Abstract
An experimental study was conducted to evaluate the damage progress of concretes containing aerogel powders subjected to sodium sulfate (\({\mathrm{Na}}_{2}{\mathrm{SO}}_{4}\)) and combination of sodium sulfate and sodium chloride (\(\mathrm{NaCl})\) attack under wetting–drying cycles. The amount of aerogel was considered at levels of 0.0, 1.75, 3.5, 5.25, and 7.0% of the concrete volume. The mechanical and physical properties of the concretes as well as the compressive strength and weight changes, and electrical resistivity were measured under the corrosive environments up to 12 months of exposure. Results indicated that the use of 1.75% aerogel improves the mechanical and physical properties of the concrete. However, these properties declined by further increase of aerogel percentage. Also, the aerogel usage up to 5.25% can increase the durability of concretes under sulfate attack. Furthermore, the presence of chloride ions reduces the concrete deterioration under the sulfate attack, but it increases the probability of steel corrosion in the sulfate environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Concrete is the most widely used material for the construction of structures exposed to different types of environments [1]. However, as the demands for the construction in harsh environments are increased, the concern towards long service life of reinforced concrete structures is also increased [2]. Therefore, degradation of concrete structures in harsh environments such as its exposure to harmful chemicals, which are normally found in groundwater, soil, and seawater is one of the major problems in construction industry [3,4,5]. Among the aggressive chemicals, sulfates and chlorides are reported to be the most aggressive ions that influence the durability of concrete structures [6].
Since seawater contains high concentrations of chlorides and sulfates, marine environments are very aggressive to concrete structures [7]. When concrete structures are exposed to marine environments, those corrosive ions will heavily devastate their durability and safety [8]. Furthermore, drying–wetting cycles accelerate the sulfate and chloride attacks against concrete caused by splash and tide [9]. Sulfate attack in concrete leads to conversion of cement hydration products to ettringite, gypsum, and other possible phases [10, 11]. Volume increase of gypsum and ettringite within concrete cannot only reduce the porosity of concrete but may also cause damage and cracking of the concrete [12]. These cracks in the hardened cement paste provide additional transport channels into the concrete for penetration of aggressive ions [13]. The presence of sulfate ion also affects the diffusivity of concrete by changing the capillary microstructure of material [14]. Further, chloride ions also exist concomitantly with sulfate ions in marine environment. It has been a well-known fact that the chloride-induced corrosion of steel rebars in concrete structure takes the first place of the severe durability problems, affecting the lifecycle of concrete structures exposed to the marine environment [15].
Extensive researches have been carried out on the degradation of concretes induced by sulfate or sulfate–chloride combined attacks [16,17,18,19]. Generally, concretes subjected to sulfate–chloride combined attack suffer less damage than concrete that suffers only sulfate attack [20, 21]. However, some reports claim that the concomitant presence of chloride and sulfate ions considerably reduces the chloride binding capacity of cements, thereby releasing the chloride ions into the pore solution and cause depassivation of steel rebars [22]. In this regard, Chen et al. reported that mineral admixtures, such as fly ash and ground granulated blast slag, can improve the resistance of concrete under combined sulfate–chloride attack [9]. Xu et al. found that the presence of sulfate ion decelerated the chloride diffusion in cracked concrete with a lower concentration of both free and total chloride ions [15]. Research conducted by Maes and De Belie showed that chloride penetration increases when the sulfate content increases at short immersion periods [23]. Studies conducted by Sotiriadis et al. indicated that chlorides inhibit sulfate attack on concrete, thus the deterioration is delayed [24]. Zhao et al. investigated the effects of sulfate–chloride combined attack on degradation of cast in situ concrete [25]. Their results indicated that cast in situ concrete suffers more severe damage and greater strength loss when subjected to sulfate–chloride combined attack than when subjected to sulfate only. The diffusion and accumulation of sulfates are accelerated by the coexisting chlorides, especially in the early stages.
However, studies on the influence of combined chloride and sulfate ions under drying–wetting cycles, which is closer to actual marine environments, are limited in the literature [26, 27]. Also, very limited researches have been conducted on the addition of aerogel particles in the concrete mix designs [28, 29], but Gao et al. introduced the aerogel particles as stable material during the hydration of cementitious materials [30]. Aerogel as an extremely low-density nanoporous material (density of 3–100 kg/m3 depending on the porosity) and typically made of silica, containing 94–99% of air voids has a low thermal conductivity (0.003–0.02 W/mK) and suitable fire and acoustic resistance [31, 32]. These characteristics make aerogel as one of the most remarkable super-insulative materials in building applications [33, 34].
