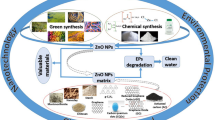
Abstract
Environmental remediation is an alternative field of science that can solve various environmental challenges based on numerous treatment methods. In particular, heterogeneous photocatalysis is an advanced oxidative process that has been the subject of many studies related to environmental control. Globally, pollution by organic pollutants represents risks to environmental health that compromise public health and directly affect the scientific knowledge of public policies that potentially improve quality of life from a sustainable point of view. ZnO systems are receiving special attention due to their attractive characteristics (non-toxic nature, high surface area, thermal/chemical stability), availability of being chemically modified by various strategies, and particularly good environmental remediation. This review focuses on the efforts of ZnO-based photocatalysts, such as the methods, chemical modifications, operational parameters, and the effects after the release of effluents in aquatic matrices. Therefore, we investigated the recent advances in zinc systems aimed at treating contaminated water and their direct application in environmental remediation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The increase in water consumption and the correspondingly high levels of pollution have generated a prominent need for water management and quality (Speight 2020). Urban rivers, lakes, and streams are being gradually affected and reduced in many countries and regions due to scaled discharge of industrial and agricultural effluents, mining tailings, which includes; mining sewer systems, and wastewater treatment plants, emerging contaminants, and toxic metals, which causing negative impacts on the ecosystem (Jiang 2009; Sabater et al 2018).
Annually, thousands of different molecules with toxic potential are released into the environment, generating adverse effects on aquatic biota, food safety, water quality, and environmental sustainability (Riva et al. 2019). Emerging contaminants, for example, are chemicals of natural or synthetic origin that need to be monitored and regulated in the environment (Vashisht et al. 2020). These contaminants include; pharmaceuticals, pesticides, metals, surfactants, industrial effluents, solvents, plasticizers, and hormones. The threat of these contaminants and their complex mixtures is concentrated in the occurrence, destination, transport, toxicity, and recovery. It lies in the risks and undesirable effects for aquatic and terrestrial organisms, potentially, humans given the high biological activity, given the high biological activity and the significant deficit of treatment (Brausch and Rand 2011; Ternes et al. 2015), and the difficulty of recovery due to the nature and degree of risk posed by the contamination (Vashisht et al. 2020). Furthermore, when these contaminants are subjected to cleaning treatment, by-products are generated whose chemical properties are undetermined (Vashisht et al. 2020).
The Handbook of Environmental Chemistry cited by Rosenfeld and Feng (2011) emphasizes the danger of these compounds by stating the following point. Every day new emerging contaminant is discovered, and new disinfection by-products are also generated during treatment, with complete ignorance of their toxicity with potential or effect on human health (Rosenfeld and Feng 2011), sustainable development goals (SDGs) (Ghiasi and Malekzadeh 2014; Yusuff et al. 2019; Rai 2022). Several techniques for the remediation of contaminated areas are used to achieve the established goals. Among the main advanced remediation techniques is the remediation of contaminated areas by multiphase extraction (MPE), by chemical oxidation in situ, by removal of contaminated soil, by thermal remediation, addition to physical, chemical, electrochemical and biological processes discussed and developed around the world (Silva et al. 2018; Yusuff et al. 2019; Wu et al. 2020). Advanced Oxidative Processes (AOP) requirements for the purification and cleaning of contaminated waters due to their high performance and simplicity (Lado Ribeiro et al. 2019; Eshaq et al. 2020). It is advantageous over other competing methods as it provides complete mineralization, does not result in solid waste disposal problems, and does not generate thermal pollution (Yusuff et al. 2019).
Heterogeneous photocatalysis has gained prominence as an oxidation technique among the most popular photochemical treatment methods. Due to its heterogeneous nature, that allows it to treat water and industrial effluents in a wide pH range (Chen et al. 2020; Lum et al. 2020; Ahmad et al. 2020b), through reactions usually regulated by a free radical mechanism (OH∙, O2∙−, HO2∙)(Colmenares et al. 2017). The main attributes of this technology include the ability to use solar and/or artificial energy, low temperature and pressure conditions, low process cost, energy feasibility, susceptibility, and development of environmentally friendly and economically viable technologies with low production of tailings (Honorio et al. 2019; Mohamed Isa et al. 2021).
The demand for efficient photocatalysts is growing due to their ease and cost-effectiveness. The zinc oxide (ZnO) has been a constant focus of research and reviews in the photocatalytic area, being a promising photocatalyst with high visibility in the photochemical industry (Sanzone et al. 2018; Ruiz-Hitzky et al. 2019). By comparing important aspects, such as low cost, low toxicity, and optical properties, ZnO has contributed to advances in this segment (Mohamed et al. 2020). Based on this context, the following sections will describe the fundamentals of zinc oxide nanoparticles (pure, doped, heterostructured, immobilized, and supported) applied in degradation processes and their operational conditions for the oxidation of substances defined as organic contaminants, for example, synthetic dyes (Ahmad et al. 2021; Freitas et al. 2022), drugs (Mohamed Isa et al. 2021; Nasseh et al. 2022), and pesticides (Behera et al. 2021; Ahmad et al. 2022).
Fundamentals of heterogeneous photocatalysis
Photocatalysis has attracted great attention in many research fields due to its potential to combat environmental impacts (Chen et al. 2020). Historically, photocatalysis has been the principle of photochemistry with reports since 1911, using titanium oxide (TiO2) as a semiconductor for the first time (Serpone et al. 2012; Ahmad et al. 2020b). Its epistemological origin is attributed to the combination of “fos and katalyo” from the Greek family, which means light and decompose/degrade, respectively (Lum et al,. 2020; Ahmad et al. 2020b). In 1972, Fujishima and Honda described the process of separating water using TiO2 electrodes under UV irradiation, generating hydrogen and oxygen (Fujishima and Honda 1972; Saravanan et al. 2017). Since then, several studies have explored the photoactive properties of semiconductors, especially TiO2. Focusing mostly on pollutant degradation, water purification, and H2 production obtained through water splitting (Ahmad et al. 2020b; Rodríguez-González et al. 2020), the number of publications in the field of photocatalysis has increased annually. Figure 1 charts the linear profile of the use of this term (heterogeneous photocatalysis) as a technology for environmental management over the last decade in the Web of Science and Scopus database.
The main attributes of this technology basically include the ability to use solar and/or artificial energy, low temperature and pressure conditions, low process cost, energy feasibility, susceptibility, and development of environmentally friendly and economically viable technologies with low production of tailings. The feasibility of using photochemical technologies is emphasized by the possibility of reusing photocatalysts (operational recycling) and by the high reactive yield. It often allows the complete destruction of target pollutants of different chemical classes, forming residues with less environmental impact (Cavalcanti et al. 2019; Khan and Narula 2019; Wetchakun et al. 2019; Liu et al. 2020), thus encompassing the concept of “green treatment”(Honorio et al. 2019; Mohamed Isa et al. 2021).
A photocatalysis is defined as a technology that involves a photoinduced reaction accelerated by the presence of a semiconductor bombarded by photons of a natural or artificial nature (Kabra et al. 2004; Qian et al. 2019). In addition, its reactions and mechanisms vary and can be described in stages (Zhu and Wang 2017; Al-Mamun et al. 2019). Its general concept is understood by mechanisms of excitations and generations of hydroxyl radicals (Al-Mamun et al. 2019). The fundamental characteristic of semiconductors is the energy discontinuity between the valence band (VB—lowest energy region) and the conduction band (CB—highest energy region), with the difference being called “band gap” (Wei et al. 2016; Saravanan et al. 2017). When the absorption of a photon (hυ) is equal to or greater than the energy of the band gap, electrons (e−) are promoted from VB to CB, leaving a positive hole (h+) in VB. Thus creating an electron/hole pair, (eCB−/hVB+), or exciton(Byrne et al. 2018; Hasanpour and Hatami 2020), whose schematic representation of the mechanism is displayed in Fig. 2.
The reductive-oxidative capacity of electrons and photogenerated holes is determined by the conduction band and the potential of the valence band of the respective semiconductor particle (Molinari et al. 2017; Saravanan et al. 2017). Oxidation reactions can occur through the VB hole and H2O, producing hydroxyl radicals (Teixeira and Jardim 2004). Reduction reactions may occur between the CB electron and molecular oxygen, producing the superoxide ion. This free radical can decompose and produce hydrogen peroxide, which in turn produces hydroxyl radicals (Teixeira and Jardim 2004; Saravanan et al. 2017).
According to Qian et al. (2019), photocatalytic efficiency is comprised of two parts. The competitiveness between charge carrier recombination and entrapment (part one) and the interfacial charge transfer (part two). Both are important to understand the degradation kinetics and therefore the photocatalytic mechanism. Such mechanisms are reported in the literature and classified as direct and indirect (Rauf and Ashraf 2009; Rauf et al. 2011).
The photoactivity of a semiconductor in a photocatalytic process will depend greatly on characteristics. Such as the composition of crystals (crystalline structure), surface area, particle size distribution (adequate morphology), porosity, hydroxyl density on the surface, and adsorption characteristics/desorption, with band gap energy being an important property in the photogeneration of electron–hole pairs in semiconductors (Hisatomi et al. 2014; Qayyum et al. 2018).
Zinc dioxide (ZnO) has been a constant focus of research and reviews in the photocatalytic area. It is a promising photocatalyst with high visibility in the photochemical industry (Sanzone et al. 2018; Ruiz-Hitzky et al. 2019). By comparing important aspects, such as low cost, low toxicity, and optical properties, ZnO has contributed to advances in this segment (Qi et al. 2017; Vishnukumar et al. 2018; Mohamed et al. 2020). Under UV irradiation, both TiO2 and ZnO are highly efficient photocatalysts, since their electrons and photogenerated holes are oxidizing and reducing species, respectively (Janotti and Van de Walle 2009; Kavitha and Kumar 2019).
