Abstract
Proliferation of citrus canker disease caused by Xanthomonas citri subsp. citri (Xcc) has increasingly become a serious threat and has resulted in a significant loss in citrus production worldwide. This research aimed to identify efficient antagonistic bacteria for the biological control of canker disease in Citrus aurantifolia cultivar Pan (Pan-lime) and their antagonistic characteristics by experimental analysis. With the dual culture method, twenty isolates inhibited Xcc. Eight out of over fifty isolates showed clear inhibition zones and were further analyzed for plant growth-promoting characteristics. After the screening process, Bacillus velezensis isolate SWUA08 and Pseudomonas aeruginosa isolate SWUC02 were selected for further analysis of bacterial canker resistance in Pan-lime seedlings and trees. Experimental results show that both antagonists increased canker disease resistance. Furthermore, their cell-free cultures reduced canker disease severity index in Pan-lime tree. Our experimental results demonstrating that the antagonists offer an alternative perspective to evaluate a method of canker disease inhibition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Citrus canker disease is prevalent in citrus fruit worldwide. Xanthomonas citri subsp. citri (Xcc), which is the causative agent of citrus canker, invades citrus host plant mainly through stomata, hydathode opening and wounds (Ference et al. 2018). When Xcc enters and colonizes to apoplast, it induces cellular hyperplasia. The proliferation of Xcc at infected regions causes raised necrotic corky lesion. This canker symptom can be found in the leaves, stem and fruit surface. Xcc is dispersed from tree to tree mainly by strong wind and rain. Xcc is capable of producing biofilms, making it attaching to plant surfaces efficiently (De Oliveira et al. 2016). Prematurely falling leaves and fruits and dieback caused by this disease results in economic loss (Das 2003).
Citrus aurantifolia or lime, a major economic fruit in Thailand, faces a serious threat of declining crop yield due to citrus canker. The lime cultivar Pan or Pan-lime is the most popular lime in Thailand because of its aroma; however, this cultivar is severely susceptible to canker disease. Bactericides, such as copper oxychloride and copper hydroxide, are the typical method of control for this disease. However, the use of bactericides results in chemical residues on the fruit and in the environment as well as chemical-resistant Xcc (Behlau et al. 2011). Copper-resistant Xcc contains plasmid-borne cluster genes, namely, copL, copA and copB (Behlau et al. 2011; Richard et al. 2017a, b). This plasmid can be transferred by conjugation to copper-susceptible Xcc strain in nature, leading to the control failure. Therefore, for controlling citrus canker, an integrated approach is suggested. For example, the use of copper spray combined with windbreak or with acibenzolar-S-methyl (a systemic disease resistance inducer) has been found to be more efficient (Ference et al. 2018). Growing resistant cultivars is an alternative to avoid this problematic disease. Unfortunately, resistant cultivars (e.g., Tahiti-lime) have diminished aroma compared to Pan-lime, thus lowering the market demand (Das 2003). Currently, the control of canker disease with biocontrol agents is a better strategy because it is safe for both consumers and the environment and usually does not have side effects on the quality of the fruit.
The use of microbial antagonists to control citrus canker disease is currently an alternative technology. This is because the antagonists show characteristics that effectively challenge Xcc. For example, Pseudomonas protegens CS1 produces pyochelin, a type of siderophore, to inhibit Xcc, and it can trigger reactive oxygen species (ROS) in plants to combat pathogens (Michavila et al. 2017). Bacillus subtilis TKS1-1 and Bacillus amyloliquefaciens WG6-14 can inhibit Xcc in the colonization process (Huang et al. 2012). Pseudomonas chlororaphis PA23 produces HCN to suppress phytopathogens by obstruction of electron transport chain activity, leading to cell death (Nandi et al. 2017).
In this study, we aimed to identify antagonistic bacteria that efficiently control canker disease in Pan-lime and study their antagonistic characteristics. We validated the effectiveness of antagonists on canker disease control in the seedling stage to ensure that the antagonist is safe to use even in the weakest plant conditions and also in the stage of fully grown nature tree in their natural environment.
