Abstract
Lime stabilization remains the most commonly adopted method to improve expansive soil; however, lime production has drawbacks such as depleting natural resources, high energy consumption, and substantial greenhouse gas emissions. To address these issues, this study introduces a novel approach involving the chemically converted waste marble powder (CCWMP) treatment of soil. In this process, sodium hydroxide (NaOH) solution is used to convert waste marble powder (WMP), containing calcium carbonate, into lime. This conversion occurs without the emission of greenhouse gases, ultimately enhancing the performance of expansive soils. Additionally, WMP is blended with expansive soil without the addition of NaOH, to understand and compare the underlying mechanisms in CCWMP treatment of soil. This approach is referred to as inactive waste marble powder (IAWMP) treatment of soil. Geotechnical and mineralogical studies were conducted with varying dosages of WMP (5–20%) in both the IAWMP and CCWMP treated soil. The results indicate that in the CCWMP treated soil, calcium carbonate is first converted into lime without emitting carbon dioxide, followed by short- and long-term reactions with expansive soil. It is also inferred that there is a significant enhancement in unconfined compressive strength (7.26 times that of untreated soil with 15% WMP addition after 28 days of curing) and the complete elimination of swelling, due to the addition of CCWMP as compared to the IAWMP. Therefore, this study strongly concludes that CCWMP treatment represents a superior alternative for chemically stabilizing the expansive subgrade in pavement construction. Furthermore, it provides an effective solution for the substantial utilization of WMP.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Expansive soils possess an inherent volume change behavior and higher moisture retention capacity. These soils are subjected to repetitive wetting and drying cycles, which cause swelling and shrinkage, respectively. In addition to that, high compressibility, low shear strength, and differential settlement causes severe stress and even damage to lightly loaded structures built on them. Such instability or volume change is due to the presence of a smectite group of minerals, amount of clay, moisture content, external loading, and pore water chemistry [1, 2].
Over the years, various techniques have evolved to inhibit the volume instability of expansive soil, such as controlled compaction, soil replacement, innovative foundations, and chemical stabilization [3]. Among these techniques, chemical stabilization attracted many field geotechnical engineers, due to its effectiveness and versatility. Lime and cement are the conventional stabilizers to inhibit volume change and enhance the engineering properties of the expansive soil [4,5,6,7,8,9,10]. However, the production of each tonne of cement and lime results in 0.95 and 0.79 tonnes of CO2 emission, respectively, and also requires 5000 MJ and 3200 MJ of energy, respectively [11, 12]. In addition to that, non-renewable resource mining has increased dramatically in recent years to fulfill the demands of the construction sector, which results in the depletion and overexploitation of natural resources.
Utilizing industrial/agricultural residue, and locally available materials provides sustainable and economical solutions to the problems with conventional binders in soil stabilization [13,14,15,16]. Additionally, the utilization of industrial waste is also required to control environmental pollution from being dumped in landfills [17]. In the context of industrial waste generation, it is important to note that India is producing a significant amount of major industrial by-products. These include pond ash (comprising fly ash and bottom ash from coal combustion) and GGBS (ground granulated blast furnace slag) from iron production. Several researchers have initiated the utilization of these wastes to enhance the physical and engineering properties of expansive soil while also, controlling its volume change [18,19,20,21]. Ladle furnace slag is another byproduct obtained by refining carbon and low alloy steels in the ladle furnace, in the steel industry. This slag has been researched for its potential in stabilizing subgrade and subbase materials in pavement construction [22,23,24]. Manso et al. [25] conducted a study on the solidification impact of ladle furnace slag (LFS) on clayey soils. Their findings indicated that the incorporation of LFS into clayey soils resulted in improved bearing capacity compared to that of the natural subgrade soil. Waste dust, containing calcium, has also been employed for soil stabilization in subbase layers for pavement construction [26]. However, using these wastes alone for soil stabilization resulted in slow hydration, low early strength, and inadequate swelling control [27, 28].
As a result, treating expansive soil with alkali-activated wastes is one potential approach to address challenges such as depletion of natural resources, high energy consumption, and substantial greenhouse gas emissions associated with the production of conventional additives. [29,30,31,32,33]. Wastes such as fly ash, slag, silica fume, rice husk ash, bagasse ash, and palm oil fuel ash (POFA) are precursors for alkali-activated stabilization, which provide aluminosilicates for geopolymerization [34]. In alkali-activated stabilization, sodium hydroxide, potassium hydroxide, and sodium silicate are the activators that facilitate the dissolution of aluminosilicates. Among these, the utilization of sodium silicate causes environmental issues compared to other activators [32].
Based on previous research, soils with a plasticity index exceeding 30% are found to be more suitable for stabilization when incorporating calcium-rich additives such as lime [35]. Typically, calcium-based chemicals are the preferred materials for stabilizing expansive soil [36]. In that context, marble waste, derived from the cutting and subsequent polishing of marble blocks, is a calcium carbonate-containing waste. Around 200 million tonnes of marble waste are produced annually worldwide [35]. In particular, India produces 16 million tonnes of marble waste per year [37]. Water is used for controlling dust and heat during the cutting and polishing of marble stones. This method generates a large amount of marble slurry. The developing countries lack proper methods for the disposal of marble waste, and it is dumped on agricultural lands or discharged into water bodies [38]. The dumping of marble waste is causing serious environmental issues [39]. In India, Rajasthan, Gujarat, Andhra Pradesh, and Madhya Pradesh are the major marble-producing states. The state of Rajasthan alone produces 95% of marble blocks, creating major air and land pollution in the nearby villages [40, 41].
Hence, the waste from the marble quarry needs to be utilized effectively and fully. There are numerous industrial applications, specifically in the construction field, for the utilization of marble powder due to its non-hazardous nature [42]. Initially, the marble powder was used along with cement and also utilized as a constituent of bricks, a replacement for aggregates, and in the manufacturing of ceramic tiles [38, 39, 43, 44]. However, there is still no proper method for the bulk and effective utilization of marble waste [38, 41]. Various researchers have undertaken studies on the specialized application of marble waste in soil stabilization for bulk utilization [45,46,47,48,49,50]. For the construction of pavements as a base material, the addition of marble dust in red tropical soil does not provide adequate strength [45]. Also, studies suggest that the mixing of marble waste in expansive soil controls volume change and improves shear strength [47, 48]. However, marble waste is chemically inert by nature. As a result, there is no beneficial chemical reaction (cation exchange and pozzolanic reaction) with clayey soil due to the inactive nature of marble powder.
To address the problems associated with conventional soil stabilizers, the current study aims to provide a novel approach: converting the WMP into lime (Ca(OH)2) by using NaOH for the stabilization of expansive soil without the emission of CO2. The objective of the current study is to understand the mechanisms involved in the stabilization of expansive soil with chemically converted waste marble powder (CCWMP). The short and long-term behavior of treated expansive soil with 1, 7, 14, and 28 days of curing is studied by evaluating the index properties, swell potential, and unconfined compressive strength (UCS). Further, the presence, conversion, and utilization of CaCO3 in the stabilized expansive soil with WMP are studied by mineralogical, microstructural, and thermal analysis. The results of the CCWMP treatment of soil are compared with those of inactive waste marble powder (IAWMP) treatment of soil and existing conventional methods (lime and cement stabilization) and other chemical stabilization methods (alkali activated waste stabilization) for expansive soil [33].
Materials
In the stabilization of expansive soil using the CCWMP and IAWMP, the WMP is used in both treatments. In the CCWMP treatment of soil, NaOH is specifically used to convert WMP into lime, thereby contributing to the stabilization of expansive soil. The subsequent section provides information on the properties of these materials and their characterization.