Strategies to improve the photocatalytic activity of ZnO
ZnO is one of the main photocatalysts applied in the management of environmental waste due to its optoelectronic, piezoelectric, catalytic, and photochemical properties (Zheng et al. 2019; Le et al. 2020), and its high quantum efficiency, which provides a significant advantage. It is an n-type semiconductor with a band gap around 3.37 eV (UV region) and a considerable free-exciton binding energy (so that excitonic emission processes can persist at or even above room temperature) (Hanh et al. 2019; Le et al. 2020). ZnO’s most common crystalline phase is the wurtzite (hexagonal) structure at room temperature. In this structure, the Zn2+ cation is surrounded by four O2−anions (Lee et al. 2016; Le et al. 2020). This tetrahedral atomic arrangement causes a non-centrosymmetric reaction in the ZnO crystal structure (Le et al. 2020). One of the relevant characteristics of ZnO is the performance of optical devices with piezoelectric properties covering applications in the field of energy technology, used in the production of closed diodes, laser diodes and photodetectors, field-effect transistors, pressure-based sensors, gas sensors, biomedical sensors, solar cells, gas detection, photovoltaic devices, and photocatalyst (Hou and Liu 2020; Bhati et al. 2020; Pinho et al. 2020). Figure 3 illustrates some functions and applications of ZnO.
From a photocatalytic point of view, the literature has addressed several strategies (doping of metal ions, doping of non-metallic immobilization, deposition, incorporation, heterojunctions, surface sensitization) to extend its light absorption to the visible range, modifying the surface of the compounds and improving photocatalytic efficiency (Abebe et al. 2020; Ahmad et al. 2020a; Ates et al. 2020; Peng et al. 2021; Sá et al. 2021). In addition, such modifications improve performance by changing the stability of particles to create structures that function as excitonic traps and regions with different redox potentials (Zhu et al. 2020).
Velmurugan et al. reported that ZnO nanoparticles from zinc oxalate dihydrate are more efficient than commercial ZnO for degrading reactive red 120 (RR120) under sunlight. Those authors attributed the efficiency of ZnO nanocrystals to the transfer of electrons from the sunlight-sensitized dye molecule to the nano-ZnO CB, which promoted an increase in the generation of free radicals and consequent degradation of the dye (Velmurugan and Swaminathan 2011).
Fe2O3 nanoparticles were selected as a sample to manufacture the multi-composite photocatalyst to improve the photocatalytic activity of ZnO and TiO2 (TiO2/Fe2O3 and ZnO/Fe2O3 based on clinoptilolite) to extend its band gap to the visible region (Davari et al. 2017). Davari et al. synthesized and evaluated the operational parameters in the degradation of the drug diphenhydramine (DPH). In the photocatalytic evaluation, ZnO/Fe2O3/Zeolite exhibited a more effective performance for the degradation of DPH than TiO2/Fe2O3/Zeolite. The photocatalytic degradation of DPH with ZnO/Fe2O3/Zeolite reached 95% at optimal conditions (Davari et al. 2017).
ZnO nanoparticles doped with lanthanum were used in the photocatalytic degradation of paracetamol. The doping process is essential in the optical absorption of visible light due to reducing particle size and gap energy. The doped photocatalyst showed more excellent photocatalytic activity than pure ZnO, reaching 99% of paracetamol degradation after three hours of irradiation (Thi and Lee 2017). Furthermore, the HPLC and GC–MS analyses demonstrated that the detected photocatalytic products aided the proposed mechanism, including the generation of radicals and subsequent reaction pathways (Thi and Lee 2017).
Sanad et al. prepared ZnO, ZnS, and ZnO@ZnS nanocomposites by the sol–gel method. They evaluated the degradation of methylene blue and eosin dyes based on the use of scavengers of active species to understand the pathways in degradation. The incorporation of ZnO onto the surface of ZnS alters the stabilization of the oxide particles, reducing the effect of recombination between the charges (e−/h+) and favoring the destruction of dyes (Sanad et al. 2018).
Bozkurt Çırak et al. manufactured ZnO nanocomposite syntheses decorated on TiO2 nanotubes using two stages. In addition, different ZnO deposition cycles were used to degrade rhodamine B (RhB) dye under UV radiation. The decorated films exhibited improved photocatalytic activity (UVC, UVB, UVA, and visible region after 10 minutes were 100, 44, 22, and 4%, respectively) and good photostability after four consecutive degradation cycles (Bozkurt Çırak et al. 2019).
Abukhadra et al. (2020) synthesized kaolinite nanotubes anchored by ZnO nanoparticles (ZnO/KNTs) and evaluated the photocatalytic oxidation of the antibiotic levofloxacin under a visible light source. ZnO/KNTs showed excellent results of 99.3, 99.6, and 99.8% by varying the dosages of photocatalyst and pH of the reaction medium. In addition, degradation was confirmed by the detection of residual TOC after analysis of the tests, and the photogenerated intermediates served to elucidate the steps of the mechanism since hydroxyl radicals were the main oxidizing species of the system (Abukhadra et al. 2020).
The g-C3N4/ZnO nanorods were prepared by simple hydrothermal, grinding, and calcination methods. The nanobasts were evaluated to degrade several essential pollutants such as MB, RhB, Cr (VI), and eosin (Zhong et al. 2020). The improved photocatalytic performance of photocatalytic nano bonds maintained high stability after five degradation cycles reaching 97% degradation (for MB) and consequently the great potential for the treatment of water pollutants. Furthermore, the tests of radical scavengers and ESR proved the degradation by proposing a photocatalytic mechanism that indicates the O2•− and •OH species as majorly significant in the degradation process (Zhong et al. 2020).
Among the methods of modifying ZnO, doping aims to improve photocatalytic performance by replacing and/or introducing a doping agent into the matrix network (Hanh et al. 2019; Shah et al. 2020). The doping effect, described by Reddy et al. (Neelakanta Reddy et al. 2018), can improve the electrical and optical properties of the semiconductor through the accumulation of impurities or the construction of intrinsic defects. In addition, doping involves forming new electronic levels inside the semiconductor, facilitating the narrowing of the bandgap and contributing to the separation of charges from the original material (Neelakanta Reddy et al. 2018). Several other dopants, including Fe, Ag, Ni, Co, Mn, Ce, and Cu (Kharatzadeh et al. 2021; Yang et al. 2021), are used to enhance the photocatalytic activity of ZnO nanoparticles (Al Abri et al. 2019; Jyothi and Ravichandran 2020).
The complexity of producing the desired property is due to phase purity, crystallinity, stoichiometry, size, and morphology (Wojnarowicz et al. 2020). Many conventional routes for preparing ZnO-based nanostructures employ physico-chemical methods (co-precipitation, precipitation, hydrothermal, thermal decomposition, microemulsion, sonochemical, ultrasonic, solid-state, sol–gel) that require specific configuration, high cost, high temperature–pressure conditions, and non-ecological chemicals (Akhtar et al. 2020; Gu et al. 2020; Wojnarowicz et al. 2020; Weldegebrieal 2020; Masmali et al. 2021). Fig. 4 presents synthesis methods used in the preparation of ZnO-based photocatalysts according to the literature (Shirdel and Behnajady 2020; Tsai et al. 2021; Kampalapura Swamy et al. 2021; Bharathi et al. 2022; Dineshbabu et al. 2022).
Another method that has been widely used is the green synthesis approach. Due to the elimination of chemical products of toxic nature and the application of environmentally correct and viable routes since it eliminates the use of toxic chemicals and applies ecological routes (Isık et al. 2019; Weldegebrieal 2020). Weldegebrieal synthesized biomolecules based on ZnO using plant extracts through the biosynthesis method. They pointed out that both the bactericidal efficacy of the compounds and the photodegradation efficiency is strongly affected by several factors such as the size, shape of the particles and the method of synthesis employed (Weldegebrieal 2020).
Parameters affecting the photoactivity of ZnO
Photocatalysts are materials whose VB and CB are separated by an energy gap widely used in photoinduced processes (Araujo et al. 2020a; Wang et al. 2020; de Sousa Filho et al. 2020; Miranda et al. 2020). Since the texture, size, and surface characteristics significantly influence the photocatalytic efficiency of metals and semiconductors, as well as their electronic and optical properties, synthetic methods are expected to lead to reliable results that obtain particles with low polydispersity, uniform size, and morphology defined in dimensions 1D, 2D, and 3D (Radhika et al. 2019). Photocatalytic degradation performance is also closely related to operational conditions that vary according to established physical–chemical tests and parameters. Such parameters that positively and/or negatively affect the process can also be optimized to ensure more excellent catalytic conversion and lower economic costs (Malato et al. 2009; Reza et al. 2017; Anwer et al. 2019; Faisal et al. 2019).
The effect of the initial concentration of the contaminant
The primary mechanism of the photocatalytic process involves the absorption of light and the formation of radical species that attack the contaminant. Therefore, the greater the concentration of contaminants, the higher the degradation rate (which is expected from the kinetic point of view), taking into account the adsorption phenomena that make the degradation more effective. After reaching the critical concentration value, which is the maximum value supported by the charge of the photocatalyst, the degradation rate is reduced due to the many layers by the adsorption of contaminant on the catalyst surface. This blocking layer makes it impossible to produce new radicals. Strategies are needed significantly to modify the photocatalyst surface structures, to avoid or minimize this negative effect caused by the high concentration of pollutants (Teixeira and Jardim 2004; Chakraborty et al. 2017; Honorio et al. 2019).
The effect of the amount of catalyst
The general trend is that greater concentration increases activity due to the more excellent radiation absorption, thus creating radicals (Ebrahiem et al. 2017; Chen et al. 2020). At high catalyst concentrations, the aggregation of the particles with a decrease in the effective surface is a limiting factor due to light scattering with a decrease in UV light penetration (Chen et al. 2020). Light scattering phenomena caused by the accumulation of suspended particles hinder light infiltration and the production of radical species (Saravanan et al. 2017). Wang and coworkers (2007) found that with the considerable increase in the amount of catalyst, the surface that adsorbs the photon is not increasing in a geometrical ratio due to inclination towards aggregation (Wang et al. 2007). Therefore, the quantity in mass ratio, or the fraction and mass, between the photocatalyst and the contaminant is significant to achieve the best photocatalytic conversion rates (Rodrigues et al. 2008; Çalışkan et al. 2017).
The effect of pH
Understanding the real influence of pH is complex due to its multiple functions (Akpan and Hameed 2009). For example, changes in pH can influence the adsorption of organic molecules by modifying the surface charge of photocatalysts (Ajmal et al. 2014; Reza et al. 2017) and the adsorption of contaminants being controlled or dominated by electrostatic forces.
Zhu et al. proved that the effect of pH is directly related to the surface charge properties of the photocatalysts and can be explained based on the point of zero charges (PZC) (Zhu et al. 2012). For ZnO pHpzc is in the pH range 8–9 (Sukriti et al. 2020). At pH values less than pHpzc or in an acidic solution, the surface of the photocatalyst is positively charged, whereas it is negatively charged under alkaline conditions (i.e.pH > pHpzc) (Akpan and Hameed 2009; Anwer et al. 2019).