Therefore, the aim of this study is to investigate the effect of aerogel powder on the reducing the invasion of sulfate ions into the concrete under wetting–drying cycles by evaluation of the compressive strength and weight changes, and the electrical resistivity measurements during the immersion of the specimens up to 12 months of exposure. Also, the effect of chloride ions on the resistance of aerogel-modified concretes under sulfate attack was investigated. Moreover, compressive strength and water absorption of the concretes were determined for evaluating the mechanical and physical properties of the concrete mixtures.
2 Experimental Program
2.1 Materials
The cement used in the mix was ordinary Portland cement of 42.5 (CEM II 42.5R) grades produced by Abyek Cement Co. in Iran. The coarse aggregates used were the crushed limestone with maximum particle size of 12.5 and 19 mm (CA-I and CA-II). The fine aggregate used was a mix of natural and crushed limestone sand with a maximum particle size of 4.75 mm. Density and water absorption capacity of the used aggregates were determined according to ASTM specifications [35, 36] and the results are described in Table 1. A liquid poly carboxylate-based superplasticizer with a density of 1.12 g/cm3 and pH of 8 was also used to keep the slump of the mixtures constant.
Aerogel used in the mixtures was highly porous and transparent powder with an average particle size of 150 µm (see Fig. 1). Average mass density of used aerogel, which is composed of pure silica and has a super-hydrophobic property, was \(\sim\) 0.1 g/cm3.
2.2 Mixture Details
In this study, five types of concrete mixtures with constant cement content of 400 kg/m3 and water to cement ratio (W/C) of 0.50 were used where the slump of concretes was kept constant (85–100 mm). The proportion of the aggregates was selected as 58% FA, 20% CA-I, and 22% CA-II corresponding to the composition of aggregates in the national method of Iranian concrete mix design [37]. Proportions of the control mixture together with superplasticizer demand to obtain the required slump are given in Table 2. In other mixtures, aerogel was used in the mixtures similar to the use of air entraining agents. Since the total volume of air content in severe exposure has been commonly proposed between 5 and 7.5% for freezing–thawing cycles [38, 39], the maximum volume of the aerogel was considered 7% of concrete volume and the interval between 0 and 7% was divided into five considered mixtures in which the values of 0%, 1.75%, 3.5%, 5.25% and 7% of aerogel particles were used in the mixtures.
As seen in Fig. 2, when the aerogel powder was used, the slump of the mixtures was increased despite of the decrease in the superplasticizer usage. Since aerogel particles act as a surfactant and have a ball-bearing effect, the slump of the mixtures was remarkably increased by increasing the aerogel content.
2.3 Casting and curing the specimens
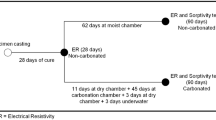
All concrete mixtures were prepared by dry mixing of the fine aggregates and cement in a pan mixer for 1 min. Then aerogel powder and a part of the mixing water and superplasticizer were added and mixed for another 1.5 min. Finally, the coarse aggregates and the rest of the mixing water and superplasticizer were added to the mixture and mixing was continued for another 2 min. Cubes having dimensions of 100 × 100 × 100 mm were casted for the compressive strength, water absorption, weight loss, changes in the compressive strength and electrical resistivity experiments. Following the casting and 24 h of humid curing, the specimens were demolded and cured in a water tank at the room temperature for 28 days. Then, the specimens were placed in three different solutions (see Table 3), where the first one was considered as a control solution and the other solutions were aggressive solutions containing different concentrations of sodium sulfate (\({\mathrm{Na}}_{2}{\mathrm{SO}}_{4}\)) and sodium chloride (\(\mathrm{NaCl}\)). It should be noted that two acceptable results were collected from the experiments and their average was reported accordingly. When the difference between the results was less than 10%, they were considered as acceptable results. Therefore extra specimens were made for each experiment.
Also wetting and drying cycles were applied for the specimens immersed in the aggressive solutions at the room temperature (23 ± 2 °C). The setup was programmed for applying 12 h of wetting and 12 h of drying where the periods were controlled using two separate timers in which the pumps started to drain the connected container when the drying or wetting period was finished (see Fig. 3). These wetting and drying cycles were continuously repeated for 12 months.
2.4 Experiments
2.4.1 Compressive Strength
The 100 mm concrete cubes were used to determine the compressive strength after 7 and 28 days of initial curing and after 3, 6 and 12 months of immersing in corrosive solutions according to the recommendations of BS1881-part118 [40].
2.4.2 Water Absorption
Specimens, after 28 and 120 days of moist curing, were wiped out and were allowed to dry superficially for about 2 h in the laboratory. Then, the specimens were placed in a well-ventilated drying oven at 105 ± 5 °C (for about 72 ± 2 h). At the end of this period, the specimens were cooled at the laboratory condition for 24 ± 2 h and then 0.5 and 48 h water absorption of the specimens were measured according to the recommendations of BS1881-part122 [41].
