Abstract
Smectite-based geosynthetic clay liners (GCLs) are popularly employed as hydraulic barriers due to their low permeability and high swelling capacity. The exposure of GCLs to aggressive inorganic permeants is inevitable in the majority of field applications. GCLs exhibit inferior hydraulic properties and low swelling in these scenarios due to the concomitance of various physico–chemical interactions. To confront such aggressive environmental conditions, bentonites are modified chemically to enhance their resistance against increased permeability. Several polymer-modified clays have been developed with improved hydraulic properties; however, the efforts made to comprehend the overview of available literature are scarce. Limited studies have focussed on addressing the fundamental mechanism ascribing to their enhanced hydraulic performance. Given this, the present review article comprehends the insights into different types of modifications on smectite-based GCLs from their hydraulic performance perspective. Osmotic swell enhancement and pore clogging phenomena were found to be the primary mechanisms responsible for the improved hydraulic performance of polymer-treated GCLs. Further, the study reviewed the variation of permeability of various polymer-modified GCLs with the swell index based on the data published in the literature till date and proposed a relationship correlating them considering the wide range of permeants. The regression analysis results evidenced the suitability of the swell index as one of the index properties for ascertaining its hydraulic performance. Also, the study advocated the use of the dielectric constant of permeant for correlating the hydraulic behaviour of polymer-modified GCLs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Geosynthetic clay liners (GCLs) are factory-made products typically consisting of either powdered or granular sodium bentonite (Na-bentonite) sandwiched between the cover and carrier geotextiles. The bonding method and the type of geosynthetic material used give rise to a variety of GCL products. GCLs gained popularity owing to several advantages such as ease of construction, cost-effectiveness, rapid installation, flexibility concerning the differential settlement, low permeability (k value), and high self-healing capacity [1]. GCLs are extensively used in liner applications such as dams, canals, reservoirs, waste containment facilities, heap leach pads, wastewater ponds, tailings impoundment, fly ash lagoons, and other geotechnical and geoenvironmental engineering practices [2,3,4,5]. Subsequently, GCLs are expected to be exposed to aggressive chemical solutions and extreme pH conditions, at which the Na-bentonite failed to perform its intended barrier function. The studies on the chemical compatibility of GCL using various chemical permeants indicated the inability of Na-bentonite in resisting the change in k value and swelling capacity [6,7,8]. The magnitude of increased k value depends on the purity and amount of smectite mineral, aggregate size, bentonite mass per unit area, type of GCL, nature of the permeant, thickness of the adsorbed water layer, composition of bound cation, and confining stress [6, 9, 10]. Several laboratory studies have evidenced the reduction in the hydraulic performance of GCL upon permeation with high ionic strength solutions [1, 11,12,13]. When GCL interacts with multivalent ions, cation exchange reactions replace the monovalent sodium ions from the exchange complex. Cation exchanged bentonite does not undergo adequate swelling even upon re-hydration and results in a large k value. The adverse conditions that promote severe cation exchange viz., permeants having high ionic strength, extreme pH conditions; environmental distress including the variation of temperature and moisture content, wet-dry cycles, freeze–thaw effects; and low overburden pressure together exacerbates the problem associated with GCL [14]. Various field investigations revealed cation exchange along with desiccation as the cause for inferior hydraulic performance [15,16,17,18,19]. The studies demonstrated the inability of virgin Na-bentonite to contain aggressive contaminants and emphasized the need for an innovative barrier material to protect the geoenvironment from plausible contamination. This led to the invention of modified bentonites in which additives or polymers are added for improving their chemical compatibility and hydraulic performance. Several modified clays were developed, namely organoclays, multi-swellable bentonite (MSB), HYPER clay (HC), dense-prehydrated (DPH) clay, contaminant resistant clay (CRC), and bentonite polymer nanocomposite (BPN). It is apparent from the previous studies that the bentonite treated with polymer was propitious in improving its hydraulic performance. However, the phenomenon of clay–polymer interaction and the fundamental mechanism attributing to their improvement was not completely understood. The critical review of the literature suggests that the efforts made to comprehend the different types of polymer-modified bentonites are limited.
With this in view, the present review article aims to provide a comprehensive overview of the preparation of various polymer-modified bentonites. The mechanisms ascribed to the improved hydraulic properties of amended bentonites are discussed in detail. The enhanced hydraulic performance of treated bentonites over a wide range of chemical environments is reviewed. Further, the study attempted to correlate the swell index and permeability of polymer-modified bentonite-based GCLs using the data previously reported in the literature. In addition, the variation of k value with the dielectric constant (ε) of the permeant is investigated.
Modified Bentonites
The swelling of clays generally involves the following two phases: crystalline and osmotic swelling. During the crystalline swelling, the internal and external clay surface is hydrated along with the cations present in the exchange complex. This results in the step-wise formation of water layers in the interlayer surrounding the exchangeable cation. Crystalline swelling occurs in all types of clay minerals despite the type of cation satisfying the net negative charge and takes place prior to osmotic swelling. On the other hand, osmotic swelling can occur only when the exchange sites contain monovalent cations. The difference in solute concentration between the interlayer and the interparticle pore fluid produces an osmotic gradient. This difference in chemical potential drives the additional water molecules into the clay interlayer resulting in the osmotic swelling [1]. Figure 1 depicts the schematic of crystalline and osmotic swelling in a 2:1 clay mineral. In the crystalline phase, hydration of clay occurs from a completely dry state and the interlayer expands until it encompasses four layers of water molecules. As a result, the basal spacing of clay (d001) typically increases from 0.9 to 2.2 nm, whereas the osmotic swelling can accommodate several layers of water molecules and results in a large increase in the basal spacing of clay, i.e., d001 > 2.2 nm [20]. The low k value exhibited by GCLs is generally associated with the osmotic swelling of Na-bentonite because it contributes to a significant fraction of adsorbed water molecules compared to the crystalline phase.
GCLs deployed in most of the applications are exposed to permeants of not only water but also other inorganic liquids comprising a wide range of concentrations from an aqueous dilute to a more aggressive, highly concentrated solution. When GCLs are exposed to aggressive inorganic solutions, osmotic swelling does not occur due to the cation exchange reactions. As a result, the thickness of the diffused double layer (DDL) is compressed, and the clay structure becomes more flocculated. These microstructural changes increase the pore space for the flow of permeant and result in a high k value [21, 22]. To overcome this, Na-bentonite present in the GCLs is modified using polymers which in turn helps in absorbing numerous water molecules and retain the osmotic swelling. In general, for improving the k value of Na-bentonite, a variety of polymers are used in practice depending on the application. However, the scope of the present study is limited to the polymer-modified bentonites belonging to GCL applications. In this regard, the following section provides a concise review of previous investigations on various polymer-modified bentonites concerning their genesis, method of preparation, the mechanism responsible for their enhanced barrier properties, hydraulic performance to several permeants, and the pitfalls associated with their long-term performance.
Organoclays
GCLs deployed as a part of petroleum waste containment systems, organic liquid storage tanks, and barrier systems for containing pollutants from accidental spills are expected to attenuate organic pollutants for alleviating their transport into the surrounding environment. However, the Na-bentonite in GCL has a limited sorption affinity towards the organic molecules. The insignificant adsorption capacity is attributed to the hydrophilic nature of the bentonite and the large molecular size of the organic compounds [23]. The retention capacity of the clay towards organic contaminants can be improved by increasing its interlamellar space and altering the clay surface to organophilic. This was achieved by replacing the existing interlayer cations with a suitable organic molecule via ion exchange reactions. This modification resulted in organoclays with reduced hydrophilicity and an extremely organophilic surface. Cationic surfactants, such as quaternary alkylammonium compounds, are the most commonly used modifiers for organoclays. These organic compounds contain both hydrophilic and hydrophobic functional groups. The former binds to the negatively charged clay surface, and the latter enables the adsorption of hydrophobic organic pollutants. The resulting sorption aids in the improved performance of organoclay compared to the Na-bentonite. The surfactants absorb irreversibly onto the clay surface through a covalent bond, hydrogen bond, hydrophobic interaction, solvation, desolvation, and Coulombic forces [24]. The treatment aids in improved sorption of organic compounds in the clay interlayer and results in increased interlattice spacing [25]. This in turn leads to the improved swelling capacity of organoclay compared to the untreated bentonite. The intercalation of organic molecules within the clay lattice contributes to its increased internal surface area. However, the extent of intercalation and adsorption of organic compounds depends on the carbon chain length, surfactant loading, and the cation exchange capacity of clay [26, 27]. Modifying the clay with alkylammonium compounds imparted high adsorption and swelling capacity upon interaction with organic pollutants when compared to the untreated Na-bentonite [28, 29]. This resulted in enhanced immobilization of organic compounds and reduced k value, which helped in impeding the migration of organic contaminants [30, 31]. Several laboratory studies performed on organoclays have identified an enhanced swelling with high affinity and a low k value towards organic pollutants [32,33,34,35,36]. The sorption affinity of the modified clays was found to increase with the extent of organic modification [28]. A considerable amount of research on the adsorption capacity of organoclays can be found in the literature related to clay nanocomposites. Even though there are many organic additives, only selective modifiers with bentonite are shown to perform well in attenuating organic pollutants. One such commonly used organic additive is hexadecyl-trimethylammonium (HDTMA).
Gitipour et al. [25] investigated the hydraulic performance of GCL containing organoclay modified with HDTMA for containing the crude oil. Organoclay maintained a low k value of 5.2 × 10–11 m s−1, whereas the untreated clay exhibited a k value as high as 1.15 × 10–8 m s−1. The improved hydraulic performance of the organoclay has been substantiated in terms of its increased interlattice space, enhanced swelling, and reduced effective porosity. Further, the sorption of volatile organic compounds (VOCs) on organoclays was shown to increase several times compared to untreated bentonite [37, 38]. Lo and Yang [39] highlighted the ability of organoclay to resist weathering damages induced by freeze–thaw and wet–dry cycles. The bentonite modified with dicetyldimethylammonium exhibited a k value of magnitude two to five orders lower than that of the virgin clay upon permeation with gasoline. Besides, organoclay maintained a k value less than 1 × 10–10 m s−1 even after three wet–dry cycles. This was attributed to the high swelling capacity of the bentonite upon interaction with organic molecules that helped in sealing the desiccation cracks.