Expansive Soil
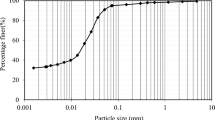
The natural expansive soil samples from the district of Nagapattinam, in the state of Tamil Nadu in India, were collected from 1.0 m below the surface of the ground. The collected soils were oven-dried at 105 °C under laboratory conditions and pulverized. The pulverized soils were sieved through a 425 μm Indian Standard (IS) sieve to remove large particles for all tests. The index and physical properties of the expansive soil are determined as per IS and summarized in Table 1. The grain size distribution curve is shown in Fig. 1. The collected soil is classified as high plastic clay CH and also based on the free swell index, it has a high degree of expansion as per the Indian standard soil classification system (IS 1498–1970 Reaffirmed: 2021). The X-ray diffraction (XRD) analysis of expansive soil is shown in Fig. 2a, which indicates the presence of montmorillonite, kaolinite, and non-clay minerals such as quartz and calcite. In Fig. 2b, the results of thermogravimetric analysis (TGA) and differential thermal analysis (DTA) are presented. The DTA of the expansive soil reveals significant endothermic peaks at distinct temperature ranges. Peaks at 100–200 °C signify the removal of hygroscopic water, with single endothermic peaks in this range linked to the loss of structural hydroxyls in montmorillonite clay. Peaks between 350 and 500 °C indicate the removal of adsorbed water, contributing to a nearly 14% mass loss up to 500 °C. The overall mass loss of the expansive soil up to 1000 °C is approximately 16% [51]. Consequently, the geotechnical properties and mineralogical examination confirm the presence of montmorillonite in the collected expansive soil. The chemical composition of expansive soil is analyzed through X-ray fluorescence (XRF) technique and is listed in Table 2.
Waste Marble Powder (WMP)
The marble waste used in the present study were collected from marble waste slurry dumping sites in the Udaipur district, in the state of Rajasthan, India. The slurry was oven-dried at 105 °C, then the larger lumps were crushed and sieved through a 75 μm IS sieve. The specific gravity of WMP is 2.72, and it is non-plastic. The index and physical properties of the WMP are determined as per IS and are summarized in Table 1. The XRD analysis of WMP indicates the presence of calcite and quartz, as shown in Fig. 2c. The TGA and DTA results of WMP as shown in Fig. 2d, indicate a major loss of mass at 700–800 °C, due to the decarbonation of CaCO3 [51]. The initial mass loss of WMP, at 30–100 °C is due to the removal of water [41, 48]. The XRF analysis of WMP indicates the presence of calcium and silica in its chemical composition, with no heavy metals detected (Table 2). So, no pretreatment is required for the collected waste marble powder.
Sodium Hydroxide (NaOH)
NaOH (98% purity) has been used to convert the inactive WMP into hydrated lime for the stabilization of expansive soil. Recently, the use of NaOH for soil stabilization has become well-established, with methods such as alkali-activation [32], lime precipitation techniques [52], magnesium hydroxide precipitation techniques [53, 54], and magnesium alkalinization [55]. A concentration of 5 M of NaOH was fixed to understand the stabilization mechanism. A majority of studies have utilized a 5 M NaOH solution for soil stabilization using alkali-activated binders. The use of higher alkalinity can lead to environmental issues such as leaching in treated soil [32]. Before starting the experiment, a NaOH solution is prepared, and placed in a closed container for 24 h to ensure the complete dissipation of heat, as the dissolution of NaOH in water is an exothermic process. The reason for the waiting time is that the release of heat should not affect the chemical reaction with expansive soil, and hence, NaOH solution is used after cooling [33].
Experimental Program
The specimen preparation for untreated and treated expansive soil with CCWMP and IAWMP, for assessing geotechnical behavior is outlined in this section. This facilitates a comprehensive analysis of soil characteristics and behavior through mineralogical, microstructural, and thermal analysis.
Mix Proportion and Specimen Preparation
In the current investigation, two methods of expansive soil stabilization, namely IAWMP and CCWMP treatment of soil, are performed to understand the reaction mechanism of CCWMP with expansive soil and changes in the geotechnical behavior of expansive soil. In the IAWMP treatment of soil, the dry soil is mixed with inert WMP, in which distilled water is a pore solution [41, 48]. The CCWMP treatment of soil uses NaOH as a pore solution for converting the inert WMP into Ca(OH)2. The details of the experimental program are shown in Fig. 3. Four proportions of WMP (5, 10, 15, and 20% of dry soil) are used for both methods. These proportions were chosen based on the studies conducted by Jain et al. (2020). The required amount of dry soil, WMP, and pore solution was calculated based on the maximum dry density (MDD) and optimum moisture content (OMC) of each mix proportion as obtained from the standard Proctor compaction test. Initially, the required soil and WMP were mixed under dry conditions for 2–3 min to achieve homogeneity in both methods; thereafter, the respective pore solution was added and mixed for another 2–3 min [31]. The details of the mix proportion and sample description are listed in Table 3.
Compaction Test
According to IS 2720 (Part 7) – 1980 (Reaffirmed: 2021), the standard Proctor compaction test was performed to determine the OMC and MDD of expansive soil with varying proportions of WMP (0, 5, 10, 15, and 20%). Distilled water was used for the tests.
Index Properties
Index properties such as liquid limit and plastic limit were determined for the untreated expansive soil and treated expansive soil with different proportions of WMP in the IAWMP and CCWMP treatment of soil, as per IS 2720 (Part 5) 1985 (Reaffirmed: 2020). The oven-dried expansive soil is thoroughly mixed with WMP, as mentioned in Sect. 3.1. Then, the respective pore solutions were added and mixed separately. The wet, mixed sample was kept for 2 h in plastic airtight bags for moisture equilibrium and chemical reactions. The Casagrande method and standard thread rolling method were used to determine the liquid limit and plastic limit, respectively.
Swell Potential Test
According to IS 2720 (Part 41), 1977 (Reaffirmed: 2021), one-dimensional swell potential tests were conducted on both untreated and treated soil specimens with IAWMP and CCWMP. The oedometer ring used has a diameter of 60 mm and a height of 20 mm. The required mix of the soil sample was compacted at OMC, for a compacted height of 14 mm, allowing a 6 mm height to accommodate swelling. The compacted specimens were kept in air-tight bags for 1 day of curing. After curing for 1 day, filter paper and porous stones were placed on each side of the specimen. The whole setup was assembled on a loading frame. Then dial gauge readings were set to zero, and a surcharge load of 5 kPa was applied. The setup was inundated with distilled water. The changes in dial gauge readings were noted at different time intervals until equilibrium was reached. The swell potential is calculated as the ratio of the change in height to the initial height of the specimen.
Unconfined Compressive Strength Test
Unconfined compressive strength (UCS) tests were conducted on untreated and treated soil specimens as per IS 2720 (Part-10), 1991 (Reaffirmed: 2020). The 38 mm diameter and 76 mm height of cylindrical specimens were prepared for each variation in a constant volume mold. The mixed samples were compacted in the mold. Three identical treated and untreated specimens for each proportion, as listed in Table 3, were prepared at their respective OMC and MDD (Fig. 4). The compacted specimens were kept in an air-tight cover in a desiccator and cured for 1, 7, 14, and 28 days at room temperature. The mass of each specimen was measured, before and after the curing period of the test to determine the moisture loss. If the moisture content loss is more than 0.5%, the specimens are rejected and then prepared again. At the end of the curing period, the tests were conducted under a constant displacement rate of 1.25 mm/min. The average UCS value is computed from the UCS values of three identical specimens. The tests were repeated if the UCS of the specimen was 10% higher than the average value. This approach was similarly applied to specimens prepared for all proportions and different curing periods to enhance the accuracy of UCS results. After attaining the residual strength, the loading was stopped, and photos were taken to capture the failure of UCS specimens to know the behavior at failure.