2.4.3 Electrical Resistivity
Electrical resistivity test, which indirectly measures the interconnected porosity of concrete, is defined as the strength of materials to the electrical current passage [42]. Since the reinforcement corrosion is an electrochemical process, the electrical resistivity has been used as a parameter providing information about the reinforcement corrosion. The corrosion rate is inversely proportional to the resistivity, although this relationship may varies with concrete composition [43]. Alonso et al. classified the value of conventional concrete resistivity in four broad categories: < 10, 10–50, 50–100, and > 100 KΩ cm in which the risk of reinforcement corrosion was high, moderate, low and negligible, respectively [44].
The electrical resistivity of 100 mm concrete cubes was determined after 3, 6, and 12 months of immersion in water and corrosive solutions. The specimens were removed from the water or aggressive solutions and their electrical resistance was measured in the SSD condition and then the specimens were returned to the solutions. For this purpose, an electrical resistivity meter was used, which produced 1 kHz AC. Two copper plates (120 × 100 × 2 mm) with a thin layer of low slump cement paste were laid on two opposite faces of each specimen, and the resistance was measured (see Fig. 4). The electrical resistivity was then obtained through the following equation:
where ρ is the electrical resistance (Ω), A is the cross-sectional area of the specimen (mm2), and l is the length of the specimen (mm).
2.4.4 Changes in compressive strength
To better analyze the effect of sodium and chloride ions on the mechanical properties of the concretes, the relative increase or decrease in the compressive strength of the specimens were investigated. For this purpose, the relative compressive strength of the specimens after 3, 6, and 12 months of immersion in the corrosive solutions to the relevant 28-day compressive strength was evaluated using the Eq. (2).
where \({F}_{1}\) is the compressive strength after 28 days of initial moist curing (MPa), and \({F}_{2}\) is the compressive strength of the immersed specimens in the corrosive solutions (MPa).
2.4.5 Weight Change
The aim of this experiment is to determine the effect of sulfate ions and the combination of sulfate and chloride ions on the deterioration of concretes. For this purpose, the weight of the specimens in SSD condition was measured after 28 days of the initial curing (\({W}_{1}\)). The specimens were then placed in the sodium sulfate and the combination of sodium sulfate and sodium chloride solutions. They were consecutively removed from the solutions every 2 weeks and their weights were determined in the SSD condition (\({W}_{2}\)). Finally, the weight change of the specimens was determined using Eq. (3).
where \({W}_{1}\) is the weight of the specimen after 28 days of initial moist curing (g), and \({W}_{2}\) is the weight of the immersed specimens in the corrosive solutions in SSD condition (g).
Measurements of electrical resistivity, compressive strength, and weight changes of the specimens immersed in corrosive solutions were considered to investigate the effect of sulfate and combined attacks on the concretes up to 12 months of exposure. Therefore, the results of the experiments can be assumed reliable if the results confirm each other. Nevertheless, the determination of the sulfate profile is proposed to make the results more reliable for the next studied.
3 Results and Discussions
3.1 Compressive Strength
The results of moist cured samples in terms of compressive strength versus aerogel content are shown in Fig. 5. No obvious trend was identified in terms of the compressive strength difference between control samples and samples with 1.75% aerogel content, although, the compressive strength was slightly increased in aerogel samples over time which is almost contrary to the expectations. Similar results were reported by Strzałkowski et al. [45] in the use of aerogel in lightweight concretes. They attributed the increase in the strength of the samples containing aerogel to the partly filling of the pores of the cement matrix by aerogel granules and stated that the structure of composites with aerogel is more uniform in comparison with control samples. It can also be seen in Fig. 5 that further use of the aerogel clearly reduces the compressive strength where the decrease in compressive strength increases over aerogel content. Overall, these results are consistent with the research results of Adhikary et al. [46], Ng et al. [29], and Gao et al., [47]. It is mainly attributed to the increase in porosity of concretes. The behavior of aerogel in this phase is similar to the behavior of air entraining agent in concrete. This increase in porosity, which is undoubtedly the most important factor in the properties of concrete, reduces the compressive strength of concretes.
3.2 Water Absorption
The absorption capacity of the mixtures is illustrated in Fig. 6 which indicates that the effect of aerogel on the water absorption is different at the age of 28 and 120 days. At the age of 28 days, the addition of aerogel significantly reduces 0.5 h and 48 h water absorption because the hydrophobic nature of aerogel is helpful for preventing water absorption [47]. In other words, when the amount of aerogel in the mixtures increases, the water absorption decreases due to hydrophobicity of aerogel particles on the interfacial substrate. Meanwhile, the aerogel concretes exhibited water droplets remaining on their surface. This is consistent with the results of Yoon et al. [48].