These findings illustrate the beneficial characteristics of organoclay, suggesting its usefulness as an alternative liner material for containing organic contaminants. However, it has been reported that modifying the clay with organic additives in the desire to increase the sorption capacity may lead to a considerable increase in its k value [3]. In the study by Lorenzetti et al. [40], the k value of modified GCL (a mixture of bentonite and HDTMA-bentonite) increased substantially when the addition of organo-bentonite exceeded more than 20% by weight. This could be attributed to the various physico–chemical reactions resulting from the clay-permeant interaction. Hence the potential benefit of organoclay is liable to the threshold value depending on the extent of modification. Also, Lake and Rowe [38] showed a modest change in the migration of contaminants (VOCs) despite considerable improvement in its sorption capacity. Overall, the limited improvements, in addition to the high cost, affected the extensive usage of organoclays.
Multi-Swellable Bentonite
Multi-swellable bentonite (MSB) was developed by Kondo [41], in which propylene carbonate (PC), a cyclic organic carbonate, was employed to treat the bentonite. MSB-based GCLs exhibited high swell and low k value towards permeants containing multivalent and high ionic strength monovalent cations [42]. The enhanced hydraulic performance was attributed to the activation of the osmotic swell induced by PC that was intercalated within the clay lattice. Through this phenomenon, PC attracts a large amount of water molecules and tends to expand the interlayer spacing of clay to sustain osmotic swelling even in the aggressive chemical environment. This increased swelling contributed by the polymer enhanced the hydraulic properties of MSB. PC was found to interact with bentonite by forming a complex with exchangeable cation. It binds to the outer hydration shell of the exchangeable cation via intermolecular hydrogen bonding and ion–dipole interaction [43, 44]. Unlike organoclays, MSB maintained a low k value of 1 × 10–11 m s−1 for deionized (DI) water owing to the ease of formation of the hydration shell. Also, it retained a k value of 6.9 × 10–11 m s−1 towards 5 mM CaCl2 solution; this moderate increase in k value compared to DI water was attributed to the sidewall leakage [45]. Na-bentonites can undergo osmotic swelling upon interaction with NaCl solutions having an ionic strength of 300 mM or lower, beyond which the swelling in clays gets suppressed significantly. Whereas MSB exhibited a considerable osmotic swell even when exposed to 750 mM NaCl solution [46]. Mazzieri and Pasqualini [47] have reported a low k value of MSB-based GCLs ranging between 4.2 × 10−11 and 5.4 × 10–11 m s−1 upon direct permeation with seawater. However, the prehydrated MSB-based GCLs exhibited a relatively high k value due to the reduced swelling capacity and microstructural modifications that are resulted from the compression of DDL thickness upon prehydration.
Katsumi et al. [43] measured the k value of MSB-based GCLs using both monovalent and divalent permeants, considering a wide range of concentrations. MSB exhibited superior hydraulic performance (< 2 × 10–11 m s−1) relative to the untreated bentonite when permeated with NaCl solutions having concentrations of 500 mM or lesser. However, an abrupt change in k value by two orders of magnitude was reported when the permeant concentration exceeded 1000 mM. On the contrary, MSB showed a low k value (~ 1 × 10–11 m s−1) towards divalent permeants regardless of their concentration which was attributed to the precipitation of carbonates within the pore structure. Overall, the treatment of bentonite with PC was found to be effective for containing the divalent and low concentrated monovalent contaminants. In the study by Fehervari et al. [48], the bentonite was modified using glycerol carbonate (GC). The GC-modified bentonite exhibited a low swell upon hydration with water when compared to MSB and untreated bentonite. The reduction in the swelling capacity can be attributed to the partial or complete removal of hydrated water surrounding the cation from the clay structure. The removal of interlattice water was fuelled by the interaction of GC with the exchangeable cation either directly or through the hydration shell. Despite low swelling and less adsorbed water in the clay interlattice, GC-treated clays outperformed MSBs by exhibiting a low k value in the order of 1 × 10–11 m s−1 for both NaCl and CaCl2 solutions having concentrations higher than 1500 mM.
The above insights into the hydraulic properties of MSB raise questions regarding its long-term performance since propylene carbonate can downgrade to propylene glycol [49]. Also, it has been reported that the prolonged permeation of prehydrated MSB resulted in the elution of propylene carbonate [50].
HYPER Clay
HYPER clay (HC) was developed by amending the bentonite with an anionic polymer viz., sodium carboxymethyl cellulose (CMC). It is prepared by mixing the bentonite with the polymer solution consisting of CMC using a mechanical stirrer for 30 min to increase the surface area of clay for polymer adsorption. The mixture is then oven-dried at temperatures higher than 60 °C to favour irreversible adsorption of polymer onto the clay surface [51, 52]. Many researchers have investigated the interaction of the anionic polymer and the negatively charged clay platelets. Adsorption of the anionic polymer onto the clay surface has been found to occur through the ion exchange process [51], hydrogen bonding [53], cation bridging [54], or coordination bond via ligands [55]. It can also interact with the positively charged edge surface of clay through the electrostatic force of attraction [56]. The results of the Fourier transform infrared spectroscopy (FTIR) analysis performed by Qiu and Yu [57] showed a strong interaction of the ether bond and Si–O bond between the polymer and clay, which was found to be the driving force for the intercalation of the polymer between the clay lamellae. Polymer intercalation was found to be responsible for the improved performance of HC even under the influence of high ionic strength solutions, which caused the basal spacing to increase and propped open upon exposure to such solutions. The extent of basal spacing increase depends on the polymer dosage, which thereby governs the performance of HC [52].
Bentonite modified with 2% CMC was found to experience higher swelling by ~ 42% than its untreated counterpart in DI water; however, the swell index decreased as the electrolyte concentration increased. Moreover, the effect of polymer content on the swell index was appreciable when the interacting fluid happened to be dilute aqueous solutions such as CaCl2 having concentrations less than 100 mM. The k value of HC treated with 8% CMC and 2% CMC was reported as 7.03 × 10–12 m s−1 and 1.37 × 10–11 m s−1, respectively, when tested with 5 mM CaCl2 solution as the permeant. Further, when HC was permeated with seawater, it exhibited a k value of magnitude one order lower than that of the untreated bentonite despite having a low swell in seawater. This could be attributed to polymer intercalation that induced a tortuous flow path during seawater permeation [58]. Compared to MSB and DPH clay, HC showed improved chemico-osmotic efficiency and was found to be suitable for its application in marine environments [59].
In general, the long-term performance of the barriers is compromised owing to the detrimental effect of chemical compatibility, which may be further exacerbated if cation exchange gets combined with desiccation cracking. De Camillis et al. [60] examined the influence of wet–dry cycles using seawater on the hydraulic performance of HC that was treated with 2% and 8% polymer content. HYPER clay with a high polymer loading performed better than its counterpart, which was consistent with the earlier discussions. At the end of four cycles, the k value of unmodified clay increased to 2.93 × 10–7 m s−1, owing to the non-closure of desiccation cracks that were formed during drying. In contrast, the k value of HC with 2% CMC remained as low as 3.5 × 10–10 m s−1 and HC with 8% CMC had a k value of 9.11 × 10–11 m s−1. HC showed a low k value and improved healing capacity compared to the untreated bentonite. The study conducted by De Camillis et al. [61] evaluated the performance of sodium-activated bentonite and HC with 8% CMC after subjecting to wet–dry cycles with seawater. The drying cycles were performed at 110 °C although it is unrealistic concerning the anticipated field conditions. After four cycles, the k value of sodium-activated bentonite and HC with 8% CMC was reported as ~ 1 × 10–7 m s−1 and 1 × 10–10 m s−1, respectively. The results revealed that the polymer treatment helped in retaining a thick DDL by imbibing more water molecules and also aided in maintaining a dispersed clay structure which was attributed to the enhanced swelling and healing capacity of HC with consequent wet–dry cycles.
Though HC showed beneficial properties, the major problem associated with the usage of HC is the biodegradation of carboxymethyl cellulose [58]. Therefore, further research is warranted for assessing the long-term performance of HC under various biochemical conditions.
Dense-Prehydrated Clay
Dense-prehydrated (DPH) clay-based GCL is a patented product that is produced via a two-step process, namely prehydration and densification. A British company named Rawell Environmental Limited, Hoylake, UK, introduced DPH GCLs in the 1990s. The patent licensed to Flynn and Carter [62] describes the manufacturing procedure of DPH clays. In this, the bentonite is first mixed with a polymer solution in a high shear mixer. It was followed by densification, where the bentonite hydrated with polymer is extruded under vacuum into a thin sheet of 5 mm thickness, and it has an average void ratio of 1.5. The extruded bentonite sheet is then sandwiched between the geotextiles, and the GCL rolls are formed. The calendering process results in forming a dense network of uniform pores within the bentonite and orients the clay particles predominantly in an edge-to-edge direction. The polymeric solution used for prehydration consists of sodium carboxymethyl cellulose (CMC), sodium polyacrylate, and methanol, which confers workability and antifungicidal preservation. However, the exact composition of the polymer additive is proprietary. The DPH bentonite exhibited an excellent performance towards aggressive permeants due to both physical and chemical processes associated with its manufacturing. Overall, the DPH clay-based GCLs showed a superior membrane behaviour compared to the conventional GCLs when tested with KCl solution of concentrations 8.7–160 mM [63]. Kolstad et al. [64] investigated the swell and k value of DPH clay-based GCLs using 1000 mM CaCl2, 1000 mM NaCl, HCl (pH = 1.2), and NaOH (pH = 13.1). The swelling capacity was found to be comparable in both treated and untreated bentonite except in the alkaline environment, where the DPH clay swelled by more than 90%. The conventional GCL experienced severe damage upon permeation with strong electrolyte solutions, which can be attributed to the suppression of DDL thickness. In contrast, DPH clay retained a low k value in all the aqueous solutions irrespective of valence and ionic strength. It retained a k value of magnitude four to five orders lower than that of the untreated clay. The improved performance of DPH clay was because of the intercalation of polymer that helped in preventing the collapse of the interlayer of the clay lattice. The DPH clay maintained a k value of ~ 1 × 10–12 m s−1 for CaCl2 solution having concentrations up to 1000 mM and exhibited a maximum difference of five orders of magnitude compared to the untreated clay when tested with 2000 mM CaCl2 [43, 65]. In addition, the DPH clay-based GCLs exhibited an improved performance even under the synergy of low confining stress and exposure to high ionic strength permeants [66, 67]. Thus, the inhibition of interlayer swelling can be controlled by retaining the basal spacing of clay through polymer intercalation, which can help in ascertaining a low k value.