Materials and methods
Isolation of the lime canker pathogen
There are two sources of isolates that we used in this research: Xcc strain from the Department of Agriculture (DOA), Ministry of Agriculture and Cooperatives, Thailand and a strain isolated from an infected lime as a representative of the present strain pervasive in Thailand. Leaves and sticks of C. aurantifolia cultivar Pan (Pan-lime) with the canker lesion were used to isolate Xcc. The leaves and sticks were washed with tap water and cut to retain the area with the lesion. For surface sterilization, the cut leaves and sticks were soaked in 0.25% NaOCl for 5 min and then washed with sterile water three times. The cut leaves and sticks were minced in 5 mL of sterile 0.85% NaCl solution, and the suspension was spread on mTMB medium (McGuire et al. 1986). A yellow colony surrounded by a white crystalline halo was obtained from mTMB medium and streaked onto Xan-D medium (Lee et al. 2009), both of which are Xanthomonas selective media. A yellow–green colony surrounded by white crystal and a wide clear zone was selected for further species identification by 16S rDNA sequencing.
Colony PCR was used to screen Xcc with primers. As recommended by the International Standards for Phytosanitary Measures (ISPM) (2014), two primer pairs specific to the hrpW gene (Park et al. 2006), and ITS regions of 16S and 23S rDNAs (Cubero and Graham 2002) were used. The cell suspension of the isolate was swabbed onto Pan-lime seedlings with a pin lesion on leaves where the canker symptom appeared within 10-20 days. The control in our experiments was obtained from the DOA and is denoted by XccD.
Isolation of bacterial Xcc antagonists
Canker-infected trees were used to screen for antagonistic bacteria potentially capable of colonizing lime plants. The leaves were surface sterilized and minced as described above. The suspension was spread on tryptone soya agar (TSA). After incubation at 28 °C for 24 h, the colony of the isolate was selected and restreaked in TSA. Traditionally, bacterial antagonists are screened from healthy trees. However, in our case, we hypothesized that the isolate and Xcc would form strongly asymmetrical competitive interactions (amensalism).
Inhibition assay
To test whether the isolates could inhibit Xcc in vitro, a dual culture method was performed. The TSA plate was swabbed with the isolated Xcc at 108 CFU/mL. Then, the plate was punched with a cork borer. To each well, 100 µL of 108 CFU/mL of an isolate in tryptone soya broth (TSB) was added. TSB without an isolate was used as a negative control. After incubation at room temperature for 24 h, the inhibition zone was calculated by subtracting the diameter of the clear zone from its colony size.
The agar well diffusion assay was used to study whether extracellular metabolites of the isolates could inhibit Xcc in vitro. One hundred microliters of cell-free culture filtrate was added into a well. The isolates were cultured in TSB in both conditions, with and without (0.01%) CuCl2, at 28 °C with agitation at 100 rpm for 3 days, and the cell suspension was then centrifuged at 9000 × g for 10 min at 4 °C. The culture broth was filtered through a membrane filter with a 0.45 µm pore size, and then the cell-free culture filtrate (cell-free culture) was immediately used for the agar well diffusion assay. The agar well plate was incubated at room temperature for 24 h. Both the dual culture test and agar well diffusion assay were repeated three times.
Plant growth-promoting characteristics of the isolates
The isolates were further tested for plant growth-promoting characteristics. First, the isolates were cultured on both media: DF medium without a nitrogen source and DF-ACC medium containing 0.25 mM ACC as the sole nitrogen source (Jacobson et al. 1994). Nitrogen-fixing bacteria can easily grow on both media; however, ACC deaminase-producing bacteria can grow only on DF-ACC medium. The isolate was also cultured on Pikovskaya’s (PVK) agar and chrome azurol S (CAS) agar to test for their phosphate-solubilization and siderophore-production capabilities, respectively. Finally, the IAA and HCN production of the isolates was tested by the methods of Sasirekha et al. (2012) and Castric (1975), respectively.