In the case of dye degradation, its nature can undergo protonation or deprotonation and change its adsorption characteristics and redox activity (Konstantinou and Albanis 2004). Moreover, high pH often causes high hydroxylation of the catalyst surface due to a large number of OH˙ ions. Therefore, it facilitates the photogeneration of more hydroxyl radicals and consequently enhances the photocatalytic degradation efficiency (Caregnato et al. 2021). The effect of pH on the photocatalytic degradation of contaminants has been extensively investigated (Chen et al. 2017).
The nature and intensity of light
The operational principle of the photocatalytic reaction, the nature of light, or better, the electromagnetic radiation that provides energy to activate the photochemical mechanism, is one of the essential parameters of the phenomenon. The effective absorption of light by the photocatalyst will depend mainly on the distribution of wavelengths and the power of the light source. These characteristics directly affect the formation process of oxidative species and other chemical/physical factors hindering and/or favoring degradation (Colmenares et al. 2017; Sergejevs et al. 2019).
The effect of oxidizing agent
The action of oxidizing agents influences the photocatalytic degradation of pollutants such as dye, hydrogen peroxide (H2O2), ammonium persulfate ((NH4)2S2O8), and potassium bromate (KBrO3) (Akpan and Hameed 2009). These oxidants can trap electrons, preventing recombination, which generates radical oxidation and consequently increases photocatalytic degradation (Reza et al. 2017). Among oxidizing species, H2O2 is widely studied in advanced oxidation processes in wastewater treatment (Sakarkar et al. 2020). H2O2 plays a dual role during the degradation process, as it acts by accepting electrons from CB (Conduct Band), promoting the separation of charge carriers, and forming radical OH (Ajmal et al. 2014).
Whether through hydroxyl radicals or direct oxidation through holes, the preferred mechanism of photooxidation remains a matter of debate. The critical point is that many studies evaluate not only the efficiency of the reaction process but also the influence of active species based on the use of inhibitors and/or scavengers of electrons and holes. It indicates a possible orientation and the importance of each species in the targeted reaction in suppressing the recombination of charges (Gusmão et al. 2019; Araujo et al. 2020b; Honorio et al. 2020; Freitas et al. 2022).
The choice of organic contaminants in the photocalytic reaction
The choice of pollutants is the main factor in knowing the photocatalytic materials, mainly when synthesized. This section will describe which are the most used and how materials can influence the presence of these molecules. Water pollution poses a significant threat to the survival of modern society (Rasheed et al. 2020). The thousands of water pollutants include dyes, pesticides, detergents, drugs, heavy metals, radioactive substances, polyaromatic hydrocarbons, plasticizers, phenolic compounds, and industrial solvents, categorically divided into different classes and compositions (Rasheed et al. 2020). In addition, organic pollutants, also known as emerging pollutants (EPs), are chemical compounds with a natural or synthetic origin, which are not commonly monitored in water, soil, and air but can cause severe problems to the environment and human beings (Geissen et al. 2015). Examples of emerging pollutants include drugs (ibuprofen, azithromycin, diclofenac, losartan, and ciprofloxacin), dyes (methylene blue, curcumin, lycopene, and rhodamine B), pesticides (glyphosate, dichlorophenoxyacetic acid, and atrazine Tkaczyk et al. 2020; Sun et al. 2020).
Dyes
Dyes are organic compounds that strongly absorb wavelengths in the region of visible light, which is why they are colored (Brillas and Martínez-Huitle 2015). Their molecules contain two main groups: the chromophores responsible for the color and the auxochromes that determine the intensity of the color (Arora 2014). Classification of dyes is based on the chemical class to which they belong and the applications to which they are intended (Guaratini and Zanoni 2000; Honorio et al. 2019; Benkhaya et al. 2020a; Samsami et al. 2020), as shown in Fig. 5.
Ajmal et al. and Guaratini et al. current definitions and characteristics of the dyes. Direct dyes are designated as having interactions with the fibers through electrostatic forces; acid dyes are generally applied to nitrogen fibers or tissues in solutions of organic or inorganic acid; and dispersed dyes present low solubility in water. (Ajmal et al. 2014) (Guaratini and Zanoni 2000). However, they can interact with the polyester chains forming dispersed particles, among other corresponding characteristics.
Among the main categories of dyes used in industries, the azo type has the most significant chemical production volume, playing an essential role in dyeing, especially printing production (Benkhaya et al. 2020b). Azo dyes are characterized by the group –N = N– linked to aromatic groups, benzene rings, naphthalenes, aromatic heterocyclics, or aliphatic groups and constitute 70% of the total production used in the industries(Rauf et al. 2011; Sen et al. 2016). These dyes can behave as anionic (deprotonation in the acid group), cationic (protonated in the amino group), or non-ionic, depending on the pH of the medium (Ajmal et al. 2014). Due to inefficiency during the dyeing process, they accumulate in water bodies and cause adverse effects related to dissolved oxygen (DO), biological oxygen demand (BOD), and chemical oxygen demand (COD), in addition to being highly resistant to chemical and photochemical degradation processes. This problem urgently requires new treatment routes to eliminate and/or convert them into functional and safe products (Teixeira and Jardim 2004; Benkhaya et al. 2020b).
Among the azo class, one of the most used dyes as a model pollutant is the methylene blue (MB) dye, also known as tetramethylthionine chloride, is a cationic dye that has a heterocyclic aromatic structure and is widely used as a model for the removal of dyes from aqueous solutions (Neris et al. 2019; Jaleh et al. 2021). Widely used in the textile industry, when discarded without prior and appropriate treatment, MB poses severe risks for contamination of the environment and ecosystems, intoxication, and to human beings (Phuruangrat et al. 2019; Hernández-Carrillo et al. 2020).
In 2019, ZnO and Ag-ZnO (doped) nanocomposites synthesized via ultrasound were investigated considering the degradation of methylene blue (MB) dye in an aqueous solution under sunlight irradiation (Satdeve et al. 2019). Several operational parameters were discussed, such as the effect of the initial dye concentration, catalyst dosage, and pH of the solution to observe the experiments optimization. The maximum activity reached 96.2% at the end of 120 min, confirming that ZnO doped with Ag presents better degradation concerning pure ZnO (89.77%) under similar conditions. The high activity is explained by the decrease in the recombination rate of the electron–hole pair with efficient transfer of electrons to dissolved oxygen, leading to the formation of radical species. In addition, a comparative study on MB degradation using the Ag-ZnO nanocomposite synthesized by different synthesis methods was observed, showing that the Ag-ZnO nanocomposite ultrasonically showed better degradation of the MB dye. Due to the fine dispersion of Ag in ZnO and the particle size that favored the increase in the surface area, more significant degradation of the MB dye (Satdeve et al. 2019).
Ramos-Corona et al. (Ramos-Corona et al. 2019) evaluated a new system based on ZnO doped with nitrogen (ZT) and supported on graphene oxide (GO) in the degradation of the methylene blue dye. Compared to the ZnO and ZT photocatalysts, ZTGO reached 95% degradation of the MB under UV energy in 35 min. The activity was attributed to the GO, which has a synergic effect with the N-doped ZnO as a collector and electron transporter. (Ramos-Corona et al. 2019).
In 2020, ZnO structures with flower-shaped morphology were obtained in conjunction with AG (Arabic Gum) and KG (Karaya Gum) polysaccharides, and the formation of the hexagonal structure of ZnO present in the structure was confirmed (Araujo et al. 2020c). Due to the better optical and morphological properties, the photocatalyst KGZnO showed better performance in the photodegradation of the pollutant under irradiation of visible light. Photophysical processes and photocatalytic activity were essential in interpreting the behavior between the dynamic separation of charge carriers and the recombination process (Araujo et al. 2020c). The presence of hijackers of active species and the study of toxicological assessment using artemia salina as a bioindicator were investigated (Araujo et al. 2020c).
Pharmaceutical drugs and pesticides
Although advances in the pharmaceutical industry are extremely relevant and beneficial to human and animal health, significant amounts of pharmaceutical products are released into the environment causing risks and uncertainties associated with a lack of knowledge about their fate, absorption, metabolism, excretion, incorrect dumping, and persistence. They are the main forms of contamination that impact the lives of people, the environment, and ecosystems (Mackuľak et al. 2017; Rastogi et al. 2018). Pharmaceutical products are considered emerging contaminants due to their increasing presence in environmental systems, such as surface water (rivers, oceans, and lakes), groundwater, and potable water (after conventional water treatment). Their high biological activity, limited information on their effects on aquatic ecosystems, and formation of metabolites of unknown nature (Bade et al. 2018; Castrignanò et al. 2018). In addition, to being substances absorbed by the body, cause uncertainty about their effects (Bila and Dezotti 2003).
Ebele et al. define pharmaceutical products as over-the-counter and prescription medications used for therapeutic and veterinary uses with the purpose of preventing and/or treating diseases and guaranteeing an improvement in the quality of life (Ebele et al. 2017). This same concept was reported as pseudo-persistent compounds due to their consumption, properties, and resistance in aquatic environments (Im et al. 2020).
Anti-inflammatory drugs are called “pseudo-persistent” compounds, such as diclofenac (Khetan and Collins 2007; Magureanu et al. 2015), a contaminant commonly found in aquatic environments, with its degradation elucidated in several studies (Mugunthan et al. 2019; Rueda-Salaya et al. 2020).
Another non-steroidal compound widely found in water bodies is ibuprofen (Wang et al. 2019). This anti-inflammatory, analgesic, and antipyretic medication is consumed for medical and veterinary health (Wang et al. 2019). Khedr et al. found that different proportions between the anatase/brookite TiO2 (A/B TiO2) phases synthesized by the hydrothermal process are capable of degrading 100% of ibuprofen over 5 h (Khedr et al. 2019).
Akkari et al. synthesized photocatalysts based on the assembly of ZnO nanoparticles on the surface of sepiolite (Sep-clay material) and evaluated the degradation of pollutants such as ibuprofen, acetaminophen, and antipyrine under sunlight. The ZnO/Sep heterostructure exhibited improved photocatalytic performance compared to ZnO/SiO-Sep materials, with ibuprofen being the target compound with the highest degradation rate (Akkari et al. 2018).
