Abstract
Elemental calcium plays an important role in human physiology. In order to study the relationship between Ca-intake, Ca-chemical formulation, and Ca-absorptivity, a balance experiment using a 41Ca tracer technique in SD rats was conducted to measure the endogenous fecal calcium and true absorption of calcium. Apparent absorption of calcium was measured as a control to the endogenous calcium labeling experiment. These results show that by using 41Ca labeled endogenous calcium in vivo, researchers could obtain the true calcium absorption data without extrinsic labeling. Therefore, the method was not affected by the chemical structure or type of calcium supplement and might be used in evaluating the absorptivity of marketed calcium supplements.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The important role played by calcium in mammalian organisms is now well recognized. Calcium is the major cation within bone mineral. Calcium also plays an important role as an intracellular messenger in many systems and cells [1, 2]. Although calcium is a microelement closely related to the health of the human body, the average actual calcium intake in China is, on average, 405 mg/d, which is only approximately 50% of the recommended dietary allowance [3, 4]. The calcium deficiency situation is especially serious in children, adolescents, and pregnant women, as reported by the Third National Nutritional Investigation in China (1992). However, recent studies have shown that some diseases may be caused by excess calcium supplementation (especially in patients with osteoporosis) [5,6,7,8]. Therefore, the accuracy of calcium absorptive measurements is very meaningful for reasonable calcium supplementation and prevention of diseases caused by calcium metabolism. Due to the poor distinction between calcium sources, lower measurement sensitivity, and radioactive damage that results from traditional calcium absorptivity measurement techniques, it is difficult to carry out accurate, systematic, and long-term research on this topic [9,10,11].
A common method of determining calcium absorptivity is using the relation ζ = (I − F)/I, where ζ is the apparent calcium absorptivity, I is the calcium intake, and F is the calcium content in feces. However, F can be divided into two parts according to the relation F = Fi + Fe, where Fi is calcium of dietary origin and Fe is calcium of endogenous origin [12,13,14]. Thus, ζ cannot be used to replace calcium absorptivity accurately. We used a more reliable relation, η = (I − Fi)/I = (I − F + Fe)/I, to measure the calcium absorptivity of rats, where η is the true calcium absorptivity. The primary difficulty in the determination is the measurement of Fe. A tracer should be used to label the calcium of endogenous origin and calculate the proportion of Fe in F. Because the radiation dose is small and it is not present in biological systems, 41Ca can be used for biological tracer studies [15,16,17]. Currently, AMS (accelerator mass spectrometry) is the most effective method of measuring 41Ca. Based on the high sensitivity of 41Ca measurements made using CIAE-AMS and the innovative method of labeling endogenous calcium with 41Ca, the calcium absorptivity of rats was studied in this work.
2 Experimental section
2.1 Animals treatment and 41Ca tracing
41Ca tracing and AMS were used to monitor the changes in the skeletal metabolism of rats by labeling endogenous calcium with 41Ca. A total of 48 adult male SD rats (provided by Vital River Laboratories) were caged for one week to allow them to acclimate to the laboratory environment and then fed a Ca2+-poor basic diet (low Ca2+ content of about 0.03 g/kg; the feed can be regarded as calcium-free) with deionized water ad libitum for 2 weeks to deplete their Ca2+ storage in vivo. The animals were then randomly divided into 4 groups of 12, and according to their body weight, groups A, B, and C were injected intramuscularly with a solution of CaCl2 with 41Ca (54 ng) while group D was injected intramuscularly with a solution of CaCl2 without 41Ca to establish a control group.
The animals were kept in polyethylene cages by group and provided with deionized water ad libitum. The methods of rearing and sampling are shown in Table 1.
Groups A and B were compared to study the influence of different chemical forms of calcium supplements on the absorption rate. The rats were fed with different doses of calcium carbonate and calcium citrate (70 and 11 mg) to create equivalent daily calcium contents (per 100 g rat). Groups A and C were used to compare the influence of calcium supplements dose on the absorption rate.
After the injection of 41Ca, daily stool samples were collected and the ratios of 41Ca to 40Ca in fecal samples were prepared for AMS measurement.
2.2 Samples preparation
Potassium-41 is the major source of interference in the AMS measurement of 41Ca. Injection of CaF3− into the measurement system provides a 104-greater suppression of the 41K interference than CaF− [18, 19]. Therefore, the calcium content of the fecal samples of the rats was converted to CaF2 and CaF3−, which were then extracted for AMS measurement. The procedures used for the separation of calcium from stool samples were similar to those in previous work [20, 21], with minor modifications. The ion exchange process was removed, and the recovery rate of Ca increased to over 95%. The CaF2 was then mixed with PbF2 powder (mass ratio 1:4) and pressed into an Al sample holder for AMS measurements.
