two exclusions were due to antibiotic treatment in the preceding 3 months, one exclusion was due to a HBV infection, and one exclusion was due to periodontal treatment having been received in the preceding 12 months. Moreover, one patient did not come back for any appointment after having fulfilled the informed consent. Fifty-one patients participated in the study until the end. Twenty-six patients of this sample were enrolled in the test group (average age 44, 31 +/− 7.65) and 25 patients were enrolled in the control group (average age 47.6 +/− 8.22).
The subjects’ clinical examination, periodontal status, and radiographic analysis were performed by an independent operator (AG). The diagnosis for the subjects and the sample of patients are presented in Table 1 below.
Study protocol
The patients were randomly assigned, according to the number allocated on the consent form, to either the combined 2780 nm Er,Cr:YSGG (IPLUS, Waterlase, Biolase, Inc., Irvine, USA) and InGaAsP 940 nm (EPIC 10, Biolase Inc., Irvine, USA) therapy adjunct to the subgingival debridement (SD) (experimental group) or the conventional therapy—subgingival debridement (SD) (control group). The study protocols for the experimental and the control group are presented in Table 2, whereas the laser parameters are presented in Tables 3 and 4. All the recordings of the clinical parameters and microbiological samples were made throughout the evaluation by an independent operator (AG) who was not aware of the assignment of the patients into treatment groups. Each treatment was performed by an experienced periodontist who was not aware of the assignment of the patients into groups (CC).
Periodontal probing depth (PPD) was measured with a manual Hu-Friedy PCP 15 periodontal probe (Hu Friedy Inc., Leimen, Germany) down to the closest lower millimeter. Gingival margin (GM) was measured using the same PCP 15 probe as the distance between the gingival margin and CEJ, or another reference point on the tooth surface, such as a crown or the restoration margin. A negative value was assigned where the gingival margin was below CEJ, and a positive one was assigned where the gingival margin was above it. Bleeding on probing (BOP) was scored based on the presence or absence of bleeding within 15 s after pocket probing. The plaque score was measured as positive or negative, using “+” or “−,” based on the presence or absence of plaque, by running a probe along the cervical part of the root surface in 6 points on every tooth.
Microbiological samples have been collected from 4 points: four samples were collected from each quadrant from the same indicated point throughout the entire evaluation. The targeted areas were previously isolated and dried. Sterile paper points were then inserted down to the bottom of the pocket and left there for 10 s. They were then removed by avoiding contact with saliva or the epithelium of the oral cavity. The points thus obtained were then placed in a sealed sterile tube provided by the Pet Plus Diagnostic Bereichsleiter mikro- und molekularbiologische Analytik, MIP Pharma GmbH company, for safe transportation purposes. The detection limit for each bacterium was confirmed by the company at 100 germs/ml.
The patients were informed about the necessity and the benefits of improving their oral hygiene. The treatment was performed by an experimented operator (CC). During the first therapeutic visit, the laser bacterial reduction protocol was applied to the experimental group, whereas no laser was used on the control group. Laser bacterial reduction was performed using InGaAsP (Epic 10, Biolase, Irvine, CA, USA), 2W, CW, E3-9 mm non-initiated tip. The laser was used for 30 s in the sulcus/periodontal pocket of each mono-rooted tooth and for 60 s in each sulcus/periodontal pocket of multi-rooted teeth using slight movements from the apex to the crown and backwards following the anatomy of the root surface. After the laser bacterial reduction protocol, ultrasound calculus removal was performed both on the experimental and the control groups using the ultrasonic device. Oral hygiene instructions and hygiene motivation were again discussed with the patients. Ultrasound calculus removal was performed using the ultrasonic device (Satelec, ACTEON GROUP Dental, Mérignac, France), as well as the brushing of all the dental surfaces using rotating brushes and Prophy Paste and Airflow (Kavo Dental, 11727 Fruehauf Drive Charlotte, NC 28273, Germany). The over-edged margins of any direct restorations and crowns were corrected in order to improve the gingival environment. Occlusal therapy was performed on all patients.