Carbamazepine (CBZ) is an antiepileptic drug highly persistent and resistant to biodegradation, whose global consumption is around one thousand tons per year. Harboune et al. and Martinez et al. studied the CBZ photodegradation catalyzed by TiO2 and ZnO, identified intermediates, and elucidated its degradation mechanism under UV-C/Vis irradiation (Martínez et al. 2011; Haroune et al. 2014). Caregnato et al. found that Ce-doped ZnO efficiently removed 53% of CBZ within 180 min under visible light irradiation (λexc. ≥ 475 nm) (Caregnato et al. 2021).
A huge variety of pharmaceutical products, including synthetic, anti-inflammatory, and antidepressant hormones have been detected by advanced analytical techniques in soils, surface waters, sediments, groundwater, and marine ecosystems (Gaw et al. 2014). Thus, numerous classes of pharmaceutical compounds are normally found contaminating waters (Magureanu et al. 2015).
The actions of antibiotics are essential due to the resistance of bacteria and impacts on fish fertility, which further reinforces the concern about the endocrine potential of these drugs (Overturf et al. 2015; Rosendo et al. 2020). Due to health and environmental risks ranging from antimicrobial resistance, endocrine disruption, toxicity, and carcinogenicity, triclosan (TCS) is classified as an emerging contaminant with antimicrobial and fungicidal effects, widely used in the composition of personal hygiene products (Kosera et al. 2017; Azarpira et al. 2019). Kosera et al. (2017) evaluated the degradation capacity of triclosan using zinc oxide (ZnO) immobilized in the sodium alginate biopolymer. They found that the preparation of immobilized ZnO in biopolymer is an interesting alternative with cost–benefit, considering that biodegradable substrates are attractive from the environmental point of view and improve degradation (90%—under solar irradiation)(Kosera et al. 2017).
Pesticides play an important role in agriculture, helping control and prevent plant diseases and pests (Rousis et al. 2017; Jørgensen et al. 2019). Pesticides are considered emerging contaminants due to their high toxicity, low biodegradability, and environmental persistence (Evgenidou et al. 2005; Bachmann Pinto et al. 2018). The main groups of agricultural pesticides can be categorized by mode of action and chemical composition and their target organism (Huang et al. 2018; Mojiri et al. 2020). According to Ali et al. (2019), less than 1% (representative) of the applied pesticides reach their target organisms, and most of them remain in the soil and water. (Ali et al. 2019).
Herbicides represent about 46% of all global markets (Balmer et al. 2019) and are used in agriculture to control weeds (Harrington and Ghanizadeh 2017). Fungicides are often used in agriculture as prophylaxis to prevent diseases (Santísima-Trinidad et al. 2018). These bioactive and toxic substances directly or indirectly influence the soil's productivity and the agroecosystem’s quality, possessing several modes of action and can have a wide range or be limited to a specific group of fungi (Mojiri et al. 2020). Insecticides are widely used to control insect pests, and their environmental safety remains a constant concern (Mojiri et al. 2020).
A chlorinated and highly persistent pesticide in the environment is lindane. The degradation was evaluated using Zn@ZnO CS nanocomposites as a photocatalyst (Jung et al. 2018). The authors pointed out that the hydroxyl radical (•OH) was the main reactive species for the degradation of lindane, which reached about 99.5% in 40 min under UV light. In addition, they conclude that defects, such as oxygen vacancies, can act as electron acceptors avoiding the process of recombination between charge carriers (Jung et al. 2018).
In this sense, due to the growing concern about the presence of these contaminants in the environment and their possible effects, researchers are increasingly investigating the degradation of pollutants using Zn-based photocatalysts Table 1.
Final remarks
Concomitantly, associated with concern about environmental impacts and the need for a better understanding of the occurrence and risks in water quality. This work evaluated the photocatalytic potential in the degradation of organic contaminants as an alternative to intense research in treating pollutants. The study showed that ZnO has excellent potential in the recovery of wastewater after breaking complex structures of contaminants into by-products of lesser aquatic impact, combined with photocatalysis and its various operational factors, ensuring safety for the health and well-being of people.
The reported experiments showed that the main limitation of ZnO is still to absorb only in the UV region, requiring structural modifications through effective methodologies to improve the quality of photocatalysts and their use in the visible region. Another challenge still observed is the recovery of photocatalysts for reuse, since most of the photocatalysts of ZnO and derivatives were in the form of powders. Among the main factors that affect the performance of ZnO and derivatives are parameters such as pH, the concentration of photocatalyst, concentration of the pollutant, source, and intensity of light, and others. Dyes are considered the most studied villains and are reported with effective/reliable results. However, few studies address degradation based on identifying photogenerated by-products and the proposal of appropriate mechanisms paths. There are several difficulties in developing stable photocatalysts and promising technologies due to the complexity of action plans and critical strategies for minimizing the cost–benefit of these materials (Ani et al. 2018).
Therefore, this review enhances points of scientific impact approaches, in particular the significant production and advances in the state of the art of ZnO, its structural and physical–chemical properties that represent principles of Green Chemistry, due to its non-toxicity, facile synthesis and chemical modification, low-cost and, standing out in the direct contribution to new compounds and the improvement of processes in environmental remediation.
Data availability
All data generated or analysed during this study are included in this published article.
References
Abebe B, Murthy HCA, Amare E (2020) Enhancing the photocatalytic efficiency of ZnO: defects, heterojunction, and optimization. Environ Nanotechnol Monit Manag. https://doi.org/10.1016/j.enmm.2020.100336
Abukhadra MR, Helmy A, Sharaf MF et al (2020) Instantaneous oxidation of levofloxacin as toxic pharmaceutical residuals in water using clay nanotubes decorated by ZnO (ZnO/KNTs) as a novel photocatalyst under visible light source. J Environ Manage 271:111019. https://doi.org/10.1016/j.jenvman.2020.111019
Ahmad I, Shoaib Akhtar M, Ahmed E et al (2020) Rare earth co-doped ZnO photocatalysts: Solution combustion synthesis and environmental applications. Sep Purif Technol 237:116328
Ahmad K, Ghatak HR, Ahuja SM (2020) A review on photocatalytic remediation of environmental pollutants and H2 production through water splitting: a sustainable approach. Environ Technol Innov 19:100893
Ahmad M, Abbas G, Tanveer M, Zubair M (2022) ZnO and TiO2 Assisted Photocatalytic Degradation of Butachlor in Aqueous Solution under Visible Light. In: The 1st International Conference on Energy, Power and Environment. MDPI. Basel Switzerland. 77
Ahmad M, Rehman W, Khan MM et al (2021) Phytogenic fabrication of ZnO and gold decorated ZnO nanoparticles for photocatalytic degradation of Rhodamine B. J Environ Chem Eng 9:104725. https://doi.org/10.1016/j.jece.2020.104725
Ajmal A, Majeed I, Malik RN et al (2014) Principles and mechanisms of photocatalytic dye degradation on TiO2 based photocatalysts: a comparative overview. RSC Adv 4:37003–37026. https://doi.org/10.1039/C4RA06658H
Akhtar J, Tahir MB, Sagir M, Bamufleh HS (2020) Improved photocatalytic performance of Gd and Nd co-doped ZnO nanorods for the degradation of methylene blue. Ceram Int 46:11955–11961. https://doi.org/10.1016/j.ceramint.2020.01.234
Akkari M, Aranda P, Belver C et al (2018) Reprint of ZnO/sepiolite heterostructured materials for solar photocatalytic degradation of pharmaceuticals in wastewater. Appl Clay Sci 160:3–8. https://doi.org/10.1016/j.clay.2018.02.027
Akpan UG, Hameed BH (2009) Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: a review. J Hazard Mater 170:520–529. https://doi.org/10.1016/j.jhazmat.2009.05.039
Al-Mamun MR, Kader S, Islam MS, Khan MZH (2019) Photocatalytic activity improvement and application of UV-TiO2 photocatalysis in textile wastewater treatment: a review. J Environ Chem Eng 7:103248. https://doi.org/10.1016/j.jece.2019.103248
Al Abri R, Al Marzouqi F, Kuvarega AT et al (2019) Nanostructured cerium-doped ZnO for photocatalytic degradation of pharmaceuticals in aqueous solution. J Photochem Photobiol A Chem 384:112065. https://doi.org/10.1016/j.jphotochem.2019.112065
Alam U, Shah TA, Khan A, Muneer M (2019) One-pot ultrasonic assisted sol-gel synthesis of spindle-like Nd and V codoped ZnO for efficient photocatalytic degradation of organic pollutants. Sep Purif Technol 212:427–437. https://doi.org/10.1016/j.seppur.2018.11.048
Ali I, Alharbi OML, ALOthman ZA, et al (2019) Modeling of fenuron pesticide adsorption on CNTs for mechanistic insight and removal in water. Environ Res 170:389–397. https://doi.org/10.1016/j.envres.2018.12.066