In contrast, at the age of 120 days, further use of aerogel increases water absorption which is generally consistent with the compressive strength test results. The reason for the increase in water absorption by increasing the aerogel content is attributed to the formation of macropores in the aerogel cement composite [49]. The formation of pores that increase the porosity of concrete has a major role in facilitating the movement of water into the concrete both in the initial (0.5 h) and final (48 h) water absorption. Also, it should be noted that the water movement into concrete is not a smile function of the porosity and depends on the pore diameter and distribution, and the pore continuity and tortuosity [50].
3.3 Electrical Resistivity
The data concerning the variation of electrical resistivity versus aerogel content are plotted in Fig. 7. The higher values were obtained from the specimens cured in water, whereas the lower values were observed from the samples immersed in sulfate–chloride solution. It can be seen that the presence of soluble ions in pore solution and the increase in their concentration significantly reduces the electrical resistivity. It is evident that the presence of chloride ions along with sulfate ions causes a sharp decrease in electrical resistivity. As ionic conduction is the main phenomenon of electricity transport in concrete [51, 52] the presence of ions in the pore solutions changed the behavior of the electrical resistivity of the concrete when compared to the reference condition. The electrical resistivity of the concrete is characterized by the movement of ions such as \({\mathrm{Cl}}^{-}\) and \({\mathrm{SO}}_{4}^{2-}\) in the pore solution and, therefore, the higher amount and movement of these ions inside concrete results in the lower electrical resistivity. Reductions in the electrical resistivity with the ingress of chloride ions into concrete were previously reported [52, 53], but, Medeiros-Junior et al. found that the concomitant action of chloride and sulfate ions is complex and shows divergent results [26].
In addition, the aerogel particles, which are effective in the concrete pore structure, also affect the electrical resistivity. According to the compressive strength test results and the hydrophobicity property of aerogel, the use of 1.75% aerogel increases the electrical resistivity while more use of aerogel significantly reduces the electrical resistivity because the porosity of concretes is increased by increasing aerogel content. Moreover, electrical resistivity increases over time because of the cement hydration and concrete hardening, but the increase in electrical resistivity is more significant for samples in water. Since the hydration process of the cement particles simply continues in water saturated media, the electrical resistivity of the concretes immersed in water is more increased in comparison to the concretes immersed in the other corrosive solutions.
3.4 Changes in Compressive Strengths
3.4.1 Specimens Placed in Sodium Sulfate Solution
The results of the compressive strength of the retained specimens in sodium sulfate solution are presented in Table 4. Also, relative compressive strength (RCS) to the 28-day moist cured compressive strength is given in the table to better comparison of the results.
As the results show, all concrete mixtures exhibited relative increase in the compressive strength after 3 months of immersion due to the formation of gypsum and ettringite and consequently filling the pores. It is even inferred that the specimens containing more aerogel, which contains more macropores in the aerogel cement matrix [49], showed further increase up to a point where the entire pores volume were filled. As seen in the table, the compressive strength of the sample containing 7% aerogel continuously increased up to 6 months of exposure while the compressive strength of the other mixes started to reduce after 3 months of exposure. Reduction in the compressive strength started when the excessive volume of the ettringite and gypsum crystals created micro cracks [54]. After 12 months of exposure, all samples exhibited lower compressive strength than those of measured after 3 months of exposure. Comparing the results between the control sample and the samples containing aerogel at different exposure time, it is concluded that the presence of aerogel at early ages and up to a certain time (depending on the amount of consumption) prevents a decrease in the concrete strength in sulfate environment, but in the long term, it does not have an acceptable performance in preventing further decrease in the concrete strength in sulfate environment. Among the entire pool of the samples, the 3.5% aerogel samples produced the best performance because of the sulfate ions repulsion, on one hand, and showing the lowest 28-day water absorption, on the other hand, consequently led to limit the diffusion of sulfate ions. The compressive strength of the samples containing 3.5% aerogel was 10% more than its 28-day compressive strength while the compressive strength of the sample containing 7% aerogel was less than its 28-day compressive strength which had the weakest performance.
A general comparison of the relative compressive strength between the water-submerged and sulfate-submerged samples (see Fig. 8) revealed that the increase in the compressive strength was considerable for the sulfate-submerged samples up to 3 months exposure. However, the increase in the compressive strength of the sulfate-submerged samples either terminated or even declined after a particular time.