However, some studies reported equivalent or less improvement compared to that of the untreated bentonite. For example, Mazzieri et al. [68] reported the k value of DPH clay-based GCL to distilled water as 3.7 × 10–12 m s−1, which is only one order magnitude lesser than that of the conventional GCL (1.5 × 10–11 m s−1). The ability of DPH clay-based GCLs to attenuate metal ions was studied by Mazzieri et al. [68]. The GCLs were first prehydrated with distilled water and then permeated with a 25-mM acidic solution containing metal ions such as Pb, Zn, and Cu. The retention capacity of DPH clay-based GCLs was found to be better than that of the traditional GCLs, owing to the fixation of metal cations through ion exchange reactions. However, the k value of DPH clay (3.3 × 10–11 m s−1) was close to that of the untreated bentonite (1.6 × 10–10 m s−1). Few attempts have been made to investigate the effect of wet–dry cycles on the hydraulic performance of DPH clay-based GCLs. Mazzieri [69] used distilled water and 12.5 mM CaCl2 solution for assessing the combined effect of desiccation and ion exchange reactions. When water was used as the permeant, no appealing changes were observed concerning the k value during successive cycles. In other words, desiccation has an insignificant effect on the k value of DPH clay if the permeant happens to be water, whereas the k value for 12.5 mM CaCl2 solution increased from 6.8 × 10−9 to 5.5 × 10–5 m s−1 after the second wet–dry cycle. It was owing to the formation of unsealable desiccation cracks that have resulted from the severe drying. It was also found that the DPH clay can maintain self-healing ability and regain its hydraulic performance if the extent of drying is limited to the threshold water content of 45%. Mazzieri and Pasqualini [70] conducted wet–dry cycling on DPH clay–based GCLs using 12.5 mM CaCl2. After the second cycle, the k value increased substantially to 5.5 × 10–7 m s−1, which is three orders of magnitude higher than that reported by Mazzieri [69]. The increase in k value can be attributed to the exchange of sodium ions from the exchange complex, as witnessed from the swell index test, which gave a value similar to calcium bentonite. It can be inferred that this method of modification is incapable of preventing cation exchange reactions. Mazzieri et al. [71] conducted wet–dry cycles on DPH clay–based GCLs using seawater. After five cycles, the increased k value was found to be in the range of 2 × 10−10 to 1 × 10−9 m s−1. Moreover, the wet–dry cycles compromised the amendment technique that was used for treating bentonite. The successive cycles resulted in the removal of additives, as witnessed by Mazzieri [69], Mazzieri et al. [71], and Mazzieri and Pasqualini [70]. As mentioned earlier in HC, DPH clay also encounters the problem of biodegradation of sodium carboxymethyl cellulose. Thus, the performance of DPH clay-based GCLs may be impaired in the long-term because of the polymer’s instability. Systematic investigations in similar lines are required to ascertain their long-term performance.
Contaminant Resistant Clay
To reduce the impact of aggressive permeants on the performance of conventional GCL, Na-bentonite is treated with an additive to develop contaminant-resistant clays (CRCs). The information regarding the chemical composition of additives is proprietary. Many types of CRCs are available commercially with the undisclosed chemical formulation. CRCs are shown to have excellent performance compared to Na-bentonite on exposure to high ionic strength solutions and extreme pH conditions [72]. McKelvey [73] used CRC for containing calcium sulfate sludge generated from the treatment of acid mine drainage in the lime neutralization method. The sludge contained concentrated solutes with high pH. The conventional Na-bentonite-based GCLs were anticipated to undergo severe ion exchange in such aggressive conditions. But the proposed CRC was likely to confront the calcium-rich leachate. The swell index and k value of CRC were comparable with that of the unmodified bentonite tested with the DI water. However, CRC exhibited a low k value of less than ~ 1 × 10–10 m s−1 to leachate rich in calcium. Benson et al. [74] measured the k value of GCL that contained a chemically modified bentonite using a mixture of 1.3 mM CsCl and 1000 mM NaOH solution representing the aluminum refining leachate. Permeation of GCL with a hyperalkaline solution resulted in a higher concentration of aluminum ions in the effluent, indicating the dissolution of clay minerals. Though the swelling was suppressed, it retained a low k value because of the precipitation of hydrous silicate phases that helped in occluding the pores. This indicates that the low swelling of treated clays does not imply a high permeability. Athanassopoulos et al. [75] reported superior hydraulic performance (1 \(\times\) 10–11 m s−1) of polymer-modified GCLs when permeated with hyperalkaline solutions viz., trona ash leachate (pH = 11 and ionic strength = 1050 mM) and bauxite residue liquor (pH = 13 and ionic strength = 2350 mM). The k value of treated GCLs was three orders of magnitude lower than that of the untreated GCL upon permeation with bauxite residue liquor. Similarly, permeation with trona ash leachate showed a difference of more than five orders of magnitude in k value relative to the untreated Na-bentonite. Also, CRC exhibited a low k value of ~ 1 × 10–11 m s−1 for leachates produced by coal combustion products (CCP) having ionic strength less than 1000 mM [76]. Further, the k value towards low-level radioactive waste leachate was found to be as low as ~ 1 × 10–10 m s−1 [77]. Based on the above findings, it can be inferred that the chemical modification of clays has resulted in enhanced hydraulic performance.
In contradiction to the above discussion, few studies have reported the inferior performance of CRC-based GCLs. Ashmawy et al. [78] investigated the hydraulic performance of CRC-based GCLs for three leachates derived from incinerator ash landfills. In this study, both treated and untreated bentonite exhibited a large k value ranging from 1 × 10−5 to 1 × 10–9 m s−1. Also, permeation with synthetic leachate simulating the chemical composition of acid drainage generated from copper and zinc ores caused the k value of CRC-based GCL to increase by a factor of 200 to 7600 relative to the k value towards groundwater [79]. Despite the limited improvement, prehydration was found to enhance the hydraulic resistance of CRC-based GCLs. It has also been reported in a recent study by Chen et al. [80] that the prehydration caused the k value to decrease from 5.0 × 10−7 to 4.3 × 10–11 m s−1 when permeated with trona leachate. Pore clogging was found to be the primary mechanism for rendering a low k value in CRC-based GCLs.
Some studies showed promising results of CRC-based GCLs for barrier application, while a few others had reported comparable performance as that of the untreated GCLs. This could be ascribed to the limited polymer content, which is less than the threshold limit required for providing adequate chemical modification. Also the difference arising from the proprietary types of polymer used for modifying the bentonite contributes to a great extent. However, the mechanism of improved performance of CRCs is not completely understood due to the unknown chemical composition of the polymer involved in the modification of clays. Further, the hydraulic performance of CRC-based GCLs reported in various studies cannot be directly compared given the uncertainty regarding the clay–polymer interactions that occur under the diverse chemical environmental conditions considered in these studies.
Bentonite Polymer Nanocomposite
It is evident from the above discussion that the clay treated with polymer has the propensity to improve bentonite’s hydraulic performance. However, factors such as the selection of suitable polymer, type of modification method, polymer adsorption, and so on have contributed to the limited performance of modified bentonite. Recently, a new type of modified bentonite has been developed, named bentonite polymer nanocomposite (BPN), to retain a low k value under aggressive chemical conditions. Trauger and Darlington [81] were the first to introduce the polymer-modified clay named bentonite polymer alloy (BPA), produced by polymerizing an organic solution within the bentonite slurry. The modified bentonite is referred to as BPN since the treatment method resembled the manufacturing of polymer nanocomposite, where the change was expected to occur at the nanoscale. The geotextile impregnated with BPA showed a low k value (5 × 10–12 m s−1) for seawater, which is approximately four orders of magnitude lower than the conventional GCL (2 × 10–8 m s−1). The study was further extended by Scalia et al. [82], where the hydraulic performance of BPN was investigated, which was produced by in-situ polymerization of acrylic acid within the bentonite slurry. It is also referred to as bentonite polymer composite (BPC) or polymerized bentonite. For producing this, Na-bentonite was first mixed with a neutral solution of acrylic acid and sodium hydroxide at about 30–50% by weight, followed by the addition of sodium persulfate (initiator). The mixture was agitated vigorously to increase the surface area for polymer adsorption. Polymerization was then commenced by elevating the temperature of the mixture above the decomposition temperature of the initiator. The free radicals generated by the initiator tend to attack the double bond of the acrylic monomer to generate further radicals for propagating the polymer chain. After the reaction was complete, the mixture was oven-dried at 105 °C, milled, and screened till its particle size distribution matches with the untreated bentonite present in GCLs. The polymer produced by this method is a superabsorbent polymer (SAP) named sodium polyacrylate, and it tends to form a polymer hydrogel upon hydration [83]. SAP possesses a high swelling capacity rendered by hydrophilic groups (COO−) present in the polymer chain and associates with bentonite through hydrogen bonding. It is relatively inexpensive, non-biodegradable, and similar to the ones used in baby diapers [84]. Na-bentonite treated with SAP was more resistant and less permeable to high ionic strength solutions [82, 85]. Irrespective of concentration, polymer-treated GCL maintained a low k value for CaCl2 solutions having concentrations ranging from 50 to 500 mM [86]. Compared to the conventional bentonite, a difference of approximately four orders of magnitude was observed upon permeation with 50 mM CaCl2 solution. A similar difference in k value was observed when BPN was permeated with a synthetic permeant representing the ocean water [87].
