Abstract
Restless legs syndrome (RLS) is characterized by an uncomfortable urge to move the legs, worsened in the evening, occurring at rest, and relieved temporarily by movement. Although its pathophysiology remains incompletely understood, oxidative stress has been suggested. Uric acid (UA) is a marker associated with oxidative stress, and its reduced levels pose a risk for certain neurodegenerative diseases. In this study, we aimed to assess serum UA concentrations in RLS patients to gain insights into its role in the etiopathogenesis of the condition.: This study involved 200 individuals. Serum UA levels were compared with clinical parameters. Disease severity was assessed, categorizing patients into "mild," "moderate," "severe," and "very severe" subgroups. Comparative analysis of UA levels was conducted between these subgroups and the control group. Patients exhibited a statistically significant reduction in UA levels compared to controls (p = 0.001; p < 0.01). No significant disparities in UA levels were observed among patients based on RLS scores (p > 0.05). The generalized linear model in which UA serves as the dependent variable revealed statistically significant associations with the "moderate" and "severe" stages of RLS, as well as age (p < 0.05). Additionally, a ROC curve analysis was executed to evaluate the potential of UA as a biomarker. The ROC analysis, focusing on the patient-control classification, revealed a statistically significant area under the curve (AUC = 0.848, p < 0.001). Our study supports the hypothesis implicating serum UA levels in RLS pathogenesis. Further understanding of UA and its physiological effects will clarify on its role in RLS pathophysiology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Restless legs syndrome (RLS) presents as a disquieting sensory-motor ailment marked by concurrent disruptions in sleep patterns. Its manifestations typically intensify during the evening hours and surface during periods of repose or inactivity. Alleviation, whether partial or complete, is often attainable through ambulation or leg movement. Prevalence rates fluctuate between 5 and 10%, exhibiting a twofold higher occurrence in females compared to males [1, 2]. The complete understanding of the pathophysiological mechanisms underlying the disease remains elusive; however, research suggests its association with both the central and peripheral nervous systems. Proposed factors include reduced iron reserves within the central nervous system, alterations in the dopaminergic system, disruptions to circadian rhythms, and potential involvement of the thalamus [1,2,3,4,5]. Notably a reaction implicating dopamine metabolism and oxidative stress is observed, given the sensitivity of dopaminergic neurons to cellular stressors. Various factors, such as toxins, free radicals, and functional deficiencies, in ubiquitin/proteasome coordination could precipitate the apoptosis of dopaminergic neurons. Additionally, uric acid, the last outcome of purine breakdown, is posited to confer a protective function against oxidative stress [5,6,7]. Oxidative stress represents a dynamic redox cycle characterized by the interplay between oxidants and antioxidants, often leaning heavily toward oxidative potentials. Elevated levels of reactive oxygen radicals within the body can overwhelm protective mechanisms, resulting in cellular injury and the initiation of protein and lipid peroxidation processes. In essence, antioxidants play a crucial role in managing and mitigating oxidative stress by modifying the concentrations of reactive oxygen species (ROS) within body. There exists significant interest in investigating the correlation between antioxidant levels and the onset of emerging diseases. Uric acid serves as a laboratory metric linked with oxidative stress, and diminished serum levels of UA present a potential risk factor for various neurodegenerative disorders [5, 6].
In our investigation, the primary objective was to examine the serum UA concentrations among individuals diagnosed with RLS, with the intention of evaluating the possible involvement of oxidative stress in the etiology and severity of the condition. Furthermore, our aim encompassed the collection of data to ascertain the viability of UA as a biomarker for RLS.
