Abstract
The preparation of nuclear-grade zirconium and hafnium is very important for nuclear power. The separation of hafnium from zirconium in a hydrochloric acid solution by solvent extraction was investigated with di(2-ethylhexyl)phosphoric acid (D2EHPA). The effects of hydrochloric acid concentration, extractant concentration, diluents, and temperature on the distribution coefficient of hafnium and zirconium were studied. The species extracted were ZrOA2·2HA and HfOA2·2HA. In this process, the separation factors varied with different diluents and followed the order octane > hexane > toluene > chloroform. A high separation factor value of 4.16 was obtained under the conditions of a solution containing 0.05 mol/L HCl and 0.01 mol/L D2EHPA for the separation of hafnium from zirconium. The extraction reaction was endothermic.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In the nuclear industry, zirconium and hafnium have completely different abilities to transmit thermal neutrons [1,2,3,4]. As we all know, zirconium is put into use in the nuclear industry [3, 5]. In contrast, hafnium is applied to a nuclear reactor as a control material [6,7,8]. Thus, nuclear-grade zirconium and hafnium have important applications in the nuclear industry. Due to their different nuclear indexes, it is essential to purify them when both metals are applied in the nuclear industry. However, the separation of them is a difficult thing due to the similarities of their ionic radii and valence states [1, 9, 10]. At present, liquid–liquid extraction is a relatively mature separation technology for separating zirconium and hafnium.
Many extraction processes [8, 9, 11,12,13,14,15,16,17] have been implemented in order to purify zirconium and hafnium. She Chen et al. [18] separated zirconium and hafnium with a novel agent, bis(2-ethylhexyl)-1-(2-ethylhexylamino)propylphosphonate (BEAP). The maximum separation factor of zirconium over hafnium was around 6.8, and the extracted complexes in the organic phase were suggested to be ZrO(HSO4)2·3B and HfO(HSO4)2·2B. Banda et al. [19] proposed a technology for the effective extraction of two metals from hafnium under 2.5–3 mol/L HCl conditions using trioctylphosphine oxide (TOPO) as the extractant in kerosene. Moreover, mixtures of TOPO with di(2-ethylhexyl)phosphoric acid (D2EHPA), methyl trioctylammonium chloride (Aliquat 336), and tri-n-octylamine (Alamine 336) were applied to find optimum purification conditions for zirconium and hafnium in hydrochloric acid. Taghizadeh et al. [12] suggested solvent extraction utilizing a tri-n-butylphosphate (TBP) and bis(2,4,4-trimethylpentyl)phosphinic acid (Cyanex 923) mixture to extract them. Also, several conditions, including TBP/Cyanex 923 volume ratio, extractant amount, and nitric acid and NaNO3 amounts, were investigated. According to the results, the advantages of the new purification process were low acid amounts and high extraction percentage of zirconium.
In nature, the maximum percentage of hafnium in zircon ore is about 1–3%. So, it is more economical to explore systems that preferentially extract hafnium. Currently, there are a low number of extraction systems [1, 20] that preferentially extract hafnium in zircon ore. The commonly used methyl isobutyl ketone (MIBK) process has many shortcomings in industrial production, such as low extraction ability (only 21.65%) and the easy flammability, high volatilization, and water solubility of MIBK. Xu et al. [1] suggested a purification process of zirconium and hafnium from a HSCN medium utilizing diisobutyl ketone (DIBK) and D2EHPA as extractants. The effects of the aqueous acidity and the amounts of NH4SCN, (NH4)2SO4, and NaCl used were investigated. They found that hafnium gathered in the organic phase. The complexation ability of hafnium with thiocyanate is better than that of zirconium, so it can set apart hafnium from zirconium. However, it needs a high amount of ammonium thiocyanate to improve the extraction. Reddy [20] used bis(2,4,4-trimethylpentyl)monothiophosphinic acid (Cyanex 302) as an extractant to extract hafnium in acidic chloride solutions and the extraction followed an ion exchange mechanism. The extraction is exothermic, and sulfuric acid is the best stripping agent. Extraction performances for other metals such as Zr, Ti, and Fe were also studied, and the IR spectra were displayed. As we all know, ammonium thiocyanate systems are frequently hampered by the challenges associated with wastewater, poison gas, and byproducts of ammonium thiocyanate in an acid medium. Therefore, it is of great significance to develop new extraction systems to realize the preferential extraction of hafnium.