BPN was also shown to exhibit superior performance in various aggressive chemical environments. Tian and Benson [88] conducted permeability tests on GCLs containing BPC using bauxite liquor (pH = 13, ionic strength = 700 mM) as the permeant. The k value of conventional GCL increased from 3.4 × 10−11 to 1.5 × 10–7 m s−1 during permeation, whereas BPC maintained a low k value of 4.3 × 10–12 m s−1, which is five orders lower than the former. Scalia et al. [85] investigated the hydraulic behaviour of BPN permeated with hyperacidic (1000 mM HNO3 having pH = 0.3) and hyperalkaline solutions (1000 mM NaOH having pH = 13.1). Na-bentonite was found to exhibit a large k value in the range between ~ 3 × 10–7 and 5 × 10–7 m s−1. In contrast, BPN had a k value ranging from 1 × 10−11 to 4 × 10–11 m s−1, which is less permeable by more than three orders of magnitude. Permeation of GCL containing BPC loaded with 12.7% polymer to CCP leachate resulted in the permeability of two to three times lower than the permeability of BPC to DI water [80]. Tian et al. [77] measured the k value of GCL encompassing 90% sodium bentonite and 10% polymerized bentonite with low-level radioactive waste synthetic leachate. As expected, it proved its excellence by retaining a low k value of ~ 5 × 10–12 to 6 × 10–12 m s−1. Besides, polymerized bentonite showed improved hydraulic performance, superior membrane behaviour, and remarkable diffusion characteristics compared to natural bentonite [89,90,91].
Scalia and Benson [92] assessed the effect of the treatment method by evaluating the k value of wet and dry blended mixtures of Na-bentonite and sodium polyacrylate for the permeants such as 5 mM-, 50 mM-, and 500 mM-CaCl2 solutions. BPC produced by the wet method maintained high swell and low k value (1.6 × 10–10 m s−1) compared to that of the dry blended composite (6.1 × 10–8 m s−1). This variation was primarily attributed to the changes associated with the fabric structure induced during the process of mixing and it signifies the strong influence of polymer properties and treatment methods on clay–polymer interaction [93]. BPC in DI water swelled more than two times the swell of the Na-bentonite. The swell index was found to decrease with the increasing concentration of CaCl2 solution, and the values were comparable to the swell of calcium bentonite (8–10 mL 2 g−1) at concentrations greater than 200 mM [85]. Similar to other modified bentonites, polymerized bentonite exhibited a low k value to aggressive permeants despite their low swell, signifying that the k value of modified bentonites cannot be ascertained by knowing their swelling behaviour alone [82, 85, 87, 88, 94]. This indicates that the hydraulic behaviour of modified bentonite is not only ascertained by swelling but also by the clay–polymer interaction that plays a key role in deciding the performance of BPC. Trauger and Darlington [81] hypothesized that polymer intercalated in the clay interlayer tends to prevent the escape of sodium cations from the exchangeable sites and is responsible for maintaining the high swell and low k value in polymer-treated bentonites. However, Scalia et al. [85] reported a complete exchange of sodium cations for calcium ions after the permeation of the CaCl2 solution. Also, the results of X-ray diffraction (XRD) analysis showed no change in the crystal lattice spacing of bentonite upon treatment, which indicates the inability of polyacrylate to intercalate owing to the large hydrated radius [95]. Later, the mechanism controlling the hydraulic behaviour of polymerized bentonite was identified as the polymer hydrogel, which clogged the intergranular pore space of bentonite to impart a lower permeability [85, 88, 94, 96]. Hence it can be concluded that compared to Na-bentonite, the hydraulic performance of BPC appears to be promising, suggesting its usefulness as an alternative barrier material.
Given this, Scalia et al. [85] observed polymer elution from BPC, a concern upon permeation with high ionic strength solutions. The amount of polymer eluted was found to decrease with the increasing CaCl2 concentration. However, Tian et al. [94] identified an increase in polymer elution with the increasing CaCl2 concentration upon permeation with 20–500 mM CaCl2 solution. A possible reason for these contradicting observations could be the use of different polymers employed in GCLs in the above studies, which contribute to different interactions with the clay surface in a given chemical environment. These findings strongly indicate the influence of permeant chemistry on polymer conformation and its interaction with bentonite [84]. Besides having all the above discussion, it is believed that the persistent attention paid to studies on polymerized bentonite is envisaged to spur development for future advancements. In view of witnessing this, different formulations of chemical solutions have been developed recently in producing polymerized bentonites, and they have evidenced promising results for barrier applications [97,98,99,100].
Relationship Between the Swell Index and Permeability of Modified GCLs
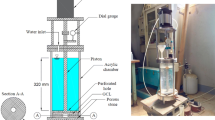
The free swell index is one of the widely used index properties of GCL as they reflect the chemical compatibility between the Na-bentonite present in the GCL and the permeants of interest. The swell index of bentonite is determined as per the ASTM D5890 codal provisions [101]. For testing, 2 g of oven-dried mass of bentonite that is ground to pass 100% through a 150-µm sieve with a minimum of 65% passing a 75-µm sieve is used. The bentonite is added in increments of 0.1 g at 10-min intervals into a 100 mL measuring cylinder that was initially filled with 90 mL of the test solution. After the last addition, the sides of the cylinder are rinsed and filled up to 100 mL using the same test solution. After 24 h, the volume in mL corresponding to the top surface of the settled clay mineral is measured. This value of swollen clay volume in mL for the given 2 g is reported as the free swell index and its unit is noted as mL 2g−1.
As the time required for conducting permeability tests is longer, the swell index is used as an indicator for ascertaining the hydraulic behaviour of GCLs, indirectly. Also, the swell index can be used as an indicator for the quick assessment of permeability testing. However, the true hydraulic behaviour cannot be ensured until performing the permeability tests because the swell index alone may not be an appropriate parameter for predicting its hydraulic behaviour. The Na-bentonites with high swelling capacity are generally associated with a low k value since the k value is a function of the thickness of DDL and the interlattice spacing [1, 43]. Given this, Katsumi et al. [7] and Katsumi et al. [43] have correlated the swell index of bentonite and the permeability of non-prehydrated GCLs for the permeation of a wide range of polar inorganic solutions tested at lower confining stresses (20–30 kPa), as shown in Eqs. 1 and 2, respectively, as follows:
where k value is the permeability of GCL (m s−1) and SI is the swell index of bentonite (mL 2 g−1) obtained by employing the corresponding test solution used for permeability. It can be inferred from the above relationships that the higher the swell index the lower the k value. Incidentally, Liu et al. [102] have demonstrated the applicability of the relationship developed by Katsumi et al. [43], Eq. 2, for the prehydrated GCLs tested over a wide range of effective stresses (35–200 kPa). These relationships predict the hydraulic performance of GCLs for a wide range of polar inorganic permeants, the mineralogical composition of the bentonite, and gradation of bentonite used for manufacturing GCLs. In general, for polymer-modified bentonites, no such definite relationships were reported in the literature. However, few studies have reported the variation of k value with the swell index for BPN, but no correlation was advocated. Scalia et al. [82], Scalia et al. [85], Scalia and Benson [87] have reported that the BPN maintained a low k value even at a low swell index. The k value was found to be consistently less regardless of the magnitude of the swell index. This implies that a lower swell index does not always correspond to a larger permeability. Further, Wireko et al. [103] attempted to develop a relationship between the swell index and k value of CRC-based GCLs using a power law, as illustrated in Eq. 3.
where k value is the permeability (m s−1) and SI is the swell index of bentonite (mL 2 g−1) obtained by employing the corresponding test solution used for permeability. Till date, no attempts have been made to develop a correlation between the swell index and the permeability of polymer-treated GCLs by considering the diverse permeants, type of bentonite, type of polymer used, polymer content, and effective stress. In view of the above discussion, the present study attempted to comprehend the variation of the k value of different polymer-amended bentonite-based GCLs with their corresponding swell index. For this purpose, the published data in the literature are collected for each polymer-modified bentonite and for the sake of completeness, the same is presented in Online Resource 1. Further, non-linear regression analysis was performed on the collected data to obtain the relationship, as shown in Eq. 4 [7]. The efficacy of the developed relationship (Eq. 4) was assessed in terms of its goodness of fit and the summary of the results is illustrated in Table 1.
where k value is the permeability (m s−1) of GCL, SI is the swell index of bentonite (mL 2 g−1) obtained by employing the corresponding test solution used for permeability, k value* is the constant k value corresponding to the large values of SI. A, B and k values* are the fitting parameters. These parameters which describe the shape of the fit curve illustrate the variation of SI with k value. The steepness of the initial portion of the curve is governed by the parameter A. The parameter B is defined such that the k value always corresponds to the positive value of the swell index, i.e., SI > 0 and the parameter, k value* describes the asymptotic nature of the curve. In Eqs. 1–4, the k value and SI were measured using the same permeant. The results of untreated Na-bentonite-based GCLs were not used for fitting; however, these values were superimposed while presenting the variation of permeability of modified GCLs with swell index. This exercise was performed to demonstrate the superior hydraulic performance of modified bentonites compared to the untreated Na-bentonite. The above described relationship between the permeability and swell index can be used as a precursor for the quick assessment of the hydraulic behaviour and a surrogate for the selection of the modified bentonites required for a specific application. Besides, the swell index can be used to check if the clay is susceptible to likely increase in the k value or not when it is exposed to the permeant of interest.