A week later, the patients in the experimental group underwent a second therapeutic session for laser bacterial reduction. The full mouth plaque score was evaluated again based on the O’Leary index. Scaling and root planning were performed after in both groups for all the periodontal pockets that exceed 4 mm in depth. Manual Gracey curettes (Hu-Friedy Inc., Leimen, Germany) were used.
In the test group, Er,Cr:YSGG laser therapy was performed on all the pockets measuring at least 4 mm in depth versus nothing in the control group. Er,Cr:YSGG laser (Waterlase, Biolase, Irvine, CA, USA) has 2780 nm wavelength, RFTP 14 mm tip, 2 W, 40 Hz, 50 μs, 15–45% air, 15–35% water with 10 s/mm depth in mono-rooted teeth, and 15 s/mm depth in multi-rooted teeth. The aim of this complex laser therapy was to complete the deep scaling, to perform curettage of the internal pocket wall, deepithelization of the internal soft wall of the periodontal pocket and deepithelization of the gingival margin to prevent epithelial ingrowth. With this therapy, we can obtain the root surface modification and detoxification, the bone decontamination, and a bactericidal effect that is reflected upon all the involved tissues. A secondary beneficial effect of laser therapy is photobiomodulation of the involved vascularized tissues.
One month after the baseline, microbiological samples were collected again intending to evaluate bacteria dynamics. Three months after the baseline, the clinical parameters were recorded in periodontal charts, to assess pocket depth dynamics in order to establish indication for the second erbium laser therapy. Laser therapy was performed in the test group 3 months after the baseline. Both wavelengths were used at the same settings in all pockets, with depths of 4 mm or more. Six months after the enrolment, all the recordings and samplings were done again. The therapy protocol is presented in Table 2. Laser parameter settings are presented in Tables 3 and 4.
The microbiological analysis was performed for Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Treponema Denticola, Tannerella Forsythia, Fusobacterium nucleatum, Prevotella intermedia, Peptostreptococcus micros, Eubacterium nodatum, and Capnocytophaga gingivalis. The expected results of the therapy were changes in the microbiological profile: the decrease of the total number of bacteria below the individual’s threshold for disease, the recolonization of the sulcus being delayed after the laser treatment, and the restoring of the balance between the bacterial species: a relative symbiotic proportion among the species after the treatment.
Statistical analysis
The statistical analysis was performed using SPSS statistics 21, IBM, Armonk, NY, and the included tests are as follows: quantitative analysis median and percentiles, qualitative analysis. The descriptive statistics analysis, Wilcoxon test, was performed to assess data validity. Considering the absence of statistically different values in the initial data for the control and experimental group, we consider that an important risk of bias has been excluded for eight variables (out of ten).
Results
Considering the literature data on the microbiological analysis in periodontics and the data distribution being nonparametric, the median and percentiles are relevant to analyze these variables. Table 5 presents the median and percentiles for the microbiological variables at baseline and respectively 1 and 6 months after the baseline for control group and experimental group.
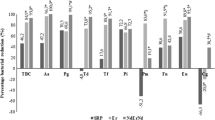
As we can see in Table 5, with the exception represented by Aa and En 1 month after, we found statistically strongly significant differences between the experimental and the control groups in favor of the experimental group, at a degree of significance ranging between 0.00 and 0.03 and showing a significant reduction immediately after the therapy in the lased group. That result was also maintained 6 months after the baseline.
Major pathogens Pg, Td, and Tf have shown a strongly significant reduction immediately after the laser therapy. These results were still maintained 6 months after the baseline. Also, Pm, Fn, Pi, and Cg have shown a significant reduction after the laser therapy. The significant differences between groups were also maintained or increased 6 months after the baseline. En also showed a significant reduction immediately after the treatment. It was more significant in the lased group than in the control group, but the differences were not statistically significant between the two group (p = 0.053). During the 6-month evaluation of the differences between the groups, the En value in the lased group was statistically significantly lower than the value in control group (p = 0.006).