Materials and methods
Participant recruitment
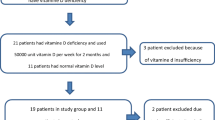
In this cross-sectional investigation, a cohort of 200 individuals, matched for age and sex, underwent assessment. Patients diagnosed with RLS by two neurologists adhering to the criteria outlined in the International Classification of Sleep Disorders Diagnostic Criteria (ICSD-3), were selected based on demographic data extracted from medical records [8]. Disease severity was gaged utilizing the Turkish version of the International RLS Study Group Rating Scale (IRLSSG), which categorizes patients into four subgroups: "mild (1–10)", "moderate (11–20)", "severe (21–30)", and "very severe (31–40)" [9]. Exclusion criteria encompassed individuals with deficiencies in iron or ferritin. The reference ranges in our laboratory were 65–175 µg/mL for iron and 30–400 ng/mL for ferritin. All patients included in the study fell within these ranges, and those with lower values were excluded. Also, those afflicted with neurological, rheumatologic, renal, metabolic, infectious, or cardiovascular ailments. Furthermore, individuals undergoing treatment with medications known to influence serum UA levels, such as anti-inflammatory drugs, neuroleptics, antidepressants, or steroids, were excluded [10, 11]. Additionally, individuals with a body mass index (BMI) falling below 19 or exceeding 25 and neuroimaging revealing focal lesions in the basal ganglia were also excluded, as illustrated in Fig. 1. In accordance with these criteria, a cohort of one hundred subjects was enrolled in the patient group, while an equivalent number of healthy subjects comprised the control group. Blood samples were obtained from the medial cubital vein following an 8-h fasting period. Subsequent laboratory analysis of iron, ferritin, and UA concentrations was conducted utilizing the Architect CI8200 system manufactured by Abbott Laboratories, located in Abbott Park, Illinois, USA. Iron levels were quantified in micrograms per milliliter (μg/mL), ferritin in nanograms per milliliter (ng/mL), and UA in milligrams per deciliter (mg/dL). The study received ethical permission from the Bahcesehir University Ethics Committee, assigned protocol number 2023–20/02.
Statistical analyses
Numeric (n) and percentage (%) representations were utilized to delineate the distribution of individuals across demographic parameters, such as gender, IRLSSG subgroups, and medication use. The normality of continuous variables was assessed graphically and through the Shapiro–Wilk test, revealing a lack of adherence to normal distribution for any of the continuous variables. Descriptive statistics encompassed mean±SD (standard deviation) and median (minimum–maximum) values. Mann–Whitney U tests were employed for comparison age, iron, ferritin, UA values between patient and control groups. Kruskal–Wallis tests were utilized to compare iron, ferritin, and UA values among IRLSSG subgroups. Gender distribution disparities between patient and control groups were analyzed through cross-tabulations, incorporating number (n), percentage (%), and chi-square (χ^2) test statistics. Spearman's nonparametric correlation coefficient was employed for correlation analyses involving UA values, age, disease duration, and IRLSSG subgroup scores. Attempted multiple linear regression analysis involving UA and other parameters was impeded by the non-normal distribution of UA values and unmet assumptions. Consequently, the Generalized Linear Models (GLM) method was applied, facilitating the analysis of non-normally distributed continuous dependent variables such as UA. Receiver Operating Characteristic (ROC) analysis was conducted to assess variables anticipated to clinically influence the identification of high-risk groups relative to the Patient–Control dichotomy. ROC plots were generated, and the area under the curve (AUC) and corresponding 95% confidence intervals (CI) were determined. Variables with AUC values exceeding 0.500 were identified as effective indicators of outcomes surpassing random chance. The sensitivity and specificity of these variables for identifying high-risk groups were documented. Data analyzing were managed using IBM SPSS Statistics 21.0 (IBM Corp., published in 2012, Armonk, NY: IBM Corp.). A predefined threshold for statistical significance was established at p<0.05.
Results
The study comprised a total of 200 individuals, with 111 (55.5%) females and 89 (44.5%) males, consisting of 100 patients and 100 controls. Their ages ranged from 21 to 78 years, with a mean age of 42.38±13.24 years. The average duration of the disease was recorded as 3.09±2.64 years, and the mean RLS score among patients was 19.81±9.11. No alcohol consumers or smokers were included. Uric acid levels in the patient group were found to be significantly lower than those in the control group (p<0.001). Analysis revealed no significant differences in age, gender, iron, and ferritin levels between individuals in the patient and control groups (p>0.05). Further demographic details and laboratory findings are presented in Table 1.
In accordance with the classification of RLS severity stages, 18.0% (n=18) of individuals were categorized as "mild", 35.0% (n=35) as "moderate", 33.0% (n=33) as "severe", and 14.0% (n=14) as "very severe". Within the patient group, there were no statistically significant differences observed in the iron, ferritin, and UA values corresponding to the various RLS Scores (p>0.05). Detailed laboratory findings pertaining to RLS scores are presented in Table 2.