Mineralogical Studies
Mineralogical studies were carried out on treated expansive samples on the fractured portion of 7 days cured UCS tested specimen. The sample was oven-dried for 6 h at 50 °C. Using a mortar and pestle, the samples were thoroughly pulverized and sieved through a 75 μm IS sieve [31]. X-ray diffraction analysis (XRD) was carried out to confirm mineral transitions, particularly from calcium carbonate to hydrated lime, subsequent changes in the expansive soil with converted lime, and the determination of newly formed cementitious compounds. The X-ray diffraction (XRD) analysis was conducted using a Rigaku ULTIMA III X-ray diffractometer with CuKα radiation at a rate of 1°/min for Bragg’s angle range of 5° to 70°. The diffractometer data is graphically represented using Origin software, wherein the intensities are plotted against 2θ values and subsequently analyzed with the Joint Committee on Powder Diffraction Standards (JCPDS) database. The formation of cementitious particles in treated expansive soil with IAWMP and CCWMP was determined using microstructural images obtained from a VEGA3 TESCAN scanning electron microscope (SEM). Before SEM examination, a thin layer of gold-palladium is coated over the sample using a sputter coater and polaron E5100 at a vacuum of 10− 3 Torr. Thermal analyses, such as TGA and DTA, are performed on treated samples. Approximately 20–25 mg of sample were analysed using a Labsys Evo 1150 thermal analyzer (Setaram, France) over a range of 30 °C to 1000 °C at a heating rate of 10 °C/min.
Results and Discussions
The behavior of the treated expansive soil was examined and discussed through geotechnical tests and mineralogical studies. Based on the findings, the reaction mechanism of the CCWMP treatment of soil was determined and subsequently discussed.
Compaction Characteristics
The compaction characteristics of expansive soil and soil mixed with WMP were determined using the standard Proctor compaction test. The compaction curves for expansive soil mixed with WMP, including MDD and OMC values are shown in Fig. 4. The results show that the MDD value of expansive soil increases with the addition of WMP to the soil. This is due to the higher specific gravity of the WMP (2.72) mixed with the lower specific gravity of expansive soil (2.58). The substantial increase (1.5 g/cc to 1.64 g/cc) in MDD of expansive soil mixed with WMP up to 10% is attributed to the filling of voids with WMP, followed by a gradual increase (1.64 g/cc to 1.67 g/cc) due to an increase in the specific gravity of the mixture. Another reason for the increase in MDD of the soil is attributed to the improvement in soil gradation. The untreated expansive soil has a higher clay content than silt content as shown in Fig. 1. The addition of WMP (silt) results in improved gradation characteristics, leading to a transition towards a well-graded composition containing silt and clay [48]. The WMP added to expansive soil, acts as a filler material. However, the addition of WMP to expansive soil decreases the OMC value (from 25 to 21.9%) of the mixture, as shown in Fig. 4, because of the decrease in porosity of the mixed matrix and the replacement of WMP in the soil. A similar observation was also reported by previous researchers [48, 56].
Index Properties
The index properties of treated expansive soils with IAWMP and CCWMP were determined to understand the plasticity behavior changes and short-term reactions involved in the CCWMP treatment of soil. The liquid limit and plastic limit values obtained for treated expansive soil are listed in Table 4. The plasticity behavior of expansive soil is primarily controlled by the diffused double layer, fabric arrangement, and interparticle shear resistance [8]. The liquid limit and plasticity index of treated soil decreases with the increase in WMP content in both methods. The implementation of the IAWMP treatment of soil led to a decrease of only 17.9% in the plasticity index. However, the addition of WMP has the opposite effect on the plastic limit of expansive soil, causing it to decrease (30–20%). This trend contrasts with the observed behavior during lime stabilization. This confirms that neither of the chemical reactions like cation exchange nor flocculation occur in the IAWMP method because of the chemically inert nature of WMP. The addition of WMP, which is a non-plastic silt, replaces some of the clay particles in expansive soil, resulting in a decrease in the liquid limit and plasticity index [48, 56].
However, the abrupt changes in the plasticity index and liquid limit of expansive soil in the CCWMP treatment of soil are primarily associated with strong short-term chemical reactions. A reduction of 76.9% in the plasticity index was observed (39–9%) due to the CCWMP treatment of soil. The addition of NaOH to the WMP-mixed expansive soil converts the CaCO3 into Ca(OH)2 which is confirmed by XRD analysis (Fig. 10). The produced lime dissociates in the pore solution as calcium and hydroxyl ions and is responsible for short-term reactions. The replacement of exchangeable ions on the clay surface by calcium ions suppresses the diffuse double layer thickness and hence leads to a decrease (69–47%) in the liquid limit of expansive soil [5, 8, 57]. It is interesting to note that, with a further increase in WMP beyond 5%, no additional change in the plasticity index occurred. The reason behind this is that the required Ca(OH)2 is converted and utilized for short-term reactions at 5% WMP itself.
The soil classification changes in expansive soil treated with IAWMP and CCWMP are represented in Casagrande’s plasticity chart, as shown in Fig. 5. As mentioned earlier, untreated expansive soil is classified as high plastic clay (CH). In the IAWMP method, it is observed that there are no changes in soil classification; it remains classified as CH type. However, with the addition of 20% WMP, the soil is shifting towards intermediate plastic clay (CI) with a 32% plasticity index. This is due to the replacement of WMP silt particles in the expansive soil. However, in the CCWMP treatment of soil, the soil classification is shifted to intermediate plastic silt (MI) with a plasticity index of 9% on the addition of 5% WMP itself. This is attributed to the chemical changes of expansive soil, such as cation exchange followed by flocculation of clay particles. Also, a slighter pozzolanic reaction in a short time leads to changes in the particle size [52].
Swell Potential
Figure 6 shows the variation of swell potential with time in untreated and treated expansive soil with IAWMP and CCWMP. The maximum swell potential of untreated expansive soil is 9.6% due to the presence of montmorillonite minerals. The 1 day cured specimens were tested in the one-dimensional swell potential setup. It is observed that the swell potential of expansive soil was not controlled by the addition of IAWMP. As discussed above in Sect. 4.2, the addition of WMP alone to expansive soil does not cause a chemical reaction. The swell potential values of the treated expansive soil with IAWMP are 11.7%, 10.3%, 8.8%, and 8.0% due to the incorporation of 5%, 10%, 15%, and 20% WMP, respectively. It can be observed that the swell potential of expansive soil with 5% and 10% WMP is greater than that of untreated expansive soil, due to its compaction characteristics. As discussed in Sect. 4.1, the addition of WMP to the soil decreases the OMC. The swelling behavior of expansive soil depends on the initial moisture content. Expansive soil exhibits higher swelling with lower initial water content compared to soil with higher initial water content. [58, 59]. This is the reason, the swell potential of IAWMP treated expansive soil with 5% and 10% are higher than untreated soil. Similar results were observed in treated expansive soil with silica fume in the existing literature [60]. However, with the further addition of WMP, the swell potential is reduced below that of the untreated expansive soil. The slight reduction in swell potential with an increasing amount of WMP is attributed to the replacement of clay particles with WMP, and it is not caused by a chemical reaction between WMP and the expansive soil. The IAWMP treated soil took about 7 days for swelling to reach equilibrium, which is similar to untreated expansive soil. However, this method requires a higher amount of WMP to eliminate the swell potential of expansive soil.
In contrast to the IAWMP method of stabilization, the CCWMP treated soil eliminated the swell potential of expansive soil with the addition of 5% WMP itself. As explained in Sect. 4.2, this is due to the short-term reaction and slighter pozzolanic reactions within a 1 day curing period [52].