After the quantitative analysis, we also considered performing the qualitative analysis of the bacteria distribution in the subjects at all moments: at the baseline, after 1 and after 6 months respectively, in order to have a more complete image of all the processed variables and their clinical significance (Table 6).
As we can see in Table 6, with the exception of Aa and Cg, we have a statistically significant reduction of the germs in almost all the evaluated bacteria. Aa was no longer detected in any patient in the lased group. Pg was still present in all cases in the control group, even if the quantity of germs was reduced after the therapy. In the lased group, in 15 out of 25 patients, we noticed the absence of Pg right after the laser therapy. The differences between the groups were statistically strongly significant. During the 6-month evaluation, in 18 patients in the control group, Pg was still present in the microbiological sample, as compared to only 9 in the lased group.
In what Td is concerned, out of 24 patients in control group for which Td was found in the initial microbiological sample, 22 still displayed the same bacteria after the therapy. In the lased group, only 9 subjects out of a total number of 25 still displayed Td at the microbiological analysis after the therapy. With regard to Tf, out of 25 cases tracked at the baseline, this bacterium was present in only in 4 patients in a month’s time and in 3 patients after 6 months, as compared to 21 in a month’s time and 20 after 6 months in the control group. Similar results were also obtained for Pi, Pm, Fn, and En.
Discussions
The ultimate goal of any effective periodontal therapy is not only to achieve the reduction of the periodontal pockets to less than 4 mm of depth, but also to restore a microbiome that is balanced and fully compatible with the local and general health. In the last decade, lasers have become increasingly common in periodontal therapy, despite the highly controversial evidences due to the poor and inconsistent standard of research [11, 13,14,15].
Different therapy protocols result into important differences in the use of different wavelengths, different settings for the same wavelength, laser application times, dosages, initiated tip, and irradiated area that lead to very heterogeneous results in the reported studies related to the effectiveness of lasers in periodontal therapy [15].
The use of dual wavelength is supported by the fact that each wavelength has different mechanisms of action. The Er,Cr:YSGG lasers have limited penetration depth, but are reliable to perform root detoxification and root surface modification, the interruption of the epithelial down-growth, curettage of the soft pocket and bone, and biostimulation due to the water chromophore. The InGaAsP lasers have a deeper penetration into the tissue; they exhibit absorption in the pigmented chromophores and a significant photobiomodulatory effect. The potential benefits of using two laser wavelengths together may be greater than one alone [11, 13, 14]. This laser combination uses the synergic antibacterial effects given by the absorption in melanin and water [11, 13].
In this study, at all moments of the evaluation after the treatment (1 and 6 months after), with the exception represented by Aa and En during the 1-month evaluation, we found strong statistically significant differences between the experimental and the control groups in favor of the experimental group, at a degree of significance ranging between 0.000 and 0.032. The total number of germs was significantly reduced immediately after the laser therapy and that was also confirmed at the 6-month evaluation. The differences between the lased group and the control group had a degree of significance of p ≤ 0.05 in favor of the lased group, supporting the hypothesis of the efficiency of the dual wavelength laser therapy in the bacterial reduction during the periodontal therapy. These results may also consider differences in the full mouth plaque score (FMPS) between the groups.
Aa was still present in six cases in the experimental group, but no longer present in any case at the 1-month and 6-month evaluations. Pg was still present in 23 out of 24 cases in the control group, as compared to only 10 cases out of 25 in the experimental group immediately after the treatment. After 6 months, only 9 patients in lased group still had this bacterium, as compared to 18 in the control group. After 6 months, Cg was still present in all the patients in the control and the experimental groups, even if the amount was significantly lower in the lased group.
Gojkov-Vukelic M. et al. (2013) demonstrated a statistically significant decrease in Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis immediately and 3 months after the baseline [12]. The significant antibacterial effects against major pathogens such as Prevotella intermedia, Tannerella forsythia, Treponema denticola, and Porphyromonas gingivalis were demonstrated for Er,Cr:YSGG laser-assisted periodontal therapy in a 6-month follow-up study conducted by Gutknecht et all [13].