The results obtained from the generalized linear model (GLM) revealed UA to be the dependent variable, with RLS stage, gender, medication usage, age, duration of the disease, RLS score, iron, and ferritin serving as independent variables. Within this model, the "moderate" and "severe" stages, along with age, exhibited statistically significant associations (p<0.05). Notably, individuals categorized with "moderate" and "severe" stages demonstrated lower UA levels compared to those classified with "mild" severity (p<0.05). Furthermore, a one-unit alteration in age correlated with a 0.029-unit change in UA values, as depicted in Table 3.
A ROC curve analysis was executed to evaluate the potential of UA as a biomarker. The ROC analysis, focusing on the patient–control classification, revealed a statistically significant area under the curve (AUC=0.848, p<0.001), as shown in Figure 2. With the patient–control classification serving as a benchmark, the UA variable's cut-off point was established at 3.75. Sensitivity and specificity for the UA value were computed as 65.00% and 93.00%, respectively, as presented in Table 4.
Discussion
The objective of this work was to assess the correlation among UA levels and Restless Legs Syndrome, emphasizing the potential implication of oxidative stress in both the etiology and severity of the condition. Furthermore, our investigation aimed to assess the viability of UA as a biomarker for RLS. The outcomes of our research provide insights into several significant dimensions of this association. Initially, our findings reveal a statistically noteworthy reduction in UA values among the patients in comparison to the control cohort. This discernment implies a plausible association between diminished UA levels and the manifestation of RLS. UA is synthesized through the breakdown of purines within the body and serves a protective function against oxidative stress. Moreover, UA serves as a laboratory indicator linked with oxidative stress, and depleted UA levels in the circulatory system heighten susceptibility to various neurodegenerative conditions [6, 7, 12]. Schirinzi et al. have underscored the importance of diminished serum UA levels as a prevalent risk factor associated with tauopathies, involving progressive supranuclear palsy, frontotemporal dementia, and Alzheimer's disease [12]. The investigation conducted by Wang et al. unveiled a notable decline in both serum UA and albumin levels within individuals afflicted with Parkinson's disease, thereby identifying these factors as independent risk determinants for the condition [13].
Further investigations have brought attention to a noteworthy correlation between heightened disease severity and serum UA levels among individuals diagnosed with Parkinson's disease (PD) [14, 15]. Both in vitro and in vivo studies have demonstrated that uric acid plays a protective role in dopaminergic neurons [16,17,18]. The prevailing hypothesis posits that decreased uric acid levels heighten oxidative stress, leading to the degeneration of dopaminergic neurons and triggering the onset of disease [18]. Research examining postmortem samples from PD patients found lower urate levels in the substantia nigra and caudate nucleus, alongside a higher rate of dopamine oxidation in PD striatum homogenates compared to controls, suggesting a pro-oxidative stress state in PD [16, 19]. Interestingly, adding urate reduced dopamine oxidation rates in PD nigral homogenates, whereas introducing urate oxidase to control homogenates increased these rates, underscoring urate's potential role in PD [16, 20]. Urate's neuroprotective effects have been further substantiated in cellular and rodent models of PD. Administration of urate prevented the degeneration of cultured nigral neurons and protected dopaminergic cells from oxidative and mitochondrial toxins [16, 21, 22]. Moreover, in a rotenone toxicity model, co-treatment with urate prevented membrane depolarization and dopaminergic cell death, highlighting its potential therapeutic role in PD [23]. Furthermore, multiple investigations exploring the impact of diminished antioxidants and heightened oxidative stress on Alzheimer's pathophysiology have demonstrated a concurrent decrease in UA levels [24, 25]. The body of research examining the correlation between RLS and UA remains relatively scant; nevertheless, our outcomes align with existing literature findings [3]. Moreover, we observed an association between RLS severity and UA levels, a departure from previous investigations. Additionally, we postulated that oxidative stress may have a part in RLS etiopathology, given uric acid's recognized role as a potent endogenous antioxidant.
Based on our current knowledge, the first study investigating the relationship between RLS and uric acid was conducted by Yazar and colleagues [3]. In both studies, uric acid levels were similarly found to be low. Unlike our study, they also found low levels of ferritin and iron. According to our criteria, we did not include patients with iron and ferritin deficiencies. While they found the uric acid level to be related to age and disease duration, we found it to be related to age and disease stage. Our exclusion criteria were relatively strict, and we used regression and ROC analysis for statistical. Despite the methodological and statistical differences, we concur that uric acid can be used as a biomarker for RLS.