Effect of the isolates on Pan-lime seedling growth
To test the effect of the isolates on plant growth, we used Pan-lime seedlings that grew in tissue culture conditions to eliminate other external factors while preserving sufficient nutrition. First, the lime seeds were surface sterilized with 0.25% NaOCl and washed with sterile water. The seeds were placed on Murashige and Skoog (MS) medium, one seed per bottle. Seedlings with 4–7 mature leaves were used and divided into five groups for inoculation with four selected isolates and one negative control. The selected isolates at 108 CFU/mL in TSB were swabbed on the leaves of lime seedlings. The leaves were punched by sterile needle, with 3 lesions per leaf. Sterile TSB was used as a negative control. The following scoring scheme was used to observe seedling health at 15 and 30 days post-inoculation (dpi). “0” indicated a healthy plant, “1” indicated seedlings with 1–2 leaves that showed yellowing, necrosis or defoliation, “2” indicated seedlings with > 3 leaves that showed yellowing, necrosis or defoliation, “3” indicated seedlings without healthy leaves, and “4” indicated dead seedlings.
Assessment of the antagonistic bacteria for canker disease control in lime seedlings
We assessed the potential of selected isolates to control canker disease in tissue culture conditions. The lime seedlings were divided into six groups: a negative control, a positive control and 4 isolates. Each seedling was inoculated twice. The first inoculation was with an isolate, and the second inoculation was with Xcc after 24 h of incubation. The isolates at 108 CFU/mL and Xcc at 106 CFU/mL were used to swab the punched leaves. The negative control was swabbed with sterile TSB twice, and the positive control was swabbed with sterile TSB and then inoculating with Xcc. Due to the effects of Xcc, a different scoring scheme was used to observe seedling health at 15 and 30 dpi. “0” indicated a healthy plant, “1” indicated seedlings with 1–2 leaves that showed yellowing, necrosis or defoliation, “2” indicated seedlings with raised corky lesions only, “3” indicated seedling with 1–2 leaves that showed raised corky lesions with yellowing, necrosis or defoliation, “4” and “5” were similar to 1 and 3, respectively, except that the symptoms appeared on the leaves more than 3 leaves, “6” indicated seedlings without healthy leaves, and “7” indicated a dead seedling.
Assessment of the antagonistic bacteria and their cell-free culture for canker disease control in lime tree
Pan-lime trees with approximately 1.2 meters high were used in this study. The tree was grown in a ten liters plant pot in outdoor condition. The lime trees were divided into ten groups. Each group was inoculated twice in order as follows: (1) Phosphate buffer saline (PBS) and PBS, (2) PBS and Xcc05, (3) SWUC02 and PBS, (4) SWUA08 and PBS, (5) SWUC02 and Xcc05, (6) SWUA08 and Xcc05, (7) cell-free culture from SWUC02 (CFSWUC02) and PBS, (8) cell-free culture from SWUA08 (CFSWUA08) and PBS, (9) CFSWUC02 and Xcc05 and (10) CFSWUA08 and Xcc05. Three young leaves per tree were used for the inoculation. The isolates at 108 CFU/mL and Xcc at 106 CFU/mL were used. There is a 24-hour interval between any two inoculations. The inoculated leaves were covered with clear plastic bag for two days. At 30 days after Xcc05 inoculation, canker lesions per leaf were count in stereo microscope. Disease severity index (DSI) was calculated by the number of canker lesions per leaf divided by leaf area (cm2) (Huang et al. 2012). The leaf area was measured by ImageJ analysis software version 1.52a (NIH, USA). Percentage of disease control efficacy (%CE) was calculated by % CE = [(DSI of positive control—DSI of treatment)/DSI of positive control] × 100.