3.4.2 Specimens Placed in Sodium–Chloride Solution
The results of the compressive strength test performed on the specimens placed in the combined sodium sulfate and chloride solution are presented in Table 5. Also in this table, the results of the relative compressive strength of the specimens with respect to the relevant 28-day compressive strengths are given.
Regarding to Tables 4 and 5, it seems that the process of degradation after 3 months of exposure in both solutions is almost the same while the degradation process declined for the sulfate–chloride solution in comparison with the sulfate solution. Figure 9 shows relative compressive strength to the relevant 28-day compressive strength in the water and aggressive solutions after 12 months of exposure. The specimen containing 5.25% aerogel exhibited 6% growth in the compressive strength after 12 months of exposure in the sulfate solution, whereas this specimen exhibited 17% growth in the compressive strength in the sulfate–chloride solution. These values for the samples containing 7% aerogel are − 2% and 8%, respectively. The difference is due to the presence of chloride ions in the environment which reduces the damage of the concretes under sulfate attack. When chloride ions are combined with sulfate ions, it is supposed that the penetration of sulfate ions will be decreased in the depth of the concretes, and as a results, ettringite and gypsum formations as expansive material will be reduced. Therefore, concrete specimens immersed in combined solutions suffered low deteriorations. Finally, it can be concluded that the presence of 50 g/l \(\mathrm{Nacl}\) in the 50 g/l \({\mathrm{Na}}_{2}{\mathrm{SO}}_{4}\) solution can significantly reduce the sulfate attack in the concretes.
When the results of this section are compared with the results of electrical resistivity, it is inferred that although chloride ions can reduce the destructive effects of sulfate ions, but it can significantly reduce the electrical resistivity of concretes which is undesirable in terms of durability of concrete structures against corrosion of steel rebars. This can be clearly seen in Fig. 10 which shows the relative decreases in the compressive strength and the electrical resistivity of the concretes after 12 months of exposure to the solutions in comparison to the relevant concretes immersed in water. It can be seen that the presence of chloride ions prevents further reduction of the compressive strength of the concretes, while the presence of chloride ions significantly reduces the electrical resistivity of the concretes. For instance, the compressive strength of the concrete containing 7% of aerogel decreased about 21.6% after 12 months exposure to sulfate solution while, at the same time, 14% decrease in the compressive strength was observed in the combined solution. However, electrical resistivity of 7% aerogel concrete decreased 10.8% and 55.6% in the sulfate and combined solutions, respectively, after 12 months of exposure.
3.5 Weight Change
The weight change of the specimens immersed in the sodium sulfate and the combined sodium sulfate and sodium chloride solutions in comparison to the relevant 28-day initial weight is given in Tables 6 and 7, respectively.
As seen in the tables, generally, the specimens exhibited an initial increase in the weight and then the weight of the specimens started to reduce after a while. Although the effect of the used aerogel on the weight changes during the test period was unclear and meaningless, what is clear is that the samples in the combined solution generally had less weight loss than the samples in the sulfate solution. The reason is that the chloride ions act as a barrier against the sulfate ions and prevent further diffuse of sulfate ions in the concrete and consequently the attack is weakened.
As the results show since the weights of the specimens are very close to each other, it could be stated that the results of the changes in the compressive strength are more reliable. Figure 11 shows the damaged surface of the cube specimens after 48 weeks exposure to the sodium sulfate solution where the differences are negligible.
4 Conclusions
An experimental program on the resistance of concrete specimens containing different percentages of aerogel content (0.0, 1.75, 3.5, 5.25, and 7.0 volume percent) under sulfate and combination of sulfate and chloride ions including wetting and drying cycles were carried out in this study. Also, additional concrete specimens were cured in water as the control solution. The following conclusions are drawn based on the results and discussions:
-
Use of 1.75% aerogel caused a slight increase in the compressive strength. In contrast, further use of the aerogel clearly reduced the compressive strength. A similar trend was observed in electrical resistivity as well as water absorption at the later ages. However, at the age of 28 days, the addition of aerogel reduced water absorption due to its hydrophobic properties.
-
The relative compressive strength of the concrete samples in aggressive solutions increased after 3 months of exposure. However, porous samples showed an increase at the longer age of exposure. Also, when the aggressive solution was changed to the combination of sulfate and chloride solution, an increase in the compressive strength happened in a longer age of exposure. The decreasing trend of relative compressive strength of the samples in the combined sulfate and chloride solution was less than the samples in sulfate solution.
-
Adding aerogel to the concrete mix design prevents a decrease in the compressive strength against sulfate invasion, but more aerogel consumption (more than 3.5%) does not prevent further decrease in the long term exposure.