The diminished UA levels detected in individuals with RLS may signify an imbalance between oxidative stress and antioxidant mechanisms, which could potentially exacerbate the onset and advancement of the disorder. A study examining oxidative stress in RLS patients reported heightened concentrations of oxidative reaction by products, such as malondialdehyde, thiol, and nitric oxide [4]. Another independent investigation examining the prevalence of RLS and biomarkers indicative of oxidative stress and inflammatory response among dialysis-treated patients unveiled notably elevated blood levels of 8-hydroxy-2'-deoxyguanosine (8-OHdG) within the RLS cohort in contrast to the control group [2, 26]. Elevated serum levels of 8-OHdG are commonly observed in conditions marked by heightened reactive oxygen species production, including but not limited to diabetes, amyotrophic lateral sclerosis, cancer, radiation injury, smoking, normal aging, and renal diseases [2, 3, 26, 27]. These observations indicate a potential involvement of oxidative stress in the etiology of the condition. Additionally, our analysis unveiled a noteworthy correlation between serum UA levels and the severity of RLS symptoms. Specifically, individuals experiencing moderate to severe RLS symptoms demonstrated lower levels of UA compared to those with milder manifestations. The fluctuations in UA concentrations may correspond with the severity of RLS symptoms, thereby reinforcing the hypothesis of oxidative damage play part in the pathophysiology of disorder. Future investigations should delve into the underlying mechanisms elucidating the relationship between UA levels and RLS severity to provide deeper insights into this association.
RLS has the potential to manifest at any phase of life, with several investigations indicating a heightened prevalence among older individuals [28, 29]. Research has demonstrated a gradual elevation in serum UA levels with advancing age, particularly evident in middle-aged and elderly populations [30, 31]. Nonetheless, the relationship between UA levels and age can be influenced by diverse factors. In certain instances, older individuals may exhibit lower UA levels due to diminished muscle mass, thereby impacting purine metabolism. Conversely, in specific populations, such as postmenopausal women, hormonal alterations may lead to an increase in UA levels [29,30,31]. Our investigation identified age as a significant predictor of UA levels, with each unit increase in age corresponding to a 0.029-unit rise in UA levels among RLS patients. Although the precise mechanism underlying this relationship necessitates further exploration, it underscores the necessity of considering age-related variables when interpreting UA levels in RLS.
Ultimately, discerning potential effects of oxidative stress in RLS patients could offer opportunities to mitigate the clinical impacts of the condition. In line with this objective, our ROC curve analysis revealed that UA holds promise as a biomarker for RLS, with a determined cut-off point of 3.75 for distinguishing between patients and controls. This discovery suggests that assessing UA levels may facilitate suspicion, diagnosis, and management of RLS, furnishing clinicians with a valuable tool for identifying individuals at risk of RLS [3].Moreover, Cipriani et al. highlighted in a 2010 study the significance of serum UA levels as an essential biomarker for Parkinson's disease risk and prognosis. This underscores the notion that UA, as a biomarker associated with favorable central nervous system outcomes in neurodegeneration, may not be exclusive to PD or other neurodegenerative disorders but could also find utility in conditions such as RLS [32].
Our research has some circumscriptions. The agents used in treating RLS may have influenced the severity of symptoms and the stage of the disease. Although these agents are not directly listed among those affecting uric acid levels, they may have had an indirect effect. Therefore, measuring uric acid levels and examining their relationship in a group of untreated patients could provide more objective data. This can be considered a limitation of our study. Future research could address this by including a larger cohort of de novo patients.
Additionally, our study offers new perspectives on the association between serum UA levels and RLS, indicating the potential involvement of oxidative stress in both the onset and severity of the condition. While routine clinical utilization of UA testing as a biomarker for RLS is not yet recommended, its strong correlation with RLS outcomes justifies its immediate integration into RLS-focused research endeavors. Our findings emphasize the necessity for further investigation to elucidate the underlying mechanisms and clinical significance of this association, with the ultimate aim of enhancing diagnostic and therapeutic approaches for RLS.
Code availability
Not applicable.
References
Manconi M, Garcia-Borreguero D, Schormair B, et al. Restless legs syndrome. Nat Rev Dis Primers. 2021;7(1):80. https://doi.org/10.1038/s41572-021-00311-z.