When comparing the effect of diode 980 nm laser-assisted plus SRP with SRP alone, Caruso et al. (2008) [16] and Euzebio A et al. (2013) [17] found an improvement in the clinical parameters, but no significant differences in the reduction of periodontal pathogens [16, 17]. Crispino et al. proved that adding 940 nm diode laser therapy to SRP leads to a more effective decontamination of the pocket as well as a delayed recolonization [14]. Despite the rapid decrease of the pathogen load inside the periodontal pockets, demonstrated by many research groups after applying conventional nonsurgical periodontal therapy, these studies also showed the early recolonization of the periodontal pockets within 5 to 7 days after the conventional periodontal therapy [4, 18].
Having assessed the results of this research, we must also consider that DNA-based techniques can detect bacteria even at a very low level, but the procedure is very sensitive. Van Stenbergeer (1996) has proven that the DNA probe method can result in about 16% false positive results and 40% of the samples with PG stain remaining undetected in his study. The data in his study suggest that the value of the DNA probe method for the periodontal pathogen detection could be questionable at a certain point [19]. We may also consider new other species that are not included into the standard microbiological test now being associated with the start and progression of the periodontal disease (Filifactor alocis, Desulfobulbus sp., Bacteroidetes sp., Veillonella atipica, Brevundimonas diminuta, Gemella sanguinis, Mogibacterium tidminum, Mycoplasma salivarium, Aneroglobus Geminatus, Megasphera micronucoformis, Streptococcus parasanguinis, Pasteurella, F. naviforme, T. Medium) [10, 19]. No research is yet available with regard to these species’ behavior in interaction with lasers. Nevertheless, due to the important differences in the values of the total number of germs detected and the amount of periodontal germs detected in each sample, we may take into consideration some possible interaction between these new bacteria and lasers, while also considering nonpathogenic bacteria as part of the total number of germs detected in the samples.
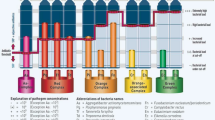
Even under these circumstances, the results of this study reveal a major population shift after the treatment: Aa, Pg, Td, and Tf associated with advanced periodontitis were dramatically reduced; a slight presence of the orange complex was noticed that is usually associated with the initial regression of the disease or with the progression from a mild to an advanced form, but also a shift to the green complex typically associated with the phase of biological healing has been shown. The average load of all the known pathogens has decreased dramatically. The most important and striking results were seen immediately after the laser treatment, but they were considerably satisfactory after 6 months too.
In the interpretation of these results, we must consider the conclusion of Tomasi et al. (2007) about multilevel analysis in periodontal studies, as the response to treatment can vary markedly both from a patient to another and depending on the tooth sites in the patients [20].
The interpretation of the results is difficult to do by comparing with literature data, due to the various combination of parameters and protocols used in these researches. The sample patient is quite homogenous in a private practice. Despite the limitations represented by the low number of studies and the inconsistencies in the protocols and methodology, the support of laser therapy in the treatment of periodontitis is considerable and may be taken into consideration as a reliable alternative to antibiotic prescriptions. Research offers moderate to poor long-term results in terms of the local use of antibiotics and antiseptics in the treatment of periodontitis [21, 22]. Despite the fact that the administration of systemic antibiotics plus SRP has proven to be more beneficial than SRP alone, we must consider the adverse effects and the possible development of the resistance to antibiotics of many aggressive bacteria [23,24,25]. There are limitations of this study due to the small sample of patients, the use of commercial microbiological tests, limited access to a private practice for the population, and short follow-up (6 months).
Conclusions
The present study suggests that a combined therapy of Er,Cr:YSGG and InGaAsP lasers is beneficial in the significant and rapid reduction of periodontal pathogens. The additional use of dual wavelength laser therapy combined with nonsurgical periodontal therapy supports the turning of the subgingival microbiome into a beneficial bacterial community that can support a stable clinical result. Also, it stands as an alternative to antibiotic prescriptions in periodontitis. The positive photobiomodulatory effects of the dual wavelength laser-assisted therapy should also be taken into consideration.