Unconfined Compressive Strength
The unconfined compressive strength of the treated soil with CCWMP was determined at 1, 7, 14, and 28 days of curing, as shown in Fig. 7. The average UCS values, along with standard deviation values, are provided for each treated group at different curing periods. For a curing period of 1 day, the addition of WMP at levels of 5%, 10%, 15%, and 20% resulted in UCS improvements of 114.1%, 140%, 180%, and 149%, respectively, compared to untreated soil (UCS = 283 kPa). When the curing period was increased to 28 days, similar additions of WMP led to UCS enhancements of 434%, 566%, 626%, and 578%, respectively, relative to untreated soil (283 kPa). The improvement in the strength of expansive soil is observed with an increase in curing periods. The improvement in UCS is due to the chemical reaction between expansive soil and converted Ca(OH)2 as confirmed in Fig. 11 which is discussed later. Another interesting observation is that a substantial improvement in strength is achieved during the shorter curing period of 1 day. This is due to modifications in the soil (cation exchange and flocculation/agglomeration) and some degree of pozzolanic reactions initiated [4, 7]. A continuous increase in the strength of expansive soil is observed with further increase in the curing periods. This phenomenon is attributed to the pozzolanic reaction, particularly during the long-term curing process. The addition of 5 M NaOH in expansive soil raises the pH of the pore solution, which dissolutes the silica and alumina. The free calcium available in the pore solution combines with the dissolution of silica and alumina to produce cementitious products such as calcium silicate hydrate (CSH) and calcium aluminate hydrate (CAH), which bind the clay particles and increase their strength [57]. The continuous development of cementitious products contributes to an increase in UCS over the curing period. But when 20% of WMP is added, the UCS is reduced to 1919 kPa from that corresponding to 15% of WMP addition (2054 kPa). The reduction in UCS may be due to the insufficient addition of a converter (NaOH) compared to additions of WMP at 5%, 10%, and 15%. As mentioned earlier, NaOH acts as a converter, converting calcium carbonate into hydrated lime for soil stabilization. The concentration of NaOH has not been changed in the current study. This needs to be studied further with various molarity of alkaline solutions in future research work.
In the IAWMP method, the UCS of treated soil was determined after 7 days of curing (Fig. 8). The strength improvement of expansive soil with the addition of 5%, 10%, 15%, and 20% WMP is 76.7%, 108%, 123%, and 100%, respectively, compared to untreated soil (283 kPa). The addition of WMP increases the MDD of soil by replacing some of the clay particles with non-plastic marble particles, thereby increasing the shear strength of expansive soil due to improved packing density, up to 15% addition of WMP. However, with further addition of WMP, there is a subsequent decrease in UCS. This reduction is a result of the replacement of non-plastic marble particles in the clayey soil, which in turn diminishes the cohesive strength of the soil. [41, 45, 48].
In the CCWMP treatment of soil, the addition of 5%, 10%, 15%, and 20% of WMP shows, respectively, 1.74, 1.83, 2.0, and 1.94 times higher UCS than the IAWMP method (Fig. 8). It confirms that the addition of NaOH to WMP-mixed expansive soil induces chemical reactions. The UCS values of the treated expansive soil with the CCWMP are higher than the minimum strength (750 kPa) requirement for treated subgrade as per Indian Road Congress (IRC) 37 (2018), whereas the IAWMP treated soil does not satisfy the requirement.
Further, to understand the material behavior, the failure pattern was observed after the UCS test on 7 days of cured specimens, as shown in Fig. 9. In the IAWMP treated soil, while loading, the specimen started to fail by bulging, followed by shear failure. The angle of the failure plane increases with the increase in WMP content (Fig. 9a and d). However, in the CCWMP treated soil specimens, sudden failure occurred due to axial splitting (Fig. 9e and h). This indicates that soil cementation occurred in the CCWMP treatment but not in the IAWMP treatment of soil. The samples tested at 14 and 28 days of curing also exhibited axial splitting failure. A similar failure pattern is reported in lime stabilization [61].
Mineralogical, Microstructural Examination and Chemical Composition
Mineralogical Characteristics by XRD
X-ray diffraction (XRD) spectra were investigated to understand the conversion of CaCO3 into Ca(OH)2 and Na2CO3, mineral changes, and products formed by pozzolanic reactions in CCWMP treated soil. The diffractometer data is graphically represented using Origin software, and the resulting diffractograms are compared with the JCPDS database, as represented in Fig. 10. The Bragg’s angle and its d-spacing for existing minerals and formed compounds in soil are listed in Table S1 (Supplementary file).
The XRD patterns of the IAWMP and CCWMP treated expansive soil, with 7 days of curing are shown in Fig. 10. The following changes occurred and are confirmed by the XRD pattern. In IAWMP, when WMP content increases from 5 to 20%, an increase in the calcite peak is observed. The reason for this is the replacement of the expansive soil by the calcite contained in the WMP. In the CCWMP treatment of soil, the addition of NaOH to soil mixed with WMP produces Ca(OH)2, which is confirmed by the peaks of XRD patterns as shown in Fig. 11e and h. This converted lime aids in stabilizing the expansive soil. Additionally, the carbonates in the WMP react with NaOH to form Na2CO3, which is identified in the XRD pattern in the CCWMP treated soil. However, the above chemical reactions were not encountered in the IAWMP treatment of soil due to the presence of inert WMP.
As previously discussed, in the CCWMP treatment, expansive soil utilizes converted lime for cation exchange reactions within a short period. This results in a reduction in the diffused double layer thickness of clay. This is reflected in the suppression of monmorillonite peaks in the XRD patterns. Nevertheless, the X-ray diffraction (XRD) analysis confirmed that the peaks corresponding to montmorillonite remained unaltered in the soil treated with IAWMP (Fig. 11a and d).
Furthermore, cementitious products like CSH and CAH are formed in the CCWMP treatment of soil and are evident in the peaks observed in the XRD data [51, 62, 63]. In contrast, in the IAWMP treated soil, no hydration products are found. These findings align closely with the experimental results.
Microstructural Characteristics by SEM
Figure 12 illustrates the microstructural characteristics of treated expansive soil with IAWMP and CCWMP using SEM after 7 days of curing. The scanned images of the untreated soil and treated soil were observed at 20 μm scale and particle sizes were compared across all images. From the SEM images of the treated expansive soil with CCWMP, the aggregated soil particles were visible when chemically converted WMP was added. The pozzolanic reaction produces the cementitious compounds that aggregate the soil particles [51]. From Fig. 12e and f, as the WMP increases from 5 to 10%, the formation of aggregated soil particles increases due to the reaction between the soil and the converted lime from WMP. This confirms the findings of the experimental results, which are indicated by the increase in UCS. However, IAWMP treated expansive soil has not produced any cemented particles (Fig. 12c and d). The WMP is a chemically inert material and doesn’t react with water, which is confirmed in the SEM images. Therefore, there is no cementation of soil particles, which is the reason for the lower strength improvement in the UCS tests conducted on IAWMP treated soil specimens compared to CCWMP treated soil specimens (Fig. 8).
Thermal Analysis by TGA and DTA
The TGA and DTA are commonly used techniques to estimate the consumption of lime, the formation of hydration products, and carbonation in lime stabilization [51]. In the current study, the addition of NaOH into the WMP-mixed soil results in the conversion of CaCO3 into Ca(OH)2 during the CCWMP treatment of the soil, and the utilization of converted lime for stabilizing expansive soil is studied by thermal analysis. To understand the mechanism of CCWMP treatment of soil, the TGA and DTA of treated expansive soil with CCWMP and IAWMP for 10% additive at 7 days of curing are shown in Fig. 13a and b.