-
Samples in both aggressive solutions were initially associated with weight gain and then with weight loss. Although the weight loss of the all samples was very small (up to 0.08%), but the samples in the combined solution had less weight loss than samples in the sulfate solution.
-
In terms of durability against sulfate diffusion, concretes containing 3.5% aerogel showed the best performance, but it can be generally said that the concretes with 1.75% aerogel will have a better performance particularly against corrosion of embedded rebars based on the electrical resistivity results of the concretes where 1.75% aerogel concretes showed about 3.5% and 19.6% more electrical resistivity than 3.5% aerogel concretes after 12 months of exposure in sulfate and combine solutions, respectively.
-
The results showed that the presence of chloride ions in the solution can significantly reduce destructive effects of the sulfate ions on the concrete, while the presence of these ions significantly reduces the electrical resistivity of concretes. For instance, the compressive strength of the concrete containing 7% of aerogel decreased about 21.6% after 12 months of exposure to the sulfate solution while 14% decrease in the compressive strength was observed after 12 months of exposure to the combined solution. However, electrical resistivity of 7%-aerogel concrete decreased 10.8% and 55.6% in sulfate and combined solutions, respectively, after 12 months of exposure.
Data availability
All data used during the study appeared in the published article.
References
Pradhan B (2014) Corrosion behavior of steel reinforcement in concrete exposed to composite chloride-sulfate environment. Constr Build Mater 72:398–410. https://doi.org/10.1016/j.conbuildmat.2014.09.026
Monteiro PJM, Kurtis KE (2003) Time to failure for concrete exposed to severe sulfate attack. Cem Concr Res 33(7):987–993. https://doi.org/10.1016/S0008-8846(02)01097-9
Bader MA (2003) Performance of concrete in a coastal environment. Cem Concr Compos 25:539–548. https://doi.org/10.1016/S0958-9465(02)00093-8
Santhanam M, Cohen M, Olek J (2006) Differentiating seawater and groundwater sulfate attack in Portland cement mortars. Cem Concr Res 36(12):2132–2137. https://doi.org/10.1016/j.cemconres.2006.09.011
Loudon N (2003) A review of the experience of thaumasite sulfate attack by the UK Highways Agency. Cem Concr Compos 25(8):1051–1058. https://doi.org/10.1016/S0958-9465(03)00146-X
Zhao G, Li J, Shao W (2018) Effect of mixed chlorides on the degradation and sulfate diffusion of cast-in-situ concrete due to sulfate attack. Constr Build Mater 181:49–58. https://doi.org/10.1016/j.conbuildmat.2018.05.251
Chen Y, Gao J, Tang L, Li X (2016) Resistance of concrete against combined attack of chloride and sulfate under drying-wetting cycles. Constr Build Mater 106:650–658. https://doi.org/10.1016/j.conbuildmat.2015.12.151
Honglei C, Zuquan J, Tiejun Z, Benzhen W, Zhe L, Jian L (2020) Capillary suction induced water absorption and chloride transport in non-saturated concrete: the influence of humidity, mineral admixtures and sulfate ions. Constr Build Mater 236:117581. https://doi.org/10.1016/j.conbuildmat.2019.117581
Chen F, Gao J, Qi B, Shen D, Li L (2017) Degradation progress of concrete subject to combined sulfate-chloride attack under drying-wetting cycles and flexural loading. Constr Build Mater 151:164–171. https://doi.org/10.1016/j.conbuildmat.2017.06.074
Macphee DE, Barnett SJ (2004) Solution properties of solids in the ettringite-thaumasite solid solution series. Cem Concr Res 34(9):1591–1598. https://doi.org/10.1016/j.cemconres.2004.02.022
Brown PW (2002) Thaumasite formation and other forms of sulfate attack. Cem Concr Compos 24:301–303. https://doi.org/10.1016/S0958-9465(01)00081-6
Zhang M, Chen J, Lv Y, Wang D, Ye J (2013) Study on the expansion of concrete under attack of sulfate and sulfate-chloride ions. Constr Build Mater 39:26–32. https://doi.org/10.1016/j.conbuildmat.2012.05.003
Yang Z et al (2019) The influence of sodium sulfate and magnesium sulfate on the stability of bound chlorides in cement paste. Constr Build Mater 228:116775. https://doi.org/10.1016/j.conbuildmat.2019.116775