On average and long term, a balanced subgingival microbiome dramatically reduces the risk of rebound of the disease after the completion of the periodontal treatment. Future research may be conducted to identify the behavior of new species associated with the periodontal disease and their interaction with the laser wavelengths used in periodontics. Research could also be conducted to compare different laser protocols used in periodontology, different dosages, and a combination of parameters to identify the protocols that can bring superior long-term clinical results in the treatment of periodontal diseases.
References
Demmer R, Papapanou P (2010) Epidemiologic patterns of chronic and aggressive periodontitis. Periodontol 2000 53(1):28–44. https://doi.org/10.1111/j.1600-0757.2009.00326.x
Teles R, Teles F, Frias-Lopez J, Paster B, Haffajee A (2013) Lessons learned und unlearned in periodontal microbiology. Periodontol 62(1):95–162. https://doi.org/10.1111/prd.12010
Lappin-Scott HM, Bass C (2001) Biofilm formation: attachment, growth, and detachment of microbes from surface. Am J Infect Control 29(4):250–251. https://doi.org/10.1067/mic.2001.115674
Haffajee AD, Patel M, Socransky SS (2008) Microbiological changes associated with four different periodontal therapies for the treatment of chronic periodontitis. Oral Microbiol Immunol 23(2):148–157. https://doi.org/10.1111/j.1399-302X.2007.00403.x
Heitz-Mayfield LJ, Trombelli L, Heitz F, Needleman I, Moles D (2002) A systematic review of the effect of surgical debridement vs non-surgical debridement for the treatment of chronic periodontitis. J Clin Periodontol 29(Suppl 3):92–102. https://doi.org/10.1034/j.1600-051X.29.s3.5.x
Darveau RP (2010) Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microb 8(7):481–490. https://doi.org/10.1038/nrmicro2337
Suzuki N, Yoneda M, Hirofuji T (2013) Mixed red-complex bacterial infection in periodontitis. Int J Dent 587279. Published online 2013 Mar 6. https://doi.org/10.1155/2013/587279
Bodet C, Chandad F, Grenier D (2007) Pathogenic potential of Porphyromonas gingivalis, Treponema denticola and Tannarella forsythia, the red bacterial complex associated with periodontitis. Pathol Biol (Paris) 55(3–4):154–162. https://doi.org/10.1016/j.patbio.2006.07.045
Johnson JD, Chen R, Lenton PA, Zhang G, Hinrichs JE, Rudney JD (2008) Persistence of extracrevicular bacterial reservoirs after treatment of aggressive periodontitis. J Clin Periodontol 79(12):2305–2312. https://doi.org/10.1902/jop.2008.080254
Martelli FS, Fanti E, Rosati C, Martelli M, Bacci G, Martelli ML, Medico E (2016) Long-term efficacy of microbiology driven periodontal laser assisted therapy. Eur J Clin Microbiol Dis 35(3):423–431. https://doi.org/10.1007/s10096-015-2555-y
Al-Falaki R, Hughes FJ, Wadia R (2016) Minimally invasive treatment of infrabony periodontal defects using dual-wavelength laser therapy. Int Sch Res Notices 25(2):131–139
Gojkov-Vukelic M, Hadzic S, Dedic A, Konjhodzic R, Beslagic E (2013) Application of a diode laser in the reduction of targeted periodontal pathogens. Acta Inform Med 21(4):237–240. https://doi.org/10.5455/aim.2013.21.237-240
Gutknecht N, Van Betteray C, Ozturan S, Vanweersch L, Franzen R (2015) Laser supported reduction of specific microorganisms in the periodontal pocket with the aid of an Er,Cr:YSGG laser: a pilot study. Sci World J 450258. https://doi.org/10.1155/2015/450258
Crispino A, Figliuzzi MM, Iovane C, DelGiudice T, Lomnano S, Pacifico D, Fortunato L, Del Giudice R (2015) Effectiveness of a diode laser in addition to non-surgical periodontal therapy: study of intervention. Anm Sromatologica (Roma) 6(1):15–20 Published online May 2015