Figure 2 shows the variation of k value of organoclay-based GCLs with the swell index for a wide range of permeants having varied dielectric constants. The R2 value close to one indicates a strong correlation between the swell index and the k value (Table 1), which in turn suggests the adequacy of using the swell index of organoclay as an index property for ascertaining the permeability of organoclay-based GCLs. However, its applicability on the lower range of the swell index is limited. The following three regions of k value were observed: low, intermediate and high. In the lower region, the k value was less than 1 × 10–9 m s−1 for which the swell index was greater than 7 mL 2 g−1. The data points falling in this region belong to the permeants such as gasoline, crude oil, jet fuel, diesel, dioctyl phthalate, etc. In the intermediate region, the k value varied between 1 × 10−4 and 1 × 10–9 m s−1 and the permeants that fall in this category are relatively polar alcohols such as ethanol, methanol and phenol. In the higher region, the k value was found to be very large (> 1 × 10–4 m s−1) for which the permeant tested was water. Incidentally, the dielectric constant (ε) of the permeant varied as ε < 5, 10 to 40, and ≥ 80 in the lower, intermediate and higher k value regions, respectively. In general, ε is one of the major influencing factors of the permeating fluid in determining the k value. Given this, the present study attempted to develop a relationship between ε of permeant and the k value of polymer-amended bentonite-based GCLs as shown in Eqs. 5 and 6.
where C, D, E and F are the fitting parameters.
From Figs. 2 and 3, it is observed that as ε of the permeant declined, the k value was decreased with the increase in the swell index in the case of organoclay-based GCLs, whereas in the case of untreated Na-bentonite, a reverse trend was noticed, as reported by Goodarzi et al. [104] and Mortezaei and Karimpour-Fard [105]. The observed variation in the organoclays can be attributed to the surface alteration upon treatment with organic modifiers, which favoured organic adsorption onto the clay surface and transformed them into more hydrophobic. On the contrary, the permeation of water resulted in a large k value of organoclay owing to the poor interaction between the highly polar permeant and the hydrophobic clay surface. When the permeant was changed from highly polar water to relatively non-polar alcohols, the k value of organoclay was reduced by two to three orders of magnitude. A significant decrease in the k value was noticed when ε became two, which represents the non-polar liquids. The k value of organoclay is related to ε of the permeant as per Eq. 5, and it was found to exhibit a good correlation, as illustrated in Table 2.
Similar attempts were made to develop correlations concerning the hydraulic performance of MSB-based GCLs. As illustrated in Fig. 4, the k value of MSB showed a strong correlation with the swell index evidencing a high R2 value (Table 1). When the permeant happens to be water, the k value was less than 1 × 10–10 m s−1. As the molar concentration of the permeant, as well as the valency of ions present in it, is increased, an increasing trend in the k value with the reducing swell index was observed. This behaviour is similar to that of untreated Na-bentonite and hence the hydraulic behaviour of MSB-based GCLs can be corroborated using DDL theory. The k value varies exponentially with the swell index for values less than 20 mL 2 g−1 and is in agreement with the results reported by Liu et al. [102]. The effect of increased concentration and valency of the permeating solution was reflected in the dielectric properties of the permeant. The dielectric constant of the permeant is decreased when the concentration and valency of the cation are increased. Hence the increased k value is visualized in terms of reduced ε of the permeant, as shown in Fig. 5. The k value of MSB for NaCl solution exhibited a good relationship with ε of the permeant as per Eq. 5 (Table 2) for the concentration ranging from 100 to 2000 mM and its corresponding ε varied from 55 to 77. As the dielectric constant of the permeant reduces, the repulsive force acting between the clay particles decreases. This leads to the formation of a flocculated clay structure and a thin DDL resulting in a high k value [21, 22]. A similar correlation as that of NaCl solution with a greater R2 value (Table 2) was observed for CaCl2 solution; however, it is limited for concentrations less than 500 mM and for ε of 68 to 77 owing to the dominant influence of both increased concentration and valence over dielectric effect, which is reflected in the slope of the fitted curve. It is to be noted that the obtained trend is based on the limited data available for the CaCl2 solution.
Relationship between the permeability of multi-swellable bentonite and the dielectric constant for NaCl and CaCl2 permeants having varied concentrations. The encircled cross marks denote the points of CaCl2 solution having concentration ≥ 500 mM, which is not used for fitting (Data collected from [43, 47, 48, 50, 106, 107])
The k value of DPH clay and HYPER clay is shown in Fig. 6 as a function of the swell index. The data of both DPH clay and HC were not fitted as there were only limited data points pertaining to the types of permeants and the range of concentrations reported. The data plotted for HC corresponds to the results of the tests conducted with solutions of only DI water, 5 mM CaCl2 and seawater. The k value of HC increased marginally with the decrease in the swell index. However, the k value was found to be less than 1 × 10–10 m s−1 for all the values of the swell index. In the case of DPH clays, a low k value (< 1 × 10–11 m s−1) was observed even though the swell index was less than 10 mL 2 g−1. Regardless of the type and concentration of permeant, DPH clay witnessed a k value less than 1 × 10–10 m s−1. Even though the regression analysis was not performed for the entire range of swell index, the data points of both HC and DPH clay appear to fall on the lower end of the potential fit based on Eq. 4. Therefore, it can be concluded that to capture the true dependence of the swell index for the above clays, more tests need to be conducted using permeants of different types and over a wide range of concentrations. Given this, the anticipated k value for the low swell index may fall in the shaded region of the curve. With the existing data, the swell index appears to be insensitive in capturing the potential hydraulic behaviour of HC and DPH clays, whereas from Fig. 7, it is interesting to note that the k value of both HC and DPH clay is strongly correlated to ε of the permeant based on Eq. 5 and the fitted parameters are shown in Table 2. The above inference is made based on the fit curve containing limited data points. However, the value of ε considered in this relation comprises a wide range (5–80) and envelops various permeants.
Figure 8 shows the variation of k value of CRC-based GCLs with the swell index indicating a strong correlation witnessing a high R2 value of 0.91 (Table 1). It can be seen that the data are scattered on the higher and lower ends of the fitting curve regardless of the type of permeant. This can be attributed to the difference in the polymer content used for modifying the bentonite. On the higher end, large k values are observed owing to the less polymer dose (≤ 5%) used in CRC-based GCLs, whereas on the lower side, low k values (< 1 × 10–10 m s−1) are detected even for the swell index < 9 mL 2 g−1 and it is mainly ascribed to the high polymer content (≥ 6%) used for manufacturing CRCs. Overall, the k value of CRC varied exponentially with the swell index for values less than 10 mL 2 g−1 irrespective of the type of permeant, bentonite and polymer. As ε of the permeant used for testing the k value of CRC is unknown, no correlation was developed; however, the variation of k value with ε is shown in Fig. 9 for water and 300 mM Na+ solution with different anion ratios. Further research is still needed to establish the relationship for CRC-based GCLs based on ε of the permeant.
The dependency of the k value on the swell index of BPN is illustrated in Fig. 10. A similar trend as that of other modified bentonites was observed. There was a strong relationship between the swell index and k value of BPN regardless of the type of permeant, type of polymer and type of bentonite used for manufacturing GCLs (Table 1). Figure 11 shows the relationship between the k value of BPN and ε of CaCl2 solution correlated by Eq. 6, where the concentration of CaCl2 solution ranges from 5 to 600 mM. Compared to other polymer-modified bentonites, BPN had a relatively low R2 value (Table 2). As ε is not known for other permeants used for testing the k value of BPN and also owing to limited data, they were not included for the present analysis. The relationship is valid only for concentrations less than 500 mM where ε varied from 70 to 80, beyond which the combined effect of concentration and valency of permeant plays a significant role in affecting the k value. Hence, it can be inferred that ε can be a suitable indicator for assessing the k value for ionic solutions having low valency cations and concentrations.
Variation of permeability of bentonite polymer nanocomposite with the dielectric constant of CaCl2 solution having varied concentrations. The encircled cross marks denote the points of CaCl2 solution having concentration ≥ 500 mM, which is not used for fitting (Data collected from [82, 85, 94, 100, 107])
Summary and Conclusions
Polymer-modified GCLs have been developed in the past few decades to overcome the drawbacks of conventional Na-bentonite and to meet the growing demand for geoenvironmental applications. The evolution of modified clays has spurred considerable momentum of research in the arena of barrier applications and has invoked the potential of new aged GCLs for future applications. Given this, a comprehensive review of various polymer-modified GCLs was discussed concerning their hydraulic performance. The mechanism contributing to their improved hydraulic properties was described. Further, the limitations associated with their usage were addressed with an emphasis on a need for future development. In addition, the relationship between the permeability and swell index of various polymer-modified bentonites was investigated and correlations based on the dielectric constant of the permeant were proposed. Based on the critical review of the literature and the interpretation of results of the proposed relationships, the following conclusions can be drawn:
-
The modification of smectite-based clays using polymers has resulted in superior hydraulic performance in terms of swelling and permeability. The degree of enhancement varied depending on the polymer content, polymer composition, and clay–polymer–permeant interaction.
-
The mechanism responsible for the enhanced hydraulic performance of polymer-treated GCLs is primarily ascribed to the activation of osmotic swell by polymer intercalation and pore clogging phenomenon by polymer hydrogel. However, the consequent changes in terms of macro-engineering properties of polymer-treated GCLs are not completely addressed.
-
Given this uncertainty, an empirical relationship was developed between the swell index and the k value of various polymer-treated GCLs by considering a wide range of permeants. The swell index is inferred to be a suitable index property for studying the effect of permeants on the k value only when it is > 20 mL 2g−1. However, it should be used with caution for assessing the chemical compatibility of polymer modified GCLs owing to the ambiguities associated with the k value at a lower swell index (< 20 mL 2g−1).
-
Considering this, the study advocated the use of the dielectric constant of the permeant for evaluating the k value of treated GCLs through the formulated empirical relationships. A strong correlation suggested that the dielectric constant can be an alternative indicator for the indirect assessment of the k value.