The mass loss at three major endothermic peaks in the range of temperatures from 50 to 200 °C, 200–600 °C, and 600–800 °C are observed in the TGA and DTA curves. The mass loss of treated expansive soil with IAWMP and CCWMP at 10% additive, up to a temperature of 400 °C are 9.4% and 13.87%, respectively. The higher mass loss observed in the CCWMP treatment of soil as compared to the untreated and IAWMP treated soil is due to the formation of cementitious compounds [63]. The mass loss in the IAWMP treated soil is lower than that of untreated soil up to 600 °C, which is due to the replacement of clay particles in expansive soil with WMP. Because of this, the TGA curve of the IAWMP treated soil is shifting towards the TGA curve of WMP. However, the mass loss becomes higher after 600 °C compared to untreated soil, attributed to the decomposition of CaCO3 in the added WMP. This indicates that there is no chemical reaction in the treatment of soil with IAWMP. However, in the CCWMP treatment of soil, the formed pozzolanic substances, such as CSH and CAH, were confirmed in the mass loss up to 400 °C [62, 63]. As identified in the mineralogical study (Fig. 11), Na2CO3 is produced during the conversion reaction. The mass loss at 600–800 °C in the CCWMP treated soil indicates the loss of CO2 from converted Na2CO3 and unreacted CaCO3 [64]. It can be concluded that by the addition of CCWMP, the CO2 from CaCO3 during the conversion of Ca(OH)2 is stored in the stable mineral form (Na2CO3).
Mechanism Involved in the CCWMP Treatment of Soil
Based on the findings from the obtained results, four fundamental reactions have been listed and are represented in Fig. 14. These reactions explain the mechanism of the CCWMP treatment of expansive soil.
Conversion of WMP into Hydrated Lime
The first reaction is the conversion of CaCO3 into Ca(OH)2 with the addition of NaOH after the dry mixing of WMP in expansive soil, as shown in Eq. (1). The above conversion takes place immediately after the addition of NaOH. Simultaneously, the carbonate content in WMP reacts with NaOH and forms Na2CO3 without emitting CO2 into the atmosphere. The converted Ca(OH)2 and Na2CO3 were confirmed with mineralogical studies (Fig. 11) and thermal studies (Fig. 13).
Dissociation of Hydrated Lime
The converted lime from CaCO3 dissociates in pore solution and produces calcium and hydroxyl ions, as shown in Eq. (2). The dissociated calcium and hydroxyl ions assist in the stabilization of expansive soil.
Cation Exchange and Flocculation
In a short-term reaction, the dissociated calcium ions in the pore solution are exchanged with exchangeable ions present on the clay surface. This cation exchange reaction reduces the diffused double-layer thickness of clay particles. The soil particles rearrange into a flocculated or agglomerated structure. This short-term reaction induces changes in the soil’s plasticity and engineering properties. The reactions discussed above are verified using initial tests such as index properties and swell potential tests.
Pozzolanic Reaction
In a long-term reaction, the added NaOH creates an alkaline condition in the pore solution. The silica and alumina dissolutes from the clay structures because of the alkaline condition of the pore solution. The dissolved silica and alumina combine with free calcium to form cementitious products like CSH and CAH, as shown in Eqs. (3) and (4) [4, 5, 8, 57]. The formation of pozzolanic compounds was confirmed by mineralogical and microstructural studies and also by the strength improvement achieved with curing periods.
The mechanism of the CCWMP treatment of soil is similar to that of the stabilization of expansive soil using lime after the conversion of WMP into Ca(OH)2. The test results indicate the elimination of swell potential and enhancement of shear strength in expansive soil by the CCWMP treatment of soil.
Comparison of CCWMP Treatment of soil with Different Stabilization Methods
In this study, the stabilized expansive soil achieved through the novel CCWMP method was compared with several other expansive soil stabilization methods sourced from the existing literature. The research involved comparing the swell potential and UCS of untreated and treated expansive soil utilizing the CCWMP treatment approach with conventional methods (lime and cement) [51, 65], the incorporation of waste additives (fly ash, GGBS, and volcanic ash) [18, 20, 28, 66, 67], polymer treatment (polyacrylamide and xanthan gum) [68, 69], the utilization of the microbially induced calcite precipitation method [70], lime precipitation techniques, and the application of the geopolymer method employing volcanic ash, fly ash, and GGBS [67, 71] as obtained from the literature.
Swell Potential
The comparison of the swell potential for untreated and treated expansive soil in both methods is shown in Fig. 15. Typically, the swell potential of treated expansive soil holds significant importance as it characterizes the soil’s swelling behavior. The lime treatment [51] and the lime precipitation technique [52] for stabilization were the only ones that completely controlled the swell potential, which means they effectively reduced soil swelling. However, it should be highlighted that a significant quantity of lime is necessary to completely mitigate the swell potential of soils with high levels of swelling, as demonstrated by Estabragh et al. [65]. This is considered unsustainable, due to the depletion of natural resources and environmental effects, as mentioned earlier. Moreover, the incorporation of fly ash and GGBS does not effectively control the swelling tendencies of expansive soil [18, 20, 28, 66, 67], primarily because of the insufficient presence of calcium and the slow rate of hydration. Stabilizing expansive soil with polyacrylamide only controls 43% of the swell potential of untreated soil [68], primarily due to the absence of a chemical reaction between the soil and the additives. Similarly, the geopolymer method for expansive soil stabilization does not eliminate the soil’s swell potential [67, 71], mainly due to inadequate short-term reactions like cation exchange.
However, in the CCWMP treatment of soil, the swell potential of untreated soil is entirely mitigated. This is achieved through the replacement of sufficient calcium on the clay surface and further pozzolanic reactions, as discussed in Sect. 4.6. When compared with the lime method, the production-related carbon emissions from WMP, are not released into the atmosphere. Instead, carbonates are sequestered in the form of Na2CO3 within the soil itself. This is the biggest advantage over the conventional methods (lime and cement). Hence, to effectively manage the swell potential of expansive soil, it is essential to employ calcium-based additives that exhibit active properties and robust pozzolanic reactions. This requirement is met by the current method of stabilization, namely the CCWMP treatment of soil.
Unconfined Compressive Strength (UCS)
Enhancing the strength of the subgrade is of paramount importance in the realm of subgrade stabilization. Notably, achieving a substantial increase in subgrade strength holds economic significance as it leads to the design of more cost-effective pavements. A well-improved subgrade can reduce the required thickness of the pavement’s base and subbase layers, ultimately conserving natural resources [72]. In this context, the current method has demonstrated the capability to surpass the required UCS (750 kPa) of the subgrade for pavement construction as per IRC 37 (2018) guidelines. Figure 16 shows a plot of UCS of untreated and treated expansive soil by various methods and is compared with the present CCWMP treatment of soil. The strength improvement in the CCWMP treatment of soil is greater than that of other chemical methods (lime, cement, or industrial waste) [20, 51, 73, 74] except for some geopolymer methods of stabilization [31, 75]. The additive used in the geopolymer method is an aluminosilicate precursor, which is activated by alkaline solutions like NaOH and sodium silicate for soil stabilization, but some of the geopolymer methods [71] achieve less strength than the CCWMP treatment of soil. The relatively lower leaching of silica and alumina in the CCWMP treatment of soil can be attributed reason for a lower improvement than the geopolymer method of stabilization. Consequently, there is a need for further research to explore the strength-related aspects of subgrade stabilization using silica-based additives with the CCWMP treatment of soil.
Applications, Limitations, and Future Scope of Study
Marble waste is a natural byproduct of the marble industry that does not contain heavy metals and thus does not require pretreatment. Numerous research initiatives have explored its versatile applications for various construction purposes. In the current study, the effective utilization of marble waste by chemical conversion for expansive soil stabilization yields significant improvement. Importantly, this chemical conversion process does not emit CO2 into the environment. This approach finds applicability in pavement construction, the construction of lightly loaded structures, canal lining, and other related areas on treated soil.