Cobb CM (2006) Lasers in periodontics:a review of the literature. J Periodontol 77(4):545–564. https://doi.org/10.1902/jop.2006.050417
Euzebio Alves VT, de Andrade AK, Toaliar JM, Conde MC, Zezell DM, Cai S, Pannuti CM, De Micheli G (2013) Clinical and microbiological evaluation of high intensity diode laser adjutant to non-surgical periodontal treatment: a 6-month clinical trial. Clin Oral Investig 17(1):87–95. https://doi.org/10.1007/s00784-012-0703-7
Caruso U, Nastri L, Piccolomini R, d’Ercole S, Mazza C, Guida L (2008) Use of diode laser 980 nm as an adjunctive therapy in the treatment of chronic periodontitis. A randomized controlled clinical trial. New Microbiol 31(4):513–518
Cugini MA, Haffajee AD, Smith C, Kent RL Jr, Socransky SS (2000) The effect of scaling and root planing on the clinical and microbiological parameters of periodontal diseases: 12-month results. J Clin Periodontol 27(1):30–36. https://doi.org/10.1034/j.1600-051x.2000.027001030.x
Van Steenbergen TJ, Timmerman MF, Mikx FH, de Quincey G, van der Weijden GA, van der Velden U, de Graaff J (1996) Discrepancy between culture and DNA probe analysis for the detection of periodontal bacteria. J Clin Periodontol 23(10):955–959. https://doi.org/10.1111/j.1600-051X.1996.tb00518.x
Tomasi C, Leyland AH, Wennstrom JL (2007) Factors influencing the outcome of non-surgical periodontal treatment: a multilevel approach. J Clin Periodontol 34(8):682–690. https://doi.org/10.1111/j.1600-051X.2007.01111.x
Ximenez-Fyvie LA, Haffajee AD, Som S, Thompson M, Torresyap G, Socransky SS (2000) The effect of repeated professional supragingival plaque removal on the composition of the supra- and subgingival microbiota. J Clin Periodontol 27(9):637–647. https://doi.org/10.1034/j.1600-051x.2000.027009637.x
Paolantonio M, D’Angelo M, Grassi RF, Perinetti G, Piccolomini R, Pizzo G, Annunziata M, D’Archivio D, D’Ercole S, Nardi G, Guida L (2008) Clinical and microbiologic effects of subgingival controlled-release delivery of chlorhexidine chip in the treatment of periodontitis: a multicenter study. J Periodontol 79(2):271–282. https://doi.org/10.1902/jop.2008.070308
Checchi L, Montevecchi M, Gatto MR, Trombelli L (2002) Retrospective study of tooth loss in 92 treated periodontal patients. J Clin Periodontol 29(7):651–656. https://doi.org/10.1034/j.1600-051X.2002.290710.x
Hellstrom MK, Ramberg P, Krok L, Lindhe J (1996) The effect of supragingival plaque control on the subgingival microflora in human periodontitis. J Clin Periodontol 23(10):934–940. https://doi.org/10.1111/j.1600-051X.1996.tb00514.x
Feres M, Haffajee AD, Allard K, Som S, Socransky SS (2001) Change in subgingival microbial profiles in adult periodontitis subjects receiving either systemically-administered amoxicillin or metronidazole. J Clin Periodontol 28(7):597–609. https://doi.org/10.1034/j.1600-051x.2001.028007597.x
Funding
This research was done under the support of Krondent Dental Clinic. No other funding was accessed for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Before starting this research, ethical approval of the Ethical Committee of Krondent Dental Clinic was obtained: approval no. 101E /30.11.2015. All patients had signed a consent form at the moment they were selected for this study.
Rights and permissions
About this article
Cite this article
Ciurescu, C., Vanweersch, L., Franzen, R. et al. The antibacterial effect of the combined Er,Cr:YSGG and 940 nm diode laser therapy in treatment of periodontitis: a pilot study. Laser Dent Sci 2, 43–51 (2018). https://doi.org/10.1007/s41547-017-0018-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41547-017-0018-8