Bacterial species identification
The isolates were identified by 16S rRNA and gyrase A (gyrA) gene sequencing analysis. Genomic DNA of the isolates was extracted and used as a template for PCR. The universal primers for the 16S rRNA gene, 27F and 1492R, and those of the gyrA gene, P-gyrA-F primer and P-gyrA-R primer, were used (Chun and Bae 2000). The PCR products were sent to Macrogen Company (Seoul, South Korea) for DNA sequencing analysis. Approximately 1300 nucleotide sequences of each isolate were compared for species against the similarity GenBank database and the EzTaxon database. The sequences of the isolates were used to construct a phylogenetic tree using the neighbor-joining method with the MEGA 7 program (Kumar et al. 2016). Bootstrap was replicated 1000 times. The 16S rDNA and gyrA sequences of isolates were deposited to GenBank database.
Statistical analysis
For experiments with lime seedlings, each treatment was applied to five seedlings, and the experiment was independently repeated three times. The disease severity score from treatments was subjected to a 95% confidence level with Kruskal–Wallis test and Mann–Whitney U test for multiple comparisons. For pot experiment, each treatment was applied to three trees and the experiment was repeated twice independently. The data in both experiments were pooled and analyzed by ANOVA. Tukey’s HSD was used for multiple comparisons at 95% confidence level.
Results
Screening of the bacterial pathogen and antagonists
We isolated Xcc from infected lime trees found in central Thailand and used them to evaluate the isolated antagonists. From infected fruits and leaves, seventeen yellow colonies surrounded by tiny white crystalline halos on mTMB and Xan-D media were obtained. Four of the seventeen were randomly selected to further screen for Xcc by colony PCR, denoted by isolate E02, E05, E08 and E15. Specific PCR products indicating the presence of the hrpW gene and the ITS region were obtained from all four isolates (Fig. 1a). Moreover, according to 16S rRNA gene analysis, the four isolates were 100% similar to X. citri subsp. citri strain AW12879T (GenBank Accession No. CP003778.1). Pathogenic capability was confirmed by inoculating healthy lime seedlings. All of the isolates induced canker symptoms on the seedlings (Fig. 1b), indicating that they were pathogenic citrus canker bacteria. We randomly chose Xcc isolate E05, denoted Xcc05, for subsequent analysis.
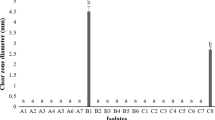
To screen bacterial antagonists, Xcc05 and XccD were used. Twenty out of fifty-five isolates inhibited XccD growth. However, eight of them, denoted SWUC02, SWUC04, SWUC08, SWUC14, SWUA08, SWUA18, SWUA23 and SWUA26, caused a clear inhibition zone against XccD in the dual culture test (Supplementary Table S1). SWUA08 had the highest antagonistic activity against XccD with an inhibition zone diameter of 30 ± 0.58 mm, followed by SWUC02 with the diameter of 20 ± 0.58 mm. Furthermore, the inhibition zones of SWUC02, SWUC04, SWUC08, SWUC14, SWUA08 and SWUA23 against Xcc05 were smaller than that of XccD. Isolation time may explain the difference in inhibition capability between XccD and Xcc05. The coevolution among bacterial pathogens, host plants and the environment might affect the virulence of Xcc05. Therefore, SWUC02, SWUA08, SWUA18 and SWUA26 were chosen for the in vivo inhibition test.
Inhibition of Xcc by cell-free culture
We found that the cell-free culture obtained from only SWUA08 could inhibit Xcc05 (16 ± 0.47 mm). Moreover, as shown in Supplementary Table S1, supplementing TSB with 0.01% CuCl2 enhanced the potential inhibition activity of both SWUA08 (18 ± 0.29 mm) and SWUC02 (23 ± 0.10 mm). These results indicated that SWUC02 and SWUA08 could produce extracellular metabolites against Xcc.