According to Pearson [21], zirconium and hafnium are categorized as hard Lewis acids owing to small ionic radii and high ionic charges, so they have powerful tendencies to complex with various anions. The stability constants of metal complexes with anions are in the following order [4, 5]: OH− > F− > SO42− > NO3− > Cl− > ClO4−. Zirconium ions usually polymerize in sulfuric acid systems at low concentrations of acid and metals [22]. So, a purification process in a hydrochloric acid system is favorable.
In this work, the process described is the selective separation of hafnium from zirconium with D2EHPA in a hydrochloric acid medium. Liquid–liquid solvent extraction programs were investigated, including the effects of the amounts of D2EHPA and hydrochloric acid used, to obtain better conditions for the separation of hafnium and zirconium.
2 Experimental
2.1 Chemicals and reagents
D2EHPA (di(2-ethylhexyl)phosphoric acid, purity > 98%) was purchased and used without any purification. HNO3 (GR) was purchased from Jingrui Chemicals, China. Octane, octanol, and all other chemicals were purchased from Sinopharm, China.
Moderate amounts of ZrOCl2·8H2O (Sinopharm, China) and HfCl4 (Sinopharm, China) were dissolved in ultrapure water, and the acidity of the aqueous solution was achieved using hydrochloric acid. Fresh solutions were prepared before the experiments to avoid hydrolysis and polymerization [23].
2.2 Extraction process
A moderate amount of D2EHPA was dissolved in octane, and a certain amount of ZrOCl2 and HfCl4 moved to the HCl medium. Then, equal volumes (2 mL) of both detection liquids were transferred into 10-mL oscillation tubes, and all tubes were shaken for 1 h at 300 rpm conditions. After reaching equilibrium at 298 ± 0.5 K, the contents were separated by centrifuge. Using inductively coupled plasma mass spectrometry (ICP-MS, Thermo Scientific, X Series), the contents of the residual metals in the water phase were tested. In view of the mass balance, the portion in the D2EHPA phase was calculated.
The distribution coefficient (D) defined the extraction ability, and D was the ratio of the metal content in the organic phase to that in water at equilibrium [24]. There is a relationship between D and extraction percentage that follows the equation E % = D × 100%/(D + 1). Based on this work, the separation factor (SF = DHf/DZr) can be regarded as the separation ability for the two metals.
3 Results and discussion
3.1 Extraction mechanism
The balance equation for the extraction of zirconium and hafnium from a HCl system using D2EHPA could be as follows:
where M = zirconium or hafnium, H2A2 represents the dimeric form of D2EHPA [25], the subscript “a” represents the water phase, and the subscript “o” represents the organic phase.
The equilibrium constant expression of this system, Kex, can be given as follows:
The distribution coefficient, D, is given as follows:
where
Taking logarithms of Eq. (4) gives the following new equation:
A series of D values were obtained by processing the data, and the number of extractant molecules (n) related to the extraction complex could be determined by the slope method.
3.1.1 Extraction of zirconium and hafnium with D2EHPA
The extraction performance for them from a hydrochloric acid system was investigated with D2EHPA in octane. The mechanism of the process with chelating reagents such as acidic extractants at a low acid concentration was a cation exchange mechanism [14, 20]. Equation (1) implies that the acidity of the aqueous phase plays a key role in the distribution coefficient. Thus, increasing the acidity of the aqueous phase can decrease the extraction distribution, because the chemical equilibrium of Eq. (1) moves to the left.