Ani IJ, Akpan UG, Olutoye MA, Hameed BH (2018) Photocatalytic degradation of pollutants in petroleum refinery wastewater by TiO2- and ZnO-based photocatalysts: recent development. J Clean Prod 205:930–954. https://doi.org/10.1016/j.jclepro.2018.08.189
Anwer H, Mahmood A, Lee J et al (2019) Photocatalysts for degradation of dyes in industrial effluents: opportunities and challenges. Nano Res 12:955–972. https://doi.org/10.1007/s12274-019-2287-0
Araujo FP, Honorio LMC, Lima IS et al (2020) New composite TiO2/naturals gums for high efficiency in photodiscoloration process. Ceram Int 46:15534–15543. https://doi.org/10.1016/j.ceramint.2020.03.100
Araujo FP, Trigueiro P, Honório LMC et al (2020) A novel green approach based on ZnO nanoparticles and polysaccharides for photocatalytic performance. Dalt Trans 49:16394–16403. https://doi.org/10.1039/D0DT01128B
Araujo FP, Trigueiro P, Honório LMC et al (2020) Eco-friendly synthesis and photocatalytic application of flowers-like ZnO structures using Arabic and Karaya Gums. Int J Biol Macromol 165:2813–2822. https://doi.org/10.1016/j.ijbiomac.2020.10.132
Arora S (2014) Textile dyes: it’s impact on environment and its treatment. J Bioremediation Biodegrad 05:6199. https://doi.org/10.4172/2155-6199.1000e146
Arunpandian M, Selvakumar K, Raja A et al (2019) Fabrication of novel Nd2O3/ZnO-GO nanocomposite: an efficient photocatalyst for the degradation of organic pollutants. Colloids Surf A Physicochem Eng Asp 567:213–227. https://doi.org/10.1016/j.colsurfa.2019.01.058
Ates M, Danabas D, Ertit Tastan B et al (2020) Assessment of oxidative stress on artemia salina and daphnia magna after exposure to Zn and ZnO nanoparticles. Bull Environ Contam Toxicol 104:206–214. https://doi.org/10.1007/s00128-019-02751-6
Azarpira H, Sadani M, Abtahi M et al (2019) Photo-catalytic degradation of triclosan with UV/iodide/ZnO process: performance, kinetic, degradation pathway, energy consumption and toxicology. J Photochem Photobiol A Chem 371:423–432. https://doi.org/10.1016/j.jphotochem.2018.10.041
Bachmann Pinto H, Miguel de Souza B, Dezotti M (2018) Treatment of a pesticide industry wastewater mixture in a moving bed biofilm reactor followed by conventional and membrane processes for water reuse. J Clean Prod 201:1061–1070. https://doi.org/10.1016/j.jclepro.2018.08.113
Bade R, White JM, Gerber C (2018) Qualitative and quantitative temporal analysis of licit and illicit drugs in wastewater in Australia using liquid chromatography coupled to mass spectrometry. Anal Bioanal Chem 410:529–542. https://doi.org/10.1007/s00216-017-0747-2
Balmer JE, Morris AD, Hung H et al (2019) Levels and trends of current-use pesticides (CUPs) in the arctic: an updated review, 2010–2018. Emerg Contam 5:70–88. https://doi.org/10.1016/j.emcon.2019.02.002
Behera M, Tiwari N, Basu A et al (2021) Maghemite/ZnO nanocomposites: a highly efficient, reusable and non-noble metal catalyst for reduction of 4-nitrophenol. Adv Powder Technol 32:2905–2915. https://doi.org/10.1016/j.apt.2021.06.005
Belaissa Y, Nibou D, Assadi AA et al (2016) A new hetero-junction p-CuO/n-ZnO for the removal of amoxicillin by photocatalysis under solar irradiation. J Taiwan Inst Chem Eng 68:254–265. https://doi.org/10.1016/j.jtice.2016.09.002
Benkhaya S, Mrabet S, El Harfi A (2020) A review on classifications, recent synthesis and applications of textile dyes. Inorg Chem Commun. https://doi.org/10.1016/j.inoche.2020.107891
Benkhaya S, M’rabet S, El Harfi A (2020) Classifications, properties, recent synthesis and applications of azo dyes. Heliyon. 6:e03271. https://doi.org/10.1016/j.heliyon.2020.e03271
Bharathi D, Thiruvengadam Nandagopal JG, Rajamani R et al (2022) Enhanced photocatalytic activity of St-ZnO nanorods for methylene blue dye degradation. Mater Lett 311:131637. https://doi.org/10.1016/j.matlet.2021.131637
Bhati VS, Hojamberdiev M, Kumar M (2020) Enhanced sensing performance of ZnO nanostructures-based gas sensors: a review. Energy Rep 6:46–62. https://doi.org/10.1016/j.egyr.2019.08.070
Bila DM, Dezotti M (2003) Fármacos no meio ambiente. Quim Nova 26:523–530. https://doi.org/10.1590/S0100-40422003000400015
Bozkurt Çırak B, Caglar B, Kılınç T et al (2019) Synthesis and characterization of ZnO nanorice decorated TiO2 nanotubes for enhanced photocatalytic activity. Mater Res Bull 109:160–167. https://doi.org/10.1016/j.materresbull.2018.09.039
Brausch JM, Rand GM (2011) A review of personal care products in the aquatic environment: environmental concentrations and toxicity. Chemosphere 82:1518–1532. https://doi.org/10.1016/j.chemosphere.2010.11.018
Brillas E, Martínez-Huitle CA (2015) Applied catalysis B : environmental decontamination of wastewaters containing synthetic organic dyes by electrochemical methods. an updated review. “Applied Catal B. Environ 166–167:603–643. https://doi.org/10.1016/j.apcatb.2014.11.016
Byrne C, Subramanian G, Pillai SC (2018) Recent advances in photocatalysis for environmental applications. J Environ Chem Eng 6:3531–3555. https://doi.org/10.1016/j.jece.2017.07.080
Çalışkan Y, Yatmaz HC, Bektaş N (2017) Photocatalytic oxidation of high concentrated dye solutions enhanced by hydrodynamic cavitation in a pilot reactor. Process Saf Environ Prot 111:428–438. https://doi.org/10.1016/j.psep.2017.08.003
Caregnato P, Espinosa Jiménez KR, Villabrille PI (2021) Ce-doped ZnO as photocatalyst for carbamazepine degradation. Catal Today 372:183–190. https://doi.org/10.1016/j.cattod.2020.07.031
Castrignanò E, Yang Z, Bade R et al (2018) Enantiomeric profiling of chiral illicit drugs in a pan-European study. Water Res 130:151–160. https://doi.org/10.1016/j.watres.2017.11.051
Cavalcanti GRS, Fonseca MG, da Silva Filho EC, Jaber M (2019) Thiabendazole/bentonites hybrids as controlled release systems. Colloids Surf B Biointerf 176:249–255. https://doi.org/10.1016/j.colsurfb.2018.12.030
Chakraborty S, Loutatidou S, Palmisano G et al (2017) Photocatalytic hollow fiber membranes for the degradation of pharmaceutical compounds in wastewater. J Environ Chem Eng 5:5014–5024. https://doi.org/10.1016/j.jece.2017.09.038
Chen D, Cheng Y, Zhou N et al (2020) Photocatalytic degradation of organic pollutants using TiO2 based photocatalysts: a review. J Clean Prod. https://doi.org/10.1016/j.jclepro.2020.121725
Chen F, Yang Q, Li X et al (2017) Hierarchical assembly of graphene-bridged Ag3 PO4 /Ag/BiVO4 (040) Z-scheme photocatalyst: an efficient, sustainable and heterogeneous catalyst with enhanced visible-light photoactivity towards tetracycline degradation under visible light irradiation. Appl Catal B Environ 200:330–342. https://doi.org/10.1016/j.apcatb.2016.07.021
Colmenares JC, Varma RS, Nair V (2017) Selective photocatalysis of lignin-inspired chemicals by integrating hybrid nanocatalysis in microfluidic reactors. Chem Soc Rev 46:6675–6686. https://doi.org/10.1039/C7CS00257B
Das S, Ghosh S, Misra A et al (2018) Sunlight assisted photocatalytic degradation of ciprofloxacin in water using Fe Doped ZnO nanoparticles for potential public health applications. Int J Environ Res Public Health 15:2440. https://doi.org/10.3390/ijerph15112440
Davari N, Farhadian M, Nazar ARS, Homayoonfal M (2017) Degradation of diphenhydramine by the photocatalysts of ZnO/Fe2O3 and TiO2/Fe2O3 based on clinoptilolite: Structural and operational comparison. J Environ Chem Eng 5:5707–5720. https://doi.org/10.1016/j.jece.2017.10.052
de Sousa Filho IA, Arana LR, Doungmo G et al (2020) SrSnO3/g-C3N4 and sunlight: Photocatalytic activity and toxicity of degradation byproducts. J Environ Chem Eng 8:103633. https://doi.org/10.1016/j.jece.2019.103633
Dineshbabu N, Jayaprakash RN, Karuppasamy P et al (2022) Investigation on Tetracycline degradation and bactericidal properties of binary and ternary ZnO/NiO/g-C3N4 composites prepared by a facile co-precipitation method. J Environ Chem Eng 10:107368. https://doi.org/10.1016/j.jece.2022.107368
Ebele AJ, Abou-Elwafa Abdallah M, Harrad S (2017) Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerg Contam 3:1–16. https://doi.org/10.1016/j.emcon.2016.12.004
Ebrahiem EE, Al-Maghrabi MN, Mobarki AR (2017) Removal of organic pollutants from industrial wastewater by applying photo-Fenton oxidation technology. Arab J Chem 10:S1674–S1679. https://doi.org/10.1016/j.arabjc.2013.06.012
Eshaq G, Wang S, Sun H, Sillanpaa M (2020) Superior performance of FeVO4@CeO2 uniform core-shell nanostructures in heterogeneous Fenton-sonophotocatalytic degradation of 4-nitrophenol. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2019.121059
Evgenidou E, Fytianos K, Poulios I (2005) Semiconductor-sensitized photodegradation of dichlorvos in water using TiO2 and ZnO as catalysts. Appl Cataly B Environ. 59:81–89. https://doi.org/10.1016/j.apcatb.2005.01.005
Faisal M, Harraz FA, Ismail AA et al (2019) Novel synthesis of Polyaniline/SrSnO3 nanocomposites with enhanced photocatalytic activity. Ceram Int 45:20484–20492. https://doi.org/10.1016/j.ceramint.2019.07.027
Freitas WA, E.C.F. Soares B, Rodrigues MS et al (2022) Facile synthesis of ZnO-clay minerals composites using an ultrasonic approach for photocatalytic performance. J Photochem Photobiol A Chem. https://doi.org/10.1016/j.jphotochem.2022.113934
Fujishima A, Honda K (1972) Electrochemical photolysis of water at a semiconductor electrode. Nature 238:37–38. https://doi.org/10.1038/238037a0