Plant growth-promoting characteristics
In addition to pathogen inhibition activity, we also validated the plant growth-promoting characteristics of the isolates. The results in Table 1 show that SWUC02 possesses all characteristics and expresses the highest ability to solubilize phosphate compared to other isolates. Moreover, only SWUC02 produced HCN, which is useful for the inhibition of pathogens. Although all of the isolates produced siderophores and IAAs, SWUA08 and SWUA18 showed relatively higher siderophore production. SWUA23 showed various plant growth-promoting abilities; however, it was not selected for subsequent in vivo study because of its inability to inhibit Xcc05 in vitro. The remaining isolates for subsequent studies were SWUC02, SWUA08, SWUA18 and SWUA26, all of which induced a clear inhibition zone against Xcc05 in vitro and had at least three plant growth-promoting characteristics.
Bacterial species identification
Based on 16S rDNA sequence analysis, SWUC02 and SWUA26 showed 100% identity to Pseudomonas aeruginosa JCM 5962T and Bacillus cereus ATCC 14579T, respectively. SWUA08 showed 99.85% similarity to Bacillus siamensis KCTC 13613T and Bacillus velezensis NRRL B-41580T, and SWUA18 showed 99.93% similarity to Bacillus subtilis subsp. inaquosorum KCTC 13429T and Bacillus tequilensis KCTC 13622T. The 16S rDNA sequences of SWUC02, SWUA08, SWUA18 and SWUA26 were deposited in GenBank under the accession numbers MN511761 MN511762, MN511763 and MN511764, respectively. For Bacillus SWUA08 and SWUA18, partial sequencing of the gyrA gene was required to identify the Bacillus species. Consequently, SWUA08 and SWUA18 were 98.88% and 99.12% similar to B. velezensis NRRL B-41580T and B. subtilis subsp. subtilis NCIB 3610T, respectively. Accession numbers of gyrA gene sequences of SWUA08 and SWUA18 are MN519795 and MN519796, respectively. In addition, the phylogenetic trees of 16S rDNA and gyrA are shown in Supplementary Figure S1.
Effect of the isolate on Pan-lime seedling growth
A common characteristic of an antagonist is that it is not pathogenic and has no phytotoxicity on plants. We performed experiments under tissue culture conditions to eliminate irrelevant factors such as malnutrition and other microbes. The disease severity score at 15 and 30 dpi of seedlings inoculated with P. aeruginosa SWUC02 was the same as that of uninoculated lime seedlings. B. cereus SWUA26 seemed to be an effective antagonist candidate when the score at 15 dpi was considered (Fig. 2a). However, the score increased significantly at 30 dpi, which disqualified it from being a good candidate. Both B. velezensis SWUA08 and B. subtilis SWUA18 had the highest phytotoxicity scores at both 15 and 30 dpi. Consequently, P. aeruginosa SWUC02 is the most likely candidate for suppressing canker disease in lime.
Assessment of the antagonistic bacteria for canker disease control in lime seedlings
The isolates were tested for whether they could control the canker disease caused by XccD or Xcc05. The results under tissue culture conditions from both XccD and Xcc05 infection were similar (Fig. 2b, c). Xcc-challenged seedlings that were preinoculated with P. aeruginosa SWUC02 showed a significantly lower disease severity score than XccD or Xcc05-infected seedlings, but the score was higher than that of the mock infection (Fig. 2b, c). Preinoculation of seedlings with B. velezensis SWUA08, B. subtilis SWUA18 and B. cereus SWUA26 did not reduce the score (the former two at both dpi and the latter at 30 dpi). Among other isolates, P. aeruginosa SWUC02 had the best performance against Xcc, even in the seedling stage.
Assessment of the antagonistic bacteria and their cell-free culture for canker disease control in lime tree
P. aeruginosa SWUC02 and B. velezensis SWUA08 were chosen to assess the potential of canker disease control in lime tree due to their capability to produce extracellular metabolites against Xcc05. We found that the use of B. velezensis SWUA08 did not impair plant health (Fig. 3d, g) in which the chlorotic and necrotic symptoms could not be observed on tested leaves. Figure 3g shows that inoculating either of the two antagonists prior to Xcc05 inoculation reduced disease severity index to the same level as the negative control. Furthermore, the use of cell-free cultures obtained from either P. aeruginosa SWUC02 or B. velezensis SWUA08 (CFSWUC02 and CFSWUA08, respectively) prior to Xcc05 inoculation reduced disease severity index significantly (P < 0.05). The disease control efficacies (%CE) obtained from the use of P. aeruginosa SWUC02, B. velezensis SWUA08, cell-free culture of P. aeruginosa SWUC02 and cell-free culture of B. velezensis SWUA08 prior to Xcc05 inoculation were 84%, 93%, 31% and 33%, respectively.