Figure 1 shows the relationship between logD and log[H+] at 0.005 mol/L D2EHPA concentration in octane. When the acid concentration is less than 0.03 mol/L (log[HCl] = −1.5), most zirconium and hafnium are extracted and a third phase is observed after centrifuging. So, the acidity in the investigation is greater than 0.03 mol/L and the third phase disappears. In Fig. 1, the slopes of the two straight lines stand for the value of n, and they are − 2, indicating the substitution of two H+ for each metal ion in the time of the extraction process.
The effect of D2EHPA amount on the extraction of zirconium and hafnium in 0.05 mol/L HCl was also studied. As shown in Fig. 2, the plot of log D versus log[D2EHPA] gives two lines with slopes of 2 for zirconium and hafnium. Hence, (n + x)/2 was determined to be 2 and the value of x is thus 2.
Therefore, the extraction expressions with D2EHPA may be given as follows:
and
It is evident from Eqs. (6) and (7) that zirconium and hafnium were extracted as ZrO2+ and HfO2+ rather than Zr4+ and Hf4+. The species are coordinated with two molecules and two acid root anions of D2EHPA. In view of the Eh–pH diagram [25, 26], ZrO2+ and HfO2+ were the predominant species in the pH range of 1.4–2.3. So, the experiments agree with the theory.
The preferential extraction of hafnium from zirconium by D2EHPA may be attributed to the fact that the polymerization tendency of zirconium is stronger than that of hafnium at lower acidity. Under these conditions, zirconium gets highly aggregated and hence forms more stable oxygen-bridged complexes than does hafnium [5]. So, hafnium was easier to extract than zirconium.
According to the hard–soft acid–base (HSAB) theory, hard Lewis acids containing zirconium and hafnium metals can have great affinity with water [10, 27, 28]. However, zirconium has a stronger tendency to interact with water than does hafnium in a low-acidity medium [27]. The bond dissociation energies of zirconium and hafnium with oxygen are 766.1 ± 10.6 kJ/mol and 801 ± 13 kJ/mol. So, the extracted complex of hafnium is more stable than zirconium. To sum up, D2EHPA can prefer to extract hafnium rather than zirconium in a low-acidity medium.
3.1.2 The effect of temperature on the extraction of zirconium and hafnium
The effects of temperature in the 303–323 K range on the extraction process of zirconium and hafnium are shown in Fig. 3.
The extraction abilities for them increased with increasing temperature, which implied that the extraction reactions are endothermic.
The thermodynamic formula was applied to determine the enthalpy change (\(\Delta H\)) of the extraction reaction [29].
Usually, the constant R is 8.314 J/mol, and the changes of enthalpy at the extraction equilibrium of zirconium and hafnium are 87.25 kJ/mol and 61.20 kJ/mol.
3.2 The influences on separating zirconium and hafnium with D2EHPA
3.2.1 The influence of HCl concentration on the separation of hafnium and zirconium
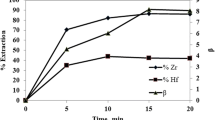
The effect of HCl content on the separation of hafnium from zirconium using 0.01 mol/L D2EHPA was examined in the 0.05–0.13 mol/L HCl range. In these programs, the initial concentrations of them were 0.001 mol/L.
The data are displayed in Fig. 4; the extraction performances for them increased with decreasing HCl amount. This is ascribed to the fact that D2EHPA gets more dissociated at low acidities. It is consistent with a cation exchange extraction reaction. The separation factor also showed a similar variation. So, a maximum separation factor value of 4.16 was obtained from 0.05 mol/L HCl solutions by using 0.01 mol/L D2EHPA in octane.
3.2.2 The influence of D2EHPA concentration on the separation of hafnium and zirconium
The extractant concentration is very important in separating them. According to the above results, a HCl concentration of 0.05 mol/L is a good index for separating them using D2EHPA. Hence, the effect of D2EHPA amount on separating them was studied with 0.05 mol/L hydrochloric acid solutions.
In Fig. 5, the D of the two metals increases with increasing D2EHPA concentration. In the meantime, the separation factors also increase. When the D2EHPA concentration was 0.01 mol/L, the separation factors reached a maximum. At this point, the percent extraction of hafnium reached above 90%; a further increase in the D2EHPA concentration will increase zirconium extraction, which will decrease the separation factor. So, a maximum separation factor value of 4.16 was obtained from a 0.05 mol/L HCl solution by using 0.01 mol/L D2EHPA in octane.