Higuchi T, Abe M, Mizuno M, et al. Association of restless legs syndrome with oxidative stress and inflammation in patients undergoing hemodialysis. Sleep Med. 2015;16(8):941–8. https://doi.org/10.1016/j.sleep.2015.03.025.
Yazar HO, Yazar T, Yildirim T, et al. Assessment of serum UA levels in patients with restless legs syndrome. Acta Neurol Belg. 2019;119(3):461–6. https://doi.org/10.1007/s13760-019-01177-5.
Baskol G, Korkmaz S, Erdem F, et al. Assessment of nitric oxide, advanced oxidation protein products, malondialdehyde, and thiol levels in patients with restless legs syndrome. Sleep Med. 2012;13(4):414–8. https://doi.org/10.1016/j.sleep.2011.11.012.
Arı BÇ, Kobak TE. Clinical usefulness of systemic inflammatory markers as diagnostic and prognostic indicators for restless Leg syndrome. Bosphorus Med J. 2022;9(1):16–22. https://doi.org/10.14744/bmj.2022.96967.
Zhong LL, Song YQ, Tian XY, et al. Level of UAand uric acid/creatinine ratios in correlation with stage of Parkinson disease. Medicine (Baltimore). 2018;97(26): e10967. https://doi.org/10.1097/MD.0000000000010967.
Ari BC, Tur EK, Domac FM, Kenangil GO. Uric acid: The role in the pathophysiology and the prediction in the diagnosis of Parkinson’s disease: a Turkish-based study. a húgysavnak a Parkinson-kór kórélattanában és a diagnózis előrejelzésében betöltött szerepe – török populáción alapuló vizsgálat. Ideggyogy Sz. 2022. https://doi.org/10.18071/isz.75.0051.
Sateia MJ. International classification of sleep disorders-third edition: Highlights and modifications. Chest. 2014;146:1387–94. https://doi.org/10.1378/chest.14-0970.
Allen RP, Picchietti DL, Garcia-Borreguero D, et al. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: Updated international restless legs syndrome study group (IRLSSG) consensus criteria–history, rationale, description, and significance. Sleep Med. 2014;15:860–73. https://doi.org/10.1016/j.sleep.2014.03.025.
Daskalopoulou SS, Tzovaras V, Mikhailidis DP, et al. Effect on serum uric acid levels of drugs prescribed for indications other than treating hyperuricaemia. Curr Pharm Des. 2005;11(32):4161–75. https://doi.org/10.2174/138161205774913309.
Ben Salem C, Slim R, Fathallah N, et al. Drug-induced hyperuricaemia and gout. Rheumatology (Oxford). 2017;56(5):679–88. https://doi.org/10.1093/rheumatology/kew293.
Schirinzi T, Di Lazzaro G, Colona VL, et al. Assessment of serum UAas risk factor for tauopathies. J Neural Transm (Vienna). 2017;124(9):1105–8. https://doi.org/10.1007/s00702-017-1743-6.
Wang L, Hu W, Wang J, et al. Impact of serum uric acid, albumin and their interaction on Parkinson’s disease. Neurol Sci. 2017;38(2):331–6. https://doi.org/10.1007/s10072-016-2738-z.
Vieru E, Köksal A, Mutluay B, et al. The relation of serum UAlevels with L-Dopa treatment and progression in patients with Parkinson’s disease. Neurol Sci. 2016;37(5):743–7. https://doi.org/10.1007/s10072-015-2471-z.
Sakuta H, Suzuki K, Miyamoto T, et al. Serum UAlevels in Parkinson’s disease and related disorders. Brain Behav. 2017;7(1): e00598. https://doi.org/10.1002/brb3.598.
Crotty GF, Ascherio A, Schwarzschild MA. Targeting urate to reduce oxidative stress in Parkinson disease. Exp Neurol. 2017;298:210–24. https://doi.org/10.1016/j.expneurol.2017.06.017.
Otani N, Hoshiyama E, Ouchi M, Takekawa H, Suzuki K. Uric acid and neurological disease: a narrative review. Front Neurol. 2023;14:1164756. https://doi.org/10.3389/fneur.2023.1164756.