Gaw S, Thomas KV, Hutchinson TH (2014) Sources, impacts and trends of pharmaceuticals in the marine and coastal environment. Philos Trans R Soc B Biol Sci 369:20130572. https://doi.org/10.1098/rstb.2013.0572
Geissen V, Mol H, Klumpp E et al (2015) Emerging pollutants in the environment: a challenge for water resource management. Int Soil Water Conserv Res 3:57–65. https://doi.org/10.1016/j.iswcr.2015.03.002
Ghiasi M, Malekzadeh A (2014) Solar photocatalytic degradation of methyl orange over La0.7Sr0.3MnO3 nano-perovskite. Sep Purif Technol 134:12–19. https://doi.org/10.1016/j.seppur.2014.07.022
Gu X, Edvinsson T, Zhu J (2020) ZnO nanomaterials: strategies for improvement of photocatalytic and photoelectrochemical activities. Current developments in photocatalysis and photocatalytic materials. Elsevier, Amsterdam Netherland, pp 231–244
Guaratini CCI, Zanoni VB (2000) Corantes têxteis. Quim Nova 23:71–78. https://doi.org/10.1590/S0100-40422000000100013
Gusmão SBS, Ghosh A, Marques TMF et al (2019) Titanate-based one-dimensional nano-heterostructure: study of hydrothermal reaction parameters for improved photocatalytic application. Solid State Sci 98:106043. https://doi.org/10.1016/j.solidstatesciences.2019.106043
Hanh NT, Le Minh TN, Van Thuan D et al (2019) Monocrotophos pesticide effectively removed by novel visible light driven Cu doped ZnO photocatalyst. J Photochem Photobiol A Chem 382:111923. https://doi.org/10.1016/j.jphotochem.2019.111923
Haroune L, Salaun M, Ménard A et al (2014) Photocatalytic degradation of carbamazepine and three derivatives using TiO2 and ZnO: Effect of pH, ionic strength, and natural organic matter. Sci Total Environ 475:16–22. https://doi.org/10.1016/j.scitotenv.2013.12.104
Harrington KC, Ghanizadeh H (2017) Herbicide application using wiper applicators—A review. Crop Prot 102:56–62. https://doi.org/10.1016/j.cropro.2017.08.009
Hasanpour M, Hatami M (2020) Photocatalytic performance of aerogels for organic dyes removal from wastewaters: review study. J Mol Liq. https://doi.org/10.1016/j.molliq.2020.113094
He J, Zhang Y, Guo Y et al (2019) Photocatalytic degradation of cephalexin by ZnO nanowires under simulated sunlight: kinetics, influencing factors, and mechanisms. Environ Int 132:105105. https://doi.org/10.1016/j.envint.2019.105105
Hernández-Carrillo MA, Torres-Ricárdez R, García-Mendoza MF et al (2020) Eu-modified ZnO nanoparticles for applications in photocatalysis. Catal Today 349:191–197. https://doi.org/10.1016/j.cattod.2018.04.060
Hisatomi T, Kubota J, Domen K (2014) Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem Soc Rev 43:7520–7535. https://doi.org/10.1039/c3cs60378d
Honorio LMC, de Oliveira ALM, da Silva Filho EC et al (2020) Supporting the photocatalysts on ZrO2: an effective way to enhance the photocatalytic activity of SrSnO3. Appl Surf Sci. https://doi.org/10.1016/j.apsusc.2020.146991
Honorio LMC, Trigueiro PA, Viana BC et al (2019) Nanostructured materials for the photocatalytic degradation of organic pollutants in water. Nanostructured materials for treating aquatic pollution. Springer International Publishing, Berlin Germany, pp 65–90
Hou Q, Liu Y (2020) Effect of Li doping and point vacancy on photocatalytic properties and mechanism of ZnO. Chem Phys 531:110657. https://doi.org/10.1016/j.chemphys.2019.110657
Huang Y, Xiao L, Li F et al (2018) Microbial degradation of pesticide residues and an emphasis on the degradation of cypermethrin and 3-phenoxy benzoic acid: a review. Molecules 23:2313. https://doi.org/10.3390/molecules23092313
Im JK, Hwang MY, Lee EH et al (2020) Pharmaceutical compounds in tributaries of the Han River watershed. Environ Res, South Korea. https://doi.org/10.1016/j.envres.2020.109758
Isik T, Elhousseini Hilal M, Horzum N (2019) Green Synthesis of Zinc Oxide Nanostructures. In: Zinc Oxide Based Nano Materials and Devices. IntechOpen.
Jaleh B, Nasrollahzadeh M, Mohazzab BF et al (2021) State-of-the-art technology: recent investigations on laser-mediated synthesis of nanocomposites for environmental remediation. Ceram Int 47:10389–10425. https://doi.org/10.1016/j.ceramint.2020.12.197
Janotti A, Van de Walle CG (2009) Fundamentals of zinc oxide as a semiconductor. Reports Prog Phys. 72:126501. https://doi.org/10.1088/0034-4885/72/12/126501
Jiang Y (2009) China’s water scarcity. J Environ Manage 90:3185–3196. https://doi.org/10.1016/j.jenvman.2009.04.016
Jørgensen LN, Kudsk P, Ørum JE (2019) Links between pesticide use pattern and crop production in Denmark with special reference to winter wheat. Crop Prot 119:147–157. https://doi.org/10.1016/j.cropro.2019.01.024
Jung HJ, Koutavarapu R, Lee S et al (2018) Enhanced photocatalytic degradation of lindane using metal–semiconductor Zn@ZnO and ZnO/Ag nanostructures. J Environ Sci 74:107–115. https://doi.org/10.1016/j.jes.2018.02.014
Jyothi NS, Ravichandran K (2020) Optimum pH for effective dye degradation: Mo, Mn, Co and Cu doped ZnO photocatalysts in thin film form. Ceram Int 46:23289–23292. https://doi.org/10.1016/j.ceramint.2020.06.076
Kabra K, Chaudhary R, Sawhney RL (2004) Treatment of hazardous organic and inorganic compounds through aqueous-phase photocatalysis: a review. Ind Eng Chem Res 43:7683–7696. https://doi.org/10.1021/ie0498551
Kampalapura Swamy C, Hezam A, Mavinakere Ramesh A et al (2021) Microwave hydrothermal synthesis of copper induced ZnO/gC3N4 heterostructure with efficient photocatalytic degradation through S-scheme mechanism. J Photochem Photobiol A Chem 418:113394. https://doi.org/10.1016/j.jphotochem.2021.113394
Kavitha R, Kumar SG (2019) A review on plasmonic Au-ZnO heterojunction photocatalysts: preparation, modifications and related charge carrier dynamics. Mater Sci Semicond Process 93:59–91. https://doi.org/10.1016/j.mssp.2018.12.026
Khan S, Narula AK (2019) Ternary photocatalyst based on conducting polymer doped functionalized multiwall carbon nanotubes decorated with nanorods of metal oxide. Mater Sci Eng B Solid-State Mater Adv Technol 243:86–95. https://doi.org/10.1016/j.mseb.2019.04.002
Kharatzadeh E, Masharian SR, Yousefi R (2021) The effects of S-doping concentration on the photocatalytic performance of SnSe/S-GO nanocomposites. Adv Powder Technol 32:346–357. https://doi.org/10.1016/j.apt.2020.12.013
Khedr TM, El-Sheikh SM, Ismail AA, Bahnemann DW (2019) Highly efficient solar light-assisted TiO2 nanocrystalline for photodegradation of ibuprofen drug. Opt Mater (amst) 88:117–127. https://doi.org/10.1016/j.optmat.2018.11.027
Khetan SK, Collins TJ (2007) Human pharmaceuticals in the aquatic environment: a challenge to green chemistry. Chem Rev 107:2319–2364. https://doi.org/10.1021/cr020441w
Konstantinou IK, Albanis TA (2004) TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: kinetic and mechanistic investigations. Appl Catal B Environ 49:1–14. https://doi.org/10.1016/j.apcatb.2003.11.010
Kosera VS, Cruz TM, Chaves ES, Tiburtius ERL (2017) Triclosan degradation by heterogeneous photocatalysis using ZnO immobilized in biopolymer as catalyst. J Photochem Photobiol A Chem 344:184–191. https://doi.org/10.1016/j.jphotochem.2017.05.014
Lado Ribeiro AR, Moreira NFF, Li Puma G, Silva AMT (2019) Impact of water matrix on the removal of micropollutants by advanced oxidation technologies. Chem Eng J 363:155–173. https://doi.org/10.1016/j.cej.2019.01.080
Le AT, Ahmadipour M, Pung SY (2020) A review on ZnO-based piezoelectric nanogenerators: Synthesis, characterization techniques, performance enhancement and applications. J Alloys Compd 844:156172. https://doi.org/10.1016/j.jallcom.2020.156172
Lee KM, Lai CW, Ngai KS, Juan JC (2016) Recent developments of zinc oxide based photocatalyst in water treatment technology: a review. Water Res 88:428–448. https://doi.org/10.1016/j.watres.2015.09.045
Liu J, Luo Z, Han W et al (2020) Preparation of ZnO/Bi2WO6 heterostructures with improved photocatalytic performance. Mater Sci Semicond Process 106:104761. https://doi.org/10.1016/j.mssp.2019.104761
Lum PT, Foo KY, Zakaria NA, Palaniandy P (2020) Ash based nanocomposites for photocatalytic degradation of textile dye pollutants: a review. Mater Chem Phys 241:122405. https://doi.org/10.1016/j.matchemphys.2019.122405
Luque PA, Garrafa-Gálvez HE, García-Maro CA, Robles CAS (2022) Study of the optical properties of ZnO semiconductor nanoparticles using Origanum vulgare and its effect in Rhodamine B degradation. Optik (stuttg). https://doi.org/10.1016/j.ijleo.2022.168937
Mackuľak T, Marton M, Radičová M et al (2017) Monitoring of micropollutants and resistant bacteria in wastewater and their effective removal by boron doped diamond electrode. Monatshefte Fur Chemie 148:539–548. https://doi.org/10.1007/s00706-016-1914-4
Magureanu M, Mandache NB, Parvulescu VI (2015) Degradation of pharmaceutical compounds in water by non-thermal plasma treatment. Water Res 81:124–136. https://doi.org/10.1016/j.watres.2015.05.037
Malato S, Fernández-Ibáñez P, Maldonado MI et al (2009) Decontamination and disinfection of water by solar photocatalysis: recent overview and trends. Catal Today 147:1–59. https://doi.org/10.1016/j.cattod.2009.06.018
Martínez C, Canle LM, Fernández MI et al (2011) Kinetics and mechanism of aqueous degradation of carbamazepine by heterogeneous photocatalysis using nanocrystalline TiO2, ZnO and multi-walled carbon nanotubes–anatase composites. Appl Catal B Environ 102:563–571. https://doi.org/10.1016/j.apcatb.2010.12.039