3.2.3 The influence of diluents on separation of zirconium and hafnium
Solvent effect was researched using various diluents; the experimental conditions featured 0.05 mol/L HCl and 0.01 mol/L D2EHPA. The results clearly show that the separation factors vary with different diluents and followed the order in Table 1:
As the polarity of the diluents increased, the extraction abilities for zirconium and hafnium increased. However, the separation factors decreased in the same conditions.
3.2.4 The influence of initial metal concentration on separation of zirconium and hafnium
The effects of initial metal amounts on the separation of hafnium and zirconium using 0.01 mol/L D2EHPA in octane were studied.
As shown in Table 2, the ratio of hafnium gradually decreases in the aqueous phase and the values of D decrease when the addition of zirconium is increased. However, according to the results, hafnium is abundant in the organic phase. Under the last conditions from Table 2, the proportion of hafnium in the organic phase is 11% higher than that in the aqueous phase. So, that was of considerable application significance.
3.3 Stripping studies
The stripping of both of them from the loaded organic phase was achieved with different acidities in the aqueous solution. The metals loaded with D2EHPA were extracted from 0.05 mol/L HCl solutions using 0.01 mol/L D2EHPA, and the amounts of zirconium and hafnium in the D2EHPA phase were 7.5 × 10−4 mol/L and 9.25 × 10−4 mol/L.
In Table 3, the stripping percentages of hafnium and zirconium increase with increasing acid concentration. This is ascribed to the fact that the extraction mechanism is changed. Zr4+ and Hf4+ are the predominant species in high-acidity aqueous solutions, and the stripping of zirconium is more difficult than that of hafnium. It can enhance the separation of hafnium and zirconium by stripping in 1-4 mol/L HCl solutions.
3.4 IR spectra of zirconium and hafnium–D2EHPA complex
The IR spectra of zirconium and hafnium with D2EHPA were obtained. In Fig. 6, D2EHPA shows an absorption band at 2337 cm−1; it belongs to the extractant’s characteristic P–O–H group. The band at 1235 cm−1 is the P=O functional group. In the spectra of the zirconium–D2EHPA complex and the hafnium–D2EHPA complex, the bands at 2337 cm−1 become weak. These suggest that the hydrogen ion of P–O–H is replaced by two metals, which agrees with the cation exchange mechanism. Also, bands at 1225 cm−1 appear, which indicates that the P=O could complex with two metals.
4 Conclusion
Hafnium was preferentially extracted over zirconium by D2EHPA in octane from hydrochloric acid at lower concentrations. The extracted species were ZrOA2·2HA and HfOA2·2HA. The extraction was an endothermic reaction. An optimum separation factor value of 4.16 was obtained from a solution containing 0.05 mol/L HCl by using 0.01 mol/L D2EHPA. In this process, the separation factors varied with different diluents and followed the order octane > hexane > toluene > chloroform. Advantages of the new process are lower necessary acid and extractant concentrations. The extraction system is of considerable application significance in the preferential extraction of hafnium.