-
Nevertheless, these correlations are applicable for monovalent permeants (NaCl) having concentrations ranging from 100 to 2000 mM and for divalent permeants (CaCl2) having concentrations less than 500 mM, which corresponds to the values of dielectric constant comprising a narrow range of 55 to 77 and 68 to 77, respectively. Their limited range of use can be attributed to the increased significance of concentration and valence effect over the dielectric properties.
-
The proposed correlations presented in this study can be considered as a surrogate for the initial guidance of selection of the modified bentonite. However, the true hydraulic behaviour of polymer-modified bentonites needs to be evaluated by performing permeability testing.
-
As there were only limited data available in the literature concerning the dielectric constant of permeants and the surface chemistry of the permeating media, systematic investigations are required for validating the proposed relationships. Further, the reviewed literature suggests that the hydraulic performance of treated GCLs may be impaired in the long run owing to the endurance characteristics of the polymer. Therefore, the durability of polymer-modified GCLs needs to be established by contemplating the long-term chemical compatibility testing protocols.
Overall, the polymer-modified GCLs are all still at the research level, and hence intense testing based on the site-specific requirements is recommended. More experimental investigations are required for deepening the understanding of the performance of innovative barriers that can assist in engineering a suitable material for a specific application.
Data Availability
All data used in the study are included in the submitted article.
References
Jo HY, Katsumi T, Benson CH, Edil TB (2001) Hydraulic conductivity and swelling of nonprehydrated GCLs permeated with single-species salt solutions. J Geotech Geoenviron Eng 127(7):557–567. https://doi.org/10.1061/(ASCE)1090-0241(2001)127:7(557)
Hornsey WP, Scheirs J, Gates WP, Bouazza A (2010) The impact of mining solutions/liquors on geosynthetics. Geotext Geomembr 28(2):191–198. https://doi.org/10.1016/j.geotexmem.2009.10.008
Di Emidio G, Verástegui-Flores RD, Van Impe WF, Bezuijen A (2012) Recent development of treated clays for geosynthetic clay liners and barriers systems. In: Proceedings of the 5th European Geosynthetic Congress (Eurogeo 5), Valencia, Spain, 16(9):124–128
Bouazza A, Gates WP (2014) Overview of performance compatibility issues of GCLs with respect to leachates of extreme chemistry. Geosynthet Int 21(2):151–167. https://doi.org/10.1680/gein.14.00006
Chen J, Benson C, Edil T, Likos W (2018) Hydraulic conductivity of geosynthetic clay liners with sodium bentonite to coal combustion product leachates. J Geotech Geoenviron Eng 144(3):1–12. https://doi.org/10.1061/(ASCE)GT.1943-5606.0001844
Shackelford CD, Benson CH, Katsumi T, Edil TB, Lin L (2000) Evaluating the hydraulic conductivity of GCLs permeated with non-standard liquids. Geotext Geomembr 18(2–4):133–161. https://doi.org/10.1016/S0266-1144(99)00024-2
Katsumi T, Ishimori H, Ogawa A, Yoshikawa K, Hanamoto K, Fukagawa R (2007) Hydraulic conductivity of nonprehydrated geosynthetic clay liners permeated with inorganic solutions and waste leachates. Soils Found 47(1):79–96. https://doi.org/10.3208/sandf.47.79
Dutta J, Mishra AK, Das P (2018) Combined effect of inorganic salts and heavy metals on the engineering behaviour of compacted bentonites. Int J Geosynth Ground Eng 4(2):1–11. https://doi.org/10.1007/s40891-018-0134-x
Chai JC, Shen SL (2018) Predicting swelling behavior of a Na+-bentonite used in GCLs. Int J Geosynth Ground Eng 4(1):1–6. https://doi.org/10.1007/s40891-018-0126-x
Reddy PS, Mohanty B, Rao BH (2020) Influence of clay content and montmorillonite content on swelling behavior of expansive soils. Int J Geosynth Ground Eng 6(1):1–12. https://doi.org/10.1007/s40891-020-0186-6
Shackelford CD, Lee JM (2003) The destructive role of diffusion on clay membrane behavior. Clays Clay Miner 51(2):186–196. https://doi.org/10.1346/CCMN.2003.0510209
Jo H, Benson C, Shackelford C, Lee J, Edil T (2005) Long-term hydraulic conductivity of a geosynthetic clay liner permeated with inorganic salt solutions. J Geotech Geoenviron Eng 131(4):405–417. https://doi.org/10.1061/(ASCE)1090-0241(2005)131:4(405)
Lee JM, Shackelford CD (2005) Impact of bentonite quality on hydraulic conductivity of geosynthetic clay liners. J Geotech Geoenviron Eng 131(1):64–77. https://doi.org/10.1061/(ASCE)1090-0241(2005)131:1(64)
Lin LC, Benson CH (2000) Effect of wet-dry cycling on swelling and hydraulic conductivity of GCLs. J Geotech Geoenviron Eng 126(1):40–49. https://doi.org/10.1061/(ASCE)1090-0241(2000)126:1(40)
Mackey R, Olsta J (2004) Performance of geosynthetic clay liners used in two landfill closures in a coastal area of Florida. Advances in Geosynthetic Clay Liners Technology: 2nd Symposium, STP 1456, R. Mackey and K. von Maugeuge, eds., ASTM International, West Conshohocken, 53–71. https://doi.org/10.1520/STP12198S
Benson CH, Jo HY, Abichou T (2004) Forensic analysis of excessive leakage from lagoons lined with a composite GCL. Geosynthet Int 11(3):242–252. https://doi.org/10.1680/gein.2004.11.3.242
Benson CH, Thorstad PA, Jo HY, Rock SA (2007) Hydraulic performance of geosynthetic clay liners in a landfill final cover. J Geotech Geoenviron Eng 133(7):814–827. https://doi.org/10.1061/(ASCE)1090-0241(2007)133:7(814)
Meer SR, Benson CH (2007) Hydraulic conductivity of geosynthetic clay liners exhumed from landfill final covers. J Geotech Geoenviron Eng 133(5):550–563. https://doi.org/10.1061/(ASCE)1090-0241(2007)133:5(550)
Scalia J IV, Benson CH (2010) Effect of permeant water on the hydraulic conductivity of exhumed GCLs. Geotech Test J 33(3):201–211. https://doi.org/10.1520/GTJ102609
Ma Z, Gamage RP, Rathnaweera T, Kong L (2019) Review of application of molecular dynamic simulations in geological high-level radioactive waste disposal. Appl Clay Sci 168:436–449. https://doi.org/10.1016/j.clay.2018.11.018
Zhang T, Deng Y, Cui Y, Lan H, Zhang F, Zhang H (2019) Porewater salinity effect on flocculation and desiccation cracking behaviour of kaolin and bentonite considering working condition. Eng Geol 251:11–23. https://doi.org/10.1016/j.enggeo.2019.02.007
Jadda K, Bag R (2020) Variation of swelling pressure, consolidation characteristics and hydraulic conductivity of two Indian bentonites due to electrolyte concentration. Eng Geol 272:105637. https://doi.org/10.1016/j.enggeo.2020.105637
Chiou CT, Porter PE, Schmedding DW (1983) Partition equilibria of non-ionic organic organic-compounds between soil organic-matter and water. Environ Sci Technol 17(4):227–231. https://doi.org/10.1021/es00110a009
Bate B, Burns SE (2010) Effect of total organic carbon content and structure on the electrokinetic behavior of organoclay suspensions. J Colloid Interface Sci 343(1):58–64. https://doi.org/10.1016/j.jcis.2009.11.009
Gitipour S, Hosseinpour MA, Heidarzadeh N, Yousefi P, Fathollahi A (2015) Application of modified clays in geosynthetic clay liners for containment of petroleum contaminated sites. Int J Environ Res 9(1):317–322. https://doi.org/10.22059/IJER.2015.903
Abbas A, Sallam AS, Usman AR, Al-Wabel MI (2017) Organoclay-based nanoparticles from montmorillonite and natural clay deposits: synthesis, characteristics, and application for MTBE removal. Appl Clay Sci 142:21–29. https://doi.org/10.1016/j.clay.2016.11.028
Chanra J, Budianto E, Soegijono B (2019) Surface modification of montmorillonite by the use of organic cations via conventional ion exchange method. In IOP Conference Series: Materials Science and Engineering, Semarang, Indonesia, 509(1). https://doi.org/10.1088/1757-899X/509/1/012057
Koh SM, Dixon JB (2001) Preparation and application of organo-minerals as sorbents of phenol, benzene and toluene. Appl Clay Sci 18(3–4):111–122. https://doi.org/10.1016/S0169-1317(00)00040-5
Sreedharan V, Sivapullaiah PV (2012) Evaluation of organically modified clays for geoenvironmental applications. In: Proceedings of GeoCongress: State of the Art and Practice in Geotechnical Engineering, Oakland, California, 1213–1222. https://doi.org/10.1061/9780784412121.125
Gates WP, Nefiodovas A, Peter P (2004) Permeability of an organo-modified bentonite to ethanol-water solutions. Clays Clay Miner 52(2):192–203. https://doi.org/10.1346/CCMN.2004.0520205
Gitipour S, Abolfazlzadeh M, Givehchi S (2008) Geo-environmental characteristics of modified and ordinary bentonitic soils exposed to MTBE. Int J Environ Studies 65(4):595–601. https://doi.org/10.1080/00207230701382016
Richards S, Bouazza A (2007) Phenol adsorption in organo-modified basaltic clay and bentonite. Appl Clay Sci 37(1–2):133–142. https://doi.org/10.1016/j.clay.2006.11.006