Masmali NA, Osman Z, Arof AK (2021) Recent developments in zinc-based two-cation oxide spinels: from synthesis to applications. Ceram Int 47:2949–2962. https://doi.org/10.1016/j.ceramint.2020.09.249
Miranda MO, Viana BC, Honório LM et al (2020) Oxide-clay mineral as photoactive material for dye discoloration. Minerals 10:132. https://doi.org/10.3390/min10020132
Mohamed Isa ED, Shameli K, Ch’ng HJ et al (2021) Photocatalytic degradation of selected pharmaceuticals using green fabricated zinc oxide nanoparticles. Adv Powder Technol 32:2398–2409. https://doi.org/10.1016/j.apt.2021.05.021
Mohamed WAA, Ibrahem IA, El-Sayed AM et al (2020) Zinc oxide quantum dots for textile dyes and real industrial wastewater treatment: solar photocatalytic activity, photoluminescence properties and recycling process. Adv Powder Technol 31:2555–2565. https://doi.org/10.1016/j.apt.2020.04.017
Mohammed R, Ali MEM, Gomaa E, Mohsen M (2020) Green ZnO nanorod material for dye degradation and detoxification of pharmaceutical wastes in water. J Environ Chem Eng 8:104295. https://doi.org/10.1016/j.jece.2020.104295
Mojiri A, Zhou JL, Robinson B et al (2020) Pesticides in aquatic environments and their removal by adsorption methods. Chemosphere 253:126646. https://doi.org/10.1016/j.chemosphere.2020.126646
Molinari R, Lavorato C, Argurio P (2017) Recent progress of photocatalytic membrane reactors in water treatment and in synthesis of organic compounds. Review Catal Today 281:144–164. https://doi.org/10.1016/j.cattod.2016.06.047
Mugunthan E, Saidutta MB, Jagadeeshbabu PE (2019) Photocatalytic activity of ZnO-WO3 for diclofenac degradation under visible light irradiation. J Photochem Photobiol A Chem. https://doi.org/10.1016/j.jphotochem.2019.111993
Nasseh N, Samadi MT, Ghadirian M et al (2022) Photo-catalytic degradation of tamoxifen by using a novel synthesized magnetic nanocomposite of FeCl2@ac@ZnO: a study on the pathway, modeling, and sensitivity analysis using artificial neural network (AAN). J Environ Chem Eng 10:107450. https://doi.org/10.1016/j.jece.2022.107450
Neelakanta Reddy I, Venkata Reddy C, Sreedhar A et al (2018) Structural, optical, and bifunctional applications: Supercapacitor and photoelectrochemical water splitting of Ni-doped ZnO nanostructures. J Electroanal Chem 828:124–136. https://doi.org/10.1016/j.jelechem.2018.09.048
Neris AM, Chantelle L, Souza JJN et al (2019) Environmental remediation and synthesis of a new pigment by irradiation-induced adsorption of methylene blue onto undoped tetragonal zirconia. Mater Lett 255:126588. https://doi.org/10.1016/j.matlet.2019.126588
Overturf MD, Anderson JC, Pandelides Z et al (2015) Pharmaceuticals and personal care products: a critical review of the impacts on fish reproduction. Crit Rev Toxicol 45:469–491. https://doi.org/10.3109/10408444.2015.1038499
Peng Z, Li Y, Wang W et al (2021) Facile synthesis of magnetic Fe@Al-ZnO nanocomposite for photocatalytic bacterial inactivation under visible-light irradiation. Mater Sci Semicond Process 123:105560. https://doi.org/10.1016/j.mssp.2020.105560
Phuruangrat A, Siri S, Wadbua P et al (2019) Microwave-assisted synthesis, photocatalysis and antibacterial activity of Ag nanoparticles supported on ZnO flowers. J Phys Chem Solids 126:170–177. https://doi.org/10.1016/j.jpcs.2018.11.007
Pinho AR, Rebelo S, de Pereira M, L, (2020) The impact of zinc oxide nanoparticles on male (In)fertility. Materials (basel) 13:849. https://doi.org/10.3390/ma13040849
Premalatha N, Rose Miranda L (2019) Surfactant modified ZnO–Bi2O3 nanocomposite for degradation of lambda- cyhalothrin pesticide in visible light: a study of reaction kinetics and intermediates. J Environ Manage 246:259–266. https://doi.org/10.1016/j.jenvman.2019.05.155
Qayyum HA, Al-Kuhaili MF, Durrani SMA et al (2018) Electromodulation of wide-bandgap semiconductors. J Alloys Compd 747:374–384. https://doi.org/10.1016/j.jallcom.2018.03.004
Qi K, Cheng B, Yu J, Ho W (2017) Review on the improvement of the photocatalytic and antibacterial activities of ZnO. J Alloys Compd 727:792–820. https://doi.org/10.1016/j.jallcom.2017.08.142
Qian R, Zong H, Schneider J et al (2019) Charge carrier trapping, recombination and transfer during TiO2 photocatalysis: an overview. Catal Today 335:78–90. https://doi.org/10.1016/j.cattod.2018.10.053
Radhika NP, Selvin R, Kakkar R, Umar A (2019) Recent advances in nano-photocatalysts for organic synthesis. Arab J Chem 12:4550–4578. https://doi.org/10.1016/j.arabjc.2016.07.007
Rai PK (2022) Novel adsorbents in remediation of hazardous environmental pollutants: Progress, selectivity, and sustainability prospects. Clean Mater. 3:100054. https://doi.org/10.1016/j.clema.2022.100054
Ramos-Corona A, Rangel R, Alvarado-Gil JJ et al (2019) Photocatalytic performance of nitrogen doped ZnO structures supported on graphene oxide for MB degradation. Chemosphere. https://doi.org/10.1016/j.chemosphere.2019.124368
Rangelova N, Aleksandrov L, Yaneva S (2022) Synthesis and structure of amorphous SiO2/ZnO composites with potential application for azo dye degradation. Mater Today Proc. https://doi.org/10.1016/j.matpr.2022.03.064
Rasheed T, Hassan AA, Bilal M et al (2020) Metal-organic frameworks based adsorbents: a review from removal perspective of various environmental contaminants from wastewater. Chemosphere 259:127369. https://doi.org/10.1016/j.chemosphere.2020.127369
Rastogi T, Mahmoud WMM, Kümmerer K (2018) Human and veterinary drugs in the environment. Encyclopedia of the anthropocene. Elsevier, Amsterdam Netherland, pp 263–268
Rauf MA, Ashraf SS (2009) Fundamental principles and application of heterogeneous photocatalytic degradation of dyes in solution. Chem Eng J 151:10–18. https://doi.org/10.1016/j.cej.2009.02.026
Rauf MA, Meetani MA, Hisaindee S (2011) An overview on the photocatalytic degradation of azo dyes in the presence of TiO2 doped with selective transition metals. Desalination 276:13–27. https://doi.org/10.1016/j.desal.2011.03.071
Ravi K, Sathish Mohan B, Satya Sree G et al (2018) ZnO/RGO nanocomposite via hydrothermal route for photocatalytic degradation of dyes in presence of visible light. Int J Chem Stud 6:20–26
Reza KM, Kurny A, Gulshan F (2017) Parameters affecting the photocatalytic degradation of dyes using TiO2: a review. Appl Water Sci 7:1569–1578. https://doi.org/10.1007/s13201-015-0367-y
Rezaei SS, Dehghanifard E, Noorisepehr M et al (2019) Efficient clean-up of waters contaminated with diazinon pesticide using photo-decomposition of peroxymonosulfate by ZnO decorated on a magnetic core/shell structure. J Environ Manage 250:109472. https://doi.org/10.1016/j.jenvman.2019.109472
Riva F, Zuccato E, Davoli E et al (2019) Risk assessment of a mixture of emerging contaminants in surface water in a highly urbanized area in Italy. J Hazard Mater 361:103–110. https://doi.org/10.1016/j.jhazmat.2018.07.099
Rodrigues AC, Boroski M, Shimada NS et al (2008) Treatment of paper pulp and paper mill wastewater by coagulation-flocculation followed by heterogeneous photocatalysis. J Photochem Photobiol A Chem 194:1–10. https://doi.org/10.1016/j.jphotochem.2007.07.007
Rodríguez-González V, Obregón S, Patrón-Soberano OA et al (2020) An approach to the photocatalytic mechanism in the TiO2-nanomaterials microorganism interface for the control of infectious processes. Appl Catal B Environ 270:118853. https://doi.org/10.1016/j.apcatb.2020.118853
Rosendo FRGV, Pinto LIF, de Lima IS et al (2020) Antimicrobial efficacy of building material based on ZnO/palygorskite against Gram-negative and Gram-positive bacteria. Appl Clay Sci 188:105499. https://doi.org/10.1016/j.clay.2020.105499
Rosenfeld PE, Feng LGH (2011) Definition of hazardous waste. Risks of hazardous wastes. Elsevier, Amsterdam Netherland, pp 1–10
Rousis NI, Gracia-Lor E, Zuccato E et al (2017) Wastewater-based epidemiology to assess pan-European pesticide exposure. Water Res 121:270–279. https://doi.org/10.1016/j.watres.2017.05.044
Rueda-Salaya L, Hernández-Ramírez A, Hinojosa-Reyes L et al (2020) Solar photocatalytic degradation of diclofenac aqueous solution using fluorine doped zinc oxide as catalyst. J Photochem Photobiol A Chem 391:112364. https://doi.org/10.1016/j.jphotochem.2020.112364
Ruiz-Hitzky E, Aranda P, Akkari M et al (2019) Photoactive nanoarchitectures based on clays incorporating TiO2 and ZnO nanoparticles. Beilstein J Nanotechnol 10:1140–1156. https://doi.org/10.3762/BJNANO.10.114
Sá AS, Feitosa RP, Honório L et al (2021) A brief photocatalytic study of zno containing cerium towards ibuprofen degradation. Materials (basel) 14:5891. https://doi.org/10.3390/ma14195891
Sabater S, Bregoli F, Acuña V et al (2018) Effects of human-driven water stress on river ecosystems: a meta-analysis. Sci Rep 8:11462. https://doi.org/10.1038/s41598-018-29807-7
Sakarkar S, Muthukumran S, Jegatheesan V (2020) Factors affecting the degradation of remazol turquoise blue (RTB) dye by titanium dioxide (TiO2) entrapped photocatalytic membrane. J Environ Manage 272:111090. https://doi.org/10.1016/j.jenvman.2020.111090
Samsami S, Mohamadi M, Sarrafzadeh M-H et al (2020) Recent advances in the treatment of dye-containing wastewater from textile industries: overview and perspectives. Process Saf Environ Prot 143:138–163. https://doi.org/10.1016/j.psep.2020.05.034