The primary limitation of the study is associated with the application of NaOH in the field, which raises environmental concerns. Further, the reaction between marble waste and NaOH is contingent on the chemical nature of the reacting substances, as well as the size and concentration of reactants, and the temperature. Hence, a more comprehensive investigation is required to optimize the concentration of NaOH and the quantity of marble powder. Before field implementation, it is crucial to conduct leaching tests and studies on durability to ensure environmental safety. Additionally, a cost-effectiveness analysis should be carried out after the optimization study, which can confirm the goals of sustainable development.
Conclusions
In order to resolve the issues associated with conventional soil stabilizers, the present study developed a novel approach for stabilizing expansive soil using the chemically converted waste marble powder method. The mechanism of CCWMP treatment of soil was examined based on geotechnical behavior, mineralogical, and microstructural studies, and it was compared with the IAWMP treated soil. Based on the results, the following overall conclusions from the current study emerge:
-
1)
The incorporation of NaOH into WMP leads to the generation of Ca(OH)2 and Na2CO3, as confirmed by XRD analysis. The mechanism of CCWMP treatment, similar to lime stabilization, follows this process after converting WMP to lime. This approach uniquely captures carbonate as Na2CO3 within the soil, distinguishing it from traditional lime stabilization. The converted Ca(OH)2 contributes towards expansive soil stabilization.
-
2)
The expansive soil, treated with CCWMP, experienced a sudden drop in plasticity index (from 39 to 9%) and liquid limit (from 69 to 47%) upon the addition of just 5% WMP. This is attributed to the substantial reaction between expansive soil and converted Ca(OH)2, leading to a reduction in the diffused double layer thickness, as confirmed by the XRD analysis showing a decrease in the montmorillonite peak. Such conversion is crucial for promoting short-term reactions in expansive soil.
-
3)
The swell potential of the expansive soil, initially at 9.6%, is completely mitigated by applying the CCWMP treatment to the soil, with just 5% WMP, even within the first day of curing. This elimination of swell potential can be attributed to a rapid short-term reaction coupled with a pozzolanic reaction in the treated soil during the initial stages of curing.
-
4)
The UCS of treated expansive soil with CCWMP increased with an increase in WMP content and curing period, attributed to short-term reactions involving cation exchange that causes flocculation and aggregation, followed by long-term pozzolanic reactions as confirmed by XRD analysis and SEM images. Based on the investigation carried out, the optimal dosage of WMP for the CCWMP treatment is determined to be 15% with 5 M NaOH, which exhibits improved strength of 1.8 and 6.26 times as compared to untreated soil (283 kPa) at 1 day and 28 days of curing, respectively.
-
5)
According to geotechnical, mineralogical, and microstructural studies, the IAWMP is not suitable for stabilizing expansive soil. The relatively lower improvement in the plasticity index, swell potential, and UCS of the expansive soil as compared to the CCWMP treatment of soil is attributed to the substitution of soil with inert WMP.
Hence, the CCWMP treatment of expansive soil provides an alternative to the conventional methods of stabilizing expansive soil. Concurrently, it also effectively addresses the utilization of substantial amounts of waste from marble quarries.
Data Availability
The data that support the findings of this study are available on request from the corresponding author.
References
Nelson JD, Miller DJ (1992) Expansive soils: problems and practice in foundation and pavement engineering. New York
Mitchell J, Soga K (2005) Fundamentals of soil behavior. John Wiley and Sons, Inc., Hoboken, NJ
Ikeagwuani CC, Nwonu DC (2019) Emerging trends in expansive soil stabilisation: a review. J Rock Mech Geotech Eng 11:423–440. https://doi.org/10.1016/j.jrmge.2018.08.013
Uppal HL, Chadda LR (1967) Physico-chemical changes in the lime stabilization of black cotton soil (India). Eng Geol 2:179–189. https://doi.org/10.1016/0013-7952(67)90017-8
Bell FG (1996) Lime stabilization of clay minerals and soils. Eng Geol 42:223–237. https://doi.org/10.1016/0013-7952(96)00028-2
Puppala AJ, Mohammad LN, Allen A (1996) Engineering behavior of lime-treated louisiana subgrade soil. Transp Res Record: J Transp Res Board 1546:24–31. https://doi.org/10.1177/0361198196154600103
Boardman DI, Glendinning S, Rogers CDF (2001) Development of stabilisation and solidification in lime–clay mixes. Géotechnique 51:533–543. https://doi.org/10.1680/geot.2001.51.6.533
Sivapullaiah PV, Sridharan A, Bhaskar Raju KV (2000) Role of amount and type of clay in the lime stabilization of soils. ICE-Ground Improv 4(1):37–45
Barman D, Dash SK (2022) Stabilization of expansive soils using chemical additives: a review. J Rock Mech Geotech Eng 14:1319–1342. https://doi.org/10.1016/j.jrmge.2022.02.011
Kiliç R, Küçükali Ö, Ulamiş K (2016) Stabilization of high plasticity clay with lime and gypsum (Ankara, Turkey). Bull Eng Geol Environ 75:735–744. https://doi.org/10.1007/s10064-015-0757-2
Lemougna PN, Wang K, Tang Q, Cui X (2017) Synthesis and characterization of low temperature (<800°C) ceramics from red mud geopolymer precursor. Constr Build Mater 131:564–573. https://doi.org/10.1016/j.conbuildmat.2016.11.108
Jiang B, Xia D, Yu B et al (2019) An environment-friendly process for limestone calcination with CO2 looping and recovery. J Clean Prod 240:118147. https://doi.org/10.1016/j.jclepro.2019.118147
Atahu MK, Saathoff F, Gebissa A (2019) Strength and compressibility behaviors of expansive soil treated with coffee husk ash. J Rock Mech Geotech Eng 11:337–348. https://doi.org/10.1016/j.jrmge.2018.11.004
Zada U, Jamal A, Iqbal M et al (2023) Recent advances in expansive soil stabilization using admixtures: current challenges and opportunities. Case Stud Constr Mater 18:e01985. https://doi.org/10.1016/j.cscm.2023.e01985
Tanyıldızı M, Uz VE, Gökalp İ (2023) Utilization of waste materials in the stabilization of expansive pavement subgrade: an extensive review. Constr Build Mater 398:132435. https://doi.org/10.1016/j.conbuildmat.2023.132435
Sharma A, Sharma RK (2021) Sub-grade characteristics of soil stabilized with agricultural waste, constructional waste, and lime. Bull Eng Geol Environ 80:2473–2484. https://doi.org/10.1007/s10064-020-02047-8
Kuntikana G, Singh DN (2017) Contemporary issues related to utilization of Industrial byproducts. Adv Civ Eng Mater 6:20160050. https://doi.org/10.1520/ACEM20160050
Kumar BRP, Sharma RS (2004) Effect of fly ash on engineering properties of expansive soils. J Geotech GeoEnviron Eng 130:764–767. https://doi.org/10.1061/ASCE1090-02412004130:7764
Mir BA, Sridharan A (2014) Volume change behavior of clayey soil-fly ash mixtures. Int J Geotech Eng 8:72–83. https://doi.org/10.1179/1939787913Y.0000000004