Irassar EF, Di Manio A, Batic OA (1996) sulfate attack on concrete with mineral admixtures. Cem Concr Res 26(1):113–123. https://doi.org/10.1016/0008-8846(95)00195-6
Xu F, Yang Z, Liu W, Wang S, Zhang H (2020) Experimental investigation on the effect of sulfate attack on chloride diffusivity of cracked concrete subjected to composite solution. Constr Build Mater 237:117643. https://doi.org/10.1016/j.conbuildmat.2019.117643
Das JK, Pradhan B (2020) Long term effect of corrosion inhibitor and associated cation type of chloride ions on chloride profile of concrete exposed to composite chloride-sulfate environment. Mater Today Proc 32:803–809. https://doi.org/10.1016/j.matpr.2020.04.014
Wang J, Cai G, Wu Q (2018) Basic mechanical behaviours and deterioration mechanism of RC beams under chloride-sulphate environment. Constr Build Mater 160:450–461. https://doi.org/10.1016/j.conbuildmat.2017.11.092
Zuquan J, Wei S, Yunsheng Z, Jinyang J, Jianzhong L (2007) Interaction between sulfate and chloride solution attack of concretes with and without fly ash. Cem Concr Res 37(8):1223–1232. https://doi.org/10.1016/j.cemconres.2007.02.016
Jarrah NR, Al-amoudi OSB, Ashiru OA, Al-mana A (1995) Electrochemical behavior of steel in plain and blended cement. Constr Build Mater 9(2):97–103. https://doi.org/10.1016/0950-0618(95)00002-W
Yin R, Zhang C, Wu Q, Li B, Xie H (2018) Damage on lining concrete in highway tunnels under combined sulfate and chloride attack. Front Struct Civ Eng 12(3):331–340. https://doi.org/10.1007/s11709-017-0421-y
Li G, Zhang A, Song Z, Liu S, Zhang J (2018) Ground granulated blast furnace slag effect on the durability of ternary cementitious system exposed to combined attack of chloride and sulfate. Constr Build Mater 158:640–648. https://doi.org/10.1016/j.conbuildmat.2017.10.062
Maslehuddin M, Page CL, Rasheeduzzafar (1997) Temperature effect on the pore solution chemistry in contaminated cements. Mag Concr Res 48(5):5–14. https://doi.org/10.1680/macr.1997.49.178.5
Maes M, De Belie N (2014) Resistance of concrete and mortar against combined attack of chloride and sodium sulphate. Cem Concr Compos 53:59–72. https://doi.org/10.1016/j.cemconcomp.2014.06.013
Sotiriadis K, Nikolopoulou E, Tsivilis S (2012) Sulfate resistance of limestone cement concrete exposed to combined chloride and sulfate environment at low temperature. Cem Concr Compos 34(8):903–910. https://doi.org/10.1016/j.cemconcomp.2012.05.006
Zhao G, Li J, Shi M, Cui J, Xie F (2020) Degradation of cast-in-situ concrete subjected to sulphate-chloride combined attack. Constr Build Mater 241:117995. https://doi.org/10.1016/j.conbuildmat.2019.117995
Medeiros-Junior RA, Gans PS, Pereira E, Pereira E (2019) Electrical resistivity of concrete exposed to chlorides and sulfates. ACI Mater J 116(3):119–130
Jiang L, Niu D (2016) Study of deterioration of concrete exposed to different types of sulfate solutions under drying-wetting cycles. Constr Build Mater 117:88–98. https://doi.org/10.1016/j.conbuildmat.2016.04.094
Fickler S, Milow B, Ratke L, Schnellenbach-held M, Welsch T (2015) Development of high performance aerogel concrete. Energy Proc 78:406–411. https://doi.org/10.1016/j.egypro.2015.11.684
Ng S, Petter B, Ingunn L, Sandberg C, Gao T, Haralds Ó (2015) Experimental investigations of aerogel-incorporated ultra-high performance concrete. Constr Build Mater 77:307–316. https://doi.org/10.1016/j.conbuildmat.2014.12.064
Gao T, Jelle BP, Gustavsen A, Jacobsen S (2014) Aerogel-incorporated concrete: an experimental study. Constr Build Mater 52:130–136. https://doi.org/10.1016/j.conbuildmat.2013.10.100
Pierre AC, Rigacci A (2011). In: Aegerter M, Leventis N, Koebel M (eds) Aerogels handbook. Advances in sol–gel derived materials and technologies, Springer, New York, pp 21–45
Pierre AC, Pajonk GM (2002) Chemistry of aerogels and their applications. Chem Rev 102:4243–4265. https://doi.org/10.1021/cr0101306
Zhang H, Yang J, Wu H, Fu P, Liu Y, Yang W (2020) Dynamic thermal performance of ultra-light and thermal-insulative aerogel foamed concrete for building energy efficiency. Sol Energy 204:569–576. https://doi.org/10.1016/j.solener.2020.04.092
Li P, Wu H, Liu Y, Yang J, Fang Z, Lin B (2019) Preparation and optimization of ultra-light and thermal insulative aerogel foam concrete. Constr Build Mater 205:529–542. https://doi.org/10.1016/j.conbuildmat.2019.01.212