2.3 Measurement method
Detailed AMS conditions for 41Ca measurements are described elsewhere [22]. Briefly, the 41CaF3− ions were selected by the injection system and injected into the accelerator. The negative molecular ions were accelerated by tandem terminal voltage (8.27 MV). Stripping foil (5 μg/cm2) was employed to break up the molecular ions and to produce atomic ions with high charge states. The resulting positively charged ions were further accelerated at the same terminal voltage. A 90° double focusing high energy analyzing magnet was used to select 41Ca7+ with an energy of 61.39 MeV. After a switching magnet, the 41Ca7+ was transported to the AMS beam line, and then the 41Ca7+ was selected using a 15o electrostatic detector and finally detected with an ionization chamber. The 41Ca optical guidance in the accelerator was simulated with a 40Ca beam using the magnetic rigidity of 41Ca.
The samples were disposed of by ashing in a muffle (the sample was prepared using an identical chemical procedure to that of the 41Ca sample) and then processed in a Type AA700CRT atom absorption spectrophotometer (Perkin ELMER, Singapore) to determine the Ca2+ contents in the feces. The results of the measurement and a linear fitting curve of standard samples are shown in Fig. 1.
A linear fit is obtained in Fig. 1:
where y is the calcium content, x is the absorbance, and the linear regression coefficient was R2 = 0.9973; this equation was used to calculate the calcium content in the actual sample.
3 Results and discussion
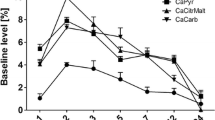
Figure 2 shows the ratio of 41Ca to 40Ca in the rat vs feces after injecting 41Ca. The ratio of 41Ca to 40Ca in feces tended to reach equilibrium on the 28th day after injection. As a result, the average value of 41Ca/40Ca in feces can be used to represent the actual value from the 35th to 49th day (including the 42nd day) after injection. On the 42nd day after injection, the rats were exclusively fed a Ca2+-poor basic diet and deionized water to obtain the ratio of 41Ca to 40Ca in feces without calcium intake. This value can be used as the 41Ca/40Ca ratio of endogenous origin.
The calcium absorption rate of each rat was replaced by the absorption rate of calcium per 100 g of rat to account for the differences in weight between rats. The results of these measurements are shown in Table 2.
Using these data, we obtained the following results in Table 3.
4 Conclusion
Generally, the actual calcium absorptivity is significantly higher than the apparent calcium absorptivity in rats (p < 0.05). Based on the actual calcium absorptivity data, the absorption rates of calcium carbonate and calcium citrate are approximately equal at the same dose, indicating that the calcium absorption rate in SD rats is not influenced by the chemical formula of calcium supplements. On the other hand, the amount of absorbed calcium in the intestine depended on the calcium intake. When intake is low, active transcellular calcium transport in the duodenum is upregulated, and a larger proportion of calcium is absorbed by the active process than by the passive paracellular process that prevails in the jejunum and ileum. Bioavailability of the calcium source—digestibility and solubilization—plays a role under low calcium intake conditions but is relatively unimportant when calcium intake is high. Our experimental results are consistent with this theory within a certain range: the greater the calcium intake, the lower the calcium absorption rate, and small doses of calcium can be nearly completely absorbed. This match between theory and experiment validates the method of measuring calcium absorptivity by 41Ca labeling endogenous calcium.
In conclusion, a monitoring method for endogenous calcium absorptivity using rats and 41Ca tracing was developed based on CIAE-AMS, and encouraging results were obtained. The method is currently restricted by factors such as limitations in biological diversity and sample size. A more detailed experimental program is being developed, and further exploration will be implemented in the near future.