Wen M, Zhou B, Chen YH, et al. Serum uric acid levels in patients with Parkinson’s disease: a meta-analysis. PLoS ONE. 2017;12(3): e0173731. https://doi.org/10.1371/journal.pone.0173731.
McFarland NR, et al. Postmortem brain levels of urate and precursors in Parkinson’s disease and related disorders. Neurodegener Dis. 2013;12(4):189–98. https://doi.org/10.1159/000346370.
Dănău A, Dumitrescu L, Lefter A, et al. Serum uric acid Levels in Parkinson’s DISEASE: a cross-sectional electronic medical record database study from a tertiary referral centre in Romania. Medicina (Kaunas). 2022;58(2):245. https://doi.org/10.3390/medicina58020245.
Jones DC, Gunasekar PG, Borowitz JL, et al. Dopamine induced apoptosis. J Neurochem. 2000;74(6):2296–304.
Zhu TG, Wang XX, Luo WF, et al. Protective effects of urate against 6-OHDA-induced cell injury in PC12 cells through antioxidant action. Neurosci Lett. 2012;506(2):175–9. https://doi.org/10.1016/j.neulet.2011.10.075.
Guerreiro S, Ponceau A, Toulorge D, et al. Protection of midbrain dopaminergic neurons by the end product of purine metabolism uric acid: potentiation by low-level depolarization. J Neurochem. 2009;109(4):1118–28.
Tana C, Ticinesi A, Prati B, et al. UAand Cognitive Function in Older Individuals. Nutrients. 2018;10(8):975.
Cankurtaran M, Yesil Y, Kuyumcu ME, et al. Altered levels of homocysteine and serum natural antioxidants links oxidative damage to Alzheimer’s disease. J Alzheimers Dis. 2013;33(4):1051–8.
Tarng DC, Huang TP, Wei YH, et al. 8-hydroxy-20-deoxyguanosine of leukocyte DNA as a marker of oxidative stress in chronic hemodialysis patients. Am J Kidney Dis. 2000;36:934–44. https://doi.org/10.3390/nu10080975.
Bagdanov M, Brown RH, Matson W, et al. Increased oxidative damage to DNA in ALS patients. Free Radical Biol Med. 2000;29:652–8. https://doi.org/10.1016/s0891-5849(00)00349-x.
Broström A, Alimoradi Z, Lind J, et al. Worldwide estimation of restless legs syndrome: a systematic review and meta-analysis of prevalence in the general adult population. J Sleep Res. 2023;32(3): e13783. https://doi.org/10.1111/jsr.13783.
Ohayon MM, O’Hara R, Vitiello MV. Epidemiology of restless legs syndrome: a synthesis of the literature. Sleep Med Rev. 2012;16(4):283–95. https://doi.org/10.1016/j.smrv.2011.05.002.
Koo BS, Jeong HJ, Son CN, et al. Distribution of serum UA levels and prevalence of hyper- and hypouricemia in a Korean general population of 172,970. Korean J Intern Med. 2021;36(Suppl 1):S264–72. https://doi.org/10.3904/kjim.2020.116.
Zitt E, Fischer A, Lhotta K, et al. Sex- and age-specific variations, temporal trends and metabolic determinants of serum UAconcentrations in a large population-based Austrian cohort. Sci Rep. 2020;10(1):7578. https://doi.org/10.1038/s41598-020-64587-z.
Cipriani S, Chen X, Schwarzschild MA. Urate: a novel biomarker of Parkinson’s disease risk, diagnosis and prognosis. Biomark Med. 2010;4(5):701–12. https://doi.org/10.2217/bmm.10.94.
Acknowledgements
The authors certify that they comply with ethical guidelines for authorship and publishing of the Sleep and Biological Rhythms.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Esma Kobak Tur: conceptualization, methodology, writing—original draft preparation, investigation, resources, writing—review and editing, formal analysis. Buse Cağla Ari: methodology, writing—original draft preparation, writing—review and editing, visualization, data curation, supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Bahcesehir University Ethics Committee, assigned protocol number 2023–20/02.
Consent to participate
We received informed consent from all participants.
Consent for publication
We received informed consent from all participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tur, E.K., Ari, B.C. The impact of uric acid levels in the pathophysiology and its contribution to the prediction of diagnosis in restless legs syndrome. Sleep Biol. Rhythms (2024). https://doi.org/10.1007/s41105-024-00549-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41105-024-00549-6