References
Z. Xu, L. Wang, M. Wu, Y. Xu et al., Separation of zirconium and hafnium by solvent extraction using mixture of DIBK and P204. Hydrometallurgy 165, 275–281 (2016). https://doi.org/10.1016/j.hydromet.2016.01.032
Z.-G. Xu, L. Wang, Y.-K. Wu et al., Solvent extraction of hafnium from thiocyanic acid medium in DIBK-TBP mixed system. Trans Nonferr. Met. Soc. 22, 1760–1765 (2012). https://doi.org/10.1016/S1003-6326(11)61384-8
M. Smolik, A. Jakóbik-Kolon, M. Porański, Separation of zirconium and hafnium using Diphonix® chelating ion-exchange resin. Hydrometallurgy 95, 350–353 (2009). https://doi.org/10.1016/j.hydromet.2008.05.010
L.Y. Wang, M.S. Lee, Development of a separation process for the selective extraction of hafnium(IV) over zirconium(IV) from sulfuric acid solutions by using D2EHPA. Hydrometallurgy 160, 12–17 (2016). https://doi.org/10.1016/j.hydromet.2015.11.013
L.Y. Wang, M.S. Lee, A review on the aqueous chemistry of Zr(IV) and Hf(IV) and their separation by solvent extraction. J. Ind. Eng. Chem. 39, 1–9 (2016). https://doi.org/10.1016/j.jiec.2016.06.004
B. Gupta, P. Malik, N. Mudhar, extraction and recovery of zirconium from zircon using Cyanex 923. Solv. Extr. Ion Exch. 23, 345–357 (2005). https://doi.org/10.1081/SEI-200050005
L.Y. Wang, H.Y. Lee, M.S. Lee, Solvent extractive separation of zirconium and hafnium from hydrochloric acid solutions by organophosphorous extractants and their mixtures with other types of extractants. Chem. Eng. Commun. 202, 1289–1295 (2015). https://doi.org/10.1080/00986445.2014.921621
M.S. Lee, R. Banda, S.H. Min, Separation of Hf(IV)–Zr(IV) in H2SO4 solutions using solvent extraction with D2EHPA or Cyanex 272 at different reagent and metal ion concentrations. Hydrometallurgy 152, 84–90 (2015). https://doi.org/10.1016/j.hydromet.2014.12.005
L.Y. Wang, M.S. Lee, Separation of Zr and Hf from sulfuric acid solutions with amine-based extractants by solvent extraction. Sep. Purif. Technol. 142, 83–89 (2015). https://doi.org/10.1016/j.seppur.2015.01.001
R. Banda, M.S. Lee, Solvent extraction for the separation of Zr and Hf from aqueous solutions. Sep. Purif. Rev. 44, 199–215 (2015). https://doi.org/10.1080/15422119.2014.920876
M. Aliakbari, K. Saberyan, M. Noaparast et al., Separation of hafnium and zirconium using TBP modified ferromagnetic nanoparticles: effects of acid and metals concentrations. Hydrometallurgy 146, 72–75 (2014). https://doi.org/10.1016/j.hydromet.2014.03.002
M. Taghizadeh, M. Ghanadi, E. Zolfonoun, Separation of zirconium and hafnium by solvent extraction using mixture of TBP and Cyanex 923. J. Nucl. Mater. 412, 334–337 (2011). https://doi.org/10.1016/j.jnucmat.2011.03.033
G. Pandey, S. Mukhopadhyay, A.U. Renjith et al., Recovery of Hf and Zr from slurry waste of zirconium purification plant using solvent extraction. Hydrometallurgy 163, 61–68 (2016). https://doi.org/10.1016/j.hydromet.2016.03.005
B.R. Reddy, J.R. Kumar, Studies on liquid–liquid extraction of tetravalent hafnium from weakly hydrochloric acid solutions by LIX 84-IC. Sep. Purif. Technol. 42, 169–174 (2005). https://doi.org/10.1016/j.seppur.2004.07.010
J.S. Gaudh, V.M. Shinde, Analytical separation of titanium(IV), zirconium(IV) and hafnium(IV) using tris(2-ethylhexyl)phosphate as an extractant. Anal. Lett. 28, 1107–1125 (1995). https://doi.org/10.1080/00032719508002682
J. Kumar, B. Reddy, J. Koduru et al., Liquid-liquid extraction of tetravalent hafnium from acidic chloride solutions using bis(2,4,4-trimethylpentyl) dithiophosphinic acid (Cyanex 301). Sep. Sci. Technol. 42, 865–877 (2007). https://doi.org/10.1080/01496390601173986