Sharma AK, Sivapullaiah PV (2016) Ground granulated blast furnace slag amended fly ash as an expansive soil stabilizer. Soils Found 56:205–212. https://doi.org/10.1016/j.sandf.2016.02.004
Singh MJ, Borana L, Weiqiang F, Xu DS (2021) Long-term swelling characteristics of montmorillonite clay with and without fly ash: wetting-drying cycle influence in 1d oedometer condition. J Test Eval 51. https://doi.org/10.1520/JTE20210248
Ayawanna J, Kingnoi N, Sukchaisit O, Chaiyaput S (2022) Utilization of ladle furnace slag from a steelwork for stabilization of soil cement. Geomech Eng 31:149–158. https://doi.org/10.12989/gae.2022.31.2.149
Radenović A, Malina J, Sofilić T (2013) Characterization of ladle furnace slag from carbon steel production as a potential adsorbent. Adv Mater Sci Eng 2013. https://doi.org/10.1155/2013/198240
Chaiyaput S, Ayawanna J (2021) Stabilization of lateritic soil by ladle furnace slag for pavement subbase material. Geomech Eng 26:323–331. https://doi.org/10.12989/gae.2021.26.4.323
Manso JM, Ortega-López V, Polanco JA, Setién J (2013) The use of ladle furnace slag in soil stabilization. Constr Build Mater 40:126–134. https://doi.org/10.1016/j.conbuildmat.2012.09.079
Chaiyaput S, Sertsoongnern P, Ayawanna J (2022) Utilization of waste dust from asphalt concrete manufacturing as a sustainable subbase course material in pavement structures. Sustain (Switzerland) 14. https://doi.org/10.3390/su14169804
Yi Y, Al-Tabbaa A, Liska M (2014) Properties and microstructure of GGBS-magnesia pastes. Adv Cem Res 26:114–122. https://doi.org/10.1680/adcr.13.00005
Al-Rawas A, Taha R, Nelson J et al (2002) A comparative evaluation of various additives used in the stabilization of expansive soils. Geotech Test J 25:199. https://doi.org/10.1520/GTJ11363J
Murmu AL, Jain A, Patel A (2019) Mechanical properties of alkali activated fly ash geopolymer stabilized expansive clay. KSCE J Civ Eng 23:3875–3888. https://doi.org/10.1007/s12205-019-2251-z
Consoli NC, Daassi-Gli CAP, Ruver CA et al (2021) Lime–ground glass–sodium hydroxide as an enhanced sustainable binder stabilizing silica sand. J Geotech GeoEnviron Eng 147. https://doi.org/10.1061/(ASCE)GT.1943-5606.0002624
Sahoo S, Prasad Singh S (2022) Strength and durability properties of expansive soil treated with geopolymer and conventional stabilizers. Constr Build Mater 328:127078. https://doi.org/10.1016/j.conbuildmat.2022.127078
Maheepala MMALN, Nasvi MCM, Robert DJ et al (2022) A comprehensive review on geotechnical properties of alkali activated binder treated expansive soil. J Clean Prod 363. https://doi.org/10.1016/j.jclepro.2022.132488
Miraki H, Shariatmadari N, Ghadir P et al (2022) Clayey soil stabilization using alkali-activated volcanic ash and slag. J Rock Mech Geotech Eng 14:576–591. https://doi.org/10.1016/j.jrmge.2021.08.012
Cruz N, Rios S, Fortunato E et al (2017) Characterization of soil treated with alkali-activated cement in large-scale specimens. Geotech Test J 40:20160211. https://doi.org/10.1520/GTJ20160211
Puppala AJ (2016) Advances in ground modification with chemical additives: from theory to practice. Transp Geotechnics 9:123–138. https://doi.org/10.1016/j.trgeo.2016.08.004
Behnood A (2018) Soil and clay stabilization with calcium- and non-calcium-based additives: a state-of-the-art review of challenges, approaches and techniques. Transp Geotechnics 17:14–32. https://doi.org/10.1016/j.trgeo.2018.08.002
Thakur AK, Pappu A, Thakur VK (2018) Resource efficiency impact on marble waste recycling towards sustainable green construction materials. Curr Opin Green Sustain Chem 13:91–101. https://doi.org/10.1016/j.cogsc.2018.06.005
Alyamac KE, Ghafari E, Ince R (2017) Development of eco-efficient self-compacting concrete with waste marble powder using the response surface method. J Clean Prod 144:192–202. https://doi.org/10.1016/j.jclepro.2016.12.156
Singh M, Srivastava A, Bhunia D (2017) An investigation on effect of partial replacement of cement by waste marble slurry. Constr Build Mater 134:471–488. https://doi.org/10.1016/j.conbuildmat.2016.12.155
Pappu A, Saxena M, Asolekar SR (2007) Solid wastes generation in India and their recycling potential in building materials. Build Environ 42:2311–2320. https://doi.org/10.1016/J.BUILDENV.2006.04.015
Jain AK, Jha AK, Shivanshi (2020) Improvement in subgrade soils with marble dust for highway construction: a comparative study. Indian Geotech J 50:307–317. https://doi.org/10.1007/s40098-020-00423-5
Mashaly AO, El-Kaliouby BA, Shalaby BN et al (2016) Effects of marble sludge incorporation on the properties of cement composites and concrete paving blocks. J Clean Prod 112:731–741. https://doi.org/10.1016/j.jclepro.2015.07.023
Munir MJ, Kazmi SMS, Wu Y-F et al (2018) Thermally efficient fired clay bricks incorporating waste marble sludge: an industrial-scale study. J Clean Prod 174:1122–1135. https://doi.org/10.1016/j.jclepro.2017.11.060
Kuoribo E, Mahmoud H (2022) Utilisation of waste marble dust in concrete production: a scientometric review and future research directions. J Clean Prod 374:133872. https://doi.org/10.1016/j.jclepro.2022.133872
Okagbue CO, Onyeobi TUS (1999) Potential of marble dust to stabilise red tropical soils for road construction Eng Geol 53:371–380. https://doi.org/10.1016/S0013-7952(99)00036-8
Chandra S, Kumar P, Feyissa BA (2002) Use of marble dust in road construction. Road Mater Pavement Des 3:317–330. https://doi.org/10.1080/14680629.2002.9689928
Öncü Ş, Bilsel H (2018) Utilization of waste marble to enhance volume change and strength characteristics of sand-stabilized expansive soil. Environ Earth Sci 77. https://doi.org/10.1007/s12665-018-7638-5
Jain AK, Jha AK, Shivanshi (2020) Geotechnical behaviour and micro-analyses of expansive soil amended with marble dust. Soils Found 60:737–751. https://doi.org/10.1016/j.sandf.2020.02.013
Çadir CC, Vekli M (2022) Usage of waste marble powder and pumice powder to improve the engineering properties of soft clays. Int J Environ Sci Technol 19:6481–6490. https://doi.org/10.1007/s13762-022-04071-5
Umar IH, Lin H, Ibrahim AS (2023) Laboratory testing and analysis of clay soil stabilization using waste marble powder. Appl Sci 13:9274. https://doi.org/10.3390/app13169274
Jha AK, Sivapullaiah PV (2015) Mechanism of improvement in the strength and volume change behavior of lime stabilized soil. Eng Geol 198:53–64. https://doi.org/10.1016/j.enggeo.2015.08.020
Thyagaraj T, Sudhakar, Rao M (2012) Laboratory studies on stabilization of an expansive soil by lime precipitation technique. J Mater Civ Eng 24. https://doi.org/10.1061/(ASCE)MT.1943-5533.0000483
Xeidakis GS (1996) Stabilization of swelling clays by mg(OH)2. Factors affecting hydroxy-Mg-interlayering in swelling clays. Eng Geol 44:93–106. https://doi.org/10.1016/S0013-7952(96)00046-4
Xeidakis GS (1996) Stabilization of swelling clays by Mg(OH)2. Changes in clay properties after addition of Mg-hydroxide. Eng Geol 44:107–120. https://doi.org/10.1016/S0013-7952(96)00047-6