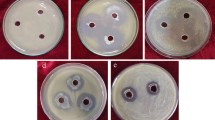
Inoculated lime leaves with a mock, bXcc05, c SWUC02 + Xcc05, d SWUA08 + Xcc05, e Cell-free culture obtained from SWUC02 (CFSWUC02) + Xcc05, f Cell-free culture obtained from SWUA08 (CFSWUA08) + Xcc05, all of which were observed under stereo microscope (× 12 magnification), and g disease severity index of lime tree at 30 dpi. The bars representing the means with SE
Discussion and conclusion
The bacteria in genera Bacillus and Pseudomonas, such as B. subtilis, B. amyloliquefaciens and P. protegens, were reported to efficiently control citrus canker disease in citrus plants (Huang et al. 2012; Michavila et al. 2017). In this study, we found that a variety of Bacillus species could efficiently inhibit Xcc in vitro, especially B. velezensis SWUA08, which produced extracellular metabolites against Xcc. Palazzini et al. (2016) reported that the genome of B. velezensis RC 218 contains several antibiotic genes, such as ericin, surfactin, iturin, fengycin, bacillibactin, bacilysin, amylocyclicin, macrolactin, bacillaene and difficidin. Despite being effective at inhibiting Xcc, full-blown proliferation of B. velezensis SWUA08 over lime seedlings showed a high phytotoxicity score on the plant. Supporting evidence for this finding demonstrated that the growth of the Bacillus spp. lowered the pH around plant roots (Leifert and Waites 1992). However, B. velezensis SWUA08 efficiently reduced canker disease severity in lime tree without observable phytotoxic effect on the plant. The reasons that B. velezensis SWUA08 is a potential antagonist suitable used for canker disease control in Pan-lime in the field condition are threefold. First, the trees in the maturity stage are stronger than those in the seedling stage. Second, population of B. velezensis SWUA08 under outdoor condition might be decreased so that it is no longer a danger to the lime tree. Third, B. velezensis (formerly B. amyloliquefaciens subsp. plantarum) is a Bacillus-based biofertilizer also known as biocontrol agent which is safe to be use in agriculture (Fan et al. 2018).
The antagonists in this study were chosen to efficiently inhibit pathogens and enhance plant growth. According to our results in vivo, P. aeruginosa SWUC02, with the most plant growth-promoting characteristics, reduced canker disease incidence in lime both seedlings and trees efficiently, possibly due to its ability to produce antibiotics such as phenazine-1-carboxylic acid, phenazine-1-carboxamide, organohalogen compound, pyocin and pyocyanin (Zhou et al. 2016; De Oliveira et al. 2016; Naz et al. 2015; El-Fouly et al. 2015). With the presence of CuCl2 in the culture medium, P. aeruginosa SWUC02 produced extracellular metabolites, which could specifically include a bioactive organometallic compound. With the presence of CuCl2 in the culture medium, P. aeruginosa SWUC02 produced extracellular metabolites, which could specifically include a bioactive organometallic compound. Gionco et al. (2017) reported that when P. aeruginosa LV was exposed to CuCl2, several genes involving in the biosynthetic pathway of organometallic compound were upregulated. Furthermore, some microorganisms such as bacteria and fungi can produce new secondary metabolites with antagonistic activity against other bacteria or parasite in culture medium supplemented with copper (Bedoya et al. 2019; De Oliveira et al. 2016; Fill et al. 2016). Organometallic compound produced by P. aeruginosa LV possesses high and broad antimicrobial activity against Xcc in Citrus sinensis cv. Valence under greenhouse condition (De Oliveira et al. 2016). This compound also could inhibit Xanthomonas arboricola pv. pruni in peach tree (Prunus persica L. Batsch) (Vasconcellos et al. 2014). However, this study combats canker disease using only cell-free culture obtained from P. aeruginosa SWUC02. An upside of cell-free culture helps avoid concern of P. aeruginosa being an opportunistic species.