R. Banda, H.Y. Lee, M.S. Lee, Separation of Zr from Hf in hydrochloric acid solution using amine-based extractants. Ind. Eng. Chem. Res. 51, 9652–9660 (2012). https://doi.org/10.1021/ie3008264
S. Chen, Z. Zhang, S. Kuang et al., Separation of zirconium from hafnium in sulfate medium using solvent extraction with a new reagent BEAP. Hydrometallurgy 169, 607–611 (2017). https://doi.org/10.1016/j.hydromet.2017.04.001
R. Banda, H.Y. Lee, M.S. Lee, Separation of Zr from Hf in acidic chloride solutions by using TOPO and its mixture with other extractants. J. Radioanal. Nucl. Ch. 298, 259–264 (2013). https://doi.org/10.1007/s10967-012-2349-y
B. Ramachandra Reddy, J. Rajesh Kumar, K. Phani Raja et al., Solvent extraction of Hf(IV) from acidic chloride solutions using Cyanex 302. Miner. Eng. 17, 939–942 (2004). https://doi.org/10.1016/j.mineng.2004.04.004
R.G. Pearson, Hard and soft acid and bases. J. Am. Chem. Soc. 85, 3533–3539 (1963). https://doi.org/10.1016/B978-0-12-395706-1.50007-8
A. Boussaha, J.C. Abbe, A. Haessler, Influence of the acidity on the polymerisation of Hf(IV) in aqueous solutions of HfOCl2 observed by the time differential perturbed angular correlation technique. J. Inorg. Nucl. Chem. 39, 853–855 (1977). https://doi.org/10.1016/0022-1902(77)80168-1
R.K. Biswas, M.A. Hayat, Solvent extraction of zirconium(IV) from chloride media by D2EHPA in kerosene. Hydrometallurgy 63, 149–158 (2002). https://doi.org/10.1016/S0304-386X(01)00220-1
L.Y. Wang, M.S. Lee, Separation of zirconium and hafnium from nitric acid solutions with LIX 63, PC 88A and their mixture by solvent extraction. Hydrometallurgy 150, 153–160 (2014). https://doi.org/10.1016/j.hydromet.2014.10.009
H. Lee, S. Gyu Kim, J. Kee Oh, Stoichiometric relation for extraction of zirconium and hafnium from acidic chloride solutions with Versatic Acid 10. Hydrometallurgy 73, 91–97 (2004). https://doi.org/10.1016/j.hydromet.2003.08.004
M. Taghizadeh, R. Ghasemzadeh, S.N. Ashrafizadeh et al., Determination of optimum process conditions for the extraction and separation of zirconium and hafnium by solvent extraction. Hydrometallurgy 90, 115–120 (2008). https://doi.org/10.1016/j.hydromet.2007.10.002
B.V. Pershina, D. Trubert, C. Le Naour et al., Theoretical predictions of hydrolysis and complex formation of group-4 elements Zr, Hf and Rf in HF and HCl solutions. Radiochim. Acta 90, 869–877 (2002). https://doi.org/10.1524/ract.2002.90.12_2002.869
B. Reddy, R.K. Jyothi, A. Reddy, Solvent extraction of tetravalent hafnium from acidic chloride solutions using 2-ethyl hexyl phosphonic acid mono-2-ethyl hexyl ester (PC-88A). Miner. Eng. 17, 553–556 (2004). https://doi.org/10.1016/j.hydromet.2003.07.002
X.-J. Peng, Y. Cui, J.-F. Ma et al., Extraction of lanthanide ions with N, N, N′, N′-tetrabutyl-3-oxa-diglycolamide from nitric acid media. Nucl. Sci. Tech. 28, 87 (2017). https://doi.org/10.1007/s41365-017-0229-4
Author information
Authors and Affiliations
Corresponding authors
Additional information
This work was supported by the Natural Science Foundation of Shandong Province (No. ZR201702160381).
Rights and permissions
About this article
Cite this article
Yang, T., Sun, GX., Qian, Y. et al. Separation of hafnium from zirconium in hydrochloric acid solution with di(2-ethylhexyl)phosphoric acid by solvent extraction. NUCL SCI TECH 30, 22 (2019). https://doi.org/10.1007/s41365-019-0548-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41365-019-0548-8