Sanad MF, Shalan AE, Bazid SM, Abdelbasir SM (2018) Pollutant degradation of different organic dyes using the photocatalytic activity of ZnO@ZnS nanocomposite materials. J Environ Chem Eng 6:3981–3990. https://doi.org/10.1016/j.jece.2018.05.035
Santísima-Trinidad ABL, del Montiel-Rozas MM, Diéz-Rojo MÁ et al (2018) Ecotoxicology and environmental safety impact of foliar fungicides on target and non-target soil microbial communities in cucumber crops. Ecotoxicol Environ Saf 166:78–85. https://doi.org/10.1016/j.ecoenv.2018.09.074
Sanzone G, Zimbone M, Cacciato G et al (2018) Ag/TiO2 nanocomposite for visible light-driven photocatalysis. Superlattices Microstruct 123:394–402. https://doi.org/10.1016/j.spmi.2018.09.028
Saravanan R, Gracia F, Stephen A (2017) Basic principles, mechanism, and challenges of photocatalysis. In: Khan MM, Pradhan D, Sohn Y (eds) Nanocomposites for visible light-induced photocatalysis. Springer International Publishing, Cham, pp 19–40
Satdeve NS, Ugwekar RP, Bhanvase BA (2019) Ultrasound assisted preparation and characterization of Ag supported on ZnO nanoparticles for visible light degradation of methylene blue dye. J Mol Liq 291:111313. https://doi.org/10.1016/j.molliq.2019.111313
Sen SK, Raut S, Bandyopadhyay P, Raut S (2016) Fungal decolouration and degradation of azo dyes: a review. Fungal Biol Rev 30:112–133. https://doi.org/10.1016/j.fbr.2016.06.003
Sergejevs A, Clarke CT, Allsopp D, et al (2017) A calibrated UV LED light source for photocatalytic experimentation. Photochem Photobiol Sci 16:1690–1699
Serpone N, Emeline AV, Horikoshi S et al (2012) On the genesis of heterogeneous photocatalysis: a brief historical perspective in the period 1910 to the mid-1980s. Photochem Photobiol Sci 11:1121. https://doi.org/10.1039/c2pp25026h
Serrano-lázaro A, Verdín-betancourt FA, Jayaraman VK et al (2020) Efficient photocatalytic elimination of Temephos pesticide using ZnO nanoflowers. J Photochem Photobiol A Chem. https://doi.org/10.1016/j.jphotochem.2020.112414
Shah AA, Bhatti MA, Tahira A et al (2020) Facile synthesis of copper doped ZnO nanorods for the efficient photo degradation of methylene blue and methyl orange. Ceram Int 46:9997–10005. https://doi.org/10.1016/j.ceramint.2019.12.024
Shirdel B, Behnajady MA (2020) Visible-light-induced degradation of Rhodamine B by Ba doped ZnO nanoparticles. J Mol Liq 315:113633. https://doi.org/10.1016/j.molliq.2020.113633
Silva LS, Ferreira FJL, Silva MS et al (2018) Potential of amino-functionalized cellulose as an alternative sorbent intended to remove anionic dyes from aqueous solutions. Int J Biol Macromol 116:1282–1295. https://doi.org/10.1016/j.ijbiomac.2018.05.034
Speight JG (2020) Remediation technologies. Natural water remediation. Elsevier, Amsterdam Netherland, pp 263–303
Sukriti CP, Singh V, Kumar D (2020) Rapid visible light-driven photocatalytic degradation using Ce-doped ZnO nanocatalysts. Vacuum 178:109364. https://doi.org/10.1016/j.vacuum.2020.109364
Sun S, Jiang T, Lin Y et al (2020) Characteristics of organic pollutants in source water and purification evaluations in drinking water treatment plants. Sci Total Environ 733:139277. https://doi.org/10.1016/j.scitotenv.2020.139277
Teixeira CPDAB, Jardim WDF (2004) Processos Oxidativos Avançados: conceitos teóricos. Campinas, SP: Universidade Estadual de Campinas (UNICAMP). 3:83
Ternes T, Joss A, Oehlmann J (2015) Occurrence, fate, removal and assessment of emerging contaminants in water in the water cycle (from wastewater to drinking water). Water Res 72:1–2. https://doi.org/10.1016/j.watres.2015.02.055
Thi VHT, Lee BK (2017) Effective photocatalytic degradation of paracetamol using La-doped ZnO photocatalyst under visible light irradiation. Mater Res Bull 96:171–182. https://doi.org/10.1016/j.materresbull.2017.04.028
Tkaczyk A, Mitrowska K, Posyniak A (2020) Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: a review. Sci Total Environ 717:137222. https://doi.org/10.1016/j.scitotenv.2020.137222
Tsai C-K, Yu-Chin L, Nguyen TT, Horng J-J (2021) Levofloxacin degradation under visible-led photo-catalyzing by a novel ternary Fe-Zno/Wo3 nanocomposite. SSRN Electron J. https://doi.org/10.2139/ssrn.3994411
Vashisht D, Kumar A, Mehta SK, Ibhadon A (2020) Analysis of emerging contaminants: a case study of the underground and drinking water samples in Chandigarh. India. Environ Adv. 1:100002. https://doi.org/10.1016/j.envadv.2020.100002
Velmurugan R, Swaminathan M (2011) An efficient nanostructured ZnO for dye sensitized degradation of reactive red 120 dye under solar light. Sol Energy Mater Sol Cells 95:942–950. https://doi.org/10.1016/j.solmat.2010.11.029
Verma S, Tirumala Rao B, Jayabalan J et al (2019) Studies on growth of Au cube-ZnO core-shell nanoparticles for photocatalytic degradation of methylene blue and methyl orange dyes in aqueous media and in presence of different scavengers. J Environ Chem Eng 7:103209. https://doi.org/10.1016/j.jece.2019.103209
Vishnukumar P, Vivekanandhan S, Misra M, Mohanty AK (2018) Recent advances and emerging opportunities in phytochemical synthesis of ZnO nanostructures. Mater Sci Semicond Process 80:143–161. https://doi.org/10.1016/j.mssp.2018.01.026
Wang H, Xie C, Zhang W et al (2007) Comparison of dye degradation efficiency using ZnO powders with various size scales. J Hazard Mater 141:645–652. https://doi.org/10.1016/j.jhazmat.2006.07.021
Wang X, Sun M, Murugananthan M et al (2020) Electrochemically self-doped WO3/TiO2 nanotubes for photocatalytic degradation of volatile organic compounds. Appl Catal B Environ 260:118205. https://doi.org/10.1016/j.apcatb.2019.118205
Wang Z, Srivastava V, Ambat I et al (2019) Degradation of Ibuprofen by UV-LED/catalytic advanced oxidation process. J Water Process Eng. https://doi.org/10.1016/j.jwpe.2019.100808
Wei Z, Spinney R, Ke R et al (2016) Effect of pH on the sonochemical degradation of organic pollutants. Environ Chem Lett 14:163–182. https://doi.org/10.1007/s10311-016-0557-3
Weldegebrieal GK (2020) Synthesis method, antibacterial and photocatalytic activity of ZnO nanoparticles for azo dyes in wastewater treatment: a review. Inorg Chem Commun 120:108140. https://doi.org/10.1016/j.inoche.2020.108140
Wetchakun K, Wetchakun N, Sakulsermsuk S (2019) An overview of solar/visible light-driven heterogeneous photocatalysis for water purification: TiO2- and ZnO-based photocatalysts used in suspension photoreactors. J Ind Eng Chem 71:19–49. https://doi.org/10.1016/j.jiec.2018.11.025
Wojnarowicz J, Chudoba T, Lojkowski W (2020) A review of microwave synthesis of zinc oxide nanomaterials: reactants. Proc Paramet Morphol Nanomater 10:1086. https://doi.org/10.3390/nano10061086
Wu Z, Liang Y, Yuan X et al (2020) MXene Ti3C2 derived Z–scheme photocatalyst of graphene layers anchored TiO2/g–C3N4 for visible light photocatalytic degradation of refractory organic pollutants. Chem Eng J. https://doi.org/10.1016/j.cej.2020.124921
Yadav S, Kumar N, Kumari V et al (2019) Photocatalytic degradation of Triclopyr, a persistent pesticide by ZnO/SnO2 nano-composities. Mater Today Proc 19:642–645. https://doi.org/10.1016/j.matpr.2019.07.746
Yang NQ, Li J, Wang YN, Ma J (2021) Investigation of photocatalytic properties based on Fe and Ce Co-doped ZnO via hydrothermal method and first principles. Mater Sci Semicond Process 131:105835. https://doi.org/10.1016/j.mssp.2021.105835
Yusuff AS, Olateju II, Adesina OA (2019) TiO2/anthill clay as a heterogeneous catalyst for solar photocatalytic degradation of textile wastewater: catalyst characterization and optimization studies. Materialia 8:100484. https://doi.org/10.1016/j.mtla.2019.100484
Zheng K, Liu H, Nie C et al (2019) Controllable synthesis of honeycomb-structured ZnO nanomaterials for photocatalytic degradation of methylene blue. Mater Lett 253:30–33. https://doi.org/10.1016/j.matlet.2019.06.017
Zhong Q, Lan H, Zhang M et al (2020) Preparation of heterostructure g-C3N4/ZnO nanorods for high photocatalytic activity on different pollutants (MB, RhB, Cr(VI) and eosin). Ceram Int 46:12192–12199. https://doi.org/10.1016/j.ceramint.2020.01.265
Zhu H, Jiang R, Fu Y et al (2012) Effective photocatalytic decolorization of methyl orange utilizing TiO2/ZnO/chitosan nanocomposite films under simulated solar irradiation. Desalination 286:41–48. https://doi.org/10.1016/j.desal.2011.10.036
Zhu S, Qian X, Lan D et al (2020) Accelerating charge transfer for highly efficient visible-light-driven photocatalytic H2 production: In-situ constructing Schottky junction via anchoring Ni-P alloy onto defect-rich ZnS. Appl Catal B Environ 269:118806. https://doi.org/10.1016/j.apcatb.2020.118806
Zhu S, Wang D (2017) Photocatalysis: basic principles, diverse forms of implementations and emerging scientific opportunities. Adv Energy Mater 7:1700841. https://doi.org/10.1002/aenm.201700841
Acknowledgements
The authors would like to thank the funding agencies CAPES, CNPq, FAPESP, and FAPEPI for all the financial support. Conflicts of Interest: The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Osajima, J.A., Sá, A.S., Feitosa, R.P. et al. Improved remediation of contaminated water using ZnO systems via chemical treatment: applications, implications and toxicological mitigation. Sustain. Water Resour. Manag. 9, 42 (2023). https://doi.org/10.1007/s40899-023-00818-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40899-023-00818-1