In summary, P. aeruginosa SWUC02 and B. velezensis SWUA08 were efficient biocontrol agents for controlling citrus canker in Pan-lime, and their cell-free cultures were also marginally effective in reducing the disease. Both antagonists demonstrated the ability to produce, IAA and siderophores, solubilize phosphate and fix nitrogen to promote plant growth. The ability to produce HCN and extracellular metabolites against Xcc supports the potential use of extracellular metabolites of the antagonist to control the disease. Future study of mechanisms and relationship between plant and plant growth-promoting bacteria (PGPB), especially induce systemic resistance (ISR), favors the use of P. aeruginosa SWUC02 as it has a variety of plant growth-promoting characteristics and possesses no threat to seedlings even in tissue culture condition.
References
Bedoya JC, Dealis ML, Silva CS et al (2019) Enhanced production of target bioactive metabolites produced by Pseudomonas aeruginosa LV strain. Biocatal Agric Biotechnol 17:545–556
Behlau F, Canteros BI, Minsavage GV, Jones JB, Graham JH (2011) Molecular characterization of copper resistance genes from Xanthomonas citri subsp. citri and Xanthomonas alfalfae subsp. citrumelonis. Appl Environ Microbiol 77:4089–4096
Castric PA (1975) Hydrogen cyanide, a secondary metabolite of Pseudomonas aeruginosa. Can J Microbiol 21:613–618
Chun J, Bae KS (2000) Phylogenetic analysis of Bacillus subtilis and related taxa based on partial gyrA sequences. Ant van Leeuwen 78:123–127
Cubero J, Graham JH (2002) Genetic relationship among worldwide strains of Xanthomonas causing canker in citrus species and design of new primers for their identification by PCR. Appl Environ Microbiol 68:1257–1264
Das AK (2003) Citrus canker-a review. J Appl Hort 5:52–60
De Oliveira AG, Spago FR, Simionato AS et al (2016) Bioactive organocopper compound from Pseudomonas aeruginosa inhibits the grow of Xanthomonas citri subsp. citri. Front Microbiol 7:113
El-Fouly MZ, Sharaf AM, Shahin AAM, El-Bialy HA, Omara AMA (2015) Biosynthesis of pyocyanin pigment by Pseudomonas aeruginosa. J Radiat Res Appl Sci 8:36–48
Fan B, Wang C, Song X et al (2018) Bacillus velezensis FZB42 in 2018: the gram-positive model strain for plant growth promotion and biocontrol. Front Microbiol 9:2491
Ference CM, Gochez AM, Behlau F, Wang N, Graham JH, Jones JB (2018) Recent advances in the understanding of Xanthomonas citri ssp. citri pathogenesis and citrus canker disease management. Mol Plant Pathol 19:1302–1318
Fill TP, Pallini HF, Amaral LDS et al (2016) Copper and manganese cations alter secondary metabolism in the fungus Penicillium brasilianum. J Braz Chem Soc 27:1444–1451
Gionco B, Tavares ER, De Oliveira AG et al (2017) New insights about antibiotic production by Pseudomonas aeruginosa: a gene expression analysis. Front Chem 5:66
Huang TP, Tzeng DDS, Wong ACL et al (2012) DNA polymorphisms and biocontrol of Bacillus antagonistic to citrus bacterial canker with indication of the interference of phyllosphere biofilms. PLoS ONE 7:e42124
International Standards for Phytosanitary Measures (ISPM) (2014) ISPM 27 Diagnostic protocols, DP 6: Xanthomonas citri subsp. citri. IPPC, FAO, Rome