Muhammad N, Siddiqua S, Latifi N (2018) Solidification of subgrade materials using magnesium alkalinization: a sustainable additive for construction. J Mater Civ Eng 30. https://doi.org/10.1061/(ASCE)MT.1943-5533.0002484
Gurbuz A (2015) Marble powder to stabilise clayey soils in sub-bases for road construction. Road Mater Pavement Des 16:481–492. https://doi.org/10.1080/14680629.2015.1020845
Cherian C, Arnepalli DN (2015) A critical appraisal of the role of clay mineralogy in lime stabilization. Int J Geosynth Ground Eng 1. https://doi.org/10.1007/s40891-015-0009-3
Gens A, Alonso EE (1992) A framework for the behaviour of unsaturated expansive clays. Can Geotech J 29:1013–1032. https://doi.org/10.1139/t92-120
Cui YJ, Yahia-Aissa M, Delage P (2002) A model for the volume change behavior of heavily compacted swelling clays. Eng Geol 64:233–250. https://doi.org/10.1016/S0013-7952(01)00113-2
Tiwari N, Satyam N (2019) Experimental study on the influence of polypropylene fiber on the swelling pressure expansion attributes of silica fume stabilized clayey soil. Geosci (Basel) 9:377. https://doi.org/10.3390/geosciences9090377
Jha AK, Sivapullaiah PV (2020) Lime stabilization of soil: a physico-chemical and micro-mechanistic perspective. Indian Geotech J 50:339–347. https://doi.org/10.1007/s40098-019-00371-9
Al-Mukhtar M, Lasledj A, Alcover JF (2014) Lime consumption of different clayey soils. Appl Clay Sci 95:133–145. https://doi.org/10.1016/j.clay.2014.03.024
Al-Mukhtar M, Khattab S, Alcover J-F (2012) Microstructure and geotechnical properties of lime-treated expansive clayey soil. Eng Geol 139–140:17–27. https://doi.org/10.1016/j.enggeo.2012.04.004
Siriwardane RV, Poston JA, Robinson C, Simonyi T (2011) Effect of additives on decomposition of sodium carbonate: precombustion CO2 capture sorbent regeneration. Energy Fuels 25:1284–1293. https://doi.org/10.1021/ef101486m
Estabragh AR, Pereshkafti MRS, Parsaei B, Javadi AA (2012) Stabilised expansive soil behaviour during wetting and drying. Int J Pavement Eng 14:418–427. https://doi.org/10.1080/10298436.2012.746688
Mypati VNK, Saride S (2022) Feasibility of alkali-activated low-calcium fly ash as a binder for deep soil mixing. J Mater Civ Eng 34. https://doi.org/10.1061/(ASCE)MT.1943-5533.0004047
Miao S, Shen Z, Wang X et al (2017) Stabilization of highly expansive black cotton soils by means of geopolymerization. J Mater Civ Eng 29. https://doi.org/10.1061/(ASCE)MT.1943-5533.0002023
Soltani A, Deng A, Taheri A, O’Kelly BC (2022) Intermittent swelling and shrinkage of a highly expansive soil treated with polyacrylamide. J Rock Mech Geotech Eng 14:252–261. https://doi.org/10.1016/j.jrmge.2021.04.009
Singh SP, Das R (2020) Geo-engineering properties of expansive soil treated with xanthan gum biopolymer. Geomech Geoeng 15:107–122. https://doi.org/10.1080/17486025.2019.1632495
Tiwari N, Satyam N, Sharma M (2021) Micro-mechanical performance evaluation of expansive soil biotreated with indigenous bacteria using MICP method. Sci Rep 11:10324. https://doi.org/10.1038/s41598-021-89687-2
Khadka SD, Jayawickrama PW, Senadheera S (2018) Strength and shrink/swell behavior of highly plastic clay treated with geopolymer. Transp Res Record: J Transp Res Board 2672:174–184. https://doi.org/10.1177/0361198118797214
Barmade S, Dhamaniya A, Patel S (2023) Evaluating the structural performance of stabilized expansive soil as subbase layer for sustainable pavements. Int J Geosynth Ground Eng 9:23. https://doi.org/10.1007/s40891-023-00440-3
Sezer A, İnan G, Yılmaz HR, Ramyar K (2006) Utilization of a very high lime fly ash for improvement of Izmir clay. Build Environ 41:150–155. https://doi.org/10.1016/j.buildenv.2004.12.009
Li W, Yi Y, Puppala AJ (2019) Utilization of carbide slag-activated ground granulated blastfurnace slag to treat gypseous soil. Soils Found 59:1496–1507. https://doi.org/10.1016/j.sandf.2019.06.002
Murmu AL, Patel A (2020) Studies on the properties of fly ash–rice husk ash-based geopolymer for use in black cotton soils. Int J Geosynth Ground Eng 6:38. https://doi.org/10.1007/s40891-020-00224-z
Bureau of Indian Standards 1970 (Reaffirmed (2021) Methods of test for soils: Classification and identification of soils for general engineering purposes, Indian Standards Institute. IS 1498. New Delhi, India: Bureau of Indian Standards
Bureau of Indian Standards 1973 (Reaffirmed (2020) Methods of test for soils: Determination of unconfined compressive strength, Indian Standards Institute. IS 2720, Part 10. New Delhi, India: Bureau of Indian Standards
Bureau of Indian Standards 1977 (Reaffirmed (2021) Methods of test for soils: Determination of swelling pressure of soils, Indian Standards Institute. IS 2720, Part 41. New Delhi, India: Bureau of Indian Standards
Bureau of Indian Standards 1980 (Reaffirmed (2021) Methods of test for soils: Determination of specific gravity. Section 1: Fine grained soils, Indian Standards Institute. IS 2720, Part 3. New Delhi, India: Bureau of Indian Standards
Bureau of Indian Standards 1980 (Reaffirmed (2021) Methods of test for soils: Determination of water content-dry density relation using light compaction, Indian Standards Institute. IS 2720, Part 7. New Delhi, India: Bureau of Indian Standards
Bureau of Indian Standards 1985 (Reaffirmed (2020) Methods of test for soils: Determination of liquid limit and plastic limit, Indian Standards Institute. IS 2720, Part 5. New Delhi, India: Bureau of Indian Standards
Bureau of Indian Standards 1985 (Reaffirmed (2020) Methods of test for soils: Grain size analysis, Indian Standards Institute. IS 2720, Part 4. New Delhi, India: Bureau of Indian Standards
Bureau of Indian Standards 1977 (Reaffirmed (2021) Methods of test for soils:Determination of free swell index of soils, Indian Standards Institute. IS 2720, Part 40. New Delhi, India: Bureau of Indian Standards
Acknowledgements
The first author Mr. S Chandru would like to express gratitude to the Ministry of Education (MoE), India, formerly the Ministry of Human Resource Development (MHRD), for providing financial support. The authors would like to extend their gratitude to the National Institute of Technology Tiruchirappalli, India for providing financial support for analytical expenses and access to the laboratory facilities used in this study. The authors would like to thank the anonymous reviewers for their valuable comments, which significantly enhanced the quality of the manuscript.
Author information
Authors and Affiliations
Contributions
S Chandru: Conceptualization, Investigation, Methodology, Data curation, Writing – original draft, Review & editing. S Jayalekshmi: Conceptualization, Methodology, Supervision, Review & editing.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chandru, S., Jayalekshmi, S. Effective Utilization of Waste Marble Powder by Chemical Conversion for Stabilization of Expansive Soil: An Alternative to Conventional Methods. Int. J. of Geosynth. and Ground Eng. 10, 81 (2024). https://doi.org/10.1007/s40891-024-00590-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40891-024-00590-y