References
J. Purkiss, M. Welch, S. Doward et al., Capsaicin-stimulated release of substance P from cultured dorsal root ganglion neurons: involvement of two distinct mechanisms. Biochem. Pharmacol. 59, 1403–1406 (2000). https://doi.org/10.1016/S0006-2952(00)00260-4
Y.P. Xu, J.W. Zhang, L. Li et al., Complex regulation of capsaicin on intracellular second messengers by calcium dependent and independent mechanisms in primary sensory neurons. Neurosci. Lett. 517, 30–35 (2012). https://doi.org/10.1016/j.neulet.2012.04.011
J. Wang, Advance in the research of calcium in the prevention and treatment of osteoprosis. Chin J. Clin. Nutr. 12, 213–217 (2004). https://doi.org/10.3760/cma.j.issn.1674-635X.2004.03.016
Z.G. Wang, Preliminary discussion of related problems on supplemental calcium. Adverse Drug React. J. 8, 326–329 (2006). https://doi.org/10.3969/j.issn.1008-5734.2006.05.002. (in Chinese)
M.J. Bolland, P.A. Barber, R.N. Doughty et al., Vascular events in healthy older women receiving calcium supplementation: randomised controlled trial. BMJ 336, 262–266 (2008). https://doi.org/10.1136/bmj.39440.525752.be
M.J. Bolland, A. Avenell, J.A. Baron et al., Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta-analysis. BMJ 341, c3691 (2010). https://doi.org/10.1136/bmj.c3691
M.R. Tadross, I.E. Dick, D.T. Yue, Mechanism of local and global Ca2+ sensing by calmodulin in complex with a Ca2+ channel. Cell 133, 1228–1240 (2008). https://doi.org/10.1016/j.cell.2008.05.025
G.N. Farhat, A.B. Newman, K.S. Tyrrell et al., The association of bone mineral density measures with incident cardiovascular disease in older adults. Osteoporosis Int. 18, 999–1008 (2007). https://doi.org/10.1007/s00198-007-0338-8
J.R. Southon, M.S. Bishop, G.J. Kost, 41Ca as a tracer for calcium uptake and deposition in heart tissue during ischemia and reperfusion. Nucl. Instrum. Method B. 92, 89–491 (1994). https://doi.org/10.1016/0168-583X(94)96060-7
H. Gu, S.R. Shi, L.L. Chang et al., Safety evaluation of daidzein in laying hens: part II. Effects on calcium-related metabolism. Food Chem. Toxicol. 55, 689–692 (2013). https://doi.org/10.1016/j.fct.2012.12.064
H.T. Shen, S. Jiang, M. He, Research on analysis methods for calcium absorptivity. Chin J. Osteoporosis Bone Miner Res. 2, 59–64 (2009). https://doi.org/10.3969/j.issn.1674-2591.2009.01.011. (in Chinese)
T. Matsumoto, T. Takano, S. Yamakido et al., Comparison of the effects of eldecalcitol and alfacalcidol on bone and calcium metabolism. J. Steroid Biochem. 121, 261–264 (2010). https://doi.org/10.1016/j.jsbmb.2010.03.035
S.P.H.T. Freeman, B. Beck, J.M. Bierman et al., The study of skeletal calcium metabolism with 41Ca and 45Ca. Nucl. Instrum. Method B. 172, 930–933 (2000). https://doi.org/10.1016/S0168-583X(00)00341-4
C.S. Kovacs, Calcium, phosphorus, and bone metabolism in the fetus and newborn. Early Hum. Dev. 91, 623–628 (2015). https://doi.org/10.1016/j.earlhumdev.2015.08.007
D. Elmore, M.H. Bhattacharyya, N.S. Gibson et al., Calcium-41 as a long-term biological tracer for bone resorption. Nucl. Instrum. Method B. 52, 531–535 (1990). https://doi.org/10.1016/0168-583x(90)90471-6
T.S. Rogers, M.G. Garrod, J.M. Peerson et al., Is bone equally responsive to calcium and vitamin D intake from food vs. supplements? Use of 41calcium tracer kinetic model. Bone Rep. 5, 117–123 (2016). https://doi.org/10.1016/j.bonr.2016.05.001
S.K. Hui, J. Prior, Z. Gelbart et al., A pilot study of the feasibility of long-term human bone balance during perimenopause using a 41Ca tracer. Nucl. Instrum. Method B. 259, 796–800 (2007). https://doi.org/10.1016/j.nimb.2007.02.003
C. Vockenhuber, T.S. König, H.A. Synal et al., Efficient 41Ca measurements for biomedical applications. Nucl. Instrum. Method B. 361, 273–276 (2015). https://doi.org/10.1016/j.nimb.2015.05.014
X.L. Zhao, A.E. Litherland, J. Eliades et al., Partial fragmentation of at low MeV energies and its potential use for 41Ca measurement. Nucl. Instrum. Method B. 294, 369–373 (2013). https://doi.org/10.1016/j.nimb.2012.01.046
L. Dou, M. He, K.J. Dong et al., Preliminary study of 41Ca-AMS measurement in rock samples. Atomic Energy Sci. Technol. 47, 2322–2326 (2013). https://doi.org/10.7538/yzk.2013.47.12.2322. (in Chinese)
H.T. Shen, F.F. Pang, S. Jiang et al., Study on 41Ca-AMS for diagnosis and assessment of cancer bone metastasis in rats. Nucl. Instrum. Method B. 361, 643–648 (2015). https://doi.org/10.1016/j.nimb.2015.05.034
M. He, X.D. Ruan, S.L. Wu et al., 41Ca analysis using CaF− in CIAE–AMS system. Nucl. Instrum. Method B. 268, 804–806 (2010). https://doi.org/10.1016/j.nimb.2009.10.035
Acknowledgements
We wish to thank experimentalist Lu Zheng from Beijing Union University for her help with the 41Ca biological tracer.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by the National Nature Science Foundation of China (No. 11375272).
Rights and permissions
About this article
Cite this article
Zhang, H., Dou, L., Wang, XM. et al. Methodological study on endogenous calcium absorptivity using rats and 41Ca tracing. NUCL SCI TECH 29, 56 (2018). https://doi.org/10.1007/s41365-018-0387-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41365-018-0387-z