Jacobson CB, Pasternak JJ, Glick BR (1994) Partial purification and characterization of 1-aminocyclopropane-1-carboxylate deaminase from the plant growth promoting rhizobacterium Pseudomonas putida GR12-2. Can J Microbiol 40:1019–1025
Kumar S, Stecher G, Tamura K (2016) Mega7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874
Lee YA, Sung AN, Liu TF, Lee YS (2009) Combination of chromogenic differential medium and estA-specific PCR for isolation and detection of phytopathogenic Xanthomonas spp. Appl Environ Microbiol 75:6831–6838
Leifert C, Waites WM (1992) Bacterial growth in plant tissue culture media. J Appl Microbiol 72:460–466
McGuire RG, Jones JB, Sasser M (1986) Tween media for semiselective isolation of Xanthomonas campestris pv. vesicatoria from soil and plant material. Plant Dis 70:887–891
Michavila G, Adler C, De Gregorio PR et al (2017) Pseudomonas protegens CS1 from the lemon phyllosphere as a candidate for citrus canker biocontrol agent. Plant Biol 19:608–617
Nandi M, Selin C, Brawerman G, Fernando WGD, De Kievit T (2017) Hydrogen cyanide, which contributes to Pseudomonas chlororaphis strain PA23 biocontrol, is upregulated in the presence of glycine. Biol Control 108:47–54
Naz SA, Jabeen N, Sohail M, Rasool SA (2015) Production and purification of pyocin from a soil associated Pseudomonas aeruginosa strain SA 188. Pak J Agri Sci 52:873–879
Palazzini JM, Dunlap CA, Bowman MJ, Chulze SN (2016) Bacillus velezensis RC 218 as a biocontrol agent to reduce Fusarium head blight and deoxynivalenol accumulation: genome sequencing and secondary metabolite cluster profiles. Microbiol Res 192:30–36
Park DS, Hyun JW, Park YJ, Kim JS, Kang HW, Hahn JH, Go SJ (2006) Sensitive and specific detection of Xanthomonas axonopodis pv. citri by PCR using pathovar specific primers based on hrpW gene sequences. Microbiol Res 161:145–149
Richard D, Boyer C, Vernière C, Canteros BI, Lefeuvre P, Pruvost O (2017a) Complete genome sequences of six copper-resistant Xanthomonas citri pv. citri strains causing Asiatic citrus canker, obtained using long-read technology. Genome Announc 5:e00010–e00017
Richard D, Tribot N, Boyer C et al (2017b) First report of copper-resistant Xanthomonas citri pv. citri pathotype A causing Asiatic citrus canker in Réunion, France. Plant Dis 101:503
Sasirekha B, Shivakumar S, Sullia SB (2012) Statistical optimization for improved indole-3-acetic acid (IAA) production by Pseudomonas aeruginosa and demonstration of enhanced plant growth promotion. J Soil Sci Plant Nutr 12:863–873
Vasconcellos FCS, De Oliveira AG, Lopes-Santos L et al (2014) Evaluation of antibiotic activity produced by Pseudomonas aeruginosa LV strain against Xanthomonas arboricola pv. pruni. Agric Sci 5:71–76
Zhou L, Jiang HX, Sun S, Yang DD, Jin KM, Zhang W, He YW (2016) Biotechnological potential of a rhizosphere Pseudomonas aeruginosa strain producing phenazine-1-carboxylic acid and phenazine-1-carboxamide. World J Microbiol Biotechnol 32:1–12
Acknowledgements
This work was supported by Srinakharinwirot University [Grant Numbers 762/2558].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sudyoung, N., Tokuyama, S., Krajangsang, S. et al. Bacterial antagonists and their cell-free cultures efficiently suppress canker disease in citrus lime. J Plant Dis Prot 127, 173–181 (2020). https://doi.org/10.1007/s41348-019-00295-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41348-019-00295-9