ASTM C127-15 (2015) Standard test method for relative density (Specific Gravity) and absorption of coarse aggregate, ASTM International, West Conshohocken, PA
ASTM C128-15 (2015) Standard test method for relative density (Specific Gravity) and absorption of fine aggregate, ASTM International, West Conshohocken, PA
Building and Housing Research Center (2008) The national method for concrete mix design, BHRC publication, No. S-479
ACI 211.1-91 (2002) Standard practice for selecting proportions for normal, heavyweight, and mass concrete. American Concrete Institute, Detroit
ACI 31R-19 (2019) Building Code Requirements for Structural Concrete. American Concrete Institute, Detroit
BS 1881-118 (1983) Testing concrete-method for determination of compressive strength of concrete cubes. British Standard Institution
BS 1881-122 (1983) Testing concrete-method for determination of water absorption. British Standard Institution
Mendes SES, Oliveira RLN, Cremonez C, Pereira E, Pereira E, Medeiros-Junior RA (2018) Electrical resistivity as a durability parameter for concrete design: Experimental data versus estimation by mathematical model. Constr Build Mater 192:610–620. https://doi.org/10.1016/j.conbuildmat.2018.10.145
ACI (2019) Guide to protection of reinforcing steel in concrete against corrosion. American Concrete Institute, Detroit
Alonso C, Andrade C, González JA (1988) Relation between resistivity and corrosion rate of reinforcements in carbonated mortar made with several cement types. Cem Concr Res 18(5):687–698. https://doi.org/10.1016/0008-8846(88)90091-9
Strzałkowski J, Garbalinska H (2016) Thermal and strength properties of lightweight concretes with the addition of aerogel particles. Adv Cem Res 28(9):567–575. https://doi.org/10.1680/jadcr.16.00032
Adhikary SK, Rudžionis Ž, Vaičiukynienė D (2020) Development of flowable ultra-lightweight concrete using expanded glass aggregate, silica aerogel, and prefabricated plastic bubbles. J Build Eng 31:1–10. https://doi.org/10.1016/j.jobe.2020.101399
Gao T, Petter B, Gustavsen A, Jacobsen S (2014) Aerogel-incorporated concrete: an experimental study. Constr Build Mater 52:130–136. https://doi.org/10.1016/j.conbuildmat.2013.10.100
Yoon HS, Lim TK, Jeong SM, Yang KH (2020) Thermal transfer and moisture resistances of nano-aerogel-embedded foam concrete. Constr Build Mater 236:117575. https://doi.org/10.1016/j.conbuildmat.2019.117575
Shah SN, Mo KH, Yap SP, Radwan MKH (2021) Effect of micro-sized silica aerogel on the properties of light weight cement composite. Constr Build Mater 290:123229. https://doi.org/10.1016/j.conbuildmat.2021.123229
McCarter WJ, Ezirim H, Emerson M (1992) Absorption of water and chloride into concrete. Mag Concr Res 44(158):31–37. https://doi.org/10.1680/macr.1992.44.158.31
Whittington HW, McCarter J, Forde MC (1981) The conduction of electricity through concrete. Mag Concr Res 33(114):48–60. https://doi.org/10.1680/macr.1981.33.114.48
Koleva DA, Copuroglu O, van Breugel K, Ye G, de Wit JHW (2008) Electrical resistivity and microstructural properties of concrete materials in conditions of current flow. Cem Concr Compos 30(8):731–744. https://doi.org/10.1016/j.cemconcomp.2008.04.001
Zhang D, Cao Z, Fan L, Liu S, Liu W (2014) Evaluation of the influence of salt concentration on cement stabilized clay by electrical resistivity measurement method. Eng Geol 170:80–88. https://doi.org/10.1016/j.enggeo.2013.12.010
Neville A (2004) The confused world of sulfate attack on concrete. Cem Concr Res 34(8):1275–1296. https://doi.org/10.1016/j.cemconres.2004.04.004
Author information
Authors and Affiliations
Contributions
MS: Investigation, resources, data curation, and writing. HR: Conceptualization, methodology, supervision, and review and editing.
Corresponding author
Ethics declarations
Conflict of interest
There is no competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
Rights and permissions
About this article
Cite this article
Soleimanirad, M., Rahmani, H. The Effect of Chloride Ions on the Resistance of Concretes Containing Aerogel Under Sodium Sulfate Attack. Int J Civ Eng 20, 501–512 (2022). https://doi.org/10.1007/s40999-021-00671-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40999-021-00671-3