Lee S, Ören AH, Benson CH, Dovantzis K (2012) Organoclays as variably permeable reactive barrier media to manage NAPLs in ground water. J Geotech Geoenviron Eng 138(2):115–127. https://doi.org/10.1061/(ASCE)GT.1943-5606.0000572
Benson CH, Jo HY, Musso T (2015) Hydraulic conductivity of organoclay and organoclay-sand mixtures to fuels and organic liquids. J Geotech Geoenviron Eng 141(2):04014094. https://doi.org/10.1061/(ASCE)GT.1943-5606.0001194
Zhao Q, Choo H, Bhatt A, Burns SE, Bate B (2017) Review of the fundamental geochemical and physical behaviors of organoclays in barrier applications. Appl Clay Sci 142:2–20. https://doi.org/10.1016/j.clay.2016.11.024
Jemima WS, Magesan P, Chiranjeevi P, Umapathy MJ (2019) Sorption properties of organo modified montmorillonite clay for the reclamation of chromium (VI) from waste water. SILICON 11(2):925–933. https://doi.org/10.1007/s12633-018-9887-z
Headley JV, Boldt-Leppin BE, Haug MD, Peng J (2001) Determination of diffusion and adsorption coefficients for volatile organics in an organophilic clay-sand-bentonite liner. Can Geotech J 38(4):809–817. https://doi.org/10.1139/t01-017
Lake CB, Rowe RK (2005) A comparative assessment of volatile organic compound (VOC) sorption to various types of potential GCL bentonites. Geotext Geomembr 23(4):323–347. https://doi.org/10.1016/j.geotexmem.2005.01.001
Lo IM, Yang X (2001) Use of organoclay as secondary containment for gasoline storage tanks. J Environ Eng 127(2):154–161. https://doi.org/10.1061/(ASCE)0733-9372(2001)127:2(154)
Lorenzetti RJ, Bartelt-Hunt SL, Burns SE, Smith JA (2005) Hydraulic conductivities and effective diffusion coefficients of geosynthetic clay liners with organobentonite amendments. Geotext Geomembr 23(5):385–400. https://doi.org/10.1016/j.geotexmem.2005.02.002
Kondo M (1996) Method of activation of clay and activated clay. US Patent Number 5,573,583. US Patent and Trademark Office. https://patents.google.com/patent/US5573583A/en
Katsumi T, Ogawa A, Fukagawa R (2004) Effect of chemical solutions on hydraulic barrier performance of clay geosynthetic barriers. In: Proceedings of the 3rd European Conference on Geosynthetics, Munich, Germany, 701–706
Katsumi T, Ishimori H, Onikata M, Fukagawa R (2008) Long-term barrier performance of modified bentonite materials against sodium and calcium permeant solutions. Geotext Geomembr 26(1):14–30. https://doi.org/10.1016/j.geotexmem.2007.04.003
Gates WP, Shaheen U, Turney TW, Patti AF (2016) Cyclic carbonate–sodium smectite intercalates. Appl Clay Sci 124:94–101. https://doi.org/10.1016/j.clay.2016.02.005
Mazzieri F, Van Impe PO, Di Emidio G (2005) Chemico-osmotic behaviour of modified “Multiswellable” bentonite. In: Proceedings of the 16th International Conference on Soil Mechanics and Geotechnical Engineering, Osaka, Japan, 16(4):2297–2300. https://doi.org/10.3233/978-1-61499-656-9-2297
Onikata M, Kondo M, Hayashi N, Yamanaka S (1999) Complex formation of cation-exchanged montmorillonites with propylene carbonate: Osmotic swelling in aqueous electrolyte solutions. Clays Clay Miner 47(5):672–677. https://doi.org/10.1346/CCMN.1999.0470514
Mazzieri F, Pasqualini E (2006) Evaluating the permeability of an organically modified bentonite to natural seawater. In 5th ICEG Environmental Geotechnics: Opportunities, Challenges and Responsibilities for Environmental Geotechnics: Proceedings of the ISSMGE’s fifth international congress, Cardiff, United Kingdom, Thomas Telford Publishing, 749–756
Fehervari A, Gates WP, Patti AF, Turney TW, Bouazza A, Rowe RK (2016) Potential hydraulic barrier performance of cyclic organic carbonate modified bentonite complexes against hyper-salinity. Geotext Geomembr 44(5):748–760. https://doi.org/10.1016/j.geotexmem.2016.06.002
Katsumi T, Fukagawa R (2005) Factors affecting chemical compatibility and barrier performance of GCLs. In: Proceedings of the 16th International Conference on Soil Mechanics and Geotechnical Engineering, Millpress Science Publishers, Rotterdam, Netherlands, 2285–2288. https://doi.org/10.3233/978-1-61499-656-9-2285
Mazzieri F, Di Emidio G, Van Impe PO (2010) Diffusion of calcium chloride in a modified bentonite: impact on osmotic efficiency and hydraulic conductivity. Clays Clay Miner 58(3):351–363. https://doi.org/10.1346/CCMN.2010.0580306
Ruehrwein RA, Ward DW (1952) Mechanism of clay aggregation by polyelectrolytes. Soil Sci 73(6):485–492. https://doi.org/10.1097/00010694-195206000-00007
Di Emidio G (2010) Hydraulic conductivity and chemico-osmotic performance of polymer treated clays. Ph.D. Dissertation, University of Gent, Belgium
Michaels AS, Morelos O (1955) Polyelectrolyte adsorption by kaolinite. Ind Eng Chem 47(9):1801–1809. https://doi.org/10.1021/ie50549a029
Deng Y, Dixon J, White G (2006) Bonding between polyacrylamide and smectite. Colloids Surf 281(1):82–91. https://doi.org/10.1016/j.colsurfa.2006.02.030
Tate KR, Theng BKG (1980) Organic matter and its interactions with inorganic soil constituents In: Soils with variable charge, Soil Bureau Department of Scientific and Industrial Research, New Zealand, 225–249
Heller H, Keren R (2003) Anionic polyacrylamide polymer adsorption by pyrophyllite and montmorillonite. Clays Clay Miner 51(3):334–339. https://doi.org/10.1346/CCMN.2003.0510310
Qiu H, Yu J (2008) Polyacrylate/(carboxymethylcellulose modified montmorillonite) superabsorbent nanocomposite: preparation and water absorbency. J Appl Polym Sci 107(1):118–123. https://doi.org/10.1002/app.26261
Di Emidio G, Van Impe WF, Flores RV (2011) Advances in geosynthetic clay liners: polymer enhanced clays. In: Proceedings of the Geo-Frontiers - Advances in Geotechnical Engineering, Dallas, Texas, 1931–1940. https://doi.org/10.1061/41165(397)197
Di Emidio G, Mazzieri F, Verastegui-Flores RD, Van Impe W, Bezuijen A (2015) Polymer-treated bentonite clay for chemical-resistant geosynthetic clay liners. Geosynthet Int 22(1):125–137. https://doi.org/10.1680/gein.14.00036
De Camillis M, Di Emidio G, Bezuijen A, Flores DV, Van Stappen J, Cnudde V (2017) Effect of wet-dry cycles on polymer treated bentonite in seawater: swelling ability, hydraulic conductivity and crack analysis. Appl Clay Sci 142:52–59. https://doi.org/10.1016/j.clay.2016.11.011
De Camillis M, Di Emidio G, Bezuijen A, Flores DV (2016) Wet and dry effects on the hydraulic conductivity of a polymer treated GCL prototype. In: Proceedings of the Geo-Chicago, Chicago, US, 518–527. https://doi.org/10.1061/9780784480144.051
Flynn BN, Carter GC (1998) Waterproofing material and method of fabrication thereof. US Patent Number 6,537,676. US Patent and Trademark Office. https://patents.google.com/patent/US6537676
Malusis MA, Daniyarov AS (2016) Membrane efficiency and diffusive tortuosity of a dense prehydrated geosynthetic clay liner. Geotext Geomembr 44(5):719–730. https://doi.org/10.1016/j.geotexmem.2016.05.006
Kolstad DC, Benson CH, Edil TB, Jo HY (2004) Hydraulic conductivity of a dense prehydrated GCL permeated with aggressive inorganic solutions. Geosynthet Int 11(3):233–241. https://doi.org/10.1680/gein.2004.11.3.233
Mazzieri F, Emidio GD (2015) Hydraulic conductivity of a dense prehydrated geosynthetic clay liner. Geosynthet Int 22(1):138–148. https://doi.org/10.1680/gein.14.00037
Schroeder C, Monjoie A, Illing P, Dosquet D, Thorez J (2001) Testing a factory- prehydrated GCL under several conditions. In: Proceedings of Sardinia 2001: 8th International Waste Management and Landfill Symposium, Cagliari, Italy, 1:187–196
Di Emidio G, Mazzieri F, Van Impe W (2008) Hydraulic conductivity of a dense prehydrated GCL: impact of free swell and swelling pressure. In: Proceedings of the 4th European Geosynthetics Conference, EuroGeo4, Edinburgh, UK
Mazzieri F, Di Emidio G, Fratalocchi E, Di Sante M, Pasqualini E (2013) Permeation of two GCLs with an acidic metal-rich synthetic leachate. Geotext Geomembr 40:1–11. https://doi.org/10.1016/j.geotexmem.2013.07.011
Mazzieri F (2011) Impact of desiccation and cation exchange on the hydraulic conductivity of factory-prehydrated GCLs. In: Proceedings of the Geo-Frontiers —Advances in Geotechnical Engineering, Dallas, Texas, 976–985. https://doi.org/10.1061/41165(397)100
Mazzieri F, Pasqualini E (2008) Effect of dry/wet cycles and cation exchange on the permeability of a dense prehydrated GCL. In: Proceedings of the Fourth International Conference on Geosynthetics-Eurogeo4, Edinburgh, UK
Mazzieri F, Di Emidio G, Pasqualini E (2017) Effect of wet-and-dry ageing in seawater on the swelling properties and hydraulic conductivity of two amended bentonites. Appl Clay Sci 142:40–51. https://doi.org/10.1016/j.clay.2016.10.031
Razakamanantsoa A, Djeran-Maigre I, Barast G (2016) Characterisation of bentonite polymer for bottom liner use. Environ Geotech 3(1):28–35. https://doi.org/10.1680/jenge.13.00095
McKelvey JA (1997) Geosynthetic Clay Liners in Alkaline Environments. Testing and Acceptance Criteria for Geosynthetic Clay Liners, ASTM STP 1308. American Society for Testing and Materials, West Conshohocken, Pennsylvania, 139–149. https://doi.org/10.1520/STP11799S
Benson CH, Ören AH, Gates WP (2010) Hydraulic conductivity of two geosynthetic clay liners permeated with a hyperalkaline solution. Geotext Geomembr 28(2):206–218. https://doi.org/10.1016/j.geotexmem.2009.10.002
Athanassopoulos C, Benson C, Donovan M, Chen J (2015) Hydraulic conductivity of a polymer-modified GCL permeated with high-pH solutions. In: Proceedings of the Geosynthetic Conference, Industrial Fabrics Association International, Portland, Oregon, USA, 181–186
Salihoglu H, Chen JN, Likos WJ, Benson CH (2016) Hydraulic conductivity of bentonite-polymer geosynthetic clay liners in coal combustion product leachates. In: Proceedings of the Geo-Chicago, Chicago, US, 438–447. https://doi.org/10.1061/9780784480144.043
Tian K, Benson CH, Likos WJ (2016) Hydraulic conductivity of geosynthetic clay liners to low-level radioactive waste leachate. J Geotech Geoenviron Eng 142(8):04016037. https://doi.org/10.1061/(ASCE)GT.1943-5606.0001495
Ashmawy AK, El-Hajji D, Sotelo N, Muhammad N (2002) Hydraulic performance of untreated and polymer-treated bentonite in inorganic landfill leachates. Clays Clay Miner 50(5):546–552. https://doi.org/10.1346/000986002320679288
Shackelford CD, Sevick GW, Eykholt GR (2010) Hydraulic conductivity of geosynthetic clay liners to tailings impoundment solutions. Geotext Geomembr 28(2):149–162. https://doi.org/10.1016/j.geotexmem.2009.10.005
Chen J, Salihoglu H, Benson CH, Likos WJ, Edil TB (2019) Hydraulic conductivity of bentonite - polymer composite geosynthetic clay liners permeated with coal combustion product leachates. J Geotech Geoenviron Eng 145(9):04019038. https://doi.org/10.1061/(ASCE)GT.1943-5606.0002105
Trauger R, Darlington J (2000) Next-generation geosynthetic clay liners for improved durability and performance. In: Proceedings of 14th GRI Conference, Geosynthetic Institute, Folsom, PA, USA, 255–267
Scalia J, Benson CH, Edil TB, Bohnhoff GL, Shackelford CD (2011) Geosynthetic clay liners containing bentonite polymer nanocomposite. In: Proceedings of the Geo-Frontiers: Advances in Geotechnical Engineering, Dallas, Texas, 2001–2009. https://doi.org/10.1061/41165(397)204
Piqué TM, Manzanal D, Codevilla M, Orlandi S (2019) Polymer-enhanced soil mixtures for potential use as covers or liners in landfill systems. Environ Geotech 8(7):467–479. https://doi.org/10.1680/jenge.18.00174
Buchholz F, Graham A (1998) Modern superabsorbent polymer technology. Wiley, New York
Scalia J IV, Benson CH, Bohnhoff GL, Edil TB, Shackelford CD (2014) Long-term hydraulic conductivity of a bentonite-polymer composite permeated with aggressive inorganic solutions. J Geotech Geoenviron Eng 140(3):04013025. https://doi.org/10.1061/(ASCE)GT.1943-5606.0001040
Ören AH, Öztürk M, Özdamar Kul T, Nart Z (2019) Barrier performance of geosynthetic clay liners to copper (II) chloride solutions. Environ Geotech 7(7):491–500. https://doi.org/10.1680/jenge.18.00024
Scalia IV J, Benson CH (2014) Barrier performance of bentonite-polyacrylate nanocomposite to artificial ocean water. In: Proceedings of the Geo-Congress: Geo-characterization and Modeling for Sustainability, Atlanta, US, 1826–1835. https://doi.org/10.1061/9780784413272.179
Tian K, Benson CH (2019) Containing bauxite liquor using bentonite-polymer composite geosynthetic clay liners. In: Zhan L, Chen Y, Bouazza A. (eds) In: Proceedings of the 8th International Congress on Environmental Geotechnics, Environmental Science and Engineering, 2, Springer, Singapore, 672–678. https://doi.org/10.1007/978-981-13-2224-2_83
Bohnhoff GL, Shackelford CD (2013) Improving membrane performance via bentonite polymer nanocomposite. Appl Clay Sci 86:83–98. https://doi.org/10.1016/j.clay.2013.09.017
Bohnhoff GL, Shackelford CD (2014) Hydraulic conductivity of polymerized bentonite-amended backfills. J Geotech Geoenviron Eng 140(3):04013028. https://doi.org/10.1061/(ASCE)GT.1943-5606.0001034
Bohnhoff GL, Shackelford CD (2015) Salt diffusion through a bentonite-polymer composite. Clays Clay Miner 63(3):145–162. https://doi.org/10.1346/CCMN.2015.0630301
Scalia IV J, Benson CH (2016) Evaluation of Na-bentonite-polyacrylate mixtures to enhance the chemical resistance of geosynthetic clay liners. In: Proceedings of the Geo-Chicago, Chicago, US, 388–397. https://doi.org/10.1061/9780784480144.038
Lieske W, Steudel A, Di Emidio G, Baille W (2020) Influence of constitution and mixture treatment of cationic polymers on modified bentonite. Environ Geotech 40:1–9. https://doi.org/10.1680/jenge.19.00096 (In press)
Tian K, Likos WJ, Benson CH (2019) Polymer elution and hydraulic conductivity of bentonite—polymer composite geosynthetic clay liners. J Geotech Geoenviron Eng 145(10):04019071. https://doi.org/10.1061/(ASCE)GT.1943-5606.0002097
Scalia J IV, Benson CH (2017) Polymer fouling and hydraulic conductivity of mixtures of sodium bentonite and a bentonite-polymer composite. J Geotech Geoenviron Eng 143(4):04016112. https://doi.org/10.1061/(ASCE)GT.1943-5606.0001628
Tian K, Likos WJ, Benson CH (2016) Pore-scale imaging of polymer-modified bentonite in saline solutions. In: Proceedings of the Geo-Chicago, Chicago, US, 468–477. https://doi.org/10.1061/9780784480144.046
Prongmanee N, Chai JC, Shrestha S (2018) Effect of cations on consolidation and permeability of polymerized bentonite. Lowland Tech Int 20(3):297–304
Prongmanee N, Chai JC (2019) Performance of geosynthetic clay liner with polymerized bentonite in highly acidic or alkaline solutions. Int J Geosynth Ground Eng 5(3):26. https://doi.org/10.1007/s40891-019-0177-7
Yu C, Liao R, Cai X, Yu X (2019) Sodium polyacrylate modification method to improve the permeant performance of bentonite in chemical resistance. J clean prod 213:242–250. https://doi.org/10.1016/j.jclepro.2018.12.179
Chai JC, Prongmanee N (2020) Barrier properties of a geosynthetic clay liner using polymerized sodium bentonite. Geotext Geomembr 48(3):392–399. https://doi.org/10.1016/j.geotexmem.2019.12.010
ASTM D5890 (2019) Standard test method for swell index of clay mineral component of geosynthetic clay liners ASTM D5890. ASTM, West Conshohocken. https://doi.org/10.1520/D5890-19
Liu Y, Bouazza A, Gates WP, Rowe RK (2015) Hydraulic performance of geosynthetic clay liners to sulfuric acid solutions. Geotext Geomembr 43(1):14–23. https://doi.org/10.1016/j.geotexmem.2014.11.004
Wireko C, Zainab B, Tian K, Abichou T (2020) Effect of specimen preparation on the swell index of bentonite-polymer GCLs. Geotext Geomembr 48(6):875–885. https://doi.org/10.1016/j.geotexmem.2020.06.006
Goodarzi AR, Fateh SN, Shekary H (2016) Impact of organic pollutants on the macro and microstructure responses of Na-bentonite. Appl Clay Sci 121:17–28. https://doi.org/10.1016/j.clay.2015.12.023
Mortezaei H, Karimpour-Fard M (2017) Variation of the hydraulic conductivity of clayey soils in exposure to organic permeants. Civil Eng J 3(11):1036. https://doi.org/10.28991/cej-030936
Katsumi T, Onikata M, Hasegawa S, Lin L, Kondo M, Kamon M (2001) Chemical compatibility of modified bentonite permeated with inorganic solutions. In: Proceedings of the 3rd Conference on Geoenvironmental Engineering - Geoenvironmental Impact Management, Edinburgh, London, 419–424
Mollerup JM, Breil MP (2015) Modeling the permittivity of electrolyte solutions. J AICE 61(9):2854–2860. https://doi.org/10.1002/aic.14799
Tian K, Benson CH, Likos WJ (2017) Effect of an anion ratio on the hydraulic conductivity of a bentonite-polymer geosynthetic clay liner. In: Proceedings of Geotechnical Frontiers, Orlando, USA, 180–189
Li Q, Chen J, Benson CH, Peng D (2021) Hydraulic conductivity of bentonite-polymer composite geosynthetic clay liners permeated with bauxite liquor. Geotext Geomembr 49(2):420–429. https://doi.org/10.1016/j.geotexmem.2020.10.015
Acknowledgements
This work was supported by the Prime Minister’s Research Fellows (PMRF) Scheme. The first author would like to acknowledge the financial support from the Ministry of Human Resource Development (MHRD), Government of India.
Author information
Authors and Affiliations
Contributions
Literature review, data curation, formal analysis, and original draft preparation were performed by SK. DNA had validated, visualized, critically reviewed, analysed, and edited.
Corresponding author
Ethics declarations
Conflict of Interests
The authors declare that there is no conflict of interest concerning the content of this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Keerthana, S., Arnepalli, D.N. Hydraulic Performance of Polymer-Modified Bentonites for Development of Modern Geosynthetic Clay Liners: A Review. Int. J. of Geosynth. and Ground Eng. 8, 24 (2022). https://doi.org/10.1007/s40891-022-00368-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40